Abstract
Background:
Biomechanical studies on anterior cruciate ligament (ACL) injuries and reconstructions are based on ACL transection instead of realistic injury trauma.
Purpose:
To replicate an ACL injury in vitro and compare the laxity that occurs with that after an isolated ACL transection injury before and after ACL reconstruction.
Study Design:
Controlled laboratory study.
Methods:
Nine paired knees were ACL injured or ACL transected. For ACL injury, knees were mounted in a rig that imposed tibial anterior translation at 1000 mm/min to rupture the ACL at 22.5° of flexion, 5° of internal rotation, and 710 N of joint compressive force, replicating data published on clinical bone bruise locations. In contralateral knees, the ACL was transected arthroscopically at midsubstance. Both groups had ACL reconstruction with bone–patellar tendon–bone graft. Native, ACL-deficient, and reconstructed knee laxities were measured in a kinematics rig from 0° to 100° of flexion with optical tracking: anterior tibial translation (ATT), internal rotation (IR), anterolateral (ATT + IR), and pivot shift (IR + valgus).
Results:
The ACL ruptured at 26 ± 5 mm of ATT and 1550 ± 620 N of force (mean ± SD) with an audible spring-back tibiofemoral impact with 5o of valgus. ACL injury and transection increased ATT (P < .001). ACL injury caused greater ATT than ACL transection by 1.4 mm (range, 0.4-2.2 mm; P = .033). IR increased significantly in ACL-injured knees between 0° and 30° of flexion and in ACL transection knees from 0° to 20° of flexion. ATT during the ATT + IR maneuver was increased by ACL injury between 0° and 80° and after ACL transection between 0° and 60°. Residual laxity persisted after ACL reconstruction from 0° to 40° after ACL injury and from 0° to 20° in the ACL transection knees. ACL deficiency increased ATT and IR in the pivot-shift test (P < .001). The ATT in the pivot-shift increased significantly at 0° to 20° after ACL transection and 0° to 50° after ACL injury, and this persisted across 0° to 20° and 0° to 40° after ACL reconstruction.
Conclusion:
This study developed an ACL injury model in vitro that replicated clinical ACL injury as evidenced by bone bruise patterns. ACL injury caused larger increases of laxity than ACL transection, likely because of damage to adjacent tissues; these differences often persisted after ACL reconstruction.
Clinical Relevance:
This in vitro model created more realistic ACL injuries than surgical transection, facilitating future evaluation of ACL reconstruction techniques.
Keywords: anterior cruciate ligament, injury mechanism, ACL reconstruction
The effects of anterior cruciate ligament (ACL) injury and reconstruction on knee kinematics have been studied in vitro and in vivo.6,13,25,26,44 During clinical examination, isolated ACL injury causes increased anterior tibial translation (ATT), shown as positive results from Lachman and anterior drawer tests, and increased rotational instability, presenting as a positive finding by pivot-shift test.13,19,34 These clinical tests are more pronounced when the ACL injury is accompanied by injury to the meniscus and/or peripheral soft tissues.13,19,33,34,39
Biomechanical cadaveric studies of ACL injuries and reconstructions have transected the ACL in isolation, finding increased laxity similar to clinical observations.10,12,16,25,46 However, while studies based on ligament transection do always find increased ATT, despite not simulating a realistic injury mechanism, the effect of isolated ACL transection on knee rotation is unclear. Studies that transect the ACL have reported changes of up to 2° to 3° of rotational laxity near knee extension,12,25,30,31 and this might underestimate the effects of real-life injury since stretching or injury of adjacent tissues is not present. Recent magnetic resonance imaging (MRI) studies37,38,42,46 have provided the evidence on which to base a more realistic experimental design, which would cause ACL ruptures with bone-bone displacements reflecting measurements of the locations of bone bruises after ACL ruptures.
Based on the discrepancy between significant clinical findings and the small changes in ACL transection studies, the purpose of this experiment was to simulate a realistic ACL injury and compare the effect on knee laxity with that caused by the common experimental practice of ACL transection. It was hypothesized that a simulated ACL injury would cause greater translational and rotational laxity than that caused by ACL transection, by exposing other soft tissues to stretching injury. It was also hypothesized that there would be greater residual laxity after ACL reconstruction in knees with ACL injury than in those with ACL transection.
Methods
After ethics approval by Imperial College Healthcare Tissue Bank, 11 pairs of fresh-frozen human knees were obtained from MedCure. They were stored at −20°C and thawed for 24 hours at room temperature before preparation. They were confirmed to have no evidence of previous surgery, abnormal laxity, or misalignment by visual and manual examination by an orthopaedic surgeon (L.W.). Two pairs were excluded owing to ACL insufficiency and grade IV osteoarthritis, leaving 9 pairs for testing (mean age, 49 years [range, 29-62 years]; 7 male, 2 female). Specimens within each pair were randomly allocated to the ACL injury or ACL transection group. Knees were kept moist with occasional water spray during the entire test.
Specimen Preparation
The femur and tibia were each cut to 17 cm in length, and the soft tissue was removed beyond 7 cm above and below the joint line. The fibula was cut to 7 cm long and secured to the tibia in its anatomic position by a transverse transcortical bone screw near the distal end, thus preserving normal joint laxity.23,28 The femur was embedded into a 6-cm cylindrical tube using PMMA bone cement (poly-methyl methacrylate). The tibia was consecutively cemented into a 4-cm pot with a 50-cm axial extension rod (for applying loads in the kinematics rig) and then into a 6-cm cylindrical pot to allow mounting into the testing machine.
Optical Tracking Measurements
A Polaris camera system (Vega; Northern Digital Inc) was used to track BrainLab reflective markers rigidly secured to the femur and tibia with bicortical rods. The measurement procedure had been developed and described previously20,28 and had a translational accuracy of ± 0.1 mm. 21 The medial and lateral epicondyles, the proximal end of the femur, the most medial and lateral points of the tibial plateau, and the distal end of the tibia were marked with fiducial screws. These landmarks were digitized with a stylus probe to define the femoral and tibial coordinate systems. Zero degrees of flexion was defined as when the tibial and femoral pots were parallel when viewed in the sagittal plane, and 6 degrees of freedom motion was measured as the tibial movement relative to the femur.
