Abstract
Bone matrix is predominantly made up of collagen, and in vitro and in animal models studies have shown that silicon is linked to glycosaminoglycans and plays an important role in the formation of cross-links between collagen and proteoglycans, determining the beneficial effects on strength, composition, and mechanical properties of bone. However, there are still no precise indications regarding a possible role of silicon on bone health in humans. Given this background, the aim of this narrative review was to consider the effectiveness of silicon dietary intake and silicon dietary supplementation (alone or with other micronutrients), in order to suggest a daily dosage of Si supplementation, on bone mineral density in humans. This review included eight eligible studies: four regarding dietary intake and four considering supplementation with silicon alone or with other nutrients. Despite the number of studies considered being low, the number of subjects studied is high (10012) and the results are interesting. Although to date the available scientific evidences are not considered valid enough to allow to establish an adequate level of Silicon intake, based on extrapolations from the data obtained with studies on animal and human models, it has been suggested that an adequate intake in order to promote beneficial effects for bone could be considered to be around 25 mg silicon/day. As for silicon dietary supplements, it has been shown that the combined treatment with orthosilicic acid (6 mg), calcium, and vitamin D has a potentially beneficial effect on femoral BMD compared to only use of calcium and vitamin D.
Keywords: Silicon, bone, dietary supplementation, bone mineral density, nutrients, humans
Impact statement
This narrative review helps to focus on the effectiveness of Silicon (Si) dietary intake and Si dietary supplementation (alone or with other micronutrients), in order to suggest a daily dosage of Si supplementation) on bone mineral density in humans.
Introduction
There is growing scientific interest in Silicon (Si), with more and more studies reported in vitro and animal models that suggest how this element may be essential for bone formation and maintenance. 1
In rats, the highest levels of Si were found in the bone and other connective tissues, such as skin, nails, hair, trachea, tendons, and aorta; a similar tissue distribution is also assumed in humans, although it has not been directly investigated so far. 2 Si is linked to glycosaminoglycans and plays an important role in the formation of cross-links between collagen and proteoglycans. 1 As early as the 1970s, a quantitative study carried out by Carlisle on rats had shown that Si was highly concentrated in immature osteoid tissue, but progressively reduced with the maturation of the bone mineral component, indicating its possible role as a promoter of mineralization and calcification in the preosseous tissue. 3 The same author then later observed, in a study on chicks, a significant slowdown in the growth rate, a greater susceptibility to fractures of the femur and tibia, and a thinner cortical bone in animals fed a poor Si diet compared to those who they had received an integration for 23 days. 4
Si daily intake and bone health
The greatest and most important source of Si for humans is represented by diet. The most bioavailable form, for the animal and the human body, is orthosilicic acid (OSA, Si (OH) 4), which however is present as such only in liquids, such as mineral water and beer, but not in foods (where it is present as polymeric or phytolytic silica). However, other forms of Si can be hydrolyzed to OSA at gastrointestinal level.5,6
The kidneys are the main route of excretion of absorbed food Si, which is largely filtered but only minimally reabsorbed by the renal tubules. Overall, up to 50% of the Si ingested through the diet is excreted in the urine; considering that liquids are responsible for 20–30% of the total intake of this element, these data suggest that some forms of Si food contained in solid foods are also well absorbed by the human body. In the blood, this element is present almost exclusively in the form of silicic acid and is not bound to proteins; Serum Si concentrations were significantly higher in patients with chronic renal failure (46 μmol/L) than in healthy subjects (21 μmol/L).6,7
Collecting data obtained from 35 bibliographic references, Pennington observed that, generally, the levels of Si in products of vegetable origin are higher than those of animal origin. 8 In 2005, Powell et al. developed, using the technique of inductively coupled plasma optical emission spectrometry, a database with the Si content of 207 foods and drinks commonly consumed in the United Kingdom. 9
Numerous researchers have investigated, mostly with the help of questionnaires, the extent of the intake of Si through the diet in different population samples; generally, in Europe and North America, the average consumption of this element is between 12 and 62 mg/day, while in Asia it tends to be higher due to a richer diet in foods of plant origin. 10 In particular, Pennington has estimated, for the US population aged between 25 and 30 years, an average intake of Si equal to 19 mg/day for women and 40 mg/day for men, with the lowest values for those who mainly followed a meaty diet and the highest values for those who preferred vegetarian diets. 8
