Abstract
Background
Risk-factor identification related to chronic opioid use after surgery may facilitate interventions mitigating postoperative opioid consumption. We evaluated the relationship between opioid use preceding total hip arthroplasty (THA) and total knee arthroplasty (TKA), and chronic use postoperatively, and the risk of chronic opioid use after total joint arthroplasty.
Methods
All primary THAs and TKAs performed during a 6-month period were identified. Opioid prescription and utilization data (in oxycodone equivalents) were determined via survey and electronic records. Relationship between preoperative opioid use and continued use >90 days after surgery was assessed via Chi-square, with significance set at P < .05.
Results
A total of 415 patients met inclusion criteria (240 THAs and 175 TKAs). Of the 240 THAs, 199 (82.9%) patients and of the 175 TKAs, 144 (82.3%) patients agreed to participate. Forty-three of 199 (21.6%) THA patients and 22 of 144 (15.3%) TKA patients used opioids within 30 days preoperatively. Nine of 199 (4.5%) THA and 10 of 144 (6.9%) TKA patients had continued use of opioids for >90 days postoperatively. Preoperative opioid consumption was significantly associated with chronic use postoperatively for THA (P = .011) and TKA (P = .024). Five of 43 (11.6%) THA and 4 of 22 (18.2%) TKA patients with preoperative opioid use had continued use for >90 days postoperatively. For opioid naïve patients, 2.6% (4/156) of THA and 4.9% (6/122) of TKA patients had chronic use postoperatively.
Conclusions
Preoperative opioid use was associated with nearly 5-fold and 4-fold increase in percentage of patients with chronic opioid use after THA and TKA, respectively. Surgeons should counsel patients regarding this risk and consider strategies to eliminate preoperative opioid use.
Keywords: Arthroplasty, Pain management, Elective surgical procedures, Perioperative care, Osteoarthritis, Opioids
Introduction
Opioids are commonly a part of postoperative pain management protocols after total hip arthroplasty (THA) and total knee arthroplasty (TKA). However, opioid use is associated with substantial risk in terms of side effects, accidental overdose, illicit diversion, and addiction [1], [2], [3], [4]. The United States currently represents <5% of the world’s population yet consumes approximately 80% of the global opioid supply [5]. The 1990s and early 2000s brought an increase in opioid prescriptions that was partially driven by administrative efforts of organizations, such as the Joint Commission on Accreditation of Healthcare Organizations, to redefine the conceptualization of pain management by patients and providers [[6], [7], [8]]. As of 2009, orthopedic surgeons are the third highest prescribers of opioids among physicians, responsible for an estimated 7.7% of opioid prescriptions in the United States [5].
Elective primary THA and TKA are among the most common orthopedic procedures performed in the United States with demand expected to continue to increase [9,10]. Adequate pain management after these procedures has widespread impact, including recovery of functional mobility and autonomy, utilization of health resources, and patient satisfaction [[11], [12], [13]]. Opioids remain a cornerstone of postoperative pain management protocols [4], [13], [14], [15]. It is imperative that surgeons understand patient opioid utilization after total joint arthroplasty, as strategies to minimize patient opioid requirements and reduce the risk of opioid overprescription postoperatively may be identified [16]. Moreover, it is important to understand that postoperative opioid utilization can lead to persistent use in some patients [17]. Identification of risk factors related to chronic use of opioids after surgery may facilitate interventions to mitigate postoperative opioid use. Our study sought to investigate the relationship between preoperative opioid consumption and chronic opioid use after THA and TKA. We hypothesized that the use of opioids preoperatively would increase the risk of chronic opioid utilization after THA and TKA and that patients with lower satisfaction levels of postoperative pain control would be at higher risk of chronic opioid consumption.