Tibiofemoral Joint Laxity Measurement
The specimens with intact ligaments were mounted on a 6 degrees of freedom kinematics rig20,28,41 with the shaft of the femur in a fixed anatomic 6° of valgus, so the tibia hung vertically unconstrained. The rig allowed 0° to 100° of passive flexion-extension (Figure 1).
Figure 1.
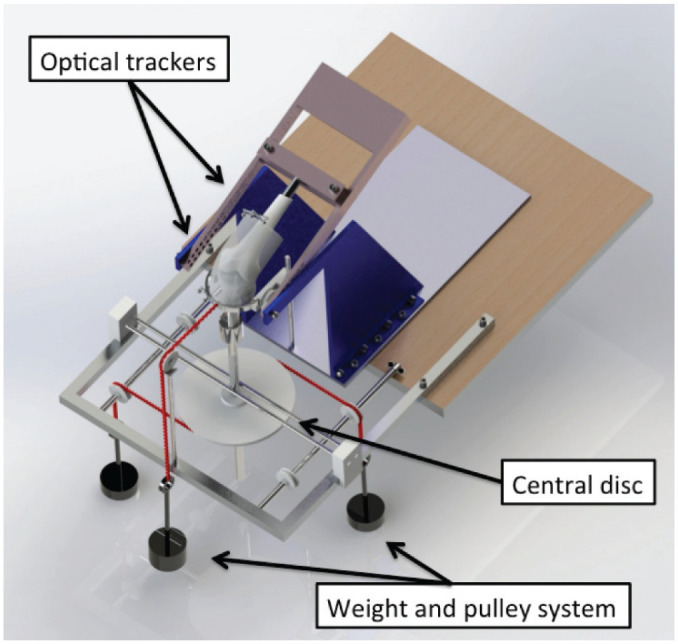
The knees were mounted in the 6 degrees of freedom rig, and optical trackers were securely drilled into bone. A pulley and weight system was used to apply external loads during the kinematic testing from 0° to 100° of knee flexion. Reprinted from Inderhaug E, Stephen JM, Williams A, Amis AA. Biomechanical comparison of anterolateral procedures combined with anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(2):347-354. © 2016 The Author(s). DOI: 10.1177/0363546516681555.
A 5.5-mm Steinmann pin was inserted mediolaterally through the proximal tibia, and 2 semicircular hoops were mounted to apply 90 N of anterior and posterior draw force by hanging weights routed by a string and pulley system while not constraining tibial internal-external rotation. Internal and external rotation torques of 5 N·m and valgus and varus angulation moments of 10 N·m were applied via a 250-mm pulley on the tibial extension rod. To simulate anterolateral laxity, a combined load of 90-N ATT force and 5-N·m internal rotation (IR) torque was applied. To simulate the tibial displacement caused by a pivot-shift test, a combined load of 5-N·m IR and 10-N·m valgus angulation was applied. The knee kinematics were measured across 0° to 100° of flexion-extension for 3 cycles while each load was applied: anterior-posterior translation, internal-external rotation, varus-valgus angulation, anterolateral laxity testing, and simulated pivot shift (SPS).
ACL Injury
To cause an ACL injury, knees were mounted onto a loading rig on a materials testing machine3,14 (model 5565; Instron Ltd) (Figure 2). The tibia was clamped horizontally to the moving Instron crosshead, and the femur was fixed to a pivot frame on linear bearings on the base of the rig. The femoral mounting allowed unconstrained varus-valgus rotation and axial joint compression. The tibial mounting allowed medial-lateral translation, while the materials testing machine imposed the ATT.
Figure 2.
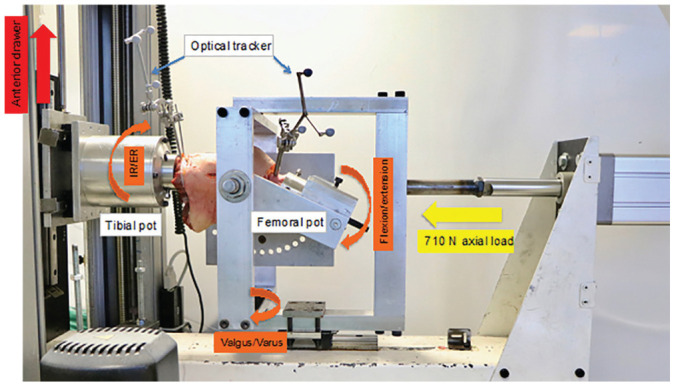
The cadaveric knee was mounted in a rig connected to a single-axis materials testing machine. The tibia was aligned horizontally and in 5° of internal rotation while the knee was fixed in 22.5° of flexion, seen as the angle of the femoral pot below horizontal. A 710-N axial joint compression force was applied in line with the tibia while a 30-mm anterior tibial translation was imposed along the vertical axis of the test machine, at the top left of the picture. The optical trackers measured the resulting displacements of the tibia against the femur. ER, external rotation; IR, internal rotation. The Appendix Video shows the test in progress.
The neutral anteroposterior position of each knee was determined first by visual approximation and then by applying ±3 mm of ATT with 10 N of axial joint compressive force and free internal-external rotation. The neutral position was defined when the point of inflection of the force-displacement hysteresis loop was symmetrical above and below the zero force axis. 3 A 710-N axial compressive force was then applied by an air cylinder (model CQ2-A100-200-DCZ; SMC Corp) to simulate weightbearing while the injury occurred; any coupled ATT effect (attributed to the posterior slope of the tibial plateau) was inhibited by the stiffness of the specimen mountings, and so it was not measured separately but was a part of the total ATT recorded at ACL failure.
An ACL injury was then caused by applying 30 mm of ATT at 1000 mm/min while the knee was held at 22.5° of flexion and 5° of IR. The knee flexion, IR, and ATT were based on an MRI study that deduced the position of the knee when a clinical ACL injury occurs. 37 The knee translations and rotations during the injury were recorded by optical tracking and a force-versus-ATT curve was logged by the Instron machine.
ACL Transection
In the ACL transection group, specimens were kept in the kinematics rig, and the ACL was transected arthroscopically across the midsubstance with a No. 11 blade.
