Abstract
Rat and mouse strains differ in behavioral and physiological characteristics, and such differences can contribute to explain discrepant results between laboratories and better select the most appropriate strain for a particular purpose. Differences in the activity of the hypothalamic-pituitary-adrenal (HPA) axis are particularly important given the pivotal role of this system in determining consequences of exposure to stressors. In this regard, Long-Evans (LE) rats are widely used in stress research, but there is no specific study aiming at thoroughly characterizing HPA activity in LE versus other extensively used strains. In a first experiment, LE showed higher resting ACTH and corticosterone levels only at certain points of the circadian rhythm, but much greater ACTH responsiveness to stressors (novel environment and forced swim) than Sprague-Dawley (SD) rats. Accordingly, enhanced corticotropin-releasing hormone (CRH) expression in the paraventricular nucleus of the hypothalamus and reduced expression of glucocorticoid receptors were observed in the hippocampal formation. Additionally, they are hyperactive in novel environments, and prone to adopt passive-like behavior when compared to SD rats. Supporting that altered HPA function has a marked physiological impact, we observed in another set of animals much lower thymus weight in LE than SD rats. Finally, to demonstrate that LE rats are likely to have higher HPA responsiveness to stressors than most strains, we studied resting and stress levels of HPA hormones in LE versus Wistar and Fischer rats, the latter considered an example of high HPA responsiveness. Again, LE showed higher resting and stress levels of ACTH than both Wistar and Fischer rats. As ACTH responsiveness to stressors in LE rats is stronger than that previously reported when comparing other rat strains and they are commercially available, they could be an appropriate model for studying the behavioral and physiological implications of a hyper-active HPA axis under normal and pathological conditions.
Keywords: Long-Evans, Strain differences, Stress responsiveness, Hypothalamic-pituitary-adrenal axis, Corticotropin-releasing hormone, Corticosteroid receptors
Highlights
-
•
Strain differences in hypothalamic-pituitary-adrenal (HPA) function were studied.
-
•
Long-Evans (LE) rats show greater HPA response to stressors than other strains.
-
•
CRH expression in critical brain areas is greater in LE than Sprague-Dawley (SD) rats.
-
•
Glucocorticoid receptor expression was lower in the hippocampal formation of LE rats.
-
•
LE rats are more active in novel environments but showed more passive coping.
1. Introduction
Behavioral and physiological differences among inbred rat or mouse strains have been repeatedly reported to be relevant for the study of the genetic and epigenetic bases of behavior and physiological processes. Particular attention has been paid to behavioral characteristics of animals that can model psychiatric diseases or make them susceptible or resilient to stress [Armario et al., 2013]. In this regard, an important number of studies has been devoted to the characterization of strain differences in the hypothalamic-pituitary-adrenal (HPA) axis [Trullás and Skolnick 1993; Armario et al., 1995; Ramos and Mormède, 1998] given the pivotal role of this system in determining behavioral and physiological consequences of stress. Two of the most studied inbred rats are Lewis and Fischer. Lewis rats showed hypo-responsiveness of the HPA axis to different types of stressors, whereas Fischer rats are likely to be in the extreme of higher responsiveness [Sternberg et al., 1989; Armario et al., 1995; Dhabhar et al., 1997]. Importantly, these differences have been demonstrated to exert a critical impact in the sensitivity to immune challenges, Lewis rats being particularly susceptible in comparison with Fischer rats [Sternberg et al., 1989].
However, if we are interested in relating the activity of the HPA axis with the behavioral consequences of stress or physiological changes in systems other than the HPA axis, the use of inbred strains can lead to erroneous conclusions as inbreeding has fixed a specific pattern of alleles in all genes influencing the function under study (e.g. function A). If we hypothesize that enhanced HPA activity can lead to detrimental consequences on function A, but the inbreeding process has also fixed some gene alleles that confer resistance of this function to stress, we would conclude that there is no relationship between HPA hyperactivity and function A. On the contrary, if the inbreeding process has fixed gene alleles unrelated to HPA activity that confer susceptibility to stress, we might conclude that there is a (false) relationship between HPA hyperactivity and function A. As an example [Armario et al., 1995], Fischer rats showed higher HPA response to the forced swim test than Lewis associated with a more active behavior, suggesting a positive relationship between active coping and HPA response. However, Lewis rats showed lower HPA response to the test than Wistar Kyoto rats, but higher levels of active behavior, suggesting a negative relationship. Fischer rats are also of great interest because they have been used in some studies searching for the genetic bases of HPA function [Solberg et al., 2003, Solberg et al., 2006; Marissal-Arvy et al., 2014].
The use of outbred animals differing in HPA function makes the problem of finding spurious relationships less likely, although not impossible, and therefore it would be more appropriate. Unfortunately, studies comparing outbred rat strains, particularly Wistar and Sprague-Dawley (SD), have demonstrated minor and inconsistent differences in HPA activity [Aulakh et al., 1988; Harbuz et al., 1994; Belda et al., 2004]. Nevertheless, evidence for genetically programmed differences in HPA function has been described. Touma et al. [Touma et al., 2008; Knapman et al., 2010] obtained, through genetic selection of CD1 outbred mice, lines showing high, intermediate or low corticosterone responsiveness to stressors. These lines have been nicely demonstrated to differ in critical components of the HPA axis, hippocampus-dependent learning and depression-like behavior, although contrary to what we would expect, high reactive mice showed greater active coping behavior in the forced swim test. Recently, rat lines differing in corticosterone responsiveness during adolescence have been generated from the commercially obtained Wistar strain, and they showed changes in the brain expression of genes related to the HPA axis [Walker et al., 2017]. These studies demonstrate an important degree of variability in the HPA axis in outbred rodents. Therefore, results obtained in outbred rodent strains are more representative of the whole population than inbred strains and consequently more generalized. However, typical albino rat strains are likely to incorporate lower and/or more biased genetic variability than wild life animals. In this regard, LE rats are of great interest because they originated from the crossing female Wistar rats with wild gray male rats, and it is likely that they maintain characteristics closer to wild animals than the typical laboratory rat strains.
LE rats have been extensively used in studies of maternal behavior and long-lasting consequences of early life experiences on behavioral and HPA responsiveness to stressors [Meaney et al., 1988; Plotsky et al., 1993]. Moreover, there is growing interest for the use of pigmented rats such as LE in tasks involving visual signals given the greater visual capabilities of the LE as compared with albino strains [e.g. Dupret et al., 2010; Meier et al., 2011]. In fact, LE rats behave similarly to wild rats in spatial memory tasks and better than other albino or pigmented laboratory rat strains, suggesting that domestication per se is not a critical factor [Harker et al., 2002]. In experiments with Long-Evans (LE) rats about early life stress done in our laboratory we noted that they showed greater stress levels of adrenocorticotropin (ACTH) and corticosterone than those typically observed in SD rats used by us in most experiments [Fuentes et al., 2014; Belda et al., 2016]. Differences were apparently much greater than those observed in rats located in the extreme of HPA responsiveness: Lewis versus Fischer [Armario et al., 1995; Dhabhar et al., 1995, 1997].
However, despite the interest for LE rats, direct and extensive comparison of HPA functioning in LE vs typical rat strains have not been done or has been restricted to corticosterone [Konkle et al., 2003; 2010]. Although Faraday et al., [2005] did compare ACTH and corticosterone levels in SD and LE rats, only the response to daily repeated restraint was studied, with no strain differences. However, repeated exposure to stressors resulted in adaptation and this might be different in the two strains. This paucity of data prompted us to directly compare HPA function in SD and LE rats, results showing higher levels of ACTH and corticosterone restricted to some times of the day, but much greater HPA responsiveness to stressors. To further demonstrate that LE rats actually have greater HPA activity than any other well-studied strains we compared in an additional study Wistar, LE and Fischer rats, which confirmed our assumption. Thus, LE rats can be used as an experimental model to relate exacerbated responsiveness of the HPA axis with relevant behavioral and physiological consequences of exposure to stressors. Our study was restricted to males as we emphasized a thorough characterization of the HPA axis that would have made it difficult to include both sexes. On the basis of the present results, further characterization of female LE rats respect to other strains is needed.
2. Material and methods
2.1. Animals and general procedures
Male rats SD, LE, Wistar and Fischer rats were purchased from Janvier Labs (France) at about 7 weeks of age. Strains were obtained from the same breeder as behavioral and hormonal differences have been reported in rats of the same strain raised in different breeding centers [Pecoraro et al., 2006]. Animals were housed in pairs with animals of the same strain and treatment, under standard conditions of temperature (22 ± 1 °C), 12 h light/dark schedule (lights on at 08:00 h), and food and water ad libitum. No specific environmental enrichment program was used in the animal facilities. The experimental protocols were approved by the Ethics Committee of the Universitat Autònoma de Barcelona and carried out in accordance with the European Communities Council Directive 2010/63/EU and Spanish legislation (RD 53/2013). Animals were allowed to acclimate to the housing conditions and habituate to procedures for 3 weeks. All experimental procedures were always done in the lights on period (09.00–13:00 h), except for the circadian rhythm. Animals were blood sampled by the tail-nick procedure, which allow obtaining true resting levels of hormones, as described previously [Belda et al., 2004].