Material and methods
This retrospective study was performed as part of an ongoing initiative to evaluate opioid prescription practices and utilization at our institution [4], [18]. Internal review board approval was obtained before the performance of this study. All English-speaking patients aged 18 years or older who underwent elective primary THAs and TKAs performed by 8 arthroplasty surgeons at our institution between March 2015 and September 2015 were identified based on billing information and included in the study. Criteria for exclusion were patients who underwent surgery in the setting of acute trauma, revision procedures, and those who experienced postoperative complications necessitating revision surgery within the first postoperative year. Patients who met inclusion criteria were contacted via telephone between December 2016 and March 2017, at a minimum of 14 months postoperatively, and asked to participate in a survey to obtain information on opioid utilization, excess opioids, and their satisfaction with postoperative pain control. Patients were queried regarding their satisfaction with pain control using a Likert scale of 1-5, with 1 representing “not satisfied at all” and 5 representing “completely satisfied”. This method of assessing satisfaction with pain management has been widely used in literature and is incorporated into various validated patient-reported outcome measures [[19], [20], [21], [22]].
For all patients included in the study, we evaluated electronic medical record and survey data pertaining to preoperative opioid use (defined as having used opioids within 30 days before surgery) and postoperative opioid prescriptions. All opioids were converted to oxycodone equivalents (1 pill = 5 mg oxycodone) using an opioid analgesic equivalence chart [23,24]. At our institution, opioids are usually prescribed upon discharge as part of a multimodal pain management strategy after total joint arthroplasty, which includes acetaminophen, gabapentin, and nonsteroidal anti-inflammatory drugs given before surgery, and continued for 1 month postoperatively. This multimodal approach is consistent across surgeons but is not standardized for each patient, as adjustments to the regimen prescribed may be made for individual patient factors at the surgeons’ discretion.
Chi-square test was used to evaluate the relationship between preoperative opioid use and continued use of opioids for >90 days after surgery. Statistical significance was set at P < .05. Statistical analysis was performed using Stata Version 14 (StataCorp., College Station, TX; StataCorp LP, 2016).
Results
A total of 415 patients met inclusion criteria (240 THAs and 175 TKAs). Of these patients, 82.9% (199/240) of THA and 82.3% (144/175) TKA patients were able to be contacted and agreed to participate. Among our sample, THA and TKA patients were similar with regard to age, race, ethnicity, preoperative opioid use, and type of opioid prescribed postoperatively, as shown in Table 1. The number of pills prescribed upon discharge was similar for both procedures, with THA patients receiving a median of 90 pills (range 20-330 pills) and TKA patients also receiving a median of 90 pills (range 10-200 pills). The THA cohort consisted of significantly fewer female patients (102/199, 51.3%) than TKA patients (93/144, 64.6%) (P = .014). THA patients also had significantly longer mean operative time than TKA patients (143.8 ± 28.8 vs 137.1 ± 24.2 minutes) (P < .023). Furthermore, there was significant variation in pain control satisfaction between THA and TKA groups, with a higher percentage of THA patients reporting that they were “very satisfied” with their pain control (P = .004).
Table 1.
Description of initial study sample.