In 2002, Jugdaohsingh et al., using the data obtained through a 126 items FFQ (food frequency questionnaire) compiled by the members of the cohorts Original Framingham Study (age 67–95 years) and Framingham Offspring Study (age 26–83 years), calculated an average dietary intake of Si equal to 29.6 ± 14.8 mg/day for men and 24.2 ± 9.5 mg/day for women belonging to the first cohort, and to 33.1 ± 19.4 mg/day for men and 25.0 ± 11.4 mg/day for women belonging to the second. For all age groups, the intake of Si in the male sex was therefore 20–33% greater than that of the female sex, and showed a progressive decrease with advancing age in both sexes (on average 0.1 mg for each additional year of age). The main contribution to Si intake was derived from beer, followed by bananas (contained in Si of the edible part 5.4 mg/100 g) and white bread (3.5 mg/100 g), in men, and by bananas, green beans, white bread, and other cereals (especially oat soup, 260 mg Si/100 g) in women. 6
Compared to the values reported in the latter study, in Belgium, (using the duplicate portion sampling technique of 24-h meals) a lower average daily intake of Si, equal to 18.6 ± 8.5 mg, was estimated; 11 in a sample of young Korean men (age 19–25 years), however, the estimate was greater, equal to 37.5 ± 22.2 mg Si/day, mainly deriving from cereals and derivatives (25.6% of the total intake), vegetables (22.7%), drinks and liqueurs (21.2%), milk, and derivatives (7%) (however, the intake of Si with drinking water was not considered). 12 These differences could be at least in part due to the different dietary patterns and the different Si contents of the foods in the various geographical areas considered, as well as to the variable quality of the data used. 12
Using a food composition database in the UK and a semi-quantitative FFQ of 150 self-administered items, McNaughton et al. calculated the average daily intake of Si in a sample of post-menopausal Scottish women older at 60, it was 18.6 mg (SD 4.6). Research has shown that cereals, mostly wholemeal bread, represented the main source of Si in the study population (about 30% of daily intake), followed by fruit (especially bananas), drinks (tea and coffee), and vegetables (leafy vegetables), responsible for more than 75% of the daily Si intake. 13
Although to date the available scientific evidence is not considered solid enough to allow the establishment of an adequate intake level (Adequate Intake, AI) for Si, it has been suggested that based on extrapolations from the data obtained with studies on animal and human models, adequate intake to promote beneficial effects for the bone can be considered to be around 25 mg Si/day. 14 Additional randomized controlled trials are probably needed to evaluate the effectiveness of any additions with Si; however, on the basis of the most recent scientific literature, it is still appropriate to recommend consumption of food and drinks such as to bring that share of Si that appears associated with human health and well-being. 14
Finally, according to the European Food Safety Authority (EFSA), due to the lack of data indicating any toxic effects of oral Si intake, it has not yet been possible to establish a tolerable upper intake level (UL) and it is unlikely that even high dietary intake of this element may cause adverse effects 15 in humans. However, some studies have been conducted on rodents in order to determine a dose without observed adverse effects (no observed adverse effects level, NOAEL): for food Si, a NOAEL equal to 50,000 ppm (mg/l) has been determined. From this, a safe upper level of 1750 mg/day was calculated for an average adult man of 70 kg, demonstrating a large safety margin. 16
Finally, that Si can play an important role for bone well-being seems to be suggested also by a study in which gastrectomized male patients, at one year after surgery, found a significant reduction in volumetric BMD (measured by quantitative CT) in the lumbar spine (at both trabecular and cortical bone levels) compared to the control group, in association with an equally significant decrease in the plasma concentration of Si (also indicating, therefore, the importance of the exocrine and endocrine functions of the stomach in maintaining Si homeostasis). 17
Si, bone formation, and mineralization: In vitro and animal studies
It has been suggested that Si may play a role in electrochemical bone mineralization processes, but the precise biological mechanism remains unclear at present. 1
In vitro studies have shown that this element stimulates the synthesis of type 1 collagen and osteoblastic differentiation (assessed by alkaline phosphatase and osteocalcin levels) in osteoblastic-like human cells 18 and increases the formation of mineralization nodules in mature osteoblast cultures. 19
Dietary Si supplementation has shown beneficial effects on strength in several animal models (study on chickens by Merkley et al.), 20 on composition (study on rats by Seaborn CD et al.), and on mechanical properties bone. 21 In particular, in the latter study, performed on mice, supplementation of the diet with soluble Si (10 ppm of sodium metasilicate, Si group) and administration of Si via desalinated deep sea water (containing 1.8 ppm of Si, DW group) associated with an increase in femoral weight, bone content of calcium and phosphorus, and the activity of tibial alkaline phosphatase (ALP) (bone formation marker) compared to the administration of desalinated surface sea water (containing 0.006 ppm of Si, group SW) or tap water (group TW). In addition, in the DW group, an increase in strength, structural rigidity, percentage deformation, and quantity of force absorbed before breaking compared to the TW group was observed at the femoral level. 21
