Abstract
Background:
The increasing cancer burden remains a public health challenge. Quality and accurate population data is important to improve cancer control, screening, and treatment programmes for the sub-Saharan Africa region.
Aim:
The aim of this study was to establish hospital-based cancer surveillance system, thereby reporting the burden that cancer diagnosis and treatment place on 3 hospitals – an approach of health systems strengthening.
Methods:
A hospital-based cancer surveillance was established in 3 public health facilities that provide oncology services in KwaZulu-Natal. An active method was used for finding cancer cases. The cancer surveillance database was evaluated according to the criteria recommended for cancer registries. Analyses of data included descriptive and crude incidence rates.
Results:
A total of 2307 newly diagnosed cancer cases were reported in 2018, with a majority from Inkosi Albert Luthuli Central hospital (65.3%), followed by Greys hospital (30.8%) and then Addington hospital (3.94%). Most of the cancer cases were from the 2 major urban areas of the province (eThekwini and uMgungundlovu district). The most commonly diagnosed cancers from all combined 3 facilities for both sexes were breast, cervix, colorectal, Kaposi Sarcoma, and lung. Approximately half of the cancer cases had no staging, and 12.8% of the cases were diagnosed at stage 4. The mostly prescribed treatments for the patients were radiotherapy and chemotherapy.
Conclusions:
Based on our hospital-based surveillance, cancer burden is high in the 3 facilities. Strengthening cancer screening and diagnostic policies and procedures that will allow expansion of accurate cancer surveillance system is essential in KwaZulu-Natal and South Africa as a whole.
Keywords: Cancer surveillance, cancer incidence, hospital-based
Introduction
The burden of cancer remains a public health challenge mainly in the sub-Saharan African region where an increase of more than 85% of cancer incidences of all cancers from 2008 to 2030 is predicted. 1 This increasing cancer burden has been recognized as a global health challenge, therefore suggesting prioritization of cancer surveillance.2,3 This increasing cancer burden is due to several factors, which include population growth and ageing as well as the changing prevalence of certain causes of cancer linked to social and economic development. 4 Early diagnosis and staging are important for cancer care and the reduction of cancer morbidity and mortality5,6; however, in low- and middle-income countries, late diagnosis of cancer is common.4,5
Cancer surveillance or registration is an important tool whose data provides a census of cancer incidence, prevalence, and mortality at the local, provincial, and country levels. The data can also be used for cancer prevention, basic research, and etiological studies.7,8 Even though cancer registration began in the 1950s in Africa, it is not fully implemented in some countries such as South Africa.7,9 The South African National Cancer Registry (NCR) is the pathology-based registry; therefore, there is an underestimation of the cancer burden namely cancer incidence and mortality.10,11 The challenges NCR has faced resulted in a backlog that goes close to 5 years on reporting of data on cancer incidence in South Africa. 12
The population-based cancer registries in South Africa are in Ekurhuleni, an urban region in the Gauteng province, and in a rural Eastern Cape. 13 KwaZulu-Natal (KZN), a province with the second largest population, does not have a population-based cancer registry, therefore limiting the reliability of estimation cancer incidence in South Africa.
The aim of this study was to establish hospital-based cancer surveillance system, thereby reporting the burden that cancer diagnosis and treatment place on 3 hospitals – an approach of health systems strengthening.
Materials and Methods
Study setting and case identification
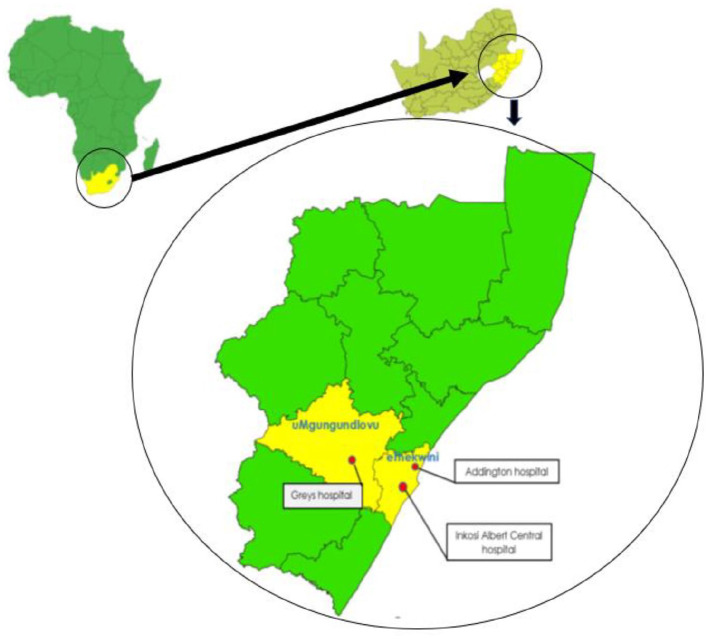
The sites for this study were 3 public health facilities that provide oncology services in KwaZulu-Natal, namely, Addington hospital (ADH), which is regional hospital; Greys hospital (GH), which is a tertiary level health care facility; and Inkosi Albert Luthuli Central hospital (IALCH), which is a central hospital ranked as tertiary facility. All these 3 health facilities function on a referral basis only (Figure 1).
Figure 1.
Map showing the districts and location of the 3 main public health facilities that provide oncology services in KwaZulu-Natal.
Data collection
Trained data collectors abstracted data of all cancer cases manually from hard and electronic medical files kept at the oncology and medical departments in the 3 health facilities. Information from the medical files was collected using a reporting form adopted from the South African National Cancer Registry (NCR). 14 The collected data included the patient’s demographics, risk factors and symptoms’ profile, detailed clinical and laboratory results, histopathology characteristics, sources of information, treatment, follow-up, and vital status (alive or dead). Details on malignant cases, including those from the unknown primary site, were also abstracted onto the hard copy instruments.