| Patient characteristics | Total hip arthroplasty (n = 199) | Total knee arthroplasty (n = 144) | P value |
|---|---|---|---|
| Age, mean (SD) | 66.5 (10.2) | 66.9 (10.0) | .757 |
| Gender, percent female | 102 (51.3%) | 93 (64.6%) | .014a |
| Race | .400 | ||
| White | 194 (97.9%) | 144 (100%) | |
| Black | 1 (0.2%) | 0 (0) | |
| Asian | 1 (0.2%) | 0 (0) | |
| Native American | 2 (0.5%) | 0 (0) | |
| Hispanic ethnicity | 1 (0.5%) | 2 (1.4%) | .383 |
| Preoperative opioid useb | 43 (21.6%) | 22 (15.3%) | .140 |
| Operative time in minutes, mean (SD) | 143.8 (28.8) | 137.1 (24.2) | .023a |
| Pain control satisfactionc | .004a | ||
| 5 (Completely satisfied) | 165 (82.9%) | 104 (72.2%) | |
| 4 | 15 (7.5%) | 19 (13.2%) | |
| 3 | 3 (1.5%) | 13 (9.0%) | |
| 2 | 5 (2.5%) | 4 (2.8%) | |
| 1 (Not satisfied at all) | 11 (5.5%) | 4 (2.8%) | |
| Postoperative opioid analgesic prescribed | .099 | ||
| Oxycodone | 124 (62.3%) | 72 (50%) | |
| Hydromorphone | 52 (26.1%) | 58 (40.3%) | |
| Hydrocodone | 6 (3.0%) | 3 (2.0%) | |
| Tramadol | 13 (6.5%) | 9 (6.3%) | |
| Morphine | 2 (1.0%) | 2 (1.4%) | |
| None | 2 (1.0%) | 0 (0%) |
Statistically significant
Pre-operative opioid use within the 30 days prior to surgery
Pain control satisfaction rated on a scale of 1-5, with 1 representing “not satisfied at all” and 5 representing “completely satisfied”
For THA patients, 21.6% (43/199) were using opioids during the 30 days preceding surgery, and these patients were similar in terms of gender, race, ethnicity, operative time, and postoperative pain control satisfaction to those who were not. The mean age of opioid users was 5 years lesser than that of nonusers, which was significant (P = .005). There was no difference in the type of postoperative opioid prescribed as shown in Table 2. Of the patients who underwent TKA, 15.3% (22/144) of patients used opioids before surgery and were similar in terms of age, gender, race, ethnicity, postoperative pain control satisfaction, and type of postoperative opioid prescribed compared with patients with no opioid use, as shown in Table 3. Operative time was significantly longer for TKA patients using opioids before surgery (148.4 ± 28.1 minutes) than that for those who were not (135.1 ± 22.9 minutes) (P = .016).
Table 2.
Comparison of patients using opioids pre-operatively and patients who were not using opioids in the 30 days prior to total hip arthroplasty.
| Variable of interest | No pre-operative opioid use (n = 156) | Pre-operative opioid use (n = 43) | P value |
|---|---|---|---|
| Age, mean (SD) | 67.6 (9.7) | 62.6 (11.0) | .005a |
| Gender, percent female | 84 (53.9%) | 18 (41.86%) | .164 |
| Race | .218 | ||
| White | 152 (98.1%) | 42 (97.7%) | |
| Black | 0 (0) | 1 (2.3%) | |
| Asian | 1 (0.7%) | 0 (0) | |
| Native American | 2 (1.3%) | 0 (0) | |
| Hispanic Ethnicity | 0 (0) | 1 (2.4%) | .053 |
| Operative Time in minutes, mean (SD) | 143.2 (28.8) | 145.9 (29.0) | .587 |
| Pain control satisfactionb | .446 | ||
| 5 (Completely satisfied) | 132 (84.6%) | 33 (76.4%) | |
| 4 | 11 (7.1%) | 4 (9.3%) | |
| 3 | 3 (1.9%) | 0 (0) | |
| 2 | 3 (1.9%) | 2 (4.7%) | |
| 1 (Not satisfied at all) | 7 (4.5%) | 4 (9.3%) | |
| Post-operative Opioid Analgesic Prescribed | .870 | ||
| Oxycodone | 96 (61.5%) | 28 (65.1%) | |
| Hydromorphone | 42 (26.9%) | 10 (23.3%) | |
| Hydrocodone | 4 (2.6%) | 2 (4.7%) | |
| Tramadol | 10 (6.4%) | 3 (7.0%) | |
| Morphine | 2 (1.3%) | 0 (0) | |
| None | 2 (1.3%) | 0 (0) |
Statistically significant
Pain control satisfaction rated on a scale of 1-5, with 1 representing “not satisfied at all” and 5 representing “completely satisfied”
Table 3.
Comparison of patients using opioids pre-operatively and patients who were not using opioids In the 30 days prior to total knee arthroplasty.