Ovariectomized female rats represent a standard model for the study of postmenopausal bone loss. Research conducted on this model has shown that the integration with 500 mg of Si/kg of food is able to significantly increase the bone mineral density (BMD) of the femur and tibia in ovariectomized rats fed on a diet low in calcium. 22 Another study, aimed at investigating the effects on the bone of supplementation for 10 weeks with genistein (a phytoestrogen) and/or Si (20 mg/kg body weight/day), has highlighted how the latter can inhibit the reduction of lumbar and femoral BMD observed following ovariectomy (with the greatest BMD values found in the group integrated with both genistein and Si), also restoring bone volume and thickness of trabeculae of the trabecular femoral bone. 23 A similar effect of Si supplementation (20 mg/kg body weight/day for four weeks) on the femoral and tibial BMD of ovariectomized rats had already been observed in a previous study by Bae et al., 24 as well as in a study by Calomme et al., in which supplementation with orthosilicic acid stabilized with choline (which increases its bioavailability; 1 mg Si/kg body weight/day for 30 weeks) has been associated with significant increase in bone mineral content (BMC) and BMD at the femoral level in ovariectomized elderly rats. 25
In Bu’s et al. study, on the other hand, supplementation with Si (in the form of metasilicate) for 15 weeks has not been shown to restore the loss of vertebral, femoral, and tibial BMD induced by ovariectomy, but high doses of Si have been associated with a significant increase in the expression of the gene for osteoprotegerin (OPG) and a reduction in the RANKL/OPG ratio, with the consequent possible stimulation of osteoblastic function (since OPG is mainly produced by osteoblasts). 26
Apparently in contrast with Carlisle's studies of the 70s (which had found a slowing of growth in chicks with a low Si diet) are the results of the research conducted by Jugdaohsingh et al., in which rats with a diet poor in Si had greater body and individual bone length (with an inverse proportionality ratio with respect to serum Si), a reduction in the thickness of the growth plate (growth cartilage) and an increase in the density of the chondrocytes compared to animals fed with diet standard. Low serum concentrations of Si, therefore, could be responsible for inhibiting the closure of the growth plate with consequent increase in longitudinal growth. 27
Si is considered an element with high biocompatibility; recent studies on Si nanoparticles have highlighted their in vitro ability to mediate powerful inhibitory effects on osteoclasts and stimulatory effects on osteoblasts, as they are able to antagonize the activation of NFkB (and therefore of the signal transduction pathway required for osteoclastic resorption and which inhibits osteoblastic bone neoformation). 28 The same Si nanoparticles have proven to significantly increase lumbar and femoral BMD 28 and to mitigate and reverse age-associated bone loss (with preferential increase in trabecular bone) by increasing the serum levels of osteocalcin, a sensitive and specific biochemical marker of bone formation, in mice in vivo. 29 In a study by Kim et al., the use of hydroxyapatite derived from cuttlefish bone replaced with Si stimulated cell proliferation, early engraftment of human mesenchymal stem cells and osteoblastic differentiation in vitro, and bone formation in rabbits in vivo, thus representing a potential material for bone transplants. 30
Given this background, the aim of this narrative review was to consider the state of the art on effectiveness of Si dietary intake and Si dietary supplementation (alone or with other micronutrients) in order to suggest a daily dosage of Si supplementation on bone mineral density in humans.
The present narrative review was performed following the steps by Egger et al. 31 as follows:
Configuration of a working group: three operators skilled in clinical nutrition (one acting as a methodological operator and two participating as clinical operators).
Formulation of the revision question on the basis of considerations made in the abstract: “effectiveness of Si dietary intake and Si dietary supplementation (alone or with other micronutrients), in order to suggest a daily dosage of Si supplementation on bone mineral density in humans.”
Identification of relevant studies: a research strategy was planned on PubMed (Public MedLine run by the National Center of Biotechnology Information (NCBI) of the National Library of Medicine of Bathesda (USA)) as follows: (a) Definition of the keywords (Si, bone health, humans, supplementation, bone mineral density), allowing the definition of the interest field of the documents to be searched, grouped in quotation marks (“…”) and used separately or in combination; (b) use of: the Boolean (a data type with only two possible values: true or false) AND operator, that allows the establishments of logical relations among concepts; (c) Research modalities: advanced search; (d) Limits: time limits: papers published in the last 20 years; humans; adults; languages: English; (e) Manual search performed by the senior researchers experienced in clinical nutrition through the revision of articles on the effectiveness of Si dietary intake and Si dietary supplementation (alone or with other micronutrients, in order to suggest a daily dosage of Si supplementation) on bone mineral density in humans.
Published in journals qualified in the Index Medicus.
Analysis and presentation of the outcomes: we create two paragraphs: a paragraph about effectiveness of Si dietary intake on bone mineral density and a second paragraph on Si supplementation (alone or in combination with other nutrients) on bone mineral density, and the data extrapolated from the “revised studies” were collocated in tables; in particular, for each study, we specified the author and year of publication and study characteristics.