2.2. Experimental designs
In Exp. 1 rats were assigned to 4 experimental groups: (i) SD – basal (n = 6); (ii) SD – stress (n = 8); (iii) LE – basal (n = 6) and (iv) LE – stress (n = 8). The average age of the animals on experimental day 1 was 70 days. On day 1, all animals were exposed to the elevated plus-maze (EPM) to study anxiety-like behavior. On day 7, the circadian rhythm of resting HPA activity was studied. Five blood samples were collected at 10.00 h, 16.00 h, 19.00 h, 22.00 h and 2.00 h. Those times were selected as representative of the early lights on and late lights on periods, the peak and the progressive decline during the active lights off period. On day 14, behavioral and HPA response to a novel environment, the hole-board (HB), was studied. The last experimental day (day 17), the behavioral and HPA response to the forced swim test was assessed in 8 subjects from each strain, whereas the others remained undisturbed in their home cages (controls, n = 6).
The EPM [Pellow and File, 1986] consisted of four white wooden arms (46 × 10 cm) at right angles to each other connected to a central square and elevated 50 cm above the floor. Two of the opposite arms had walls (43 cm), whereas the other two were open arms. The rat was placed facing a closed arm, and the subject was considered to be in a given arm when all of the paws were inside. A black curtain surrounded the EPM to minimize external influences and a white bulb (25 W) was placed 1.20 m above the center of the apparatus. The two animals of the same home-cage were tested simultaneously in two independent mazes in the same room. The procedure lasted 5 min. Behavior was videotaped from the top for subsequent manual analysis. Time spent in open and closed arms, number of entries and ambulation in each type of arm were measured. Ambulations were measured by means of the number of crossings (with the 4 feet) of any of the two imaginary lines used to divide each arm. The apparatus was carefully cleaned between animals with a water solution containing soap.
The HB [File and Wardill, 1975] consisted of a rectangular white wooden box (62 × 53 × 28 cm) with a floor divided into 16 equal squares, containing four empty holes (diameter 4.5 cm). Each animal was placed in the periphery of the apparatus facing the wall. The procedure lasted for 15 min. Both animals from the same home-cage were tested simultaneously in two independent HBs in the same room. The number of central and peripheral ambulations (with all four paws into the abovementioned squares), central and peripheral rearings and number of explored holes (head-dipping at least until the level of the eyes) were measured. The behavior was videotaped from the top. A blood sample was taken after the test to assess hormonal levels after a mild stress (novel environment). The apparatus was cleaned between animals with a solution containing ethanol in tap water (5%, v/v).
In the forced swim test (FST), rats were placed in transparent cylindrical plastic tanks (height 40 cm, internal diameter 19 cm) containing water (36 °C) to a level of 25 cm. All tanks were separated by black screens. Water was always changed for each rat. The procedure lasted for 15 min. Since the classical forced swim is performed in water at 20 °C and this results in hypothermia [Dal-Zotto et al., 2000], we exposed the animals to water at 36 °C to avoid the physical component of swim at a lower temperature. Different behavior variables were measured [Armario et al., 1988]: (i) struggling was defined as strongly moving all four limbs to break the surface of the water or scratching the walls, (ii) immobility was defined as the animal remaining motionless to keep its head out of the water and (iii) the remaining activity was considered as swimming and calculated subtracting from the total time of the test the time the animal spent struggling and immobile. The behavior was videotaped from the front. A blood sample was taken after the test to assess the hormonal response to the stressor, and immediately after that forced swim-exposed rats as well as controls were anesthetized and perfused for further histological processing.
In Exp. 2 SD and LE rats (n = 10 per strain) maintained under non-stress conditions were euthanized when 86 days old to measure adrenal and thymus weight.
In Exp. 3 Wistar, LE and Fischer rats (n = 10 per strain) were blood sampled at two times (9.00 and 19.00 h) under resting conditions (D1) and after exposure to HB (D7) and forced swim (D10) between 09:00 and 13:00 h. Animals were 65-day-old on D1.
2.3. Histological procedures
2.3.1. Perfusion
The rats were anesthetized with isoflurane (2%) and then perfused trans-cardiac, first with saline solution (0.9% NaCl) for 2 min and then for 12 min with paraformaldehyde (PFA) 4% and sodium tetra-borate 3.8% (borax, 4 °C). Their brains were removed, submerged in PFA/borax and stored at 4 °C for 24 h. After this period, the PFA/borax was exchanged for a 30% (w/v) sucrose solution in potassium phosphate-buffered saline (KPBS). They were maintained at 4 °C until they were completely embedded in the cryoprotective solution (2–3 days). Subsequently, the brains were frozen in isopentane maintained at −50 °C on dry ice and conserved at −80 °C.
2.3.2. In situ hybridization
The CRH probe was generated from a pGEM-4Z plasmid containing an EcoRI fragment (1.2 kb) of cDNA of the rat (Dr. K. Mayo, Northwestern University, Evanston, IL, USA), linearized with HindIII. The GR probe was transcribed from a 500-bp rat cDNA fragment that encodes for the N-terminal region of the rat liver glucocorticoid receptor (GR, courtesy of Dr. K.R. Yamamoto and Dr. R. Miesfeld, Department of Biochemistry, University of Arizona, Tucson, AZ, USA). This fragment was subcloned from a 2.8-kb fragment and transfected into pGEM3 plasmid (courtesy of Dr. M.C. Bohn, Department of Pediatrics, Northwestern University Medical School, Chicago, IL, USA). It was linearized with BamHI. The mineralocorticoid receptor (MR) probe was generated from a pBS SK + plasmid containing an EcoRI/HindIII fragment (550 bp) from a 3′ coding region and 3’ untranslated region of rat MR cDNA (courtesy of Dr. K.R. Yamamoto and Dr. R. Miesfeld, Department of Biochemistry, University of Arizona, Tucson, AZ, USA). It was linearized with EcoRI. Radioactive antisense cRNA copies were generated using a transcription kit (SP6/T7 Transcription Kit, Roche, Germany) in the presence of [α-35S]-UTP (specific activity 1250 Ci/mmol, PerkinElmer, Spain). The transcription process was stopped by adding 40 μL of a sodium chloride–Tris–EDTA buffer solution (STE: 0.1 M NaCl, 10 mM Tris–HCl pH 8.0, 1 mM EDTA). Then, the product was heated during 5 min at 65 °C. The probes were isolated through gel filtration columns (mini Quick Spin RNA Columns, Roche) and stored at −20 °C.
Coronal cryostat sections (14 μm, Leica Microsystems, Germany) from the paraventricular nucleus of the hypothalamus (PVN) and the central amygdala (CeA) and the dorsal hippocampal formation (HF), corresponding to Bregma coordinates [Paxinos and Watson 1998] −1.20 mm to −2.16 mm for PVN, −2.28 mm to −3.84 mm for CeA, and −3.00 mm to −3.60 mm for HF, were obtained and preserved at −20 °C in cryoprotective solution. In situ hybridization and image analysis were performed in both hemispheres using 3 to 4 sections. CRH expression was measured in the PVN (medial dorsal parvocellular division, mpdPVN) and the CeA (lateral subdivision), where CRH is highly expressed [Watts et al., 1995]. Corticosteroid receptor expression was measured in those areas with the highest expression [Van Eekelen et al., 1988; Makino et al., 1995]: GR in the mpdPVN, CA3 region and dentate gyrus (DG) of the HF and MR in all HF regions (CA1, CA2, CA2 and DG). Signal for GR expression in the CeA was too low to be accurately measured.
The protocol used for the in situ hybridization was adapted from Simmons et al., [1989]. All solutions were pretreated with DEPC and sterilized before use. Sections were post-fixed in 4% PFA + Borax, digested with 0.01 mg/ml proteinase K (Roche, Germany), acetylated in 0.25% acetic anhydride, washed in 2 saline sodium citrate (SCC), dehydrated through graded concentrations of ethanol and air-dried. Thereafter, 100 μl of hybridization buffer containing 1 × 106 dpm of the labeled probe was spotted onto each slide and sealed with a coverslip. After a 16–18 h incubation in a humid chamber at 60 °C, the slides were washed in descending concentrations of SSC containing 1 mM DTT (Sigma, Spain), including one wash at 60 °C, digested with RNase A (0.02 mg/ml, GE Healthcare, UK), dehydrated through a series of ethanol solutions and air-dried. The slides were then exposed to an autoradiography film (XAR-5 Kodak Biomax MR, Kodak, Spain), for 9 h in the case of CRH in the PVN, for 44 h in the case of CRH in the CeA, for 7 days in the case of GR in the mpdPVN and for 3 days in the case of GR/MR in the HF, to obtain optimum signal. The films were revealed with a film processor (Fuji photo film FPM–100 A).