| Variable of interest | No pre-operative opioid use (n = 122) | Pre-operative opioid use (n = 22) | P value |
|---|---|---|---|
| Age, mean (SD) | 67.4 (9.4) | 63.7 (12.8) | .111 |
| Gender, percent female | 81 (66.4%) | 12 (54.55%) | .285 |
| Race | .218 | ||
| White | 122 (100%) | 22 (100%) | NA |
| Black | 0 (0) | 0 (0) | |
| Asian | 0 (0) | 0 (0) | |
| Native American | 0 (0) | 0 (0) | |
| Hispanic Ethnicity | 2 (1.7%) | 0 (0) | .544 |
| Operative time in minutes, mean (SD) | 135.0 (22.9) | 148.4 (28.1) | .016a |
| Pain control satisfactionb | .247 | ||
| 5 (Completely satisfied) | 87 (71.3%) | 17 (77.3%) | |
| 4 | 18 (14.8%) | 1 (4.5%) | |
| 3 | 9 (7.4%) | 4 (18.2%) | |
| 2 | 4 (3.3%) | 0 (0) | |
| 1 (Not satisfied at all) | 4 (3.3%) | 0 (0) | |
| Postoperative opioid analgesic prescribed | .315 | ||
| Oxycodone | 62 (50.8%) | 10 (45.5%) | |
| Hydromorphone | 47 (38.5%) | 11 (50%) | |
| Hydrocodone | 3 (2.5%) | 0 (0) | |
| Tramadol | 9 (7.4%) | 0 (0) | |
| Morphine | 1 (0.82%) | 1 (4.6%) | |
| None | 0 (0) | 0 (0) |
SD, standard deviation.
Statistically significant.
Pain control satisfaction rated on a scale of 1-5, with 1 representing “not satisfied at all” and 5 representing “completely satisfied”.
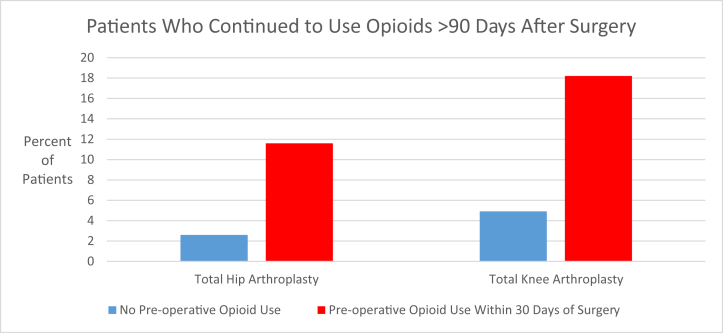
In total, 4.5% (9/199) of THA patients demonstrated continued opioid use for >90 days postoperatively, of which 5 had preoperative opioid use, constituting 11.6% (5/43) of the THA patients with opioid use within 30 days before surgery. For TKA, 6.9% (10/144) of patients demonstrated continued opioid use for >90 days postoperatively, of which 4 had preoperative opioid use, or 18.2% (4/22) of patients with preoperative use. Preoperative opioid use was found to be significantly associated with the continued use of opioids for >90 days after surgery for both THA (P = .011) and TKA (P = .024). Excluding patients with preoperative opioid use from analysis, 2.6% (4/156) of THA and 4.9% (6/122) of TKA patients demonstrated continued opioid use for >90 days postoperatively as shown in Figure 1.
Figure 1.
Percentage of patients with continued opioid use beyond 90 days after total hip arthroplasty and total knee arthroplasty.
Discussion
Our study demonstrates that <3% of THA and <5% of TKA patients that are opioid naïve before joint replacement will continue to use opioids beyond 90 days postoperatively. These findings are comparable to those seen in other major nonorthopaedic surgeries including cardiac, thoracic, abdominal, and pelvic procedures [[25], [26], [27]]. Within our sample, preoperative opioid use was significantly associated with a 5-fold increase in likelihood of chronic opioid use after THA and nearly 4-fold increased risk in TKA patients. This substantial increased risk in non–opioid-naïve patients represents an easily identifiable and potentially modifiable risk factor for opioid mitigation strategies in total joint arthroplasty. While prior studies have suggested that preoperative opioid use may be a risk factor for prolonged opioid use after total joint arthroplasty and identified risk factors such as female sex, younger age, hypnotic use, and depression [[28], [29], [30], [31]]. Our study is the first to demonstrate that any opioid use preoperatively, regardless of the origin for the prescription, places patients at a substantial increased risk of chronic opioid use postoperatively and is independent of both postoperative pain control satisfaction and type of opioid included in postoperative pain management protocols. The specific opioid used may not be as clinically significant as we believe, and the risks associated with prolonged opioid use are unlikely unique to individual medications. The lack of a significant difference between patients’ satisfaction with pain control and opioid use is a unique finding lacking in current literature. We believe our findings contribute to the current understanding of risks associated with opioid use after THA and TKA and support that long-term opioid prescriptions fail to improve pain control satisfaction.
