Abstract
Introduction
Platelet-rich plasma (PRP) therapy is used to treat pathological conditions such as degenerative inflammatory diseases including osteoarthritis (OA) by enhancing tissue repair and promoting anti-inflammatory effects. Although PRP therapy for patients with knee OA improved pain and functional scores, the association of clinical outcomes and quality of PRP including cell composition and concentration is unclear.
Methods
Therefore, this study analyzed blood cell counts, including the immature platelet fraction (IPF), in peripheral blood and PRP of 144 patients with knee OA who underwent PRP therapy. The mean leukocyte and platelet concentrations in whole blood and PRP were analyzed using an XN-1000 automated hematology analyzer. Visual analogue scale (VAS) scores and knee injury and osteoarthritis outcome scores (KOOS) before and 1 month after a single PRP injection were also determined.
Results
Higher platelet and lower leukocyte concentration rates were observed in PRP compared with whole blood. The platelet concentration in whole blood was negatively correlated with VAS improvement. The percentage of IPF (IPF%) in whole blood was positively correlated with VAS improvement and KOOS (pain) improvement, whereas the IPF% in PRP tended to correlate with VAS improvement. Furthermore, multivariate logistic regression demonstrated the high IPF% in whole blood was significantly associated with VAS improvement. The low percentage of neutrophil (neutrophil%) in PRP was significantly associated with the VAS improvement and KOOS (ADL) improvement.
Conclusions
Therefore, PRP efficacy for OA might depend on the patient's biological status.
Keywords: Platelet-rich plasma, Immature platelet fraction, Knee osteoarthritis
Abbreviations: ACD, acid citrate dextrose; EDTA, ethylenediaminetetraacetic acid; IPF, immature platelet fraction; KOOS, knee injury osteoarthritis outcome score; OA, osteoarthritis; PRP, platelet rich plasma; SFL, side fluorescence; VAS, visual analogue scale
Highlights
-
•
High percentage of IPF (IPF%) in whole blood was significantly associated with VAS improvement.
-
•
Low neutrophil% in PRP was significantly associated with VAS improvement and KOOS (ADL) improvement.
-
•
The PRP efficacy for knee OA might depend on the patient's biological status.
1. Introduction
Platelets are short-lived (8–9 days) cell-particles that are constantly generated from megakaryocytes and are released from the bone marrow to the circulating blood flow [1]. Thus, circulating platelets contain a mixture of young and old platelets. Newly produced platelets just released from the bone marrow to the cytoplasm are rich in RNA and are called reticular platelets (RP) [2] or the immature platelets [3]. Immature platelets contain higher amounts of RNA and can produce various proteins typical for active platelets such as GPIIb/IIIa and P-selectin [4]. In addition, although there is only a partial correlation with the MPV, immature platelets tend to be larger than mature platelets [4]. Therefore, the immature platelet fraction (IPF), that can be defined by means of both the forward scattered light strength, surrogate marker of the particles size in flow cytometry, and the intensity of fluorescence stained with dye for nucleic acids, can be measured reproducibly by certain automated hematology analyzers. Indeed, the percentage of immature platelet fraction to total platelets (IPF%) correlates well with the reticulated platelet count obtained from CD61 flow cytometry [3]. The IPF% in the peripheral blood reflects platelet production in the bone marrow [5]; thus, IPF% can be a useful marker of thrombopoietic activity. The most relevant clinical utility is the differential diagnosis between immune thrombocytopenia (ITP) and thrombocytopenia from thrombopoiesis hypoproduction [6]. Generally, thrombocytopenia related to platelet destruction or consumption such as ITP can be predicted by high IPF% and thrombocytopenia from thrombopoiesis hypoproduction can be predicted by low IPF% [6]. Therefore, IPF% has been used to diagnose various pathological conditions and thrombocytopenic diseases such as ITP, aplastic anemia, and liver cirrhosis, as well as the prediction of platelet recovery after chemotherapy and hematopoietic cell transplantation, and judging indications of platelet transfusion [6].
Platelets have critical roles in maintaining homeostasis as well as tissue repair [7]. Several factors secreted from the alpha-granules of activated platelets including transforming growth factor-β, vascular endothelial growth factor, and platelet-derived growth factor help to repair damaged tissue. In addition, platelets are involved in the immune system especially diverse inflammatory processes that influence normal leukocyte biology and inflammatory signals [8].