2.3.3. Image analysis
Densitometric analyses of mRNAs were done on the autoradiography films. The mRNA levels were semi-quantitatively determined in three sections per brain area (both hemisphere pooled) and animal. The sections to be analyzed were photographed under a 20 x microscope objective (Eclipse E400, Nikon, Japan) with DXM1200 digital camera (Nikon, Japan) and subsequently quantified with Image J. We set two different regions of interest (ROIs) in order to select the area in the case of mpdPVN and CeA, and from 4 to 10 ROIs in the case of CA1, CA2, CA3 and DG, subtracting the average background to the total signal of the areas. Measures were obtained in arbitrary units (square pixels average sum gray). In all cases, the intensity of the signal was within the linear range as evaluated by the 14C microscales (GE Healthcare, UK).
2.4. Biochemical analysis
Plasma ACTH and corticosterone levels were determined by double-antibody radioimmunoassay, as previously described [Muñoz-Abellán et al., 2011]. All samples to be compared were run in the same assay to avoid inter-assay variability. The intra-assay coefficient of variation was <6% for ACTH and corticosterone. The sensitivity was 12.5 pg/ml for ACTH and 1 ng/ml for corticosterone.
Plasma corticosteroid binding globulin (CBG) assay was performed as previously described [Martí et al., 1997] by ligand binding assay using tritium labeled corticosterone (corticosterone [1, 2, 6, 7 - 3H (N)], PerkinElmer, Spain) as a ligand in plasma samples taken at 10:00 h and 19:00 h on day 7.
2.5. Statistical analysis
Statistical Package for Social Sciences (SPSS, version 24 for Windows) was used for statistical analyses. In Exp. 1 comparing LE and SD rats, either t-test or two-way ANOVA was used when only between-subjects factors were considered and generalized linear models with repeated measures (generalized estimating equations model, GEE [Hardin and Hilbe, 2003] when the data included both between-subjects factor (strain) and within-subject factor (time). In Exp. 3 one-way ANOVA (strain factor, 3 levels) and post-hoc tests with Bonferroni corrections were used. Where appropriate data were log transformed to achieve homogeneity of variances. The criterion for significance was set at p < 0.05.
3. Results
3.1. Circadian pattern of SD and LE rats
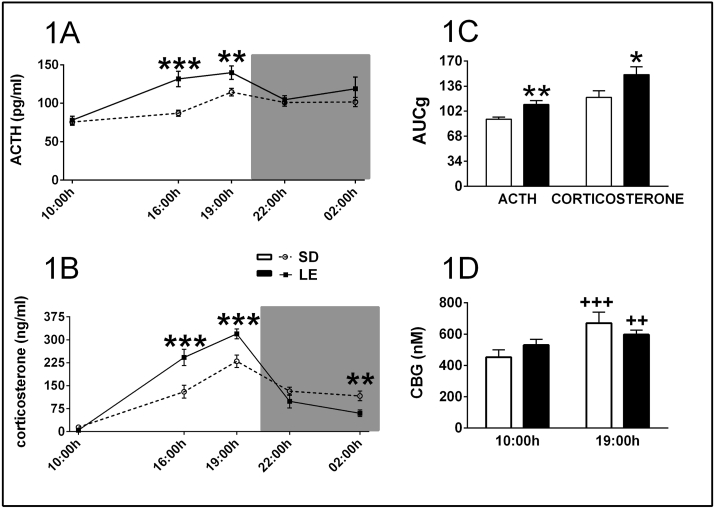
The statistical analysis (GEE) of the ACTH levels during the circadian rhythm (Fig. 1A) revealed a significant effect of strain (X2 (1) = 8.4 p ≤ 0.01), time (X2 (4) = 92.3 p ≤ 0.001) and strain × time interaction (X2 (4) = 15.3 p ≤ 0.01). The GEE analysis of the corticosterone changes showed a significant effect of time (X2 (4) = 584.6 p ≤ 0.01) and strain × time interaction (X2 (4) = 54.1 p ≤ 0.01) (Fig. 1B). The interaction strain x time suggested that strain differences in ACTH and corticosterone levels were dependent on the time of day, and therefore, the two strains were compared at each time. LE rats had higher levels of ACTH and corticosterone during the light cycle (16:00 h and 19:00 h) and lower levels of corticosterone during the night (02:00 h). The area under the curve relative to ground (AUCg) of hormone levels across the day was calculated by the Graph-Pad Prism software (version 5) using the trapezoid rule. The t-test analysis of AUC also showed a significant strain effect for ACTH (t (26) = −3.27 p ≤ 0.01) and corticosterone (t26 = −2.21 p ≤ 0.05) (Fig. 1C). Finally, the GEE analysis of the CBG levels during the circadian rhythm (Fig. 1D) revealed a significant effect of time (X2 (1) = 34.28 p ≤ 0.001) and strain × time interaction (X2 (1) = 9.58 p ≤ 0.01). Both strains showed higher CBG levels at 19:00 h respect to corresponding 10:00 h values (p ≤ 0.001 for SD and p ≤ 0.01 for LE), without statistically significant differences between the two strains at any time point.
Fig. 1.
LE and SD differences in the basal levels of peripheral pituitary-adrenal variables. It is represented Mean ± SEM (n = 14 per group) of the circadian pattern of plasma levels of ACTH and corticosterone (A and B), the area under the curve (AUCg) of the two hormones over the 24 h period (Panel C), and the plasma levels of CBG at two time points; ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 vs SD; +++ p ≤ 0.001, ++ p ≤ 0.01 vs. respective 10:00 h sampling time.
3.2. Comparison of the HPA response to stressors in SD and LE rats
After exposure to the HB, LE rats showed significantly higher levels of ACTH (t26 = −6.78 p ≤ 0.001) and corticosterone (t26 = −3.21 p ≤ 0.01) than SD rats (Fig. 2). Similar differences were observed between the two strains in the forced swim test regarding ACTH (t14 = −7.81 p ≤ 0.001), whereas no differences were observed in corticosterone (t14 = −0.905 p = 0.381) (Fig. 2).
Fig. 2.
LE and SD differences in the ACTH and corticosterone response to two acute stressors (15 min): hole-board (HB) and forced swim test. Mean ± SEM are represented (n = 14 for the HB, n = 8 for the forced swim test). Basal levels obtained on a different day were similar in the two strains and are represented with dotted lines. Blood sampling was done between 09.00 and 13.00 h. Hormone levels were higher in LE than SD rats except for corticosterone after the forced swim test; ***p ≤ 0.001, **p ≤ 0.01 vs SD.
3.3. Gene expression in SD and LE rats
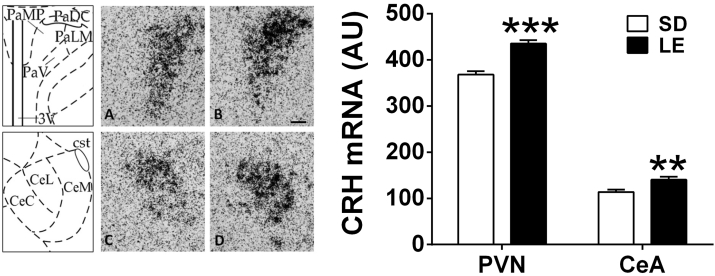
The two-way ANOVA did not reveal differences in CRH mRNA levels induced by forced swim stress exposure either in the PVN or the CeA. Therefore, data were pooled (control and acute stress) to show strain differences. LE group showed higher CRH mRNA levels in both areas, the PVN (t26 = −6.37 p ≤ 0.001) and the CeA (t25 = −3.11 p ≤ 0.01) (Fig. 3). CRH signal in the CeA was localized in the lateral part, in accordance with results from the literature [Watts and Sanchez-Watts, 1995].
Fig. 3.
LE and SD differences in CRH mRNA levels in the PVN and the CeA. Mean ± SEM of arbitrary units are represented (n = 13–14 per group). Brains were processed under non-stress conditions or immediately after the forced swim test. Since there were no differences between non-stress and stress conditions, all animals from the same strain were represented and analyzed together. Expression was studied in the areas showed in the left panels, corresponding to the mpdPVN (PaMP) and the lateral CeA (CeL). A and B: representative images of the PVN in SD (left) and LE (right) rats. C and D: representative images of CeA in SD (left) and LE (right) (100 μm scale bar); ***p ≤ 0.001, **p ≤ 0.01 vs SD.
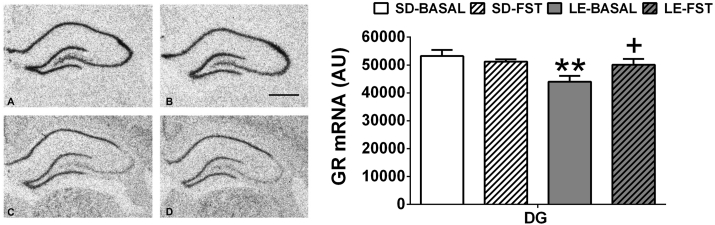
Strain and acute stress effects on GR mRNA levels were analyzed using two-way ANOVAs (Table 1 and Fig. 4). The effects were dependent on the particular area studied. In the PVN, no effects of strain or stress were found. In CA1, significantly lower levels were found in LE than SD (F1,26 = 5.87 p ≤ 0.05), whereas acute stress had no effect. In the DG, significant effects of strain (F1,26 = 7.7 p ≤ 0.05) and strain × stress interaction (F1,26 = 4.7 p ≤ 0.05) were found. Further decomposition of the interaction showed lower basal levels of GR mRNA levels in LE than SD rats (p ≤ 0.01) and a decrease after acute stress only in LE rats (p ≤ 0.05). The two-way ANOVA of MR mRNA levels revealed no effects of strain or stress in any of the areas studied (Table 1).