Of note, there are several differences between opioid naïve patients and those using opioids preoperatively in both the THA and TKA cohorts. Patients with preoperative opioid consumption were on average several years younger, which was significant for THA, but not TKA. In addition, no difference was observed in postoperative opioid pain control satisfaction compared with opioid naïve patients. Despite these differences, our data clearly identify opioid consumption within 30 days before surgery as an important, potentially modifiable risk factor for prolonged postoperative opioid use in patients undergoing THA or TKA. While there were a greater percentage of THA patients who were “completely satisfied” with their pain control, in alignment with prior arthroplasty studies demonstrating THA patients having significantly lower overall postoperative pain levels than their TKA counterparts, [32,33] a significant risk for continued consumption remains. Our study contributes to the growing body of evidence demonstrating that opioid use preceding total joint arthroplasty results in poorer clinical outcomes, increased postoperative length of stay, greater cost, higher readmission rates, and increased risk revision rates [29,[34], [35], [36]]. Identification of patients using opioids before surgery facilitates the implementation of opioid-weaning protocols and may not only limit postoperative chronic opioid use but also promote improved clinical outcomes, as described by Nguyen et al. [37].
It is important to note that previous literature has identified that most preoperative opioid prescriptions originate from nonorthopaedic providers, [36] and the conditions for which these medications are prescribed continue to expand [38]. It is not uncommon for patients to be using opioids preoperatively, and our study demonstrates the importance of surgeons counseling these patients on the risks associated with the continued use of opioids in the month before total joint arthroplasty. Furthermore, surgeons may consider collaborating with referring prescribing providers, as this offers a potential area of meaningful intervention. Our study is not without limitations. The retrospective nature of our study design precluded controlling for all patient factors that might affect opioid utilization. However, we attempted to contact all patients who underwent these procedures during the study period, and participation rate was high at approximately 82% for both THA and TKA. Therefore, we believe the included sample of patients is representative of our patient population. Furthermore, reasons for preoperative opioid use were not evaluated in this study. While this consideration merits further investigation, we believe that the value of simply identifying any opioid use, irrespective of primary reason, by patients within the 30 days before joint replacement as a risk factor for chronic use is immense, as it is something that can be quickly and easily screened for during the preoperative period. Another limitation is that the method by which pain control satisfaction was ascertained in this study relies upon patient recollection and self-reporting. While these may be subject to bias, this method has been used successfully in prior studies, and this limitation is inherent to the use of a survey for these types of subjective measures [39]. Finally, the generalizability of our findings may be limited, as the population we serve in northern New England is largely homogenous in terms of race and ethnicity, limiting our ability to assess the influence of these demographic factors on opioid utilization.