Evaluation of cancer surveillance
The established surveillance on the 3 health facilities was evaluated using comparability, validity, and completeness as recommended by World Health Organization (WHO)-International Agency for Research on Cancer (IARC) for cancer registration database. 15 The classification and coding of new cases, and basic definitions of incidence1,12,15 were elements covered when looking for comparability. The cancer cases had to:
be malignant cases that were diagnosed by medically qualified personnel, had their coding and classification of tumour site and morphology validated against the standardized coding for cancer.
meet the rule for the registration of incidence date algorithm.
be differentiated between primary cancer and an extension, recurrence, or metastasis of existing cancer.
For the validity of the cases, we looked at the proportion of histological verification in the database with higher percentage being an indicator of better quality.
Evaluating the completeness, the missing or incomplete information was assessed as a way of reflecting on the case investigation’s uncertainty or incomprehensibility of medical records used for data abstraction.
Data management and analysis
On each of the completed abstraction forms, cancer diagnosis were coded according to the primary site of origin. Data on topography and morphology were manually coded according to the third edition of the International Classification of Disease for Oncology (ICDO-3). 16 The incidence date for cancer cases was computed as the date of the first biopsy, cytology, x-ray, scans or mammogram, and death report with the date of biopsy given priority if more than one procedure was considered. 15 We used the patient’s first visit or referral date for cancer cases that did not have the information for computing incidence date. The age of each case was computed according to date of birth and age reported date of diagnosis. Four of the cases had multiple primaries and were recorded according to the different primary sites 17 and the recurrent cancers excluded. All the data from hard copy forms were captured into EpiData Version 3.1 (Epidata Association, Odense, Denmark, 2010) 18 and transferred to MS Excel to check for duplicates, missing data, and errors.
Management of data
A total of 3480 records of cancer cases were identified and data collected from them. Of these, 152 were duplicate cases and therefore were dropped from the database. We further excluded 920 and 101 cases that were either not diagnosed in 2018 or were benign or/and of uncertain tumours, respectively. This exercise left 2307 cancer cases qualifying to be retained for the 2018 surveillance database. The retained cases were further checked for completeness of the mandatory variables namely names, sex, date of diagnosis, and topography. At the end, a total of 2307 cancer cases were retained and eligible for the analysis purpose (Figure 2). Data were then transferred from Excel to Stata IC version 13 (Stata Corp, College Station, TX, USA, 2018) 19 for further analysis.
Figure 2.
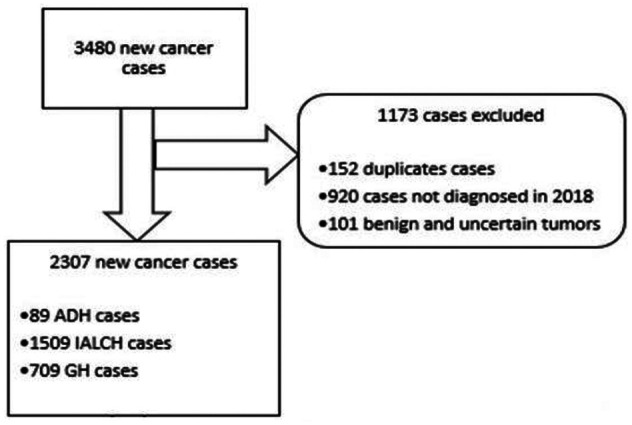
A flow diagram showing cancer surveillance data collection and elimination of cases that did not meet the criteria in 3 public health facilities in KwaZulu-Natal, South Africa.
ADH indicates Addington hospital; GH, Greys hospital; IALCH, Inkosi Albert Luthuli Central hospital.
Ethical Considerations
The project and data collection tools used were approved by the University of KwaZulu-Natal’s Biomedical Research Ethics Committee (Ref: BE533/18) and the National and Provincial Health Research & Ethics Committees of South Africa (The National Health Research Database [NHRD] serves as a repository of health-related research) (Ref: HRKM0007/18 [KZ_201B01_013]). Permission was also sorted and granted by each of the 3 public health facilities.
Results
Validity and completeness of cancer surveillance
Completeness and timeliness
The surveillance database had 2.08% (n = 32) cases with missing or with observations categorized as unknown in the mandatory variables. The ratio of cases with missing information to the total number of cases was 0.52% for the variable ‘topography’ and ‘sex’ was 0.88%. Other cases that had missing information not mandatory, such as marital status, were retained in the database and included in the analysis. The surveillance collected, processed, and reported the new cancer cases registered for 2018.
Validity
One or more information sources in the medical records were used to complete data per case, namely clinical investigation reports (such as x-ray), specific tumour marker, cytology or haematology, histology of metastasis, and history of primary site reports. The basis of diagnosis for most cases in all 3 health facilities was histology of primary site at 87.7% (n = 2026). Only 10 cases (0.43%) in the surveillance database had results that were categorized as an unknown source of diagnosis.
New cancer cases in the 3 hospitals
The highest number of newly registered cancer cases in 2018 were from IALCH, followed by GH and ADH with the lowest (65.4%, n = 1509; 30.7%, n = 709; 3.9%, n = 89, respectively) (Table 1).
Table 1.
Demographic characteristics of validated cancer patients seen in the 3 hospitals, 2018 (N = 2307).