On the basis of these platelet functions, platelet-rich plasma (PRP) therapy has been developed and used to treat various pathological conditions in motor organs such as bone, tendon, ligament, and articular cartilage [9,10]. The mechanisms of PRP action on motor organs diseases are thought to be related to the enhancement of tissue repair as well as anti-inflammatory effects [11]. Therefore, PRP therapy is frequently used for the treatment of degenerative inflammatory diseases such as osteoarthritis (OA). Several meta-analyses assessing clinical trials of PRP therapy for patients with knee OA demonstrated that PRP had favorable effects on improving pain and functional scores [12,13]. However, few reports have shown a relationship between clinical outcomes and the quality of PRP, such as cell composition and concentration.
The purpose of this study was to analyze the blood cell counts, including IPF, in peripheral blood and PRP of patients with knee OA who underwent PRP therapy, and to reveal the relationships between blood cell parameters and clinical outcomes.
2. Method
2.1. Patients
Overall, 144 consecutive patients with unilateral knee OA who underwent PRP therapy in our hospital in 2020 were enrolled in this study. Informed consent was obtained from each donor and patient before drawing peripheral blood. This study was approved by the Ethical Committee of Juntendo Hospital (approval number 2020027). The indications for PRP therapy in our hospital include chronic knee pain despite other known conservative treatments. Patients were excluded from this study if they had systematic inflammatory diseases such as rheumatoid arthritis, active infectious diseases, poorly controlled diabetes mellitus, or had platelet disorders or diseases.
2.2. PRP preparation
The protocol and ethics of PRP therapy were certified by a special committee for regenerative medicine based on a law regulating the safety of regenerative medicine in Japan (approval number PB3150023). The PRP preparation was obtained by a single centrifugation of whole blood using the MyCells autologous platelet preparation system (Kaylight Ltd., Israel). Each kit contains 1 mL of anticoagulant acid citrate dextrose (ACD). Here, 22 mL of whole blood was aspirated from the median cubital vein and 5.0 mL of PRP was obtained according to the manufacturer's instructions. In brief, centrifugation was performed at 2000×g for 7 min at 21–25 °C, and the supernatant was discarded leaving 2.5 mL. The portion of Buffy coat directly above the gel was pipetted and mixed before collection, and a total of 5 mL of leukocyte poor PRP (LP-PRP) was adjusted.
2.3. Hematological analysis
Venous peripheral blood (whole blood) samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes which is usually used for blood count measurement for peripheral blood separately from the PRP preparation kit, and part of the PRP used for treatment was used for hematological analysis. Peripheral blood and PRP samples were analyzed using an XN-1000 automated hematology analyzer (Sysmex, Kobe, Japan) according to the manufacturer's recommendations. The concentrations of erythrocytes, neutrophils, lymphocytes, monocytes, platelets, and IPF were measured from 300 μL of peripheral blood and PRP.
2.4. Analysis of IPF
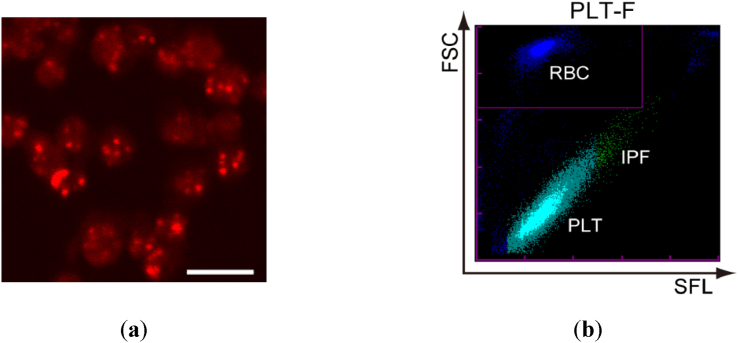
Sysmex Corporation has developed a method for measuring IPF, using technology that uses a proprietary dye of platelets and laser imaging. The Sysmex XN system uses flow cytometry with a semiconductor laser that uses fluorescent light and stains platelets specifically. The internal structures of nucleic acid-containing platelets, including mitochondria and endoplasmic reticulum, were stained using reagents containing oxazine fluorescent dyes. Platelets were irradiated with a semiconductor laser beam, and the FSC light indicated the size of platelets and the side fluorescence (SFL) indicated the RNA of platelets. Although the analyzer has three channels (PLT-I, PLT-O, and PLT-F) that can count platelets, we used the PLT-F channel only to count platelets in this study because of its accuracy (Fig. 2) [14].