The analysis was carried out in the form of a narrative review of the reports. At the beginning of each section, the keywords considered and the type of studies chosen are reported. We evaluated, as is suitable for the narrative review, studies of any design which considered the effectiveness of Si dietary intake and Si dietary supplementation (alone or with other micronutrients), in order to suggest a daily dosage of Si supplementation, on bone mineral density in humans.
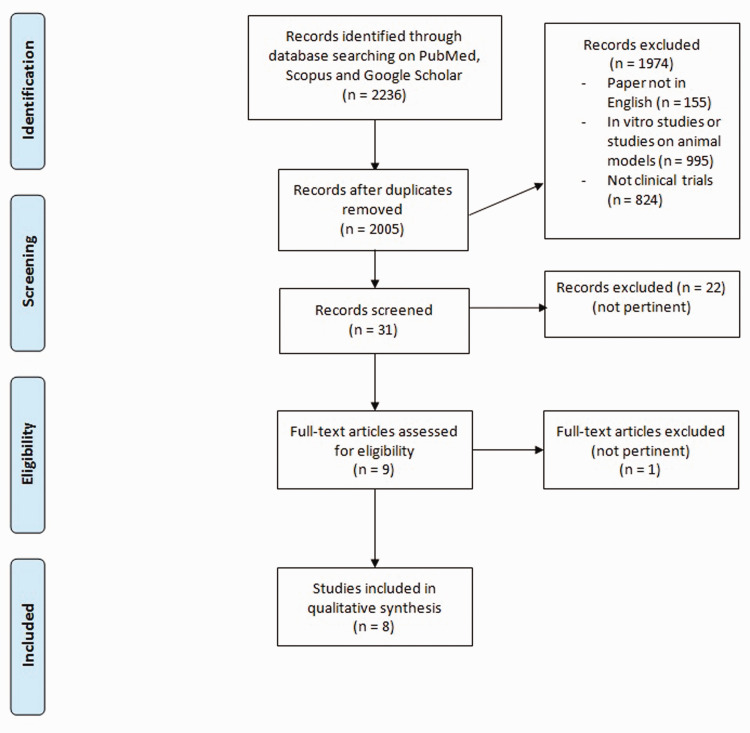
Figure 1 shows the flow chart of literature research.
Figure 1.
Flow chart of literature research. (A color version of this figure is available in the online journal.)
Results
The research for Si intake was conducted based on the keywords: “silicon intake” AND “bone” or “bone mineral density” AND “humans”.
For the present part we have analyzed a total of four studies: two cohort studies, one cross-sectional cohort study, and one cross-sectional study.
Table 1 shows the studies that evaluated the relationship between dietary intake of Si and bone mineral density.
Table 1.
Studies regarding Si dietary intake and bone health in humans.
| First author, year | Study design | Setting | Inclusion criteria | Exclusion criteria | Number of subjects (M-F)Mean age | Lowest quintile intake / RDA or EAR | % subject in lowest quintile intake / % subject <RDA or EAR | Highest Quintile intake | % subject in highest quintile intake | Primary outcomes | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jugdaohsingh et al. 32 | Cross-sectional cohort study | Free-living subjects | All participants with dietary intake data and BMD measurement were included; written informed consent. | Bone diseases, other diseases, women with premature menopause or bilateral ovariectomy, or subjects on treatments for bone diseases or other diseases. | 2847 subjects (1251 men; 1596 women)30-87 years | <14 mg Si/dayMen: 7.6–18.8 mg Si/dPre-menopausal women: 7.1–16.7 mg Si/dPost-menopausal women: 5.9–16.4 mg Si/d | / | >40 mg Si/dayMen: 34.4–118.0 mg Si/dPre-menopausal women: 30.2–63.2 mg Si/dPost-menopausal women: 29.9–83.5 mg Si/d | / | Association between dietary Si intake and BMD at the left hip (total hip, trochanter, Ward’s area, and femoral neck) and at the lumbar spine (L2–L4) assessed by DXA | Marked significant differences in BMD between the highest and lowest quintiles at the hip sites for both premenopausal women (average, 9.9 ± 2.0%) and men (5.1 ± 0.8%); suggestive differences in BMD at the lumbar spine for premenopausal women and men. No significant differences for post-menopausal women. |
| Macdonald et al. 33 | Cohort study | United Kingdom, from the APOSS study (Aberdeen Prospective Osteoporosis Screening Study) | Peri-menopause, whether or not subjected to hormone replacement therapy (HRT), with dietary intake data; written informed consent. | Other treatments for osteoporosis (e.g. bisphosphonates) at the time of this study | 3199 women (mean age 48.5 ± 2.4 years) | // | // | // | // | Association between dietary Si intake and BMD at the left hip (total hip, trochanter, Ward’s area, and femoral neck) and at the lumbar spine assessed by DXA | A significant positive association between the Si intake with the diet adjusted for energy and the BMD of the hip (femoral neck) and lumbar spine. The significance of the positive association between energy-adjusted Si intake and hip BMD was confirmed only for pre-menopausal subjects and those taking HRT |
| Macdonald et al. 34 | Cohort study | United Kingdom, from the APOSS study (Aberdeen Prospective Osteoporosis Screening Study) | Written informed consent | Other treatments for osteoporosis (e.g. bisphosphonates) at the time of the study | 3198 women (n = 1170 current HRT users, n = 1010 past HRT users, n = 1018 never used HRT) 50–62 years | Lowest quartile intake: 16.5 ± 4.0 mg Si/day | 25.02% | Highest quartile intake: 31.5 ± 7.3 mg Si/day | 24.98% | Association between dietary energy-adjusted Si intake and markers of bone health (BMD of the left femoral neck and lumbar spine measured by DXA; urinary fPYD and fDPD; serum PINP) | Mean FN BMD was 2% lower in the lowest quartile compared to the top quartile of energy-adjusted Si intake; energy-adjusted Si intake was associated with FN BMD for oestrogen-replete women only. Quartile of dietary Si intake was negatively associated with fDPD/Cr and fPYD/Cr and positively with PINP. |
| Choi et al. 12 | Cross-sectional study | Free-living subjects | Healthy Korean males of 19–25 years; informed consent. | History of prior medication use that may have led to alterations of bone metabolism; insufficient dietary report. | 400 male subjects22.68 ± 2.00 years | Minimum Si intake 8.38 mg/day | / | Maximum Si intake 183.14 mg/day | / | Relationship between dietary Si intake and calcaneus bone density (measured by quantitative ultrasound) and the bone metabolism markers (serum total alkaline phosphatase, N-midosteocalcin and type 1 collagen C-terminal telopeptide) | Daily total Si intake had no correlation with calcaneus bone density and the bone metabolism markers, but Si intake from vegetables had a positive correlation with serum total alkaline phosphatase activity. |