ACL Reconstruction
After the ACL-deficient laxity was recorded (for ACL-injured and ACL-transected specimens), an arthroscopic examination was performed to diagnose all visible intra-articular injuries in addition to the ACL deficiency, and the status of the cartilage was documented. A standardized arthroscopic ACL reconstruction was performed by an experienced orthopaedic surgeon (L.W.) while the knee remained in the kinematics rig. A 10-mm bone–patellar tendon–bone autograft was harvested, and the bone blocks were sutured (Ultrabraid; Smith & Nephew). It was pretensioned with an 80-N hanging weight for 20 minutes. The ACL remnant was resected arthroscopically, and the femoral and tibial tunnels were drilled in the centers of the anatomic attachments. After insertion of the graft, the femoral bone block was fixed with a 7 × 25–mm interference screw (RCI; Smith & Nephew). Before tibial fixation, the graft was preconditioned in its intra-articular position by cycling the knee 40 times while the graft was manually tensioned. 36 The knee was held in 30° of flexion and neutral tibial rotation while the tibia was reduced by a 70-N posterior force. The graft was tensioned with 44-N by means of a tensiometer, 18 while the tibial bone block was secured with a 9 × 35–mm interference screw (RCI). 18 To avoid slippage, a backup fixation was used on the femur and tibia by tying the bone block sutures to cortical bone screws. The kinematics of the reconstructed knees were then measured as before.
Statistical Analysis
MATLAB scripts (MathWorks) were used to process the motion capture data to calculate translations and rotations of the tibia relative to the femur at 10° intervals from 0° to 100° of knee flexion. Statistical analysis was conducted via SPSS (Version 24.0; IBM). Shapiro-Wilk tests confirmed that the data were normally distributed. Multiple 2-way repeated measures analyses of variance were used to compare knee translations and rotations within and between the ACL injury and ACL transection groups across different flexion angles. When differences were found between or within groups, post hoc paired t tests with Bonferroni correction were applied for the individual flexion angles. Statistical significance was set at P < .05.
Results
ACL Injury
The ACL injury mechanism induced an ACL injury in all 9 specimens. Under arthroscopic inspection, 6 femoral-sided midsubstance ACL injuries and 3 ACL tibial avulsion fractures were identified, while no other intra-articular injuries were found (Figure 3). The force-displacement curve of the materials testing machine revealed a peak ATT force of 1549 ± 619 N (mean ± SD) at a crosshead movement of 25.7 ± 5.1 mm at ACL rupture. The ACL rupture caused a clear and audible “crack/pop” spring-back of the knee, releasing stored energy of the ACL fibers (Appendix Video). The optical tracking showed an ATT of 23.3 ± 3.4 mm and a valgus angulation of 5.1°± 3.9°, above the 6° of anatomic valgus of the knee at the neutral position, when the ACL injury occurred.
Figure 3.
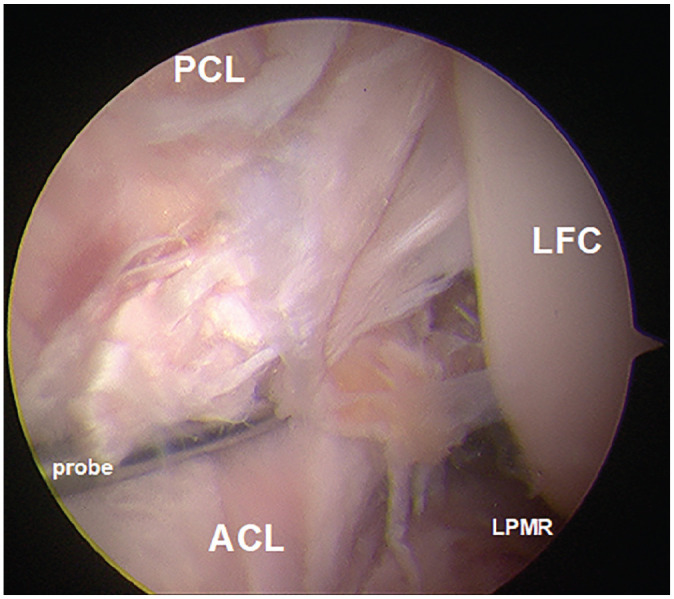
The arthroscopic view from the anterolateral portal revealed a femoral ACL detachment after injuring the ACL in the materials testing machine. ACL, anterior cruciate ligament; LFC, lateral femoral condyle; LPMR, lateral posterior meniscus root; PCL, posterior cruciate ligament.
Kinematics
Anterior Translation
Both groups (ACL injury and ACL transection) showed the same ATT with the ACL intact. The ACL injury and ACL transection each caused a significant increase in ATT (P < .001) (Figure 4). The ATT of the ACL injury group was significantly larger (P = .033) than that of the ACL transection group across the flexion arc. The mean difference of ATT was 1.4 mm (range, 0.4-2.2 mm), with the largest difference at 20° of flexion (P = .058). The ACL reconstruction reduced ATT to normal (P > .05) from 0° to 40° of flexion after ACL injury and after ACL transection, with significant residual laxity of up to 2.0 mm and 1.3 mm as compared with the intact state in deeper flexion, respectively (P < .05).
Figure 4.
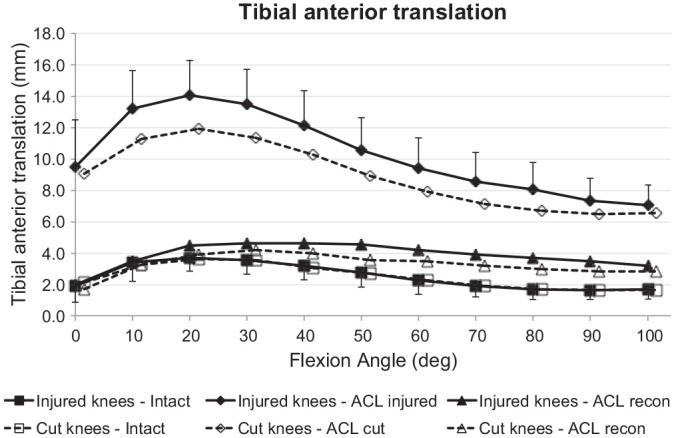
Tibial anterior translation laxity from the intact knee, from the ACL-insufficient knee, and after ACL reconstruction under a 90-N anterior drawer force for the ACL injury and ACL transection groups. Values are presented as mean ± SD (n = 9). ACL, anterior cruciate ligament; cut, ACL transected.