Table 1.
GR and MR expression in PVN and Hippocampal formation.
| GR mRNA (AU) |
MR mRNA (AU) |
|||||
|---|---|---|---|---|---|---|
| STRAIN | PVN | CA1 | CA1 | CA2 | CA3 | DG |
| SD | 346 ± 12 | 51,065 ± 1250 | 65,786 ± 1518 | 103,677 ± 1318 | 64,985 ± 1460 | 74,672 ± 1671 |
| LE | 320 ± 14 | 46,986 ± 1421* | 68,875 ± 1718 | 106,517 ± 1875 | 65,295 ± 1294 | 76,964 ± 1221 |
Mean ± SEM of arbitrary units (AU) are represented (n = 13–14). Brains were processed under non-stress conditions or immediately after the forced swim test. GR mRNA levels were quantified in PVN and CA1, and MR mRNA levels in all hippocampal formation regions: CA1, CA2, CA3 and dentate gyrus (DG). Since there were no differences between non-stress and stress conditions, all animals from the same strain were represented and analyzed together; *p ≤ 0.05 vs SD.
Fig. 4.
LE and SD differences in GR mRNA levels in the dentate gyrus (DG). Mean ± SEM of arbitrary units are represented (n = 5–6 for basal conditions; n = 7–8 for FST stress). A and B: representative images of MR mRNA expression in the hippocampal formation in SD (left) and LE (right) rats. C and D: representative images of GR mRNA expression SD (left) and LE (right). 500 μm scale bar. GR mRNA expression was lower in LE strain at basal levels in comparison to SD rats; **p ≤ 0.01 vs. SD-Basal, + p ≤ 0.05 vs. LE-Basal.
3.4. Behavioral differences between SD and LE rats
The t-test was used to analyze the behavior in the EPM (Table 2). Differences between strains were found in two variables: total arms entries (t26 = −2.33 p ≤ 0.05) and total arms ambulations (t26 = −2.50 p ≤ 0.05). LE group showed higher activity levels reflected in more entries and ambulation number in all arms. However, no differences were found in the measures related to anxiety-like behavior such as the percent time or entries into open arms in comparison to total arms.
Table 2.
Behavioral response in the elevated plus maze (5 min).
| STRAIN | OAE | CAE | TAE | % OAE | % TOA | OAA | CAA | TA |
|---|---|---|---|---|---|---|---|---|
| SD | 5.6 ± 0.6 | 5.1 ± 0.6 | 10.7 ± 0.9 | 52.2 ± 4.8 | 59.2 ± 5.4 | 23.6 ± 2.7 | 24.1 ± 3.2 | 47.7 ± 4.1 |
| LE | 8 ± 1.2 | 5.9 ± 0.7 | 13.9 ± 1.01* | 53.3 ± 6.9 | 53.8 ± 9.2 | 33.6 ± 4.6 | 28.6 ± 4.8 | 62.2 ± 4.1* |
Mean ± SEM are represented (n = 14 per group). OAE: open arm entries; CAE: closed arm entries; TAE: total arm entries; %TOA: percent time in open arms; OAA: open arm ambulations; CAA: closed arm ambulations; TA: total ambulations; *p ≤ 0.05 vs SD.
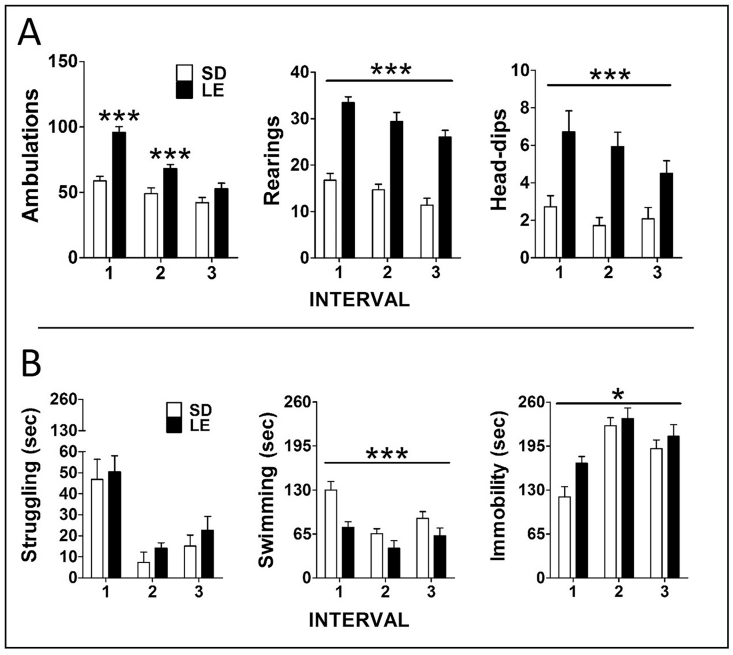
Behavior during the 15 min exposure to the HB in blocks of 5 min are depicted in Fig. 5A. The GEE analysis of total ambulation revealed significant effect of strain (X2 (1) = 26.6 p ≤ 0.001), time (X2 (2) = 106.6 p ≤ 0.001) and the interaction strain x time (X2 (2) = 22.9 p ≤ 0.001). The analysis of rearing episodes revealed significant effect of strain (X2 (1) = 114.3 p ≤ 0.001) and time (X2 (2) = 22.3 p ≤ 0.001), with no interaction. Regarding head-dips, only significant effect of strain was found (X2 (2) = 27.2 p ≤ 0.001).
Fig. 5.
Behavior of LE and SD rats during hole-board (HB) and forced swim exposure (15 min). Mean ± SEM of different behaviors (are represented in 5 min blocks (upper panel: HB; lower panel: forced swim). Number of rats per group were 14 in the HB and 8 in the forced swim test (the remaining rats were perfused under resting conditions). LE rats showed higher levels of ambulation during the first 10 min, and overall higher levels of rearing and head-dips at all times. ***p ≤ 0.01 vs SD strain. During forced swim, there were overall lower levels of swimming and higher levels of immobility in LE vs SD rats (strain effect); ***p ≤ 0.001, *p ≤ 0.05.
Behavior in the 15 min forced swim test was also analyzed in blocks of 5 min (Fig. 5B). The GEE analysis of struggling revealed no effect of strain, time or their interaction. The analysis of swimming revealed significant effect of strain (X2 (1) = 27.0 p ≤ 0.001) and time (X2 (2) = 13.4 p ≤ 0.001), with no interaction. Similar results were observed regarding immobility: strain (X2 (1) = 4.2 ≤ 0.05); time (X2 (2) = 68.7 p ≤ 0.001).
3.5. Body weight and adrenal and thymus weight in SD and LE rats
Results can be seen in Table 3. Body weight was lower in LE than SD rats (t (18) = 9.38 p ≤ 0.001). Absolute adrenal weight was lower in LE than SD rats (t (18) = 3.75 p ≤ 0.001), but no differences were observed in relative adrenal weight. Both absolute and relative thymus weight were lower in LE than SD rats (t (18) = 10.80 p ≤ 0.001; t (18) = 7.42 p ≤ 0.001, respectively).
Table 3.
Absolute and relative adrenal and thymus weight of SD and LE rats.
| STRAIN | Body weight (BW) g | Adrenal weight |
Thymus weight |
||
|---|---|---|---|---|---|
| Mg | mg/100 g BW | mg | mg/100 g BW | ||
| SD | 503.8 ± 8.4 | 71.9 ± 3.5 | 14.3 ± 0.7 | 719.8 ± 37.6 | 142.8 ± 6.9 |
| LE | 343.6 ± 4.5*** | 54.0 ± 3.3*** | 15.7 ± 0.9 | 298.7 ± 10.5*** | 87.0 ± 3.1*** |
Mean ± SEM are represented (n = 10 per group); ***p ≤ 0.001 vs SD.
3.6. Resting and stress levels of HPA hormones in Wistar, Fischer and LE rats
Results can be seen in Fig. 6. The one-way ANOVA revealed significant strain effect for resting ACTH levels at 9.00 h (F2,27 = 3.46, p = 0.046) and 19.00 h (F2,27 = 8.87, p = 0.001) and ACTH responses to HB (F2,27 = 16.44, p = 0.000) and FST (F2,27 = 28.27, p = 0.000). Post-hoc comparisons of ACTH levels showed higher lights on levels in LE than Fischer rats (p = 0.050), higher 19.00 h levels in LE than Wistar (p = 0.004) and Fischer (p = 0.003), and higher levels after HB and forced swim in LE than in the other two strains (always p < 0.001). The analysis of resting corticosterone levels revealed a significant strain effect at lights on (F2,27 = 4.32, p = 0.024) and a marginally significant effect before lights off (F2,27 = 3.05, p = 0.064), with a clear trend toward higher corticosterone levels in LE versus the other two strains. No strain differences after stress were observed.