The research for Si supplementation was conducted based on the keywords: “Silicon dietary supplementation” AND “bone” OR “bone mineral density” AND “humans”.
For the present part we have analyzed a total of four studies: one retrospective study, one randomized placebo controlled trial, and two double blind randomized placebo controlled trials.
Table 2 shows the studies that evaluated the relationship between dietary supplementation of Si and bone mineral density.
Table 2.
Studies regarding Si supplementation and bone health in humans.
| First author, year | Study design | Setting | Inclusion criteria | Exclusion criteria | Intervention | Parallel treatments | Number of subjects (M-F) | Duration of the intervention | Primary outcomes | Secondary outcomes | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eisinger et al. 35 | Retrospective study | Free-living subjects | Osteoporosis | / | Si (n = 8) | Placebo (n = 16) Fluoride (n = 10) Etidronate (n = 13) Magnesium (n = 6) | 53 osteoporotic women | 14–22 months | Vertebral and femoral bone mineral density (BMD) changes | / | Si induced a significant increase in femoral BMD. Fluoride induced a significant increase in vertebral and a slight decrease in femoral BMD. Etidronate and, to a lesser extent, magnesium induced a slight although statistically non-significant increase in vertebral BMD. |
| Li et al. 36 | Randomized, placebo-controlled trial | Free-living subjects | Postmenopausal women within 5 years of menopause; reduced bone density but no evidence of osteoporosis or osteopenia by DEXA scan (T ≥ −1.5 in the total hip and lumbar spine); written informed consent. | Women with a body mass index (BMI) > 30 kg/m2 or who were being treated with estrogens, corticosteroids, or bisphosphonates, who consumed more than one alcoholic beverage a day, or who had significant illness affecting bone metabolism. | 1200 mg of calcium + 800 IU of vitamin D + 1 liter of Si-rich artesian aquifer water (SW, 86 mg/L silica)/day | 1200 mg of calcium + 800 IU of vitamin D + 1 L of purified water of low-Si content (PW, undetectable amount of silica)/day | 17 post-menopausal women (average age 54 years) | 12 weeks | Changes in urinary Si level. Changes in serum parathyroid hormone (PTH) level and bone turnover markers (procollagen type I intact N-terminal propeptide, bone specific alkaline phosphatase and osteocalcin). Changes in urinary collagen type 1 cross-linked N-telopeptide (NTx) excretion. | / | The urinary Si for the SW group increased by 133.5%, a statistically significant increase by comparison to the PW group (no change in the urinary Si level). No change of PTH or markers of bone formation including procollagen type I intact, N-terminal propeptide, bone specific alkaline phosphatase, and osteocalcin within groups or between groups. No change in urinary NTx during the study within groups or between groups. |
| Spector et al. 37 | Double-blind randomized placebo controlled trial | St Thomas' Hospital, London | Osteopenic, but otherwise healthy women; written informed consent. | Renal failure as defined by serum creatinine > 200 μmol/L, abnormal serum ferritin level, concomitant medication, oral glucocorticoid treatment, local injectable glucocorticoid treatment if > 5 injections per year, inhaled glucocorticoid treatment if > 6 months in the previous year and more than 2 mg/day prednisone equivalent, concomitant or previous treatment for bone diseases, concomitant and previous use of food supplements containing silicon or horsetail herb extract, bamboo extract, colloidal silicic acid, or silanol derivatives in the previous 6 months | 1000 mg Ca and 20 microg cholecalciferol (Vit D3) and three different ch- orthosilicic acid (OSA) doses (3, 6 and 12 mg Si) | A choline-glycerol solution without ch-OSA | 184 women (mean age for placebo: 62.0 ± 10.9 (n = 37); mean age for 3 mg Si: 60.4 ± 11.8 (n = 33; mean age for 6 mg Si: 59.7 ± 9.4 (n = 33); mean age for 12 mg Sì: 60.8 ± 9.7 (n = 33)) | 12 months | Evaluation of the effect of oral choline-stabilized orthosilicic acid and Si on markers of bone turnover and on bone mineral density in osteopenic women | / | There were no significant changes in femoral and lumbar BMD following the intake of ch-OSA; however, a post hoc analysis of subgroup of the femoral neck BMD (subjects with baseline femoral T-score <−1) showed a significant change of this parameter in the group treated with 6 mg of Si compared to the placebo group (BMD total femur and femoral neck greater than 0.98% and 2% respectively), with possible consequent reduction in the risk of hip fractures. |