Fig. 2.
Typical PLT-F scattergram of IPF. (a) Confocal laser scanning fluorescent microscopic image of isolated platelets from a healthy volunteer. The platelets were stained with a dedicated reagent of the PLT-F channel. Bar: 5 μm. (b) A typical PLT-F scattergram of whole blood from a healthy volunteer taken with an XN-1000 automated hematology analyzer. In the scattergram, platelets are plotted in a single cluster from bottom left to top right, where IPF is at the top right. Because immature platelets tend to be larger and contain higher amount of RNA than mature platelets, IPF is at the top right part of the platelet cluster colored in green. Erythrocytes are displayed as another cluster, which is located in the inset at top left. FSC: forward scattered light; SFL: side fluorescence from nucleic acid staining dye; RBC: red blood cell; PLT: platelets; IPF: immature platelet fraction.
2.5. Clinical scores
Visual analogue scale (VAS) and knee injury and osteoarthritis outcome scores (KOOS) before and 1 month after treatment were evaluated, and the amount of improvement before and after treatment was calculated. The relationships between changes in clinical scores and cell parameters in whole blood and PRP were analyzed.
2.6. Statistical analysis
VAS and KOOS improvement before and after treatment were analyzed by paired t-test. The correlation between each cell parameter and VAS improvement was analyzed by Spearman's correlation. Univariate regression and multiple logistic regression analyses were performed using the presence or absence of one or more improvements in VAS as a dependent variable, and age, gender, KL grade, and blood cell parameters as exploratory variables. Age was converted into two categories, and blood parameters were converted into four categories so that the number of each category was uniform with reference to the 4-min displacement point. Regarding IPF, Smirnov-Grubbs’ test was performed to exclude outliers. All p-values were two-sided, and p-values <0.05 were considered statistically significant. Statistical analyses were performed with SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
2.7. Microscopy
The confocal laser scanning fluorescent microscopic image of isolated platelets from a healthy volunteer was taken as previously described [13]. Briefly, PRP from venous peripheral blood anticoagulated with EDTA was placed on poly-l-lysine (PLL) coated glass-bottomed dish (D11131H, Matsunami, Osaka, Japan) for 10 min. Immediately after residual plasma drained, platelets attached on the PLL-coated glass were covered with 1020 mL staining solution (CELLPACK® DFL (Sysmex): Fluorocell® PLT (Sysmex) = 50:1). After a minute incubation at RT, the fluorescent images were taken by a confocal laser scanning fluorescent microscope (FV-10i, Olympus, Tokyo, Japan) using 635-nm laser.
3. Result
3.1. Characteristics of patients
Table 1 shows the demographics of the patients in this study.
Table 1.
Demographics of the patients in this study.
| Number of patients | 144 |
| Age (mean ± SD) | 65.8 ± 12.0 |
| Sex (female/male) | 99/45 |
| BMI (mean ± SD) | 23.7 ± 3.4 |
| KL grade (2/3/4) | 43/64/37 |
SD, standard deviation; KL, Kellgren–Lawrence; BMI, body mass index.
3.2. Measurement of cell parameters in whole blood and PRP
The mean leukocyte concentration in whole blood was 5189 ± 1242 (/μL) and the mean platelet concentration was 22.5 ± 5.2 (×104/μL). The mean leukocyte concentration in PRP was 3019 ± 1086 and the mean platelet concentration was 36.4 ± 12.5. The leukocyte concentration rate and platelet concentration rate were 0.21 ± 0.1-fold and 1.65 ± 0.5-fold greater than those of peripheral blood, respectively (Table 2). This is classified as P2-Bβ PRP (LP-PRP) based on the PAW classification system described by DeLong et al., [15]. In the PAW classification, platelet concentration, activation method, WBC content are defined by category. Platelet concentration: P1, less than or equal to baseline levels; P2, greater than baseline levels to 750,000 platelets/μL; P3, greater than 750,000 to 1,250,000 platelets/μL; and P4, greater than 1,250,000 platelets/μL. Total WBC content is identified as either above (A) or below/equal to (B) baseline levels. If neutrophils are included in the buffy coat, then α (above) is added. If neutrophils are filtered out, then β (below) is added.