| Characteristics/category | ADH, n (%) | GH, n (%) | IALCH, n (%) | Overall, n (%) |
|---|---|---|---|---|
| Total per health facility | 89 | 709 | 1509 | 2307 |
| Age | ||||
| 0-14 | 0 (0) | 5 (0.71) | 62 (4.11) | 67 (2.90) |
| 15-24 | 3 (3.37) | 16 (2.26) | 28 (1.86) | 47 (2.04) |
| 25-34 | 24 (27.0) | 51 (7.19) | 99 (6.56) | 174 (7.54) |
| 35-44 | 32 (36.0) | 128 (18.1) | 229 (15.2) | 389 (16.9) |
| 45-54 | 7 (7.87) | 162 (22.9) | 327 (21.7) | 496 (21.5) |
| 55-64 | 13 (14.6) | 146 (20.6) | 351 (23.3) | 510 (22.1) |
| 65-74 | 7 (7.87) | 130 (18.3) | 272 (18.0) | 409 (17.7) |
| 75-84 | 2 (2.25) | 51 (7.19) | 108 (7.16) | 161 (6.98) |
| 85+ | 1 (1.12) | 17 (2.40) | 11 (0.73) | 29 (1.26) |
| 999 a | 0(0) | 3 (0.42) | 22 (1.46) | 25 (1.08) |
| Gender | ||||
| Female | 38 (42.7) | 423 (59.7) | 1031 (68.3) | 1492 (64.7) |
| Male | 51 (57.3) | 284 (40.1) | 469 (31.1) | 804 (34.9) |
| Unknown | 0 (0) | 2 (0.28) | 9 (0.60) | 11 (0.48) |
| Race | ||||
| African | 77 (86.5) | 621 (87.6) | 1032 (68.4) | 1730 (75.0) |
| Coloured | 1 (1.12) | 13 (1.83) | 39 (2.58) | 53 (2.30) |
| White | 5 (5.62) | 40 (5.64) | 119 (7.89) | 164 (7.11) |
| Asian/Indian | 5 (5.62) | 33 (4.65) | 292 (19.4) | 330 (14.3) |
| Other | 1 (1.12) | 0 (0) | 1 (0.07) | 2 (0.09) |
| Unknown | 0(0) | 2 (0.28) | 26 (1.72) | 28 (1.21) |
| HIV Status | ||||
| No | 16 (18.0) | 306 (43.2) | 531 (35.2) | 853 (37.0) |
| Yes | 67 (75.3) | 226 (31.9) | 380 (25.2) | 673 (29.2) |
| Unknown | 6 (6.74) | 177 (25.0) | 598(39.6) | 781 (33.9) |
Abbreviations: ADH, Addington hospital; GH, Greys hospital; IALCH, Inkosi Albert Luthuli Central hospital.
999 = unknown.
The demographic characteristics and HIV status of the patients with cancer are shown in Table 1. There were more females diagnosed with cancer at 64.7% (n = 1492) than males, with the mean (± standard deviation [SD]) age of 52.9 (16.8). Most (43.6%, n = 1006) of the cases were between the age of 45 and 64 years (Table 1). Seventy-five percent (75%, n = 1730) of the cancer cases were Africans followed by the Asian/Indian at 14.3% (n = 330). More than 29% (29.2%, n = 673) of the cases were people living with HIV.
Histology of primary site was the most commonly used basis of diagnosis at 87.8% (n = 2026). Death reports were the least used sources for identifying diagnosis at 0.17% (n = 4) and other 10 (0.43%) did not have a known diagnosis method. Approximately half (50.9%, n = 1175) of the cancer cases were not staged and those staged, stage 1 was the most common stage at 20.8%. Then 12.8% of the cancer cases diagnosed at stage 4 were the second most prevalent. Most cancer cases were graded as moderately differentiated at 24.3% (Table 2).
Table 2.
Diagnosis and treatment of patient with cancer from the 3 hospitals, 2018 (N = 2307).
| Characteristics/category | ADH, n (%) | GH, n (%) | IALCH, n (%) | Overall, n (%) |
|---|---|---|---|---|
| Total per health facility | 89 | 709 | 1509 | 2307 |
| Basis of diagnosis | ||||
| Death report | 0 (0) | 4 (0.56) | 0 (0) | 4 (0.17) |
| Clinical only | 0 (0) | 0 (0) | 1 (0.07) | 1 (0.04) |
| Clinical investigation | 1 (1.12) | 198 (27.9) | 54 (3.58) | 253 (11.0) |
| Cytology/haematology | 0 (0) | 3 (0.42) | 2 (0.13) | 5 (0.22) |
| Histology of metastasis | 0 (0) | 7 (1.00) | 1 (0.07) | 8 (0.35) |
| Histology of primary site | 88 (98.9) | 497 (70.1) | 1441 (95.5) | 2026 (87.8) |
| Unknown | 0 (0) | 0 (0) | 10 (0.66) | 10 (0.43) |
| Stage | ||||
| 1 | 7 (7.87) | 321 (45.3) | 151 (10.0) | 479 (20.8) |
| 2 | 3 (3.37) | 25 (3.53) | 128 (8.48) | 156 (6.76) |
| 3 | 2 (2.25) | 44 (6.21) | 155 (10.3) | 201 (8.71) |
| 4 | 2 (2.25) | 71 (10.0) | 223 (14.8) | 296 (12.8) |
| Unknown | 75 (84.3) | 248 (35.0) | 852 (56.5) | 1175 (50.9) |
| Grade | ||||
| Well differentiated | 1 (1.12) | 62 (8.74) | 41 (2.72) | 104 (4.51) |
| Moderately differentiated | 1 (1.12) | 146 (20.6) | 413 (27.4) | 560 (24.3) |
| Poorly differentiated | 2 (2.25) | 40 (5.64) | 58 (3.84) | 100 (4.33) |
| Undifferentiated | 0 (0) | 5 (0.71) | 10 (0.66) | 15 (0.65) |
| Unknown | 85 (95.5) | 456 (64.3) | 987 (65.4) | 1528 (66.2) |
| Treatment | ||||
| No treatment | ||||
| No | 84 (94.8) | 631 (89.0) | 978 (64.8) | 1693 (73.4) |
| Yes | 5 (5.62) | 78 (11.0) | 531 (35.2) | 614 (26.6) |
| Surgery | ||||
| No | 86 (96.6) | 686 (96.8) | 1336 (88.5) | 2108 (91.4) |
| Yes | 3 (3.37) | 23 (3.24) | 173 (11.5) | 199 (8.60) |
| Radiotherapy | ||||
| No | 19 (21.4) | 145 (20.5) | 1046 (69.3) | 1210 (52.4) |
| Yes | 70 (78.7) | 564 (79.6) | 463 (30.7) | 1097 (47.6) |
| Chemotherapy | ||||
| No | 34 (38.2) | 470 (66.3) | 868 (57.5) | 1372 (59.5) |
| Yes | 55 (61.8) | 239 (33.7) | 641 (42.5) | 935 (40.5) |
| Palliation | ||||
| No | 86 (96.6) | 628 (88.6) | 1350 (89.5) | 2064 (89.5) |
| Yes | 3 (3.37) | 81 (11.4) | 159 (10.5) | 243 (10.5) |
| Hormone therapy | ||||
| No | 89 (100) | 709 (100) | 1506 (99.8) | 2304 (99.8) |
| Yes | 0 (0) | 0 (0) | 3 (0.20) | 3 (0.20) |
| Combination of treatments | ||||
| No | 44 (49.4) | 448 (63.2) | 1123 (74.4) | 1615 (70.0) |
| Yes | 45 (50.6) | 261 (36.8) | 386 (25.6) | 692 (30.0) |
Abbreviations: ADH, Addington hospital; GH, Greys hospital; IALCH, Inkosi Albert Luthuli Central hospital.