| Spector et al. 35 | Double-blind randomized placebo controlled trial | St Thomas' Hospital, London | Osteopenic, but otherwise healthy women; written informed consent. | Renal failure as defined by serum creatinine > 200 μmol/L, abnormal serum ferritin level, concomitant medication, oral glucocorticoid treatment, local injectable glucocorticoid treatment if > 5 injections per year, inhaled glucocorticoid treatment if > 6 months in the previous year and more than 2 mg/day prednisone equivalent, concomitant or previous treatment for bone diseases, concomitant and previous use of food supplements containing silicon or horsetail herb extract, bamboo extract, colloidal silicic acid, or silanol derivatives in the previous 6 months | Three different ch-OSA doses (3, 6, and 12 drops) were used corresponding to 3, 6, and 12 mg Si. All subjects received calcium and vitamin D3 (1000 mg calcium and 20 mg cholecalciferol) daily | A choline-glycerol solution without ch-OSA | 114 women (average age: 61 years) | 12 months | Investigate the effect of low dose oral silicon as an adjunct to calcium/vitamin D3 on markers of bone turnover and BMD. | / | The doses of 6 and 12 mg of Si were also shown to be significantly associated with an increase in procollagen type I N propeptide, while no significant change in BMD to the column was observed |
Si intake and bone mineral density: Human studies
In order to investigate the role of food Si in bone health in humans, the study by Jugdaohsingh et al. of 2004 used the same subjects participating in the Framingham Osteoporosis Study, in turn taken from the Framingham Offspring cohort: 1251 male and 1596 female pre- (306) and post-menopausal (1325) (aged 30 and 87 years old) who had completed two semi-quantitative food frequency questionnaires (FFQ) and performed BMD (4 measurement sites) and lumbar spine measurements via DXA between 1996 and 2001. Si intake is positively related to femoral BMD in men and pre-menopausal women, but not in post-menopausal women; however, no significant association was found between Si intake and lumbar BMD in any group. By dividing the groups into quintiles according to the dietary intake of Si, there was a marked difference in BMD between the lower (<14 mg Si/day) and the upper (>40 mg Si/day) quintile in pre-menopausal men and women (again no statistically significant difference in post-menopausal women). These results suggest that a high dietary intake of Si may have beneficial effects on bone health, especially cortical health, in younger women and men. 32
In line with the results reported by Jugdaohsingh et al., the Framingham Offspring cohort is also those of a study on 3199 women in the United Kingdom (taken from the APOSS study, Aberdeen Prospective Osteoporosis Screening Study) in peri-menopause, whether or not subjected to hormone replacement therapy (HRT). The subjects underwent a first bone densitometry in the years 1990–1993 (average age 48.5 ± 2.4 years) and a second 6.3 ± 0.9 years later; they also completed a Food Frequency Questionnaire. The authors found a significant positive association between the Si intake with the diet adjusted for energy and the BMD of the hip (femoral neck) and lumbar spine. After adjusting the data also for menopausal status/HRT, age, weight, height, smoking, and level of physical activity, dietary Si was responsible for 0.1% of changes in hip BMD, while the association with lumbar BMD was no longer statistically significant. When the groups of menopausal women were analyzed separately, the significance of the positive association between energy-adjusted Si intake and hip BMD was confirmed only for pre-menopausal subjects and those taking HRT, suggesting a possible interaction between estrogenic state and effects of Si on the bone. 33 In order to support this hypothesis, the same authors, in 2012, published a second study performed on a cohort of 3198 women (also from the APOSS study) aged between 50 and 62 years, of which 1170 with hormone replacement therapy in place and 1018 have never been in HRT. The recruited subjects underwent the evaluation by DXA of the BMD of the femoral neck (FN) and of the lumbar spine (LS) and the measurement of the urinary markers of bone resorption (free pyridinoline and deoxypyridinoline, compared to the creatinine values: fPYD/Cr and fDPD/Cr) and serum bone formation markers (N-terminal pro-peptide of type I procollagen: PINP); they were also asked to fill in a semi-quantitative validated food frequency questionnaire to evaluate Si intake through the diet. From the latter emerged an average daily intake, adjusted for energy, of 23.3 mg of Si (SD: 7.5; minimum value: 5.7 mg; maximum value: 59.4 mg), and based on the data thus collected, the participants were divided into quartiles. The results showed that the femoral neck BMD of women belonging to the lower quartile (Q1: 16.5 ± 4.0 mg Si/day) was 2% lower than that of women of the upper quartile (Q4: 31, 5 ± 7.3); a similar trend was also observed for lumbar BMD.