Posterior Translation
Rupturing or transecting the ACL did not change tibial posterior translation, and neither did ACL reconstruction.
Tibial IR
The IR laxity with the ACL intact did not differ between the ACL injury and ACL transection groups. The ACL injury and the ACL transection each caused a significant increase in IR (P < .001), and the IR laxities of the 2 groups were not then significantly different (Figure 5). Transecting the ACL caused a significant increase of IR from 0° to 20° of flexion, whereas the injury model caused a significant increase from 0° to 30° (P < .05). There was no statistical difference between the IR laxity of the ACL injury and ACL transected groups after ACL reconstruction or between the IR laxity of the ACL-reconstructed joints and the ACL-intact state in either group.
Figure 5.
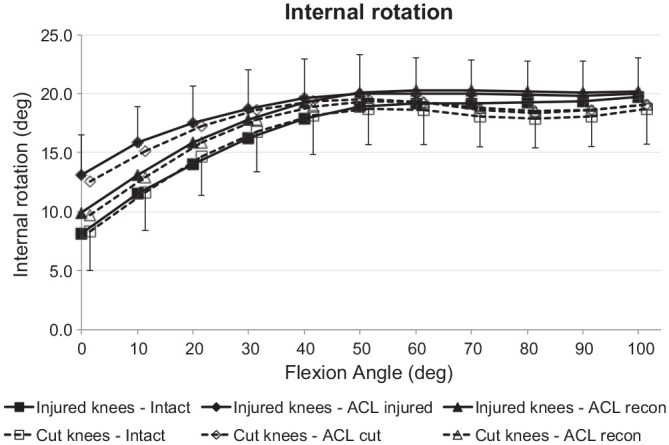
Tibial internal rotation of the intact knee and the ACL-insufficient knee and after ACL reconstruction under a 5-N·m internal rotation torque for the ACL injury and ACL transection groups. Values are presented as mean ± SD (n = 9). ACL, anterior cruciate ligament; cut, ACL transected.
Tibial External Rotation
Neither transecting nor rupturing or reconstructing the ACL caused a significant change in tibial external rotation across the range of motion, with mean differences <1°.
Valgus/Varus Angulation
Neither transecting nor rupturing or reconstructing the ACL caused a significant change of varus or valgus laxities across the range of motion, with mean differences <1°.
ATT + IR Laxity
Both groups (ACL injury and ACL transection) showed the same ATT in response to the ATT + IR loading with the ACL intact. There was a significant increase in ATT in response to the combined loading (P < .001) after ACL injury and after ACL transection (Figure 6). The ATT caused by combined loading was significantly increased in the ACL transection group from 0° to 60° of flexion (P < .05) and the ACL injury group from 0° to 80° (P < .05). There were no differences between the transection and injury models in the ACL-deficient state or after ACL reconstruction. There was residual ATT laxity after ACL reconstruction as compared with the intact state in the injury group from 0° to 40° of flexion and in the transection group from 0° to 20° (P < .05).
Figure 6.
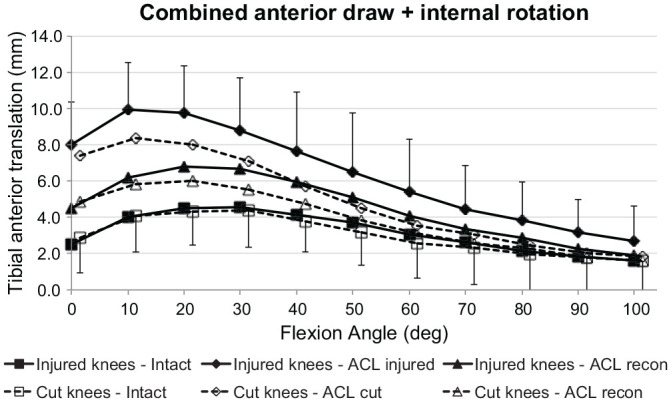
Tibial anterior translation of the intact knee and the ACL-insufficient knee and after ACL reconstruction under a 90-N anterior drawer force plus a 5-N·m internal rotation torque for the ACL injury and ACL transection groups. Values are presented as mean ± SD (n = 9). ACL, anterior cruciate ligament; cut, ACL transected.
IR with the ATT + IR loading was significantly higher than in the intact state (P < .05) after ACL transection from 0° to 20° of flexion and after ACL injury from 0° to 40°. The ACL-deficient IRs were not significantly different between the ACL injury and ACL transection groups (P > .194), with both being 3° larger than the intact laxity at 20° flexion. After ACL reconstruction, the IR remained higher (P < .05) than with an intact ACL in the ACL injury group from 0° to 30° of flexion and in the ACL transection group at 0° and 10° of flexion.
IR + Valgus: Simulated Pivot-Shift
The coupled ATT induced by the IR + valgus loading (SPS laxity) did not differ significantly between the ACL transection and ACL injury groups when the ACL was intact (Figure 7). ACL deficiency caused significant increases of coupled ATT for the ACL transection and injury groups (P < .001), with significantly (P < .05) increased ATT from 0° to 20° after ACL transection and 0° to 50° after ACL injury. After ACL reconstruction, the coupled ATT laxity persisted more in the ACL injury group (P < .05 at 0°-40° of flexion) than in the ACL transection group (0°-20°).
Figure 7.