Table 2.
Cell parameters in whole blood and PRP.
| Cell composition | Measurement parameters | Whole blood | PRP | Concentration rate | |
|---|---|---|---|---|---|
| Leukocyte | Cell concentration | Leukocyte (/μL) | 5189 ± 1242 | 3019 ± 1086 | 0.21 ± 0.10 |
| Neutrophil (/μL) | 3031 ± 936 | 54 ± 39 | 0.02 ± 0.01 | ||
| Lymphocyte (/μL) | 1654 ± 516 | 906 ± 472 | 0.56 ± 0.26 | ||
| Monocyte (/μL) | 307 ± 90 | 95 ± 56 | 0.32 ± 0.18 | ||
| Eosinophil (/μL) | 157 ± 109 | 4.2 ± 8.0 | 0.05 ± 0.10 | ||
| Basophil (/μL) | 40 ± 18 | 0.1 ± 1.2 | 0.00 ± 0.03 | ||
| Fraction | Neutrophils (%) | 57.9 ± 8.0 | 5.2 ± 2.1 | ||
| Lymphocytes (%) | 32.2 ± 7.3 | 85.3 ± 4.4 | |||
| Monocytes (%) | 6.0 ± 1.4 | 9.0 ± 3.0 | |||
| Eosinophils (%) | 3.1 ± 2.0 | 0.5 ± 1.2 | |||
| Basophil (%) | 0.8 ± 0.3 | 0.03 ± 0.3 | |||
| Platelet | Cell concentration | Platelet (×104/μL) | 22.5 ± 5.2 | 36.4 ± 12.5 | 1.65 ± 0.50 |
| IPF (×104/μL) | 0.5 ± 0.2 | 0.6 ± 0.4 | 1.38 ± 0.62 | ||
| Fraction | IPF (%) | 2.0 ± 1.0 | 1.6 ± 0.8 | ||
| H-IPF (%) | 0.6 ± 0.3 | 0.5 ± 0.3 | |||
| Others | MPV (fL) | 10.6 ± 1.0 | 10.1 ± 0.9 | ||
| PCT (%) | 0.2 ± 0.1 | 0.3 ± 0.1 | |||
| Erythrocyte | Cell concentration | Erythrocytes (×104/μL) | 444.9 ± 44.4 | 0.015 ± 0.046 | N/A |
N/A; not applicable.
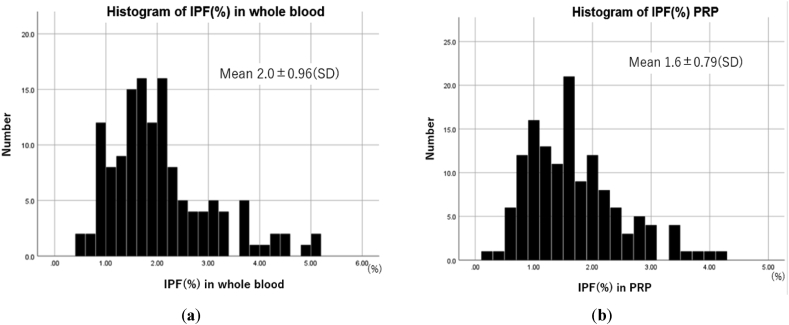
Fig. 1 shows the histogram of IPF% in the whole blood and PRP. They didn't follow a normal distribution. The mean IPF% in whole blood was 2.0 ± 0.96, and that of PRP was 1.6 ± 0.79. The median of IPF% in whole blood was 1.8, and that of PRP was 1.5. The histogram of IPF% in PRP was similar to that in the whole blood. Several studies have reported IPF% of healthy volunteers. Jung H et al. who reported in 2039 healthy adults persons in Korea, an IPF% of (0.5–3.2) and (0.4–3) in men and women, respectively [16]. KO YJ et al. who reported in 2104 healthy adults' persons in Korea, an IPF percentage of 1.6 (0.3–7.4) [17]. Although this study includes data from knee OA patients, IPF% was comparable to previously reported data from healthy volunteers.