Most of the patients were treated with either radiotherapy (47.6%, n = 1097) or chemotherapy (40.5%, n = 935). Some patients (30.0%, n = 692) received a combination of treatments in the form of chemotherapy and radiotherapy. More than a quarter (26.6%, n = 614) of the patients were not documented as receiving or having received any form of treatment. Only IALCH had treated patients with hormone therapy (0.20%) (Table 2).
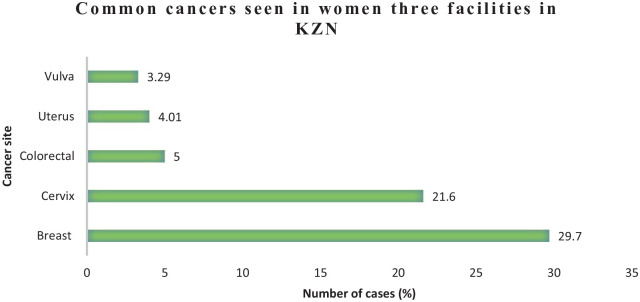
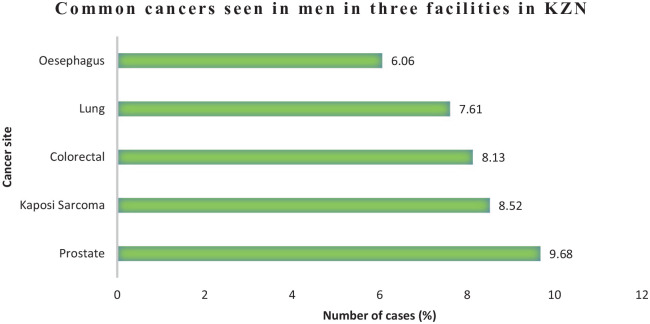
Tables 3 and 4 illustrate a detailed tabulation of total number of cases registered in 2018 for males and females. Figures 3 and 4 illustrate the proportion of the most common cancers registered in the KZN public health facilities in 2018 for females and males. Frequency of cancers is useful for determining the burden on a provincial and country’s health care systems of screening, diagnosis, and treatment of cancers. Among women, 451 new cases of breast cancer were reported. This was closely followed by cervical cancer with 329 cases. Prostate cancer was by far the commonest cancer among men with 75 cases. This was closely followed by Kaposi sarcoma with 66 cases.
Table 3.
Summary statistics for all cancers in women in 3 KwaZulu-Natal hospitals (2018).
| Site | ICD-10 | N* | ADH | GH | IALCH |
|---|---|---|---|---|---|
| All sites | All | 1521 | 38 | 425 | 1058 |
| All sites but C44 | All but C44 | 1462 (96.1) | 38 (100) | 402 (94.6) | 1022 (96.6) |
| Lip | C00 | 1 (0.07) | 0 (0) | 0 (0) | 1 (0.09) |
| Tongue | C02 | 20 (1.31) | 2 (5.26) | 3 (0.71) | 15 (1.42) |
| Mouth | C06 | 12 (0.79) | 0 (0) | 3 (0.71) | 9 (0.60) |
| Salivary gland | C07 | 2 (0.13) | 0 (0) | 1 (0.24) | 1 (0.09) |
| Naso-oropharynx | C11 | 19 (1.25) | 0 (0) | 11 (2.59) | 8 (0.76) |
| Oesophagus | C15 | 38 (2.5) | 0 (0) | 6 (1.41) | 32 (3.02) |
| Stomach | C16 | 18 (1.18) | 1 (2.63) | 5 (1.18) | 12 (1.13) |
| Small intestine | C17 | 2 (0.13) | 0 (0) | 0 (0) | 2 (0.19) |
| Colorectal | C18 | 76 (5.0) | 0 (0) | 26 (6.12) | 50 (4.73) |
| Anus | C21 | 19 (1.25) | 0 (0) | 8 (1.88) | 11 (1.04) |
| Liver and bile duct | C22 | 13 (0.85) | 0 (0) | 3 (0.71) | 10 (0.95) |
| Larynx | C32 | 8 (0.53) | 0 (0) | 2 (0.47) | 6 (0.67) |
| Lung | C34 | 36 (2.37) | 0 (0) | 15 (3.53) | 21 (1.98) |
| Bone | C41 | 15 (0.99) | 0 (0) | 5 (1.18) | 10 (0.95) |
| Skin other | C44 | 28 (1.84) | 0 (0) | 8 (1.88) | 20 (1.89) |
| BCC | C44 | 2 (0.13) | 0 (0) | 0 (0) | 2 (0.19) |
| SCC of skin | C44 | 29 (1.91) | 0 (0) | 15 (3.53) | 14 (1.32) |
| Kaposi sarcoma | C46 | 40 (2.63) | 23 (60.5) | 13 (3.06) | 4 (0.38) |
| Connective tissue | C49 | 21 (1.38) | 0 (0) | 8 (1.88) | 13 (1.23) |
| Breast | C50 | 451 (29.7) | 7 (18.4) | 104 (24.5) | 340 (32.1) |
| Vulva | C51 | 50 (3.29) | 0 (0) | 17 (4.00) | 33 (3.12) |
| Vagina | C52 | 21 (1.38) | 0 (0) | 10 (2.35) | 11 (1.04) |
| Cervix | C53 | 329 (21.6) | 2 (5.26) | 109 (25.6) | 218 (20.6) |
| Uterus | C54 | 61 (4.01) | 0 (0) | 14 (3.29) | 47 (4.44) |
| Ovary | C56 | 31 (2.04) | 0 (0) | 8 (1.88) | 23 (2.17) |
| Kidney | C64 | 10 (0.66) | 0 (0) | 2 (0.47) | 7 (0.66) |
| Bladder | C67 | 3 (0.20) | 0 (0) | 0 (0) | 3 (0.28) |
| Eye | C69 | 10 (0.66) | 1 (2.63) | 4 (0.94) | 5 (0.47) |
| Brain, CNS | C71 | 7 (0.46) | 0 (0) | 0 (0) | 7 (0.66) |
| Thyroid | C73 | 38 (2.50) | 0 (0) | 2 (0.47) | 36 (3.40) |
| Endocrine | C74 | 2 (0.13) | 0 (0) | 0 (0) | 2 (0.19) |
| Ill defined | C76 | 11 (0.72) | 0 (0) | 4 (0.94) | 9 (0.85) |
| Primary site unknown | C80 | 20 (1.31) | 0 (0) | 7 (1.65) | 13 (1.23) |
| Hodgkin lymphoma | C81 | 7 (0.46) | 0 (0) | 0 (0) | 7 (0.66) |