Fig. 6.
Resting and stress levels of HPA hormones in Wistar, Fischer and LE rats. Means ± SEM (n = 10) are represented. Please note the different scales for both hormones depending on the experimental condition (panel A: resting levels at lights on and at the peak around lights off; Panel B and C: responses to hole-board and forced swim exposure for 15 min). * Indicates differences versus Wistar, + differences versus Fischer (one symbol p ≤ 0.05; two symbols p ≤ 0.01; three symbols p ≤ 0.001.
4. Discussion
Although LE rats have been extensively studied as an alternative to typical albino SD or Wistar rats, there are few studies comparing behavioral characteristics of LE to the latter strains, and only a few of them have included measures related to the HPA axis. We then first wanted to compare basal activity of the HPA axis and behavioral and HPA response of male LE and SD rats to various tests involving some degree of stress (novel environments and forced swim test). The results demonstrated that compared with SD rats, LE rats are characterized by modestly higher resting HPA activity, but much greater responsiveness of the HPA axis to acute stressors. To demonstrate that these characteristics of the HPA axis of LE rats are also observed when compared with several other strains, we did a new experiment with LE, Wistar and Fischer rats that essentially confirmed the previous results, thus suggesting that LE rats can be a model of HPA hyper-responsiveness to stressors.
4.1. HPA function in SD vs LE rats
We observed in both strains a minimum in ACTH levels in the early lights on hours (10.00 h) and a peak before lights off (19.00 h), to decrease further during the rest of the lights off period. However, LE rats showed higher levels at 16.00 h and 19.00 h, suggesting a sooner rise when approaching to the dark period. Similar but much more marked changes were observed in plasma corticosterone, with LE rats showing clearly greater levels at 16.00 h and 19.00 h. The lower levels of corticosterone in LE rats at 02.00 h reflect a stronger post-peak decrease in LE rats. The modest daily pattern of ACTH associated to a strong increase in corticosterone levels from early lights on to lights off is a well-described phenomenon in laboratory animals. It appears to be the result of a great amplification of adrenocortical responsiveness to circulating ACTH, mediated by sympathetic adrenal innervation [Dallman et al., 1978; Ulrich-Lai et al., 2006].
Although the results were suggestive of basal HPA hyperactivity in LE rats, most of the circulating levels of glucocorticoids are bound to CBG (corticosteroid-binding globulin or transcortin). The classical view considers that CBG determines the biologically active (free) fraction of glucocorticoids, but there is some evidence that CBG might act as a reservoir of glucocorticoids and reduce glucocorticoid availability under stress [Moisan, 2010; 2013]. Regardless of the exact role of CBG, it was considered important to determine CBG activity using a binding assay. No major strain differences were observed, indicating that the higher levels of total circulating corticosterone in LE rats should result in higher corticosterone availability to tissues. Nevertheless, a moderate increase in CBG was observed in both SD and LE rats at lights off, in accordance with previous results in rats [Hsu and Kuhn, 1988]. Overall, the data showed a flatter circadian rhythm of corticosterone in SD than LE rats, a pattern that has been associated to certain pathologies such as depression and chronic fatigue syndrome in humans [Chung et al., 2011; Kronfeld-Schor et al., 2012]. In rats, exogenous corticosterone administration can flatten its circadian rhythm without affecting the total amount of plasma corticosterone over the 24 h period. These treatments have been shown to alter hippocampal and prefrontal cortex release of serotonin, probably mediated by 5-HT1A autoreceptor desensitization [Leitch et al., 2003; Gartside et al., 2003], and to increase dopamine synthesis in the ventral tegmental area and resting and K+ -evoked release of dopamine in the medial prefrontal cortex [Minton et al., 2009].
LE rats not only showed higher basal levels of ACTH and corticosterone at certain times, but also a markedly higher response to stress. We studied the response to two stressors differing in intensity: a novel environment (HB) and forced swim. After the HB and the forced swim, LE rats showed greater ACTH response than all other strains (SD, Wistar and Fischer). Similarly higher levels of corticosterone were observed in LE rats after the HB; however, no differences were observed after the forced swim. This is not surprising considering that plasma corticosterone immediately after stress does not reflect circulating ACTH when these levels are above a certain threshold (about 300–400 pg/ml) because of the saturation of the adrenal cortex with these levels of ACTH [Armario, 2006]. Under these conditions of maximum adrenocortical secretion, plasma levels of corticosterone are related to adrenal size rather than to ACTH [Márquez et al., 2004]. As relative adrenal weight is similar in SD and LE rats [Konkle et al., 2010, present results], it appears that LE rats are more sensitive to stress than SD rats, but the maximum secretion capability of the adrenal cortex appears to be similar in both strains. The overall results thus strongly suggest that the main differences between the two strains are likely to be above the adrenal cortex. It is however of note that the size of the adrenal was not higher in LE than SD rats despite the overall greater basal and stress-induced secretion of ACTH, which exerts a trophic effect in the gland. This suggests that reduced trophic response to ACTH or other trophic factors is present in LE compared with SD rats.
The higher ACTH and corticosterone levels of LE rats under basal and stress conditions might be due to a greater release of CRH and other secretagogues into the median eminence or to a greater responsiveness of corticotrope cells to such secretagogues. However, the higher CRH expression in the PVN in LE than SD rats strongly favors that enhanced CRH release is the main contributing factor. This enhanced PVN CRH expression occurs despite the greater corticosterone secretion in LE rats, suggesting that the probably enhanced negative glucocorticoid feedback is not enough to counteract the central hyperactivity of the HPA axis.
The assumption that HPA hyperactivity has a relevant impact on the physiology of LE rats is supported by the markedly reduced thymus weight of LE compared to SD rats. Thymus weight is very sensitive to the levels of glucocorticoids in rats [Akana et al., 1985]. Although factors other than circulating levels of glucocorticoids can influence thymus weight [Marissal-Arvy et al., 2007], the present results strongly support the hypothesis that the reduced thymus weight of LE rats is mainly due to HPA hyperactivity.
Importantly, evidence was found for enhanced CeA CRH expression in LE rats. Since, CRH expression in this area is positively regulated by glucocorticoids [Watts and Sanchez-Watts, 1995], the greater corticosterone secretion in LE rats might contribute to this enhanced expression, although other factors are likely to be involved. Overexpression of CRH in CeA has been associated with a more emotional/anxious phenotype in some rat strains [Altemus et al., 1994–1995; Carrasco et al., 2008] and exacerbation of certain stress-related effects such as potentiated conditioned fear [Kolber et al., 2008] and visceral nociception [Su et al., 2015].
We did not observe any significant differences in MR or GR expression between basal and acute stress conditions, in accordance with previous results in the literature using brief exposure to stressors [e.g. Herman et al., 1995; Paskitti et al., 2000; Noguchi et al., 2010], except in GR in the DG where an increase was observed in LE rats but not in SD rats. More importantly, LE showed lower expression of GR in the DG and CA1 regions of the HF, but similar levels in the PVN. MR expression did not differ in any region of the HF. Therefore, strain differences appear to be mainly restricted to GR expression in the HF. The finding that strain differences were observed in certain HF regions in GR but not MR expression is consistent with the fact that MR appear to be less sensitive than GR to exogenous administration of glucocorticoids and chronic stress [e.g. Makino et al., 1995; Gómez et al., 1996; Noguchi et al., 2010]. Similarly, the lack of strain differences in GR expression in the mpdPVN is compatible with previous reports showing that GR expression in the whole hypothalamus or the PVN was maintained under conditions that decreased GR in the HF [Pfeiffer et al., 1991; Noguchi et al., 2010]. It is unclear at present whether higher overall levels of corticosterone in LE rats were the cause or the consequence of reduced GR expression. Excessive levels of circulating corticosterone due to higher overall resting levels and greater responsiveness to mild stressful routines might contribute to down-regulate GR expression. Although such down-regulation is typically observed only with severe stressors, the markedly low thymus weight of LE rats suggests that overall higher levels of corticosterone exert a great functional impact. On the other hand, it seems likely that a defective GR expression in the HF of LE rats might contribute to the hyperactivity of the HPA axis as reduced levels of GR in the HF are typically associated with impaired negative glucocorticoid feedback after exposure to emotional stressors [e.g. Sapolsky et al., 1985; Meaney et al., 1989]. Studies in adrenalectomized rats might help to distinguish between the two alternatives.