Considering, however, separately groups based on hormonal status, the association between intake of Si and FN BMD was significant only in women not deficient in estrogen (women in pre-menopause and women currently in HRT), significance maintained even after correction for confounding variables. According to linear regression analysis, the energy-adjusted Si intake would represent a significant predictor of the FN BMD in women in HRT. The authors also highlighted a significant interaction between estrogenic and quartile status of Si intake on femoral neck BMD (but not on lumbar BMD). The Si intake quartiles proved to be associated negatively with urinary bone resorption markers and positively with PINP; finally, serum concentrations of 25 (OH) vitamin D were lower in the lower quartile than in the third and fourth quartiles. The individual's estrogenic status therefore appears to be able to influence the metabolic role of Si in bone health, but further studies are needed to clarify its mechanisms. 34
In a 2017 research, Choi et al. estimated, through two 24-h reminders, the average daily intake of Si in a sample of 400 young Korean men (aged between 19 and 25 years), investigating its possible association with calcareous BMD (assessed through the technique of quantitative ultrasound) and with serum bone metabolism markers (including total ALP and osteocalcin). The results showed an average intake of 37.54 ± 22.16 mg Si/day, without any significant correlation between the amount of Si introduced in the diet and BMD or heel bone metabolism. However, the intake of Si through vegetables was positively correlated with total serum alkaline phosphatase, an important marker of bone formation, suggesting a potential positive role of Si deriving from vegetables on bone health. 12
Dietary supplements with Si and bone mineral density
As for human intervention studies, in a retrospective study conducted on 53 osteoporotic women over a period of 14–22 months, Si treatment was more effective in inducing a significant increase in femoral BMD than treatment with fluoride (significant increase in vertebral BMD but slight decrease in femoral BMD), with etidronate, and with magnesium (in both cases increase in vertebral BMD not statistically significant). 35
In the randomized controlled pilot study of Li et al., 17 postmenopausal women of no more than 5 years (mean age 54 years), with reduced bone density but not osteopenic or osteoporotic according to DEXA, were divided into two groups: a placebo group, which was to take 1 l/day of purified water (PW, containing an undetectable amount of Si), and an intervention group, which was to take 1 l/day of artesian groundwater (SW, containing 86 mg Si/l); all the participants were then supplemented daily with 1200 mg of calcium and 800 I.U. of vitamin D, all for a period of 12 weeks. At the end of the 12th week in the SW group, a significant increase in urinary Si levels was observed with respect to the baseline, while no change was found in the PW group; in particular, urinary Si in the SW group increased by 133.5%, a statistically significant value compared to the placebo group. No significant changes, however, were observed in the levels of NTx (urinary bone resorption marker) and in the levels of serum bone formation markers (intact type I procollagen, N-terminal propeptide, bone ALP, osteocalcin), nor at within groups or between groups. Both the purified water and the water rich in Si were well tolerated, with no reported adverse events. According to the authors, therefore, water from artesian layers represents a safe and effective means of providing the human body with easily absorbable Si; although there have been no effects on short-term bone turnover in post-menopausal women, it is possible that this element exerts more pronounced effects in men or in pre-menopausal women, especially taking into consideration longer periods of treatment. 36