Fig. 1.
Histogram of the IPF% in whole blood and PRP. (a) Histogram of IPF% in whole blood: IPF% in whole blood didn't follow the normal distribution. The mean IPF% in whole blood was 2.0 ± 0.96, and the median of IPF% in whole blood was 1.8. (b) Histogram of IPF% in PRP: IPF% in PRP didn't follow the normal distribution. The mean IPF% in whole blood was 1.6 ± 0.79, and the median of IPF% in whole blood was 1.5.
Fluorescent microscopic images of platelets and a typical PLT-F scattergram of IPF are shown in Fig. 2. In the scattergram, platelets are plotted in a single cluster from bottom left to top right, and the IPF is at the top right in green. The details of the analysis of IPF are described in the Methods.
3.3. Clinical outcomes
All the patient-oriented outcomes were significantly improved at 1-month after the PRP injections (Table 3).
Table 3.
Visual Analogue Scale (VAS) scores and Knee Injury and Osteoarthritis Outcome Scores (KOOS) before and 1 month after a single PRP injection.
| Clinical score | Before | After 1 month | p value |
|---|---|---|---|
| VAS | 52.6 ± 27.9 | 40.5 ± 25.6 | <0.001 |
| KOOS (symptom) | 59.5 ± 20.9 | 66.4 ± 18.4 | <0.001 |
| KOOS (pain) | 56.6 ± 20.8 | 65.7 ± 17.7 | <0.001 |
| KOOS (activity of daily living) | 70.9 ± 18.9 | 78.9 ± 14.8 | <0.001 |
| KOOS (sports and recreation function) | 36.4 ± 25.3 | 44.3 ± 26.4 | <0.001 |
| KOOS (quality of life) | 31.6 ± 21.6 | 43.1 ± 21.8 | <0.001 |
3.4. Correlation between cell parameters and VAS and KOOS improvement
The platelet concentration in whole blood was negatively correlated with the amount of VAS improvement (R = −0.20, p = 0.025), whereas the platelet concentration in PRP was not correlated with clinical score improvement. The IPF% in whole blood was positively correlated with the amount of VAS improvement (R = 0.25, p = 0.007) and KOOS (pain) improvement (r = 0.19, p = 0.036), whereas the IPF% in PRP tended to correlate with VAS improvement (r = 0.18, p = 0.05).
All leukocyte parameters in whole blood did not correlate with the amount of VAS improvement, whereas the percentage of neutrophils (neutrophil%) in PRP was negatively correlated with the amount of VAS improvement (R = −0.19, p = 0.034) (Table 4).
Table 4.
Correlation between cell parameters and amount of VAS and KOOS improvement.
| Whole blood |
PRP |
|||||
|---|---|---|---|---|---|---|
| Platelet concentration (×104/μL) | IPF% | Platelet concentration (×104/μL) | IPF% | Neutrophil% | ||
| VAS | r | −0.20 | 0.25 | −0.75 | 0.18 | −0.19 |
| p value | 0.025∗ | 0.007∗ | 0.41 | 0.05 | 0.034∗ | |
| KOOS (symptom) | r | −0.072 | 0.049 | 0.023 | 0.059 | −0.17 |
| p value | 0.43 | 0.60 | 0.80 | 0.52 | 0.067 | |
| KOOS (pain) | r | −0.074 | 0.19 | −0.080 | 0.11 | −0.094 |
| p value | 0.42 | 0.036∗ | 0.38 | 0.23 | 0.30 | |
| KOOS (activity of daily living) | r | 0.079 | 0.60 | 0.047 | 0.034 | −0.11 |
| p value | 0.37 | 0.51 | 0.60 | 0.71 | 0.20 | |
| KOOS (sports and recreation function) | r | −0.10 | 0.094 | −0.57 | 0.14 | −0.042 |
| p value | 0.25 | 0.31 | 0.52 | 0.14 | 0.64 | |
| KOOS (quality of life) | r | 0.098 | −0.52 | 0.045 | 0.022 | −0.17 |
| p value | 0.28 | 0.57 | 0.62 | 0.81 | 0.058 | |
3.5. Logistic regression to predict VAS and KOOS improvement or deterioration
The relationships between patient factors (age, sex, KL grade, IPF% in whole blood, and neutrophil% in PRP) and changes of VAS or KOOS were analyzed by logistic regression. When the outcomes were set to VAS changes, a high IPF% in whole blood and low neutrophil% in PRP were significantly associated with an improvement in the VAS (odds ratio [OR] = 1.89, 95% confidence interval [CI] = 1.24–2.88, p = 0.003; OR = 0.67, 95% CI = 0.46–0.99, p = 0.045, respectively) (Table 5). When the outcomes were set to KOOS changes, younger age (65≤) was significantly associated with an improvement in KOOS (symptom) (OR = 0.33, 95% CI = 0.13–0.82, p = 0.017), and low neutrophil% in PRP were significantly associated with an improvement in KOOS (ADL) (OR = 0.67, 95% CI = 0.46–0.97, p = 0.034).