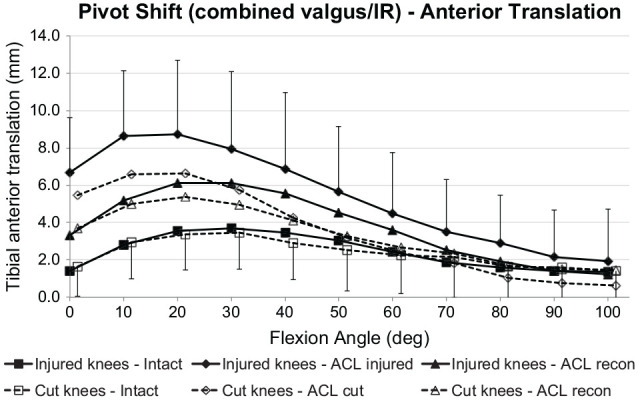
Tibial anterior translation of the intact knee and the ACL-insufficient knee and after ACL reconstruction under a 5-N·m IR torque plus 10-N·m valgus moment (simulated pivot-shift loading) for the ACL injury and ACL transection groups. Values are presented as mean ± SD (n = 9). ACL, anterior cruciate ligament; cut, ACL transected; IR, internal rotation.
The IR during the SPS loading did not differ significantly between the ACL transection and ACL injury groups when the ACL was intact (Figure 8). ACL deficiency caused significant increases of IR under SPS loading for ACL transection and injury groups (P < .001), with significant (P < .05) increases of IR from 0° to 10° after ACL transection and 0° to 30° after ACL injury. After ACL reconstruction, the IR laxity under SPS loading did not differ significantly from the ACL-intact values in the ACL injury and ACL transection groups.
Figure 8.
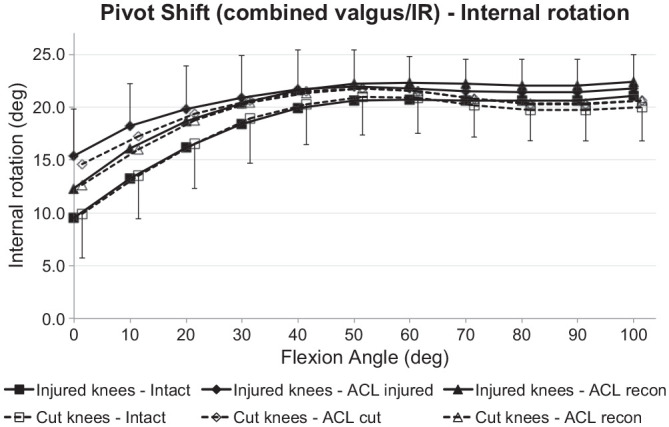
Tibial internal rotation of the intact knee and the ACL-insufficient knee and after ACL reconstruction under a 5-N·m IR torque plus 10-N·m valgus moment (simulated pivot-shift loading) for the ACL injury and ACL transection groups. Values are presented as mean ± SD (n = 9). ACL, anterior cruciate ligament; cut, ACL transected; IR, internal rotation.
Discussion
This study introduces a novel biomechanical injury mechanism that simulates the tibiofemoral bone displacements derived from imaging data 37 after real-life ACL injuries to consistently cause an isolated ACL rupture in vitro. The ACL injury model resulted in significantly larger ATT laxity when compared with conventionally performed ACL transection, supporting the first hypothesis. No injuries to the collateral ligaments, menisci, or adjacent capsule were identified during arthroscopy and knee dissection after the testing, although there must have been some stretching-out damage to cause the increased laxity. There was greater persistence of residual tibiofemoral joint laxity after ACL reconstruction in the knees that had ACL injury than in those where the ACL had been transected, supporting the second hypothesis.
The mechanism causing an ACL injury is still under debate. A complex tibiofemoral subluxation including ATT and rotation near knee extension is widely accepted as the primary mechanism of “noncontact” ACL injury.5,17,22,24,27 Depending on the degree of subluxation and the loads imposed on the knee, concomitant injuries to the joint capsule and the menisci occur in many of these patients.11,29,44 Hence, truly isolated ACL injuries are uncommon, despite the lack of other pathology found in the present study.
A number of laboratory studies in vitro have sought to replicate the ACL injury mechanism. One tried to simulate an ACL injury in a materials testing machine by imposing 30 mm of ATT in 90° of knee flexion. 1 The ACL ruptured at 14.7 ± 6.7 mm of ATT at a mean force of 673 ± 262 N, leading to a significant increase in ATT at 20° and 90° of knee flexion. Although studies of noncontact injuries have shown that that experiment used an unrealistic knee flexion angle, it is the only one to use ACL-injured specimens as the basis of a study of ACL reconstructions. However, a more recent study of patients with ACL injuries described a different knee position at the time of an ACL rupture by superimposing the tibial and femoral bone bruises on MRI. 37 The authors reported that the average knee position at the time of an ACL rupture was around 20° of knee flexion, 8° of valgus, 5° of IR, and 25 mm of anterior tibial displacement. In an earlier pilot study, 22 they found the mean injury posture to be 12° of flexion, 5° of valgus, 15° of IR, and 22 mm of ATT. Similar knee joint postures have been used in experimental studies. DeMorat et al 9 examined the mechanism where the foot impacts the ground with the knee at 20° of flexion and with high quadriceps tension. In knee specimens aged 49 to 93 years, they imposed a 4.5-kN tension (approximately 5- to 6-times body weight) on the quadriceps tendon, causing complete ACL rupture in 3 of 11 knees, with 21 mm of ATT, 2° of valgus, and 7° of IR. They noted that the patellar tendon tension has an anterior vector near knee extension, which causes ATT. Additionally, a high quadriceps tension compresses the knee axially, so the femoral condyles tend to slide down the posterior slope of the tibial plateau. Thus, Meyer and Haut 32 applied an axial compressive force to their cadaveric knees at 30° of flexion and caused ACL rupture at a joint force of 5.4 ± 2.0 kN (approximately 7-times body weight), inducing a peak ATT of 27 ± 15 mm.