Conclusions
Despite the stated limitations, we believe this study is an important evaluation of postoperative opioid prescription and utilization after THA and TKA. Our results demonstrate that preoperative opioid use within the 30 days before surgery is a significant predictor of opioid utilization for >90 days after surgery. Patients using opioids before THA or TKA should be counseled regarding their increased risk of postoperative use, and surgeons should consider strategies to eliminate preoperative opioid use in this patient population.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Appendix A. Supplementary data
References
- 1.Beletsky L., Rich J.D., Walley A.Y. Prevention of fatal opioid overdose. JAMA. 2012;308(18):1863. doi: 10.1001/jama.2012.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohnert A.B., Valenstein M., Bair M.J. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 3.Volkow N.D., McLellan T.A. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305(13):1346. doi: 10.1001/jama.2011.369. [DOI] [PubMed] [Google Scholar]
- 4.Kunkel S., Sabatino M., Pierce D., Fillingham Y., Jevsevar D., Moschetti W. What happens to unused opioids after total joint arthroplasty? An evaluation of unused postoperative opioid disposal practices. J Arthoplasty. 2020;35(4):966–970. doi: 10.1016/j.arth.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Morris B.J., Mir H.R. The opioid epidemic: impact on orthopaedic surgery. J Am Acad Orthop Surg. 2015;23(5):267. doi: 10.5435/JAAOS-D-14-00163. [DOI] [PubMed] [Google Scholar]
- 6.Holman J.E., Stoddard G.J., Higgins T.F. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. J Bone Joint Surg Am. 2013;95(12):1075. doi: 10.2106/JBJS.L.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Large R.G., Schug S.A. Opioids for chronic pain of non-malignant origin--caring or crippling. Health Care Anal. 1995;3(1):5. doi: 10.1007/BF02197187. [DOI] [PubMed] [Google Scholar]
- 8.Mehendale A.W., Goldman M.P., Mehendale R.P. Opioid overuse pain syndrome (OOPS): the story of opioids, prometheus unbound. J Opioid Manag. 2013;9(6):421. doi: 10.5055/jom.2013.0185. [DOI] [PubMed] [Google Scholar]
- 9.Kurtz S.M., Ong K.L., Lau E., Bozic K.J. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624. doi: 10.2106/JBJS.M.00285. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 11.Cheville A., Chen A., Oster G., McGarry L., Narcessian E. A randomized trial of controlled-release oxycodone during inpatient rehabilitation following unilateral total knee arthroplasty. J Bone Joint Surg Am. 2001;83-a(4):572. doi: 10.2106/00004623-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Illgen R.L., Pellino T.A., Gordon D.B., Butts S., Heiner J.P. Prospective analysis of a novel long-acting oral opioid analgesic regimen for pain control after total hip and knee arthroplasty. J Arthroplasty. 2006;21(6):814. doi: 10.1016/j.arth.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Sinatra R.S., Torres J., Bustos A.M. Pain management after major orthopaedic surgery: current strategies and new concepts. J Am Acad Orthop Surg. 2002;10(2):117. doi: 10.5435/00124635-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 14.de Beer Jde V., Winemaker M.J., Donnelly G.A. Efficacy and safety of controlled-release oxycodone and standard therapies for postoperative pain after knee or hip replacement. Can J Surg. 2005;48(4):277. [PMC free article] [PubMed] [Google Scholar]
- 15.Nowicki P.D., Vanderhave K.L., Gibbons K. Perioperative pain control in pediatric patients undergoing orthopaedic surgery. J Am Acad Orthop Surg. 2012;20(12):755. doi: 10.5435/JAAOS-20-12-755. [DOI] [PubMed] [Google Scholar]
- 16.Nota S.P., Spit S.A., Voskuyl T., Bot A.G., Hageman M.G., Ring D. Opioid use, satisfaction, and pain intensity after orthopedic surgery. Psychosomatics. 2015;56(5):479. doi: 10.1016/j.psym.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Hah J.M., Bateman B.T., Ratliff J., Curtin C., Sun E. Chronic opioid use after surgery: implications for perioperative management in the face of the opioid epidemic. Anesth Analg. 2017;125(5):1733. doi: 10.1213/ANE.0000000000002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabatino M., Kunkel S., Ramkumar D., Keeney B., Jevsevar D. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180–188. doi: 10.2106/JBJS.17.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litwin M.S., Shpall A.I., Dorey F. Patient satisfaction with short stays for radical prostatectomy. Urology. 1997;49(6):898. doi: 10.1016/s0090-4295(97)00103-9. [DOI] [PubMed] [Google Scholar]