Table 5.
Logistic regression to predict VAS improvement or deterioration.
| Predictors | Univariate |
Multivariate |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age (≤65, >65 years) | 0.90 (0.43–1.96) | 0.810 | 0.96 (0.36–2.57) | 0.940 |
| Sex (male, female) | 1.67 (0.76–3.68) | 0.200 | 2.39 (0.94–6.09) | 0.069 |
| KL classification (2, 3, 4) | 0.86 (0.52–1.44) | 0.57 | 0.72 (0.39–1.34) | 0.300 |
| IPF% in whole blood (≤1.5, 1.5 to ≤2.0, 2.0 to ≤2.5, >2.5) | 1.67 (2.43) | 0.007∗ | 1.89 (1.24–2.88) | 0.003∗ |
| Neutrophil% in PRP (≤4.0, 4.0 to ≤6.0, 6 to ≤8.0, >8.0) | 0.76 (0.54–1.09) | 0.130 | 0.67 (0.46–0.99) | 0.045∗ |
4. Discussion
The pathophysiology of OA is complex. The goal of the investigation of the molecular pathology of OA is to contribute to the development of novel therapeutic strategies for OA. However, the standard conservative care of OA has been pharmacological treatments such as the oral intake of NSAIDs and intra-articular steroids or hyaluronic acid administration. Here, we investigated a novel biological treatment for OA, PRP therapy, and demonstrated its efficacy was associated with the IPF% in the peripheral blood. To the best of our knowledge, this is the first study to report a relationship between immature platelets and the efficacy of PRP therapy. This study suggests an important concept whereby the efficacy of autologous biological therapy depends on the status of the patient's biological system.
Murray et al. reported a checklist of the minimum reporting requirements for clinical studies evaluating PRP that reached consensus through the Delphi Process. The concentrations of platelets and white blood cells, and the differential leukocyte count in the whole blood and PRP were also included [18]. Because PRP is plasma containing high concentrations of platelets, it is important to examine the concentration of platelets and determine their qualitative status. Therefore, we measured the IPF in whole blood and PRP including the quality of platelets to determine whether they are related to the effects of PRP. In this study, IPF% in whole blood correlated with VAS improvement. The IPF% in whole blood was previously reported to reflect platelet hematopoiesis in the bone marrow [19]. People with a high IPF% in whole blood may have active platelet hematopoiesis, good platelet quality, and their PRP might be highly effective for therapy. The IPF% in PRP tended to correlate with VAS improvement. However, the function of immature platelets is not clear, and how immature platelets mediate their effects in the joints of knee OA patients is poorly understood. In the future, it may be possible to elucidate the function of immature platelets by evaluating the relationship between immature platelets and growth factors and cytokines.
Anticoagulants can also affect platelet quality. In this study, EDTA was used for whole blood and ACD was used for PRP. Platelet concentrations with ACD tended to be lower than with EDTA. The mean platelet volume was larger with EDTA than ACD because the platelets changed from discoid to spherical morphology. Platelets are small particles of variable size, the smallest of which are near the limit of detection by flow cytometry. An increased volume of EDTA-treated platelets can aid their detection by a hematology analyzer, but this effect is not observed for ACD-treated platelets [20]. The different anticoagulants used for whole blood and PRP should be considered when discussing the difference in platelet quality between whole blood and PRP.