| Non-Hodgkin lymphoma | C84 | 10 (0.66) | 0 (0) | 1 (0.24) | 9 (0.85) |
| Myeloma | C90 | 7 (0.46) | 0 (0) | 0 (0) | 7 (0.66) |
| Leukaemia | C92 | 10 (0.66) | 0 (0) | 0 (0) | 10 (0.95) |
| Haematology other | C96 | 30 (1.97) | 2 (5.26) | 9 (2.12) | 19 (1.80) |
| Other | 13 (0.85) | 0 (0) | 2 (0.47) | 11 (1.04) |
Abbreviations: ADH, Addington hospital; BCC, Basal Cell Carcinoma; CNS, central nervous system; GH, Greys hospital; IALCH, Inkosi Albert Luthuli Central hospital; ICD-10, International Classification of Diseases, 10th Revision; SCC, Squamous Cell Carcinoma.
N* includes cases with unknown age.
Table 4.
Summary statistics for all cancers in men seen in the 3 hospitals in KwaZulu-Natal (2018).
| Site | ICD-10 | N* | ADH | GH | IALCH |
|---|---|---|---|---|---|
| All sites | All | 775 | 51 | 282 | 442 |
| All but C44 | All but C44 | 718 (92.6) | 51 (100) | 265 (94.0) | 402 (91.0) |
| Lip | C00 | 4 (0.52) | 0 (0) | 2 (0.71) | 2 (0.45) |
| Tongue | C02 | 20 (2.58) | 0 (0) | 8 (2.84) | 12 (2.71) |
| Mouth | C06 | 22 (2.84) | 1 (1.96) | 14 (4.96) | 7 (1.58) |
| Salivary gland | C07 | 5 (0.65) | 0 (0) | 2 (0.71) | 3 (0.68) |
| Naso-oropharynx | C11 | 26 (3.35) | 1 (1.96) | 11 (3.90) | 14 (3.17) |
| Oesophagus | C15 | 47 (6.06) | 0 (0) | 8 (2.84) | 39 (8.82) |
| Stomach | C16 | 19 (2.45) | 0 (0) | 7 (2.48) | 12 (2.71) |
| Small intestine | C17 | 6 (0.77) | 0 (0) | 0 (0) | 6 (1.36) |
| Colorectal | C18 | 63 (8.13) | 2 (3.92) | 23 (8.16) | 38 (8.60) |
| Anus | C21 | 8 (1.03) | 0 (0) | 3 (1.06) | 5 (1.13) |
| Liver and bile duct | C22 | 21 (2.71) | 0 (0) | 7 (2.48) | 14 (3.17) |
| Pancreas | C25 | 5 (0.65) | 0 (0) | 0 (0) | 5 (1.13) |
| Larynx | C32 | 31 (4.0) | 0 (0) | 11 (3.90) | 20 (4.52) |
| Lung | C34 | 59 (7.61) | 1 (1.96) | 13 (4.61) | 45 (10.2) |
| Bone | C41 | 18 (2.32) | 0 (0) | 13 (4.61) | 5 (1.13) |
| SCC of skin | C44 | 26 (3.35) | 0 (0) | 8 (2.84) | 18 (4.07) |
| BCC | C44 | 7 (0.90) | 0 (0) | 0 (0) | 7 (1.58) |
| Skin other | C44 | 24 (3.10) | 0 (0) | 9 (3.19) | 15 (3.39) |
| Kaposi sarcoma | C46 | 66 (8.52) | 42 (82.4) | 23 (8.16) | 1 (0.23) |
| Connective tissue | C49 | 9 (1.16) | 0 (0) | 3 (1.06) | 6 (1.36) |
| Penis | C60 | 20 (2.58) | 1 (1.96) | 16 (5.67) | 3 (0.68) |
| Prostate | C61 | 75 (9.68) | 2 (3.92) | 35 (12.4) | 38 (8.60) |
| Testis | C62 | 14 (1.81) | 0 (0) | 4 (1.42) | 10 (2.26) |
| Other specified | C63 | 2 (0.26) | 0 (0) | 2 (0.71) | 0 (0) |
| Kidney | C64 | 16 (2.06) | 0 (0) | 4 (1.42) | 12 (2.71) |
| Bladder | C67 | 22 (2.84) | 0 (0) | 11 (3.90) | 11 (2.49) |
| Eye | C69 | 8 (1.03) | 0 (0) | 2 (0.71) | 6 (1.36) |
| Brain, CNS | C71 | 12 (1.55) | 0 (0) | 1 (0.35) | 11 (2.49) |
| Thyroid | C73 | 8 (1.03) | 0 (0) | 1 (0.35) | 7 (1.58) |
| Endocrine | C74 | 1 (0.13) | 0 (0) | 1 (0.35) | 0 (0) |
| Ill defined | C76 | 11 (1.42) | 0 (0) | 7 (2.48) | 4 (0.90) |
| Primary site unknown | C80 | 7 (0.90) | 0 (0) | 1(0.35) | 6 (1.36) |
| Hodgkin lymphoma | C81 | 9 (1.16) | 0 (0) | 3 (1.06) | 6 (1.36) |
| Non-Hodgkin lymphoma | C84 | 7 (0.90) | 0 (0) | 3 (1.06) | 4 (0.90) |
| Myeloma | C90 | 11 (1.42) | 0 (0) | 2 (0.71) | 9 (2.04) |
| Leukaemia | C92 | 6 (0.77) | 0 (0) | 0 (0) | 6 (1.36) |
| Haematology other | C96 | 40 (5.16) | 1 (1.96) | 18 (6.38) | 21 (4.75) |
| Other | 20 (2.58) | 0 (0) | 6 (2.13) | 14 (3.17) |
Abbreviations: ADH, Addington hospital; BCC, Basal Cell Carcinoma; CNS, central nervous system; GH, Greys hospital; IALCH, Inkosi Albert Luthuli Central hospital; ICD-10, International Classification of Diseases, 10th Revision; SCC, Squamous Cell Carcinoma.