Axial compressive loading also induces a coupled tibial IR because the lateral tibial plateau has a larger posterior slope than the medial 15 and the lateral femoral condyle is less constrained than the medial. As the lateral femoral condyle moves posteriorly, a coupled tibial IR occurs, but it displaces inferiorly, causing an overall valgus alignment, as measured in this study, during the ACL injury. This motion increases ACL tension because of the increased anterior-posterior component of the joint force acting on the posterolateral rim of the tibial plateau. 35 The ACL injuries in the present study were based on a clinically measured injury mechanism derived from bone bruises on MRI of clinical patients. 37 The ACL rupture at 26 ± 5 mm of ATT in the present study replicated the MRI findings of Owusu-Akyaw et al, 37 who calculated the ATT to be 25 ± 7 mm in females and 27 ± 6 mm in males. Owusu-Akyaw et al assumed that the contusions resulted at the time of ACL injury. This major assumption is supported by the present study, suggesting that the bone bruises do occur at the time of ACL injury. The 5° of IR in this work is a small deviation from neutral tibial rotation, and ATT with a larger IR posture might cause ACL plus anterolateral injury. This study confirms previous findings that during the posterior/inferior dislocation of the lateral femoral condyle the knee moves into 5° of valgus.22,37 These displacements correspond to the common finding of bone bruises at the posterior rim of the lateral tibial plateau and at the sulcus terminalis of the lateral femoral condyle on MRI scans of patients with a ruptured ACL. 40 Video analysis of ACL injuries occurring has also revealed knee flexion, IR, and valgus angulation of the same magnitudes in biomechanical and MRI investigations.17,24,27 After the moment of injury, the knee may collapse into valgus, which is more prominent in females than males.5,17,27
Bone bruises after ACL rupture are usually more obvious in the lateral compartment, but they also occur in the medial compartment. Shi et al 38 found lateral bone bruises alone in 45% of knees and medial plus lateral bruises in 55%. The locations of the bruises showed that both compartments had large ATT near knee extension. Taken together, this suggests that ATT and abduction loads are important in ACL injury mechanisms. Zhang et al 46 reported more bone bruises in the lateral than medial compartments. They concluded that the ACL ruptures had occurred principally because of ATT near extension, as in the present study. Wittstein et al 42 found bone bruises in the medial compartment in 89% of male knees and 84% of female knees after noncontact ACL injuries. It follows that the present work has matched the ACL injury mechanism calculated by Owusu-Akyaw et al 37 and that further work may investigate additional loads, such as larger joint force, abduction moment, or IR torque, which may cause additional pathology than the isolated ACL ruptures in the present study.
In the present study, IR was significantly increased close to extension but not at larger flexion angles, with the ACL injury group having increased IR over a larger range of flexion. Injury to the capsular and meniscal structures was not identified during dissection, but stretching out of these tissues probably occurred in the ACL-injured knees. Previous studies have also shown that the ACL constrains IR principally at lower flexion angles.23,31,45
The results of this study should be interpreted in light of the limitations of working in vitro. Although the ACL injury was caused by bone-bone movements that reproduced clinical imaging data, the soft tissues were not tensed by muscle actions. However, while the bone excursions in vivo are influenced strongly by the muscle tensions, the testing setup allowed for them by accurately re-creating the resulting bone-bone movements. The test speed of the in vitro injuries was less than in a “live” injury. The effect of testing speed results from the strain rate sensitivity of the tissues. However, the properties of the rabbit ACL changed only 31% when the test speed changed ×38,000, 8 so test speed is unlikely to alter ACL failure parameters significantly. The specimen age and injury mechanism will have reduced the ACL injury force below the published 43 tensile strength of 2160 ± 157 N in young specimens (22-35 years). The specimens, aged 29 to 63 years failed at an ATT force of 1549 ± 619 N. The ATT loading will have caused ACL impingement on the edge of the femoral intercondylar notch; that would also have reduced the failure force. The axial joint compression force was approximately 1 body weight, whereas muscle tensions in vivo will likely increase that. The 710-N joint compression force was chosen to match the ASTM total knee arthroplasty stability testing conditions. 2
An ACL injury mechanism likely includes the ground-reaction force and body weight deceleration causing muscle co-contractions and eccentric lengthening in addition to the ACL tension, so injuries may impose a larger joint compression force than in the present work, causing meniscal damage and bone bruises. In an ACL injury in vivo, quadriceps muscle tension causes ATT, owing to the orientation of the patellar tendon and slope of the tibial plateau, and this ATT was simply imposed directly by the test machine. The results of Meyer and Haut 32 suggest that an increased axial joint compression force in the present tests might reduce the ATT force needed to cause ACL rupture. However, that would not change the ACL failure strain, so the injury ATT would likely not change significantly in response to changes of axial joint force.
Other groups have used more complex testing systems that imposed high-speed axial impact loads, with various combined tibial torques, abduction moments, and muscle tensions. Chen et al 7 used repetitive impacts of 4-times body weight and found disruption of the collagen ultrastructure, without causing ACL rupture. Bates et al 4 added an ATT force, among a range of axial impact and abduction moment load combinations, but did not cause ACL rupture in every knee. Comparison between transection and injury is impossible within each knee. Therefore, comparisons were between contralateral pairs of knees, and their intact laxities were not significantly different. Although injuries vary, the same tibial displacement was imposed on every injured knee, based on the MRI study described earlier. 37 A more complex model could scale these displacements to the size of the knee, but clinical data 37 have not shown significant differences. Although the test rig was heavily constructed, the optical trackers showed that the deflections of the loaded rig and the bones caused a mean difference of 2.4 mm in the ATT recorded by the test machine and the bone-mounted optical trackers, which give a more accurate measure of the relative movements of the ends of the bones.
In 6 of the 9 injured knees, a midsubstance proximal ACL rupture occurred, the most common clinical scenario. However, 3 knees sustained a tibial avulsion fracture, which occurs uncommonly. These avulsions may be related to reduced bone quality in older specimens.
This study reliably caused an “isolated” ACL rupture in cadaveric knees, by imposing bone-bone displacements that reproduced those calculated from clinical imaging. This ACL injury mechanism caused significantly greater joint laxity across a wider arc of flexion than what followed from surgical transection. However, ATT was restored to the intact values only from 0° to 40° of knee flexion by ACL reconstruction, and that did not differ between the ACL injury and ACL transection knees. The differences between the ACL injury and ACL transection laxities show that secondary restraints must have been stretched by the injury, giving a more realistic basis for evaluation of ACL reconstruction techniques. However, the reduction of those differences once the primary restraint had been restored by ACL reconstruction brings into question the value of the extra effort involved in creating an ACL injury as compared with simply transecting the ACL. If more complex injuries are simulated, with larger tibial IR, for example, then greater peripheral soft tissue damage might occur, enhancing the attraction of using an ACL injury model in future work to biomechanically evaluate reconstruction techniques.