For PRP therapy for knee OA, it was reported that LP-PRP of P2-Bβ is highly effective in terms of PRP quality according to the PAW classification [21]. When discussing the quality of PRP and its efficacy, platelet and leukocyte concentrations are often investigated; however, the leukocyte fraction is rarely studied. In this study, the LP-PRP of PAW P2Bb was used, and the leukocytes contained 85.3% lymphocytes, 9% monocytes, 5.2% neutrophils, and 1% or less eosinophils and basophils. A systematic review and meta-analysis of randomized controlled trials reported by Belk et al. showed that a pooled analysis of studies that compared leukocyte rich (LR)-PRP and LP-PRP demonstrated that LP-PRP resulted in greater improvements in Subjective International Knee Documentation Committee (IKDC) scores. Results of an indirect effects analysis estimated that LP-PRP resulted in a mean 5.1-unit-greater improvement in Subjective IKDC scores versus LR-PRP (95% CI, 210.1 to 20.2) [12]. In our study, an improvement of VAS was negatively correlated with the neutrophil% in PRP. A previous study reported that neutrophils release the catabolic enzyme matrix metalloprotease-9 [22], suggesting that the intra-articular administration of neutrophils might induce inflammation in joints. According to these observations, lower neutrophil fractions may be more suitable for the treatment of knee OA.
This study had some limitations. First, we did not evaluate growth factors in the whole blood and PRP. Therefore, it is unclear whether IPF% is related to the concentration of growth factors. Second, the clinical outcome of this study was evaluated 1 month after a single injection of PRP. Therefore, this was a short-term result and the long-term outcomes might be different. However, to clarify the relationship between blood cell parameters and clinical score changes, we examined short term score changes after a single administration of PRP.
5. Conclusion
This study demonstrated the efficacy of PRP therapy for knee OA was related to the IPF% in peripheral blood. This observation provides an important concept whereby the efficacy of autologous biological therapy depends on the patient's biological status. Further molecular biology studies might aid the development of a novel biological treatment strategy for OA.
Declaration of competing interest
This research was partially funded by Sysmex Corporation (costs related to measuring the IPF fraction using the Sysmex XN System).
Acknowledgements
This work was supported by Sysmex Corporation. The authors are grateful to the physicians and staff working at Juntendo University for supporting our work. We also thank Maya Kobayashi, Akiko Mutaguchi, and Kaori Isobe for supporting the data collection. We thank J. Ludovic Croxford, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Harker L.A., Roskos L.K., Marzec U.M., Carter R.A., Cherry J.K., Sundell B. Effects of megakaryocyte growth and development factor on platelet production, platelet life span, and platelet function in healthy human volunteers. Blood. 2000;95:2514–2522. [PubMed] [Google Scholar]
- 2.Ingram M., Coopersmith A. Reticulated platelets following acute blood loss. Br J Haematol. 1969;17:225–229. doi: 10.1111/j.1365-2141.1969.tb01366.x. [DOI] [PubMed] [Google Scholar]
- 3.Pons I., Monteagudo M., Lucchetti G., Muñoz L., Perea G., Colomina I. Correlation between immature platelet fraction and reticulated platelets. Usefulness in the etiology diagnosis of thrombocytopenia. Eur J Haematol. 2010;85:158–163. doi: 10.1111/j.1600-0609.2010.01468.x. [DOI] [PubMed] [Google Scholar]
- 4.Guthikonda S., Alviar C.L., Vaduganathan M., Arikan M., Tellez A., DeLao T. Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J Am Coll Cardiol. 2008;52:743–749. doi: 10.1016/j.jacc.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Finkel T., Serrano M., Blasco M.A. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 6.Benlachgar N., Doghmi K., Masrar A., Mahtat E.M., Harmouche H., Tazi Mezalek Z. Immature platelets: a review of the available evidence. Thromb Res. 2020;195:43–50. doi: 10.1016/j.thromres.2020.06.048. https://www.ncbi.nlm.nih.gov/pubmed/32652352 [DOI] [PubMed] [Google Scholar]