N* includes cases with unknown age.
Figure 3.
Frequency of top 5 common cancers in females in 2018 in 3 KZN health facilities.
KZN indicates KwaZulu-Natal.
Figure 4.
Frequency of top 5 common cancers in males in 2018 in 3 KZN health facilities.
KZN indicates KwaZulu-Natal.
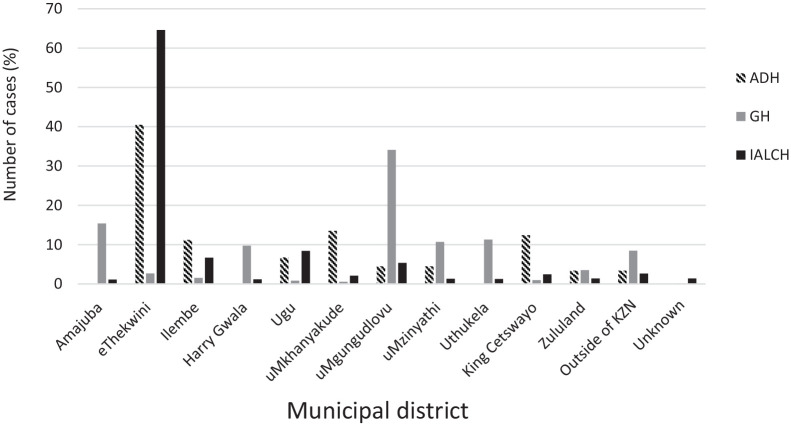
Most of the new cancer cases in 2018 were from the 2 major urban areas of the province (eThekwini with 44.8% [n = 994] and uMgungundlovu with 14.6% [n = 323]) (Figure 5). Zululand district (one of the rural districts) had the least number of new cancer diagnosis at 2.07% (n = 46). One hundred cases (4.51%) were from outside the KZN province and most of them from Eastern Cape province.
Figure 5.
Distribution of crude incidence rates by the municipal district in 2018, KwaZulu-Natal.
KZN indicates KwaZulu-Natal.
Discussion
Hospital-based cancer surveillance was successfully established in the 3 KwaZulu-Natal health facilities that have oncology services. Female cancers were the most diagnosed cancers together with colorectal in the 3 health facilities. Cancer of the crevix was the most prevalent female cancer and prostate was the most prevalent male cancer in 2018.
The number of variables and data items within the surveillance programme is an important aspect for data collection. The key is to select minimum but necessary information for each registry. 20 Without some variables, computations cannot be done, therefore affecting data accuracy, completeness, and comparability.
The missing information in our database was well below the suggested acceptable maximum by different institutions.21,22 The Centers for Disease Control and Prevention’s (CDC) data quality criteria states that the cancer surveillance data must be 95% complete, the maximum percentage for missing mandatory variables is 2% for age and sex. 22 Missing or incomplete information such as primary site uncertain (PSU) affects the validity of the database and according to Curado et al, 23 the acceptable maxima for the percentage of cases with unknown age is <20%, ill-defined sites should be <20%, and unknown basis of diagnosis also should be (<20%). 23
The basis of diagnosis in all 3 facilities was primary through histology (87.8%) which exceeds the proportion of 50% regarded as a minimum of indication of quality and completeness. 24 According to Bray et al, 25 there is no consistency with the availability of high-quality data in many transitioning countries. Using more than one basis of diagnosis increases the completeness of the cancer surveillance data. 26 Good-quality data can be found in the histopathology report which provides accurate and complete recording of pathology information, including staging.26,27
According to 2018-Globocan report, the most commonly diagnosed cancers in both males and females were lung, breast, and colorectal; however, specific cancers such as prostate cancer was common in males while breast cancer was commonly diagnosed in females. 25 Lung, breast, and colorectal cancers amount to one-third of the global cancer incidence and mortality burden. 25 In this study, the most commonly diagnosed cancers for males are prostate, Kaposi sarcoma, and colorectal; and for females, breast, cervix, and colorectal. Colorectal cancer for both genders was among the top 10 commonly diagnosed cancers. Cervical and Kaposi sarcoma are the most diagnosed cancers which is different from the global picture. The Globocan report also shows that in 2018, the top 10 cancer types accounted for more than 65% of newly diagnosed cancer cases 25 and in our surveillance the top 10 cancers accounted for 60% and 76% of males and females, respectively.
Our study findings show high number of cancer cases in females (65%) compared with males. These findings concur with the reports of 2 cancer registries in the country, that is, NCR’s 2018 report and Eastern Cape Cancer Registry report of 2003 to 2007, 24 where incidence cases were reported at 52% and 60%, respectively, in females. According to Bray et al, 25 the global incidence rate for all cancers was 20% higher in males compared with females and this is contrary to our findings. In our study, female cancers were among the 10 commonly diagnosed cancers in all 3 health facilities and this could be an indicator of country’s efforts lagging behind in addressing preventable cancers such as cervical.