Conclusion
ACL injury versus ACL transection caused larger effects on ATT, in the ATT + IR test, and in the SPS test, as hypothesized. This likely arose from damage to adjacent tissues, although dissection evidence to support that was not found. The ACL-injured knee will be a more demanding and realistic testbed than ACL transection when evaluating surgical techniques in vitro.
Acknowledgments
This study was funded by a grant paid to Imperial College London by Smith and Nephew Ltd. The Instron machine was provided by the Arthritis Research UK charity. The tissue specimens were sourced from the MedCure tissue bank under ethics permit of the Imperial College Healthcare Tissue Bank.
Footnotes
Submitted July 10, 2020; accepted February 1, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: Financial support for this work was paid by Smith & Nephew to a research account of Imperial College London. L.W. was supported by the German Foundation for Research. K.K.A. was supported by Smith & Nephew. A.W. is a director of Innovation Orthopaedics Co and Fortius Clinic London and has received speaking fees from Smith & Nephew. A.A.A. is a director of Orthonika Co and has received speaking fees from Smith & Nephew. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
A Video Supplement for this article is available online.
References
- 1. Amis AA, Scammell BE. Biomechanics of intra-articular and extra-articular reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 1993;75(5):812-817. [DOI] [PubMed] [Google Scholar]
- 2. ASTM International. ASTM-F1223: Standard Test Method for Determination of Total Knee Replacement Constraint. ASTM International; 2014. [Google Scholar]
- 3. Athwal KK, Milner PE, Bellier G, Amis AA. Posterior capsular release is a biomechanically safe procedure to perform in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2019;27(5):1587-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bates NA, Schilaty ND, Nagelli CV, Krych AJ, Hewett TE. Validation of noncontact anterior cruciate ligament tears produced by a mechanical impact simulator against the clinical presentation of injury. Am J Sports Med. 2018;46:2113-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boden BP, Torg JS, Knowles SB, Hewett TE. Video analysis of anterior cruciate ligament injury: abnormalities in hip and ankle kinematics. Am J Sports Med. 2009;37(2):252-259. [DOI] [PubMed] [Google Scholar]
- 6. Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee: a biomechanical study. J Bone Joint Surg Am. 1980;62(2):259-270. [PubMed] [Google Scholar]
- 7. Chen J, Kim J, Shao W, et al. An anterior cruciate ligament failure mechanism. Am J Sports Med. 2019;47:2067-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danto MI, Woo SL. The mechanical properties of skeletally mature rabbit anterior cruciate ligament and patellar tendon over a range of strain rates. J Orthop Res. 1993;11:58-67. [DOI] [PubMed] [Google Scholar]
- 9. DeMorat G, Weinhold P, Blackburn T, Chudik S, Garrett W. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. Am J Sports Med. 2004;32:477-483. [DOI] [PubMed] [Google Scholar]
- 10. Driscoll MD, Isabell GP, Jr, Conditt MA, et al. Comparison of 2 femoral tunnel locations in anatomic single-bundle anterior cruciate ligament reconstruction: a biomechanical study. Arthroscopy. 2012;28(10):1481-1489. [DOI] [PubMed] [Google Scholar]
- 11. Forkel P, Reuter S, Sprenker F, et al. Different patterns of lateral meniscus root tears in ACL injuries: application of a differentiated classification system. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):112-118. [DOI] [PubMed] [Google Scholar]
- 12. Goldsmith MT, Jansson KS, Smith SD, Engebretsen L, LaPrade RF, Wijdicks CA. Biomechanical comparison of anatomic single- and double-bundle anterior cruciate ligament reconstructions: an in vitro study. Am J Sports Med. 2013;41(7):1595-1604. [DOI] [PubMed] [Google Scholar]
- 13. Grassi A, Di Paolo S, Lucidi GA, Macchiarola L, Raggi F, Zaffagnini S. The contribution of partial meniscectomy to preoperative laxity and laxity after anatomic single-bundle anterior cruciate ligament reconstruction: in vivo kinematics with navigation. Am J Sports Med. 2019;47(13):3203-3211. [DOI] [PubMed] [Google Scholar]
- 14. Halewood C, Athwal KK, Amis AA. Pre-clinical assessment of total knee replacement anterior-posterior constraint. J Biomech. 2018;73:153-160. [DOI] [PubMed] [Google Scholar]
- 15. Hashemi J, Chandrashekar N, Gill B, et al. The geometry of the tibial plateau and its influence on the biomechanics of the tibiofemoral joint. J Bone Joint Surg Am. 2008;90:2724-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herbort M, Domnick C, Raschke MJ, et al. Comparison of knee kinematics after single-bundle anterior cruciate ligament reconstruction via the medial portal technique with a central femoral tunnel and an eccentric femoral tunnel and after anatomic double-bundle reconstruction: a human cadaveric study. Am J Sports Med. 2016;44(1):126-132. [DOI] [PubMed] [Google Scholar]
- 17. Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoher J, Kanamori A, Zeminski J, Fu FH, Woo SL. The position of the tibia during graft fixation affects knee kinematics and graft forces for anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29(6):771-776. [DOI] [PubMed] [Google Scholar]
- 19. Hoshino Y, Miyaji N, Nishida K, et al. The concomitant lateral meniscus injury increased the pivot shift in the anterior cruciate ligament–injured knee. Knee Surg Sports Traumatol Arthrosc. 2019;27(2):646-651. [DOI] [PubMed] [Google Scholar]
- 20. Inderhaug E, Stephen JM, Williams A, Amis AA. Biomechanical comparison of anterolateral procedures combined with anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(2):347-354. [DOI] [PubMed] [Google Scholar]
- 21. Khadem R, Yeh CC, Sadeghi-Tehrani M, et al. Comparative tracking error analysis of five different optical tracking systems. Comput Aided Surg. 2000;5:98-107. [DOI] [PubMed] [Google Scholar]