Differences in HPA function between LE and other rat strains have been addressed in some previous studies, although information was partial and hampered by certain methodologic drawbacks. The enhanced CRH expression reported here fits with a study showing enhanced CRH release into the pituitary portal blood of LE as compared with Wistar rats [Tannahill et al., 1988]. In the same study, increased levels of plasma ACTH and corticosterone were observed in LE rats during early lights on hours, although it is unclear whether they represented truly basal levels or enhanced stress responsiveness. Obtaining truly basal levels of corticosterone (10–20 ng/ml in the early lights on period) is difficult and the reported results are likely to reflect altered responsiveness to minor stressors associated to the sampling procedure rather than actual resting levels. In general, minor or null differences between LE and SD rats have been reported in resting levels of corticosterone at lights or in response to stress [Faraday et al., 2005; Konkle et al., 2003; 2010]. Faraday et al., [2005] did not observe differences in basal or stress levels of ACTH and corticosterone between LE and SD rats, although stress levels were obtained after repeated restraint, adaptation to stress perhaps precluding detection of differences in ACTH.
The higher HPA responsiveness to stressors observed here is supported by a previous study reporting enhanced corticosterone response to novelty stress in LE compared with Wistar rats [Jurcovicova et al., 1984]. Interestingly, the HPA response of LE rats to isoproterenol (β-adrenergic agonist) administration is similar to that of Wistar rats [Vermes et al., 1981; Berkenbosch et al., 1983]. Isoproterenol-induced ACTH release requires the hypothalamic-pituitary integrity [Vermes et al., 1981]. Therefore, it appears that there is not a generalized greater response of the HPA axis to any stimulus and that the exacerbated response to emotional stressors in LE rats is unlikely to be mainly due to a greater responsiveness of anterior pituitary corticotropic cells.
4.2. Behavioral differences between LE and SD rats
Exposure to the EPM, a classical test of anxiety in rodents, did not reveal differences between LE and SD in those measures associated with anxiety-like behavior (open arm entries and time). However, total entries and ambulation in all EPM arms were higher in LE rats, suggesting hyperactivity in novel environments that was confirmed in the HB. Importantly, the number of head-dips was also markedly greater in LE vs SD rats, indicating high motivation to explore. The pattern of activity over time was quite similar in the two strains, although habituation of horizontal activity was greater in LE than SD rats. Previous reports comparing LE with SD rats support that LE rats show higher levels of activity/exploration in several environments (EPM, HB, open-field) [Padilla et al., 2009; Turner and Burne, 2014].
In the FST, the main differences between the two strains appeared during the first 5 min, with LE rats showing similar levels of struggling-diving, but clearly lower levels of swimming, thus resulting in higher levels of immobility. These results suggest that LE rats are more prone to passive coping strategies than SD rats when firstly confronted with apparently inescapable situations. Previous comparisons of forced swim behavior between LE and other rat strains has led to inconclusive results [Abel et al., 1992; Vieyra-Reyes et al., 2008; Konkle et al., 2010; Koss et al., 2012], but it is of note that in all cases, behavior was only measured during the second forced swim exposure (test) carried out 24 h (or more) after a first pretest exposure and temperature of water was lower than in the present study. Although the pretest-test procedure was that originally described by Porsolt et al., [1977] to evaluate antidepressants, behavior during the pretest is better to evaluate coping strategies not contaminated by individual or strain differences in memory about prior experience [Armario et al., 1988; Martí and Armario, 1993]. Swimming appears to be more sensitive to antidepressants inhibiting serotonin reuptake whereas struggling is more sensitive to antidepressants inhibiting noradrenaline reuptake [López-Rubalcava and Lucki, 2000], and it is tempting to speculate that LE might have a defective serotoninergic system. Unfortunately, there is only one report about differences in the serotoninergic system between LE and SD rats that showed no differences in turnover in the cingulate cortex, the caudate and the nucleus accumbens [Baumann et al., 1998].
It is difficult to know whether or not some of the behavioral changes observed in LE vs SD rats are mediated or modulated by the changes in the activity of the HPA axis. However, in comparing different rat strains, a dissociation has been typically observed in our laboratory between anxiety-like behavior in novel environments or forced swim coping behavior and the activity of the HPA axis [Márquez et al., 2006; Armario et al., 1995]. In the literature, overall results point to the same conclusion at least regarding anxiety [Armario et al., 2013]. Given the important role of glucocorticoids in the consolidation of fear memory [Rodrigues et al., 2009], the study of the possible differential sensitivity of this strain to fear conditioning is warranted.
4.3. Peripheral HPA function in LE versus Wistar and Fischer rats
Comparison of resting levels of HPA hormones in the three strains confirmed that resting ACTH levels at lights off and after HB and forced swim exposure were higher in LE than the other two strains, in accordance with the previous results in LE versus SD. However, differences in corticosterone levels at lights off and after HB did not reach statistical significance, although a clear trend was found. It is likely that such differences would appear with a higher number of animals, but the data are consistent with the assumption that the main differences between LE and other strains are likely to be located within the brain.
Strain/lines comparisons of HPA activity have been repeatedly reported, and Lewis and Fischer rats were considered the extremes of low and high HPA responsiveness, respectively [Armario et al., 1995; Dhabhar et al., 1995; 1997]. However, differences between the two strains were sometime modest unless exposure to stressors was prolonged for several hours [Uchida et al., 2008]. We reported here that probably LE rats showed clearly greater HPA responsiveness than most strains, to a level at least similar to lines genetically selected for corticosterone hyper-responsiveness in mice [Touma et al., 2008] and rats [Walker et al., 2017].
5. Limitations and conclusions
The present results demonstrate that male LE rats have higher resting HPA activity, but more particularly higher HPA responsiveness to stressors when compared with SD and Wistar outbred rat strains, but also with Fischer rats, an inbred strain, considered to model HPA hyper-responsiveness. One important imitation of the present study is that we studied only males and it would be important to extend the characterization of HPA function in LE versus other strains to females.
Although the HPA hyperactivity observed in LE rats mainly affects ACTH rather than corticosterone, a modestly higher resting HPA hyperactivity combined with a greater responsiveness to regular daily stressors can result in a higher area under the curve of corticosterone release over a period of time. The clearly lower thymus weight in LE versus SD rats strongly supports this possibility and the immune consequences of such hyperactivity are likely to be important and deserve to be explored. Given these marked differences, LE rats might be considered as an excellent outbred and commercially available model to study the role of HPA hyperactivity in physiological and behavioral alterations, both under normal conditions and after stress.
6. Ethics
The experimental protocols were approved by the Ethics Committee of the Universitat Autònoma de Barcelona in accordance with the “Principles for the care of laboratory animals” and were carried out in accordance with the European Communities Council Directive 2010/63/EU and Spanish legislation (RD 53/2013).
Funding sources
The laboratory was supported by Spanish grants to AA and RN from the Ministerio de Economía y Competitividad (SAF2017-83430R) and Generalitat de Catalunya (SGR2017-457). RN was a recipient of an ICREA-ACADEMIA award (Generalitat de Catalunya). The funding sources had no role either in the design, collection, analysis, and interpretation of the data or in the decision to submit the article for publication. The Universitat Autònoma de Barcelona animal facility received funding from 2015FEDER7S–20IU16-001945.
Author contributions
M-SO: investigation, data curation, formal analysis, visualization, writing-original draft
L-SB: investigation, data curation, formal analysis, visualization, writing-original draft
SF: investigation, data curation
HG: investigation
XB: investigation
PM: investigation, editing
JC: methodology, validation
RN: conceptualization, formal analysis, data curation, writing-original draft, funding acquisition
AA: conceptualization, methodology, data curation, writing-original draft, writing-review and editing, funding acquisition
Disclosure
The authors have no conflicts of interest to declare.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Data availability
Data will be made available on request.