Kaposi sarcoma is a rare cancer at a global scale; however, it is prevalent in several countries mainly in the Southern and Eastern Africa regions. 25 This is due to the high prevalence of HIV in these regions with South Africa having the biggest population of people living with HIV and AIDS (7.7 million) in the world and the province of KwaZulu-Natal has the highest HIV prevalence (27%) in South Africa.28,29 In the 90s, the relative risk of Kaposi sarcoma in HIV-infected individuals in South Africa was 62-fold (odds ratio [OR] = 62; 95% confidence interval [CI] = 20-194) compared with individuals who are not HIV-infected. 30
It is worth nothing that 50.9% of the cancer cases did not have staging; however, in this study, only 12.8% of the diagnosed cases were at stage 4 and this is different to some studies indicating that most cancer presents at an advanced stage.5,31 Our data has indicated that in the KZN public health facilities, there is a need to improve recording of staging for cancer surveillance data quality and patient care as this information is important in treatment and cancer care. 31
The health care systems of countries in the sub-Saharan Africa are not equipped to cope with the large cancer burden 1 which results in the inadequate care for the patients. Morhason-Bello et al 1 alluded to inequality and access to treatment for an increasing number of patients with cancer in sub-Saharan Africa as being a challenge in the future for most health care systems. Our study indicates that more than a quarter (26.6%) of the patients who were diagnosed with cancer were not reported as receiving treatment. Our data indicate that even though access to treatment is a challenge, patient care is still important and a priority in these 3 facilities.
Most of the patients were from the urban districts eThekwini and uMgungundlovu. This is in agreement with a study done by Scott et al, 32 which reported eThekwini with the highest crude incidence rates and this could be due to the 2 hospitals (ADH and IALCH) that provide oncology services located in eThekwini. According to Scott et al, 32 in 1997, in KwaZulu-Natal’s 3 health facilities, the 2 hospitals in eThekwini had the highest crude incidence rate at 115 per 100 000.
The strength of this study is that it is the first multi-centre cancer surveillance system in KZN, which was set up in 3 health facilities allowing for a reasonable volume of new cancer cases. These health facilities diagnose, manage, and treat patients with cancer from the 11 municipal districts and outside KZN, which allowed for diverse population. The cancer surveillance used the requirements from WHO-IARC which assisted in achieving satisfactory data validity and completeness.
This study’s limitation is data was only collected from 3 health institutions for a 1-year period; therefore, our data could not be used to provide incidence rate for all cancers in the KZN province because some of the South African population (17%) uses private health facilities. 24 According to the Independent Clinical Oncology Network and Ahmed et al, due to the cost, some patients who sort cancer care services in private health care end up on the public health care.33,34 This implies that majority of the population of cancer cases seen in the 2 districts or province are likely to be captured on our multi-centre hospital-based cancer surveillance. Our data only abstracted cancer cases from medical records in the oncology departments within the 3 public health facilities and no vital statistics sources such as death records were used and this could underestimate the cancer burden as some cancer cases are likely not to be reported due to the lack of accessibility to facilities, poor transport networks, and low socio-economic status. 32
Conclusions
Hospital-based cancer surveillance in the public health facilities can be established successfully. The hospital-based cancer surveillance can help in estimation of cancer burden in the facilities by using the initiative to develop a population-based cancer surveillance. The information from the hospital-based cancer surveillance can improve knowledge on cancer burden, diagnosis, and treatment in KZN. Strengthening cancer policy will allow expansion of cancer surveillance system and is essential in setting such as KwaZulu-Natal and South Africa as a whole.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Bristol-Myers Squibb Foundation (Grant #1011) under the Multinational Lung Cancer Control Program (MLCCP). The funders had no role in the study design, data collection and analysis, decision to publish.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: NPM and TGG conceptualized and designed the study. NPM carried out data collection and analyses, and wrote the paper. NJ and TGG analysed data and supervised writing the manuscript. All authors have read and approved the final version of the manuscript.
Availability of Data and Materials: Data from this study are the property of the KZN-DOH and University of KwaZulu-Natal and cannot be made publicly available. All interested readers can access the data set from the DOH Research ethics committee and University of KwaZulu-Natal Biomedical Research Ethics Committee (BREC) from the following contacts: The Health Research and Knowledge Management, 330 Langalibalele Street, Private bag X9051, Pietermaritzburg, 3200, Tel: +27 33 3952805 Fax: +27 33 3943782 Email: hrk@kznhealth.gov.za. The Chairperson Biomedical Research Ethics Administration Research Office, Westville Campus, Govan Mbeki Building University of KwaZulu-Natal P/Bag X54001, Durban, 4000 KwaZulu-Natal, South Africa Tel.: +27 31,260 4769 Fax: +27 31,260 4609 Email: BREC@ukzn.ac.za
ORCID iD: Noluthando P Mbeje  https://orcid.org/0000-0003-2958-3409
https://orcid.org/0000-0003-2958-3409
References
- 1. Morhason-Bello IO, Odedina F, Rebbeck TR, et al. Challenges and opportunities in cancer control in Africa: a perspective from the African organisation for research and training in cancer. Lancet Oncol. 2013;14:e142-e151. doi: 10.1016/S1470-2045(12)70482-5. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Soerjomataram I. The changing global burden of cancer: transitions in human development and implications for cancer prevention and control. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, eds. Cancer: Disease Control Priorities. Vol. 3, 3rd ed. Washington, DC: The International Bank for Reconstruction and Development / The World Bank; 2015:23-44. doi: 10.1596/978-1-4648-0349-9_ch2. [DOI] [PubMed] [Google Scholar]
- 3. Piñeros M, Znaor A, Mery L, Bray F. A global cancer surveillance framework within noncommunicable disease surveillance: making the case for population-based cancer registries. Epidemiol Rev. 2017;39:161-169. doi: 10.1093/epirev/mxx003. [DOI] [PubMed] [Google Scholar]
- 4. WHO-IARC. Latest global cancer data. https://www.who.int/cancer/PRGlobocanFinal.pdf. Published September 12, 2018.