- 22. Kim SY, Spritzer CE, Utturkar GM, Toth AP, Garrett WE, DeFrate LE. Knee kinematics during noncontact anterior cruciate ligament injury as determined from bone bruise location. Am J Sports Med. 2015;43(10):2515-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kittl C, El-Daou H, Athwal KK, et al. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee. Am J Sports Med. 2016;44:345-354. [DOI] [PubMed] [Google Scholar]
- 24. Koga H, Nakamae A, Shima Y, et al. Mechanisms for noncontact anterior cruciate ligament injuries: knee joint kinematics in 10 injury situations from female team handball and basketball. Am J Sports Med. 2010;38(11):2218-2225. [DOI] [PubMed] [Google Scholar]
- 25. Kondo E, Merican AM, Yasuda K, Amis AA. Biomechanical comparison of anatomic double-bundle, anatomic single-bundle, and nonanatomic single-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2011;39(2):279-288. [DOI] [PubMed] [Google Scholar]
- 26. Kondo E, Yasuda K, Azuma H, Tanabe Y, Yagi T. Prospective clinical comparisons of anatomic double-bundle versus single-bundle anterior cruciate ligament reconstruction procedures in 328 consecutive patients. Am J Sports Med. 2008;36(9):1675-1687. [DOI] [PubMed] [Google Scholar]
- 27. Krosshaug T, Nakamae A, Boden BP, et al. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35(3):359-367. [DOI] [PubMed] [Google Scholar]
- 28. Lagae KC, Robberecht J, Athwal KK, Verdonk PCM, Amis AA. ACL reconstruction combined with lateral monoloop tenodesis can restore intact knee laxity. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1159-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee DW, Lee JH, Kim JN, et al. Evaluation of anterolateral ligament injuries and concomitant lesions on magnetic resonance imaging after acute anterior cruciate ligament rupture. Arthroscopy. 2018;34(8):2398-2406. [DOI] [PubMed] [Google Scholar]
- 30. Lie DT, Bull AM, Amis AA. Persistence of the mini pivot shift after anatomically placed anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2007;457:203-209. [DOI] [PubMed] [Google Scholar]
- 31. Lorbach O, Pape D, Maas S, et al. Influence of the anteromedial and posterolateral bundles of the anterior cruciate ligament on external and internal tibiofemoral rotation. Am J Sports Med. 2010;38(4):721-727. [DOI] [PubMed] [Google Scholar]
- 32. Meyer EG, Haut RC. Anterior cruciate ligament injury induced by internal tibial torsion or tibiofemoral compression. J Biomech. 2008;41:3377-3383. [DOI] [PubMed] [Google Scholar]
- 33. Musahl V, Citak M, O’Loughlin PF, Choi D, Bedi A, Pearle AD. The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament–deficient knee. Am J Sports Med. 2010;38(8):1591-1597. [DOI] [PubMed] [Google Scholar]
- 34. Musahl V, Rahnemai-Azar AA, Costello J, et al. The influence of meniscal and anterolateral capsular injury on knee laxity in patients with anterior cruciate ligament injuries. Am J Sports Med. 2016;44(12):3126-3131. [DOI] [PubMed] [Google Scholar]
- 35. Navacchia A, Bates NA, Schilaty ND, Krych AJ, Hewett TE. Knee abduction and internal rotation moments increase ACL force during landing through the posterior slope of the tibia. J Orthop Res. 2019;37(8):1730-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Noyes FR, Huser LE, Ashman B, Palmer M. Anterior cruciate ligament graft conditioning required to prevent an abnormal lachman and pivot shift after ACL reconstruction: a robotic study of 3 ACL graft constructs. Am J Sports Med. 2019;47(6):1376-1384. [DOI] [PubMed] [Google Scholar]
- 37. Owusu-Akyaw KA, Kim SY, Spritzer CE, et al. Determination of the position of the knee at the time of an anterior cruciate ligament rupture for male versus female patients by an analysis of bone bruises. Am J Sports Med. 2018;46(7):1559-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi H, Ding L, Jiang Y, et al. Bone bruise distribution patterns after acute anterior cruciate ligament rupture: implications for the injury mechanism. Orthop J Sports Med. 2020;8(4):2325967120911162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the stability of the anterior cruciate ligament–deficient knee. Am J Sports Med. 2015;43(4):905-911. [DOI] [PubMed] [Google Scholar]
- 40. Song G-Y, Zhang H, Wang Q-Q, Zhang J, Li Y, Feng H. Bone contusions after acute noncontact anterior cruciate ligament injury are associated with knee joint laxity, concomitant meniscal lesions, and anterolateral ligament abnormality. Arthroscopy. 2016;32:2331-2341. [DOI] [PubMed] [Google Scholar]
- 41. Stephen JM, Halewood C, Kittl C, Bollen SR, Williams A, Amis AA. Posteromedial meniscocapsular lesions increase tibiofemoral joint laxity with anterior cruciate ligament deficiency, and their repair reduces laxity. Am J Sports Med. 2016;44(2):400-408. [DOI] [PubMed] [Google Scholar]
- 42. Wittstein J, Vinson E, Garrett W. Comparison between sexes of bone contusions and meniscal tear patterns in noncontact anterior cruciate ligament injuries. Am J Sports Med. 2014;42:1401-1407. [DOI] [PubMed] [Google Scholar]
- 43. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur–anterior cruciate ligament–tibia complex: the effects of specimen age and orientation. Am J Sports Med. 1991;19:217-225. [DOI] [PubMed] [Google Scholar]
- 44. Yoon KH, Yoo JH, Kim KI. Bone contusion and associated meniscal and medial collateral ligament injury in patients with anterior cruciate ligament rupture. J Bone Joint Surg Am. 2011;93(16):1510-1518. [DOI] [PubMed] [Google Scholar]
- 45. Zantop T, Herbort M, Raschke MJ, Fu FH, Petersen W. The role of the anteromedial and posterolateral bundles of the anterior cruciate ligament in anterior tibial translation and internal rotation. Am J Sports Med. 2007;35(2):223-227. [DOI] [PubMed] [Google Scholar]
- 46. Zhang L, Hacke JD, Garrett Liu H, Yu B. Bone bruises associated with anterior cruciate ligament injury as indicators of injury mechanism: a systematic review. Sports Med. 2019;49:453-462. [DOI] [PubMed] [Google Scholar]