References
- Abel E.L. Response to alarm substance in different rat strains. Physiol. Behav. 1992;51:345–347. doi: 10.1016/0031-9384(92)90151-q. [DOI] [PubMed] [Google Scholar]
- Akana S.F., Cascio C.S., Shinsako J., Dallman M.F. Corticosterone: narrow range required for normal body and thymus weight and ACTH. Am. J. Physiol. 1985;249:527–532. doi: 10.1152/ajpregu.1985.249.5.R527. [DOI] [PubMed] [Google Scholar]
- Altemus M., Smith M.A., Diep V., Aulakh C.S., Murphy D.L. Increased mRNA for corticotrophin releasing hormone in the amygdala of fawn-hooded rats: a potential animal model of anxiety. Anxiety. 1994-1995;1:251. doi: 10.1002/anxi.3070010602. 157. [DOI] [PubMed] [Google Scholar]
- Armario A., Gavaldà A., Martí O. Forced swimming test in rats: effect of desipramine administration and the period of exposure to the test on struggling behavior, swimming, immobility and defecation rate. Eur. J. Pharmacol. 1988;158:207–212. doi: 10.1016/0014-2999(88)90068-4. [DOI] [PubMed] [Google Scholar]
- Armario A., Nadal R. Individual differences and the characterization of animal models of psychopathology: a strong challenge and a good opportunity. Front. Pharmacol. 2013;4:137. doi: 10.3389/fphar.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A., Gavaldà A., Martí J. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology. 1995;20:879–890. doi: 10.1016/0306-4530(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Armario A. The hypothalamic-pituitary-adrenal axis: what can it tell us about stressors? CNS Neurol. Disord. - Drug Targets. 2006;5:485–501. doi: 10.2174/187152706778559336. [DOI] [PubMed] [Google Scholar]
- Aulakh C.S., Wozniak K.M., Hill J.L., Devane C.L., Tolliver T.J., Murphy D.L. Differential neuroendocrine responses to the 5-HT agonist m-chlorophenylpiperazine in Fawn-Hooded rats relative to Wistar and Sprague-Dawley rats. Neuroendocrinology. 1988;48:401–406. doi: 10.1159/000125041. [DOI] [PubMed] [Google Scholar]
- Baumann M.H., Horowitz J.M., Kristal M.B., Torres G. Effects of cocaethylene on dopamine and serotonin synthesis in Long-Evans and Sprague-Dawley brains. Brain Res. 1998;804:316–319. doi: 10.1016/s0006-8993(98)00714-8. [DOI] [PubMed] [Google Scholar]
- Belda X., Márquez C., Armario A. Long-term effects of a single exposure to stress in adult rats on behavior and hypothalamic-pituitary-adrenal responsiveness: comparison of two outbred rat strains. Behav. Brain Res. 2004;154:399–408. doi: 10.1016/j.bbr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Belda X., Nadal R., Armario A. Critical features of acute stress-induced cross-sensitization identified through the hypothalamic-pituitary-adrenal axis output. Sci. Rep. 2016;6:31244. doi: 10.1038/srep31244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkenbosch F., Vermes I., Buijs R.M., Tilders F.J. Vasopressin is not involved in the catecholamine-induced release of ACTH, alpha-MSH and beta-endorphin from the rat pituitary gland. Neuroendocrinology. 1983;37:117–121. doi: 10.1159/000123529. [DOI] [PubMed] [Google Scholar]
- Carrasco J., Márquez C., Nadal R., Tobeña A., Fernández-Teruel A., Armario A. Characterization of central and peripheral components of the hypothalamus-pituitary-adrenal axis in the inbred Roman rat strains. Psychoneuroendocrinology. 2008;33:437. doi: 10.1016/j.psyneuen.2008.01.001. 345. [DOI] [PubMed] [Google Scholar]
- Chung S., Son G.H., Kim K. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim. Biophys. Acta. 2011;1812:581–591. doi: 10.1016/j.bbadis.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Dallman M.F., Engeland W.C., Rose J.C., Wilkinson C.W., Shinsako J., Siedenburg F. Nycthemeral rhythm in adrenal responsiveness to ACTH. Am. J. Physiol. 1978;235:210–218. doi: 10.1152/ajpregu.1978.235.5.R210. [DOI] [PubMed] [Google Scholar]
- Dal-Zotto S., Martí O., Armario A. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behav. Brain Res. 2000;114:175–181. doi: 10.1016/s0166-4328(00)00220-5. [DOI] [PubMed] [Google Scholar]
- Dhabhar F.S., McEwen B.S., Spencer R.L. Adaptation to prolonged or repeated stress – comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- Dhabhar F.S., Miller A.H., McEwen B.S., Spencer R.L. Differential activation of adrenal steroid receptors in neural and immune tissues of Sprague Dawley, Fischer 344, and Lewis rats. J. Neuroimmunol. 1995;56:77–90. doi: 10.1016/0165-5728(94)00135-b. [DOI] [PubMed] [Google Scholar]
- Dupret D., O'Neill J., Pleydell-Bouverie B., Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat. Neurosci. 2010;13:995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraday M.M., Blakeman K.H., Grunberg N.E. Strain and sex alters effects of stress and nicotine on feeding, body weight, and HPA axis hormones. Pharmacol. Biochem. Behav. 2005;80:577–589. doi: 10.1016/j.pbb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- File S.E., Wardill A.G. The reliability of the hole-board apparatus. Psychopharmacologia. 1975;44:47–51. doi: 10.1007/BF00421183. [DOI] [PubMed] [Google Scholar]
- Fuentes S., Daviu N., Gagliano H., Garrido P., Zelena D., Monasterio N., Armario A., Nadal R. Sex-dependent effects of an early life treatment in rats that increases maternal care: vulnerability or resilience? Front. Behav. Neurosci. 2014;8:56. doi: 10.3389/fnbeh.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside S.E., Leitch M.M., Young A.H. Altered glucocorticoid rhythm attenuates the ability of a chronic SSRI to elevate forebrain 5-HT: implications for the treatment of depression. Neuropsychopharmacology. 2003;28:1572–1578. doi: 10.1038/sj.npp.1300201. 2003. [DOI] [PubMed] [Google Scholar]
- Gómez F., Lahmame A., de Kloet E.R., Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–337. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- Harbuz M.S., Jessop D.S., Lightman S.L., Chowdrey H.S. The effects of restraint or hypertonic saline stress on corticotrophin-releasing factor, arginine vasopressin, and proenkephalin A mRNAs in the CFY, Sprague-Dawley and Wistar strains of rat. Brain Res. 1994;667:6–12. doi: 10.1016/0006-8993(94)91707-8. [DOI] [PubMed] [Google Scholar]
- Hardin J.W., Hilbe J.M. Chapman and Hall/CRC; Boca Raton: 2003. Generalized Estimating Equations. [Google Scholar]
- Harker K.T., Whishaw I.Q. Place and matching-to-place spatial learning affected by rat inbreeding (Dark-Agouti, Fischer 344) and albinism (Wistar, Sprague-Dawley) but not domestication (wild rat vs. Long-Evans, Fischer-Norway) Behav. Brain Res. 2002;134:467–477. doi: 10.1016/s0166-4328(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Watson S.J. Stress regulation of mineralocorticoid receptor heteronuclear RNA in rat hippocampus. Brain Res. 1995;677:243–249. doi: 10.1016/0006-8993(95)00152-g. [DOI] [PubMed] [Google Scholar]
- Hsu B.R., Kuhn R.W. The role of the adrenal in generating the diurnal variation in circulating levels of corticosteroid-binding globulin in the rat. Endocrinology. 1988;122:421–426. doi: 10.1210/endo-122-2-421. [DOI] [PubMed] [Google Scholar]
- Jurcovicová J., Vigas M., Klír P., Jezová D. Response of prolactin, growth hormone and corticosterone secretion to morphine administration or stress exposure in Wistar-AVN and Long Evans rats. Endocrinol. Exp. 1984;18:209–214. [PubMed] [Google Scholar]
- Knapman A., Heinzmann J.M., Hellweg R., Holsboer F., Landgraf R., Touma C. Increased stress reactivity is associated with cognitive deficits and decreased hippocampal brain-derived neurotrophic factor in a mouse model of affective disorders. J. Psychiatr. Res. 2010;44:566–575. doi: 10.1016/j.jpsychires.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Kolber B.J., Roberts M.S., Howell M.P., Wozniak D.F., Sands M.S., Muglia L.J. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12004–12009. doi: 10.1073/pnas.0803216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle A.T., Baker S.L., Kentner A.C., Barbagallo L.S., Merali Z., Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Konkle A.T., Kentner A.C., Baker S.L., Stewart A., Bielajew C. Environmental-enrichment-related variations in behavioral, biochemical, and physiologic responses of Sprague-Dawley and Long Evans rats. J Am Assoc Lab Anim Sci. 2010;49:427–436. [PMC free article] [PubMed] [Google Scholar]
- Koss A.W., Einat H., Schloesser J.R., Manji K.H., Rubinow R.D. Estrogen effects on the forced swim test differ in two outbred rat strains. Physiol. Behav. 2012;106:81–86. doi: 10.1016/j.physbeh.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfeld-Schor N., Einat H. Circadian rhythms and depression: human psychopathology and animal models. Neuropharmacology. 2012;62:101–114. doi: 10.1016/j.neuropharm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Leitch M.M., Ingram C.D., Young A.H., McQuade R., Gartside S.E. Flattening the corticosterone rhythm attenuates 5-HT1A autoreceptor function in the rat: relevance for depression. Neuropsychopharmacology. 2003;28:119–125. doi: 10.1038/sj.npp.1300016. [DOI] [PubMed] [Google Scholar]
- López-Rubalcava C., Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Makino S., Smith M.A., Gold P.W. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- Marissal-Arvy N., Gaumont A., Langlois A., Dabertrand F., Bouchecareilh M., Tridon C., Mormède P. Strain differences in hypothalamic pituitary adrenocortical axis function and adipogenic effects of corticosterone in rats. J. Endocrinol. 2007;195:473–484. doi: 10.1677/JOE-07-0077. [DOI] [PubMed] [Google Scholar]