- 20.Kirsh E.J., Worwag E.M., Sinner M., Chodak G.W. Using outcome data and patient satisfaction surveys to develop policies regarding minimum length of hospitalization after radical prostatectomy. Urology. 2000;56(1):101. doi: 10.1016/s0090-4295(00)00594-x. [DOI] [PubMed] [Google Scholar]
- 21.North F., Crane S.J., Ebbert J.O., Tulledge-Scheitel S.M. Do primary care providers who prescribe more opioids have higher patient panel satisfaction scores? SAGE Open Med. 2018;6 doi: 10.1177/2050312118782547. 2050312118782547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greimel F., Maderbacher G., Baier C. Multicenter cohort-study of 15326 cases analyzing patient satisfaction and perioperative pain management: general, regional and combination anesthesia in knee arthroplasty. Sci Rep. 2018;8(1):3723. doi: 10.1038/s41598-018-22146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Committee, UNC Opiate equianalgesic dosing chart. 2009. www.med.unc.edu/aging/fellowship/current/curriculum/palliative-care
- 24.CfDCa, P Opioid morphine conversion factors. 2015. https://www.cms.gov/medicare/prescription-drug-coverage/prescriptiondrugcovcontra/downloads/opioid-morphine-eq-conversion-factors-march-2015.pdf [accessed 07.11.17]
- 25.Brummett C.M., Waljee J.F., Goesling J. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke H., Soneji N., Ko D.T., Yun L., Wijeysundera D.N. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251. doi: 10.1136/bmj.g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soneji N., Clarke H.A., Ko D.T., Wijeysundera D.N. Risks of developing persistent opioid use after major surgery. JAMA Surg. 2016;151(11):1083. doi: 10.1001/jamasurg.2016.1681. [DOI] [PubMed] [Google Scholar]
- 28.Bedard N.A., Pugely A.J., Dowdle S.B., Duchman K.R., Glass N.A., Callaghan J.J. Opioid use following total hip arthroplasty: trends and risk factors for prolonged use. J Arthroplasty. 2017;32(12):3675. doi: 10.1016/j.arth.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Blevins Peratikos M., Weeks H.L., Pisansky A.J.B., Yong R.J., Stringer E.A. Effect of preoperative opioid use on adverse outcomes, medical spending, and persistent opioid use following elective total joint arthroplasty in the United States: a large retrospective cohort study of administrative claims data. Pain Med. 2019 doi: 10.1093/pm/pnz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inacio M.C.S., Hansen C., Pratt N.L., Graves S.E., Roughead E.E. Risk factors for persistent and new chronic opioid use in patients undergoing total hip arthroplasty: a retrospective cohort study. BMJ Open. 2016;6(4):e010664. doi: 10.1136/bmjopen-2015-010664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh J.A., Lewallen D. Predictors of pain and use of pain medications following primary Total Hip Arthroplasty (THA): 5,707 THAs at 2-years and 3,289 THAs at 5-years. BMC Musculoskelet Disord. 2010;11(1):90. doi: 10.1186/1471-2474-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dailiana Z.H., Papakostidou I., Varitimidis S. Patient-reported quality of life after primary major joint arthroplasty: a prospective comparison of hip and knee arthroplasty. BMC Musculoskelet Disord. 2015;16:366. doi: 10.1186/s12891-015-0814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Beer J., Petruccelli D., Adili A., Piccirillo L., Wismer D., Winemaker M. Patient perspective survey of total hip vs total knee arthroplasty surgery. J Arthroplasty. 2012;27(6):865. doi: 10.1016/j.arth.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 34.Pivec R., Issa K., Naziri Q., Kapadia B.H., Bonutti P.M., Mont M.A. Opioid use prior to total hip arthroplasty leads to worse clinical outcomes. Int Orthop. 2014;38(6):1159. doi: 10.1007/s00264-014-2298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weick J., Bawa H., Dirschl D.R., Luu H.H. Preoperative opioid use is associated with higher readmission and revision rates in total knee and total hip arthroplasty. J Bone Joint Surg Am. 2018;100(14):1171. doi: 10.2106/JBJS.17.01414. [DOI] [PubMed] [Google Scholar]
- 36.Zarling B.J., Yokhana S.S., Herzog D.T., Markel D.C. Preoperative and postoperative opiate use by the arthroplasty patient. J Arthroplasty. 2016;31(10):2081. doi: 10.1016/j.arth.2016.03.061. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen L.-C.L., Sing D.C., Bozic K.J. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(9, Supplement):282. doi: 10.1016/j.arth.2016.01.068. [DOI] [PubMed] [Google Scholar]
- 38.Schuchat A., Houry D., Guy G.P., Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318(5):425. doi: 10.1001/jama.2017.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill M.V., McMahon M.L., Stucke R.S., Barth R.J.J. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017 doi: 10.1097/SLA.0000000000001993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.