- 5. World Health Organization. The ‘unofficial’ World Health Communication Associates (WHCA) action guide to the: WHO – 70th World Health Assembly. https://www.whcaonline.org/uploads/WHEN2017/WHO.pdf. Published May 22-31, 2017. [Google Scholar]
- 6. Takaoka Md M, Okuyama A, Mekata E, et al. Staging discrepancies between Hospital-Based Cancer Registry and Diagnosis Procedure Combination data. Jpn J Clin Oncol. 2016;46:788-791. doi: 10.1093/jjco/hyw066. [DOI] [PubMed] [Google Scholar]
- 7. White MC, Babcock F, Hayes NS, et al. The history and use of cancer registry data by public health cancer control programs in the United States. Cancer. 2017;123:4969-4976. doi: 10.1002/cncr.30905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parkin DM. The role of cancer registries in cancer control. Int J Clin Oncol. 2008;13:102-111. doi: 10.1007/s10147-008-0762-6. [DOI] [PubMed] [Google Scholar]
- 9. Parkin DM, Ferlay J, Hamdi-Chérif M, et al. Cancer in Africa IARC Scientific Publication No. 153. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Cancer-In-Africa-Epidemiology-And-Prevention-2003. Published 2003.
- 10. Somdyala NI, Bradshaw D, Gelderblom WC, Parkin DM. Cancer incidence in a rural population of South Africa, 1998-2002. Int J Cancer. 2010;127:2420-2429. doi: 10.1002/ijc.25246. [DOI] [PubMed] [Google Scholar]
- 11. Singh E, Underwood JM, Nattey C, Babb C, Sengayi M, Kellett P. South African national cancer registry: effect of withheld data from private health systems on cancer incidence estimates. S Afr Med J. 2015;105:107-109. doi: 10.7196/SAMJ.8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. South African National Cancer Registry. https://www.nicd.ac.za/centres/national-cancer-registry/. Published April 23, 2020.
- 13. Singh E, Motsuku L, Khoali L, Sengayi-Muchengeti M, Chen W, Abraham N. Ekurhuleni Population-Based Cancer Registry 2018 annual report. https://www.nicd.ac.za/wp-content/uploads/2020/04/EPBCR-2018-report-Final-report.pdf. Published 2020.
- 14. Department of Health. South Africa, Department of Health. 2011. National Health Act, 2003 (Act no. 61 of 2003). Regulations relating to Cancer Registration. Government Gazette No. 34248, 26 April 2011. 2011;2003(61):34248. [Google Scholar]
- 15. Bray F, Parkin DM. Evaluation of data quality in the cancer registry: principles and methods. Part I: comparability, validity and timeliness. Eur J Cancer. 2009;45:747-755. doi: 10.1016/j.ejca.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 16. Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 17. Jensen OM, Parkin DM, MacLennan R, Muir CS, Skeet RG, eds. Cancer Registration: Principles and Methods. IARC Scientific Publication No. 95. Lyon, France: IARC Press; 1991. [Google Scholar]
- 18. EpiData, Christiansen TB, Lauritsen JM, eds. EpiData – Comprehensive Data Management and Basic Statistical Analysis System. Odense, Denmark: EpiData Association. http://www.epidata.dk. Published 2010. [Google Scholar]
- 19. STATA (Software for Statistics and Data Science). Stata Software. College Station, TX: Software for Statistics and Data Science; 2018:217-219. [Google Scholar]
- 20. Zachary I, Boren SA, Simoes E, Jackson-Thompson J, Davis JW, Hicks L. Information management in cancer registries: evaluating the needs for cancer data collection and cancer research. Online J Public Health Inform. 2015;7:e213. doi: 10.5210/ojphi.v7i2.5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parkin DM, Chen VW, Ferlay J, Galceran J, Storm HH, Whelan SL. Comparability and Quality Control in Cancer Registration. Lyon, France: International Agency for Research on Cancer; 1994:19. [Google Scholar]
- 22. Centers for Disease Control and Prevention. National Program of Cancer Registries Program Standards, 2012-2017. https://stacks.cdc.gov/view/cdc/44020. Published 2017.
- 23. Curado MP, Edwards B, Shin HR, et al. Cancer Incidence in Five Continents. Vol. IX. Lyon, France: International Agency for Research on Cancer; 2007:1-961. [Google Scholar]
- 24. Singh E, Ruff PP, Babb C, et al. Establishment of a cancer surveillance programme: the South African experience. Lancet Oncol. 2016;16:e414-e421. doi: 10.1016/S1470-2045(15)00162-X.Establishment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 26. Parkin DM, Bray F. Evaluation of data quality in the cancer registry: principles and methods part II. Completeness. Eur J Cancer. 2009;45:756-764. doi: 10.1016/j.ejca.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 27. Pinheiro ZN, Gonçalves AA, Leitão AR. Integrated Hospital-Based Cancer Registry System. Published 2008. https://www.tecsi.org/contecsi/index.php/contecsi/5contecsi/paper/viewFile/1552/823 [Google Scholar]
- 28. UNAIDS. Ending AIDS: progress towards the 90–90–90 targets. https://www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_update_2017. Published 2017.
- 29. UNAIDS. UNAIDS – South Africa. https://www.unaids.org/en/regionscountries/countries/southafrica.
- 30. Sitas F, Newton R. Kaposi’s sarcoma in South Africa. J Natl Cancer Inst Monogr. 2001;28:1-4. [DOI] [PubMed] [Google Scholar]
- 31. Curado MP. Importance of hospital cancer registries in Africa. ecancermedicalscience. 2019;13:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scott D, Curtis B, Twumasi FO. Towards the creation of a health information system for cancer in KwaZulu-Natal, South Africa. Health Place. 2002;8:237-249. doi: 10.1016/S1353-8292(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 33. The Independent Clinical Oncology Network. The challenges of suffering from cancer in South Africa. https://www.huffingtonpost.co.uk/2017/05/16/the-challenges-of-suffering-from-cancer-in-south-africa_a_22093419/. Published May 16, 2017.
- 34. Ahmed S, Shahid RK, Gesy K. Cancer care burden: aiming at the Achilles heel. Curr Oncol. 2015;22:e134-e138. doi: 10.3747/co.22.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]