- Marissal-Arvy N., Heliès J.-M., Tridon C., Moisan M.-P., Mormède P. Quantitative trait lici influencing abdominal fat deposition and functional variability of the HPA axis in the rat. Horm. Metab. Res. 2014;46:635–643. doi: 10.1055/s-0034-1383574. [DOI] [PubMed] [Google Scholar]
- Márquez C., Nadal R., Armario A. Influence of reactivity to novelty and anxiety on hypothalamic-pituitary-adrenal and prolactin responses to two different novel environments in adult male rats. Behav. Brain Res. 2006;168:13–22. doi: 10.1016/j.bbr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Márquez C., Nadal R., Armario A. The hypothalamic-pituitary-adrenal and glucose responses to daily repeated immobilisation stress in rats: individual differences. Neuroscience. 2004;123:601–612. doi: 10.1016/j.neuroscience.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Martí J., Armario A. Effects of diazepam and desipramine in the forced swimming test: influence of previous experience with the situation. Eur. J. Pharmacol. 1993;236:295–299. doi: 10.1016/0014-2999(93)90601-d. [DOI] [PubMed] [Google Scholar]
- Martí O., Martín M., Gavaldà A., Giralt M., Hidalgo J., Hsu B.R., Kuhn R.W., Armario A. Inhibition of corticosteroid-binding globulin caused by a severe stressor is apparently mediated by the adrenal but not by glucocorticoid receptors. Endocrine. 1997;6:159–164. doi: 10.1007/BF02738959. [DOI] [PubMed] [Google Scholar]
- Meaney M.J., Aitken D.H., van Berkel C., Bhatnagar S., Sapolsky R.M. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Meaney M.J., Aitken D.H., Viau V., Sharma S., Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- Meier P., Reinagel P. Rat performance on visual detection task modeled with divisive normalization and adaptive decision thresholds. J. Vis. 2011;11(1):1–17. doi: 10.1167/11.9.1. [DOI] [PubMed] [Google Scholar]
- Minton G.O., Young A.H., McQuade R., Fairchild G., Ingram C.D., Gartside S.E. Profound changes in dopaminergic neurotransmission in the prefrontal cortex in response to flattening of the diurnal glucocorticoid rhythm: implications for bipolar disorder. Neuropsychopharmacology. 2009;34:2265–2274. doi: 10.1038/npp.2009.53. [DOI] [PubMed] [Google Scholar]
- Moisan M.P. CBG: a cortisol reservoir rather than a transporter. Nat. Rev. Endocrinol. 2013;9:78. doi: 10.1038/nrendo.2012.134-c1. [DOI] [PubMed] [Google Scholar]
- Moisan M.P. Genotype-phenotype associations in understanding the role of corticosteroid-binding globulin in health and disease animal models. Mol. Cell. Endocrinol. 2010;316:35–41. doi: 10.1016/j.mce.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Muñoz-Abellán C., Rabasa C., Daviu N., Nadal R., Armario A. Behavioral and endocrine consequences of simultaneous exposure to two different stressors in rats: interaction or independence? PloS One. 2011;6 doi: 10.1371/journal.pone.0021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Makino S., Matsumoto R., Nakayama S., Nishiyama M., Terada Y., Hashimoto K. Regulation of glucocorticoid receptor transcription and nuclear translocation during single and repeated immobilization stress. Endocrinology. 2010;151:4344–4355. doi: 10.1210/en.2010-0266. [DOI] [PubMed] [Google Scholar]
- Padilla E., Barrett D., Shumake J., Gonzalez-Lima F. Strain, sex, and open-field behavior: factors underlying the genetic susceptibility to helplessness. Behav. Brain Res. 2009;201:257–264. doi: 10.1016/j.bbr.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskitti M.E., McCreary B.J., Herman J.P. Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: time-course analysis. Mol. Brain Res. 2000;80:142–152. doi: 10.1016/s0169-328x(00)00121-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. fourth ed. Academic Press; San Diego: 1998. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Pecoraro N., Ginsberg A.B., Warne J.P., Gomez F., la Fleur S.E., Dallman M.F. Diverse basal and stress-related phenotypes of Sprague Dawley rats from three vendors. Physiol. Behav. 2006;89:598–610. doi: 10.1016/j.physbeh.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Peiffer A., Lapointe B., Barden N. Hormonal regulation of type II glucocorticoid receptor messenger ribonucleic acid in rat brain. Endocrinology. 1991;129:2166–2174. doi: 10.1210/endo-129-4-2166. [DOI] [PubMed] [Google Scholar]
- Pellow S., File S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Plotsky P.M., Meaney M.J. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol. Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Le Pichon M., Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Ramos A., Mormède P. Stress and emotionality: a multidimensional and genetic approach. Neurosci. Biobehav. Rev. 1998;22:33–57. doi: 10.1016/s0149-7634(97)00001-8. [DOI] [PubMed] [Google Scholar]
- Rodrigues S.M., LeDoux J.E., Sapolsky R.M. The influence of stress hormones on fear circuitry. Annu. Rev. Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., Meaney M.J., McEwen B.S. The development of the glucocorticoid receptor system in the rat limbic brain. III. Negative-feedback regulation. Brain Res. 1985;350:169–173. doi: 10.1016/0165-3806(85)90261-5. [DOI] [PubMed] [Google Scholar]
- Simmons D.M., Arriza J.L., Swanson L.W. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radio-labeled single-stranded RNA probes. J. Histotechnol. 1989;12:169–181. [Google Scholar]
- Solberg L.C., Ahmadiyeh N., Baum A.E., Vitaterna M.H., Takahashi J.S., Turek F.W., Redei E.E. Depressive-like behavior and stress reactivity are independent traits in a Wistar Kyoto x Fisher 344 cross. Mol. Psychiatr. 2003;8:423–433. doi: 10.1038/sj.mp.4001255. [DOI] [PubMed] [Google Scholar]
- Solberg L.C., Baum A.E., Ahmadiyeh N., Shimomura K., Li E., Turek F.W., Takahashi J.S., Churchill G.A., Redei E.E. Genetic analysis of the stress-responsive adrenocortical axis. Physiol. Genom. 2006;27:362–369. doi: 10.1152/physiolgenomics.00052.2006. [DOI] [PubMed] [Google Scholar]
- Sternberg E.M., Young W.S., 3rd, Bernardini R., Calogero A.E., Chrousos G.P., Gold P.W., Wilder R.L. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc. Natl. Acad. Sci. U. S. A. 1989;86:4771–4775. doi: 10.1073/pnas.86.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Tanaka Y., Muratsubaki T., Kano M., Kanazawa M., Fukudo S. Injection of corticotropin-releasing hormone into the amygdala aggravates visceral nociception and induces noradrenaline release in rats. Neuro Gastroenterol. Motil. 2015;27:30–39. doi: 10.1111/nmo.12462. [DOI] [PubMed] [Google Scholar]
- Tannahill L.A., Dow R.C., Fairhall K.M., Robinson I.C., Fink G. Comparison of adrenocorticotropin control in Brattleboro, Long-Evans, and Wistar rats. Measurement of corticotropin-releasing factor, arginine vasopressin, and oxytocin in hypophysial portal blood. Neuroendocrinology. 1988;48:650–657. doi: 10.1159/000125077. [DOI] [PubMed] [Google Scholar]
- Touma C., Bunck M., Glasl L., Nussbaumer M., Palme R., Stein H., Wolferstätter M., Zeh R., Zimbelmann M., Holsboer F., Landgraf R. Mice selected for high versus low stress reactivity: a new animal model for affective disorders. Psychoneuroendocrinology. 2008;33:839–862. doi: 10.1016/j.psyneuen.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Trullás R., Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berlin) 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- Turner K.M., Burne T.H. Comprehensive behavioural analysis of Long Evans and Sprague-Dawley rats reveals differential effects of housing conditions on tests relevant to neuropsychiatric disorders. PloS One. 2014;9 doi: 10.1371/journal.pone.0093411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Nishida A., Hara K., Kamemoto T., Suetsugi M., Fujimoto M., Watanuki T., Wakabayashi Y., Otsuki K., McEwen B.S., Watanabe Y. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur. J. Neurosci. 2008;27:2250–2261. doi: 10.1111/j.1460-9568.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Arnhold M.M., Engeland W.C. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:1128–1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- Van Eekelen J.A., Jiang W., De Kloet E.R., Bohn M.C. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J. Neurosci. Res. 1988;21:88–94. doi: 10.1002/jnr.490210113. [DOI] [PubMed] [Google Scholar]
- Vermes I., Berkenbosch F., Tilders F.J., Smelik P.G. Hypothalamic deafferentation in the rat appears to discriminate between the anterior lobe and intermediate lobe response to stress. Neurosci. Lett. 1981;27:89–93. doi: 10.1016/0304-3940(81)90210-x. [DOI] [PubMed] [Google Scholar]
- Vieyra-Reyes P., Mineur Y.S., Picciotto M.R., Túnez I., Vidaltamayo R., Drucker-Colín R. Antidepressant-like effects of nicotine and transcranial magnetic stimulation in the olfactory bulbectomy rat model of depression. Brain Res. Bull. 2008;77:13–18. doi: 10.1016/j.brainresbull.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S.E., Zanoletti O., Guillot de Suduiraut I., Sandi C. Constitutive differences in glucocorticoid responsiveness to stress are related to variation in aggression and anxiety-related behaviors. Psychoneuroendocrinology. 2017;84:1–10. doi: 10.1016/j.psyneuen.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Watts A.G., Sanchez-Watts G. Region-specific regulation of neuropeptide mRNAs in rat limbic forebrain neurones by aldosterone and corticosterone. J. Physiol. 1995;484:721–736. doi: 10.1113/jphysiol.1995.sp020698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.