- 7.Chicharro-Alcántara D., Rubio-Zaragoza M., Damiá-Giménez E., Carrillo-Poveda J.M., Cuervo-Serrato B., Peláez-Gorrea P. Platelet rich plasma: new insights for cutaneous wound healing management. J Funct Biomater. 2018;9 doi: 10.3390/jfb9010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco A.T., Corken A., Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126:582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen R.T., Borg-Stein J., McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R. 2011;3:226–250. doi: 10.1016/j.pmrj.2010.11.007. https://www.ncbi.nlm.nih.gov/pubmed/21402369 [DOI] [PubMed] [Google Scholar]
- 10.Foster T.E., Puskas B.L., Mandelbaum B.R., Gerhardt M.B., Rodeo S.A. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. https://www.ncbi.nlm.nih.gov/pubmed/19875361 [DOI] [PubMed] [Google Scholar]
- 11.Alves R., Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. 2018;4:18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belk J.W., Kraeutler M.J., Houck D.A., Goodrich J.A., Dragoo J.L., McCarty E.C. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249–260. doi: 10.1177/0363546520909397. [DOI] [PubMed] [Google Scholar]
- 13.Campbell K.A., Saltzman B.M., Mascarenhas R., Khair M.M., Verma N.N., Bach B.R., Jr. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:2213–2221. doi: 10.1016/j.arthro.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 14.Wada A., Takagi Y., Kono M., Morikawa T. Accuracy of a new platelet count system (plt-f) depends on the staining property of its reagents. PLos One. 2015;10 doi: 10.1371/journal.pone.0141311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLong J.M., Russell R.P., Mazzocca A.D. Platelet-rich plasma: the paw classification system. Arthroscopy. 2012;28:998–1009. doi: 10.1016/j.arthro.2012.04.148. https://www.ncbi.nlm.nih.gov/pubmed/22738751 [DOI] [PubMed] [Google Scholar]
- 16.Jung H., Jeon H.K., Kim H.J., Kim S.H. Immature platelet fraction: establishment of a reference interval and diagnostic measure for thrombocytopenia. Korean J Lab Med. 2010;30:451–459. doi: 10.3343/kjlm.2010.30.5.451. [DOI] [PubMed] [Google Scholar]
- 17.Ko Y.J., Kim H., Hur M., Choi S.G., Moon H.W., Yun Y.M. Establishment of reference interval for immature platelet fraction. Int J Lab Hematol. 2013;35:528–533. doi: 10.1111/ijlh.12049. [DOI] [PubMed] [Google Scholar]
- 18.Murray I.R., Geeslin A.G., Goudie E.B., Petrigliano F.A., LaPrade R.F. Minimum information for studies evaluating biologics in orthopaedics (mibo): platelet-rich plasma and mesenchymal stem cells. J Bone Jt Surg Am. 2017;99:809–819. doi: 10.2106/JBJS.16.00793. https://www.ncbi.nlm.nih.gov/pubmed/28509821 [DOI] [PubMed] [Google Scholar]
- 19.Buttarello M., Mezzapelle G., Freguglia F., Plebani M. Reticulated platelets and immature platelet fraction: clinical applications and method limitations. Int J Lab Hematol. 2020;42:363–370. doi: 10.1111/ijlh.13177. [DOI] [PubMed] [Google Scholar]
- 20.Mannuß S. Influence of different methods and anticoagulants on platelet parameter measurement. J Lab Med. 2020;44:255–272. doi: 10.1515/labmed-2020-0037. https://www.degruyter.com/view/journals/labm/44/5/article-p255.xml [DOI] [Google Scholar]
- 21.Milants C., Bruyere O., Kaux J.F. Responders to platelet-rich plasma in osteoarthritis: a technical analysis. BioMed Res Int. 2017;2017:7538604. doi: 10.1155/2017/7538604. https://www.ncbi.nlm.nih.gov/pubmed/28904970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasterlain A., Braun H., Dragoo J. Contents and formulations of platelet-rich plasma. Operat Tech Orthop. 2012;22:33–42. doi: 10.1053/j.oto.2011.11.001. [DOI] [Google Scholar]