Considering the hypothesis of a role of Si in bone mineralization, Spector et al. have investigated the oral use of orthosilicic acid (Si (OH) 4) stabilized with choline (ch-OSA) as a useful agent in the prevention and/or treatment of osteoporosis in combination with the administration of calcium and vitamin D. In this double-blind randomized controlled trial (RCT), a total of 184 women (mainly postmenopausal, mean age 60.7 ± 10.4 years) with osteopenia (T-score <−1.5 to the spine lumbar evaluation by DEXA) were recruited, of which 136 completed the study. The subjects were divided into 4 groups: a placebo group and three groups receiving daily oral integration for 12 months with ch-OSA at a dose of 3, 6, and 12 drops, respectively (corresponding to 3, 6, and 12 mg of Si); all the volunteers also daily took 1000 mg of calcium and 20 µg of cholecalciferol. At the end of the 12-month period, significantly higher levels of PINP (N-terminal pro-peptide of type I procollagen), a type I collagen synthesis marker and early marker of bone formation, were found in groups supplemented with 6 and 12 mg of ch-OSA compared to the placebo group. On the other hand, there were no significant changes in femoral and lumbar BMD following the intake of ch-OSA; however, a post hoc analysis of subgroup of the femoral neck BMD (subjects with baseline femoral T-score <−1) showed a significant change of this parameter in the group treated with 6 mg of Si compared to the placebo group (BMD total femur and femoral neck greater than 0.98% and 2%, respectively), with possible consequent reduction in the risk of hip fractures. 37 Completely overlapping results had already been obtained by the same authors in 2005 following the recruitment of 114 women (average age: 61 years) with T-score at the column <−1. The subjects had taken 1000 mg of calcium and 800 I.U daily for 12 months of cholecalciferol with (in three intervention groups) or without (in the placebo group) the addition of 3, 6, or 12 mg of Si (in the form of ch-OSA). The doses of 6 and 12 mg of Si were also shown to be significantly associated with an increase in PINP in this case, while no significant change in BMD to the column was observed. 38
Overall, the results obtained from these studies suggest that the combined treatment with ch-OSA (6 mg), calcium, and vitamin D has a potentially beneficial effect on bone turnover, in particular on bone collagen, and perhaps also on femoral BMD compared to only use of calcium and vitamin D.37,38
In order to identify a form of Si integration readily bioavailable and safe for use in humans, Jugdaohsingh et al. developed a randomized, placebo-controlled, cross-over and double-blind study conducted on 22 healthy women, aged between 22 and 38 years, who were administered for 4 weeks, at a daily dose of 10.5 mg, monomethylsilanetriol (MMST), an oral Si supplement that has long been used for the health of bone and connective tissue, especially in continental Europe, despite the absence of official data on its use and its safety in vivo. Overall, at the end of the four weeks, this integration resulted in a significant increase in serum and urinary concentrations of Si assessed on an empty stomach, and there were no adverse effects (investigated through questionnaires) nor alterations to routine blood chemistry tests. The study therefore proved that MMST represents a form of Si well absorbed and metabolized by the human body, similarly to other forms of Si of food origin (for example orthosilicic acid), thus being considered an adequate supplement of this microelement. 39
Although, to date, available scientific evidence is not considered valid enough to allow establishment of an adequate level of intake (Adequate Intake, AI) for Si, based on extrapolations from the data obtained with studies on animal and human models, it has been suggested that an adequate intake in order to promote beneficial effects for the bone could be considered to be around 25 mg Si/day. As for Si dietary supplements, it has been shown that the combined treatment with orthosilicic acid (6 mg), calcium, and vitamin D has a potentially beneficial effect on bone turnover, in particular on bone collagen, and also on femoral BMD compared to only use of calcium and vitamin D.
Conclusions
In conclusion, this review included eight eligible studies: four regarding dietary intake and four considering supplementation with Silicon alone or with other nutrients. Despite the number of studies considered being low, the number of subjects studied is high (10012) and the results are interesting.
Although to date the available scientific evidences are not considered valid enough to allow to establish an adequate level of Silicon intake, based on extrapolations from the data obtained with studies on animal and human models, it has been suggested that an adequate intake in order to promote beneficial effects for bone could be considered to be around 25 mg silicon/day. As for silicon dietary supplements, it has been shown that the combined treatment with orthosilicic acid (6 mg), calcium, and vitamin D has a potentially beneficial effect on femoral BMD compared to only use of calcium and vitamin D.
Footnotes
AUTHORS’ CONTRIBUTIONS: All authors participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Gabriella Peroni https://orcid.org/0000-0002-1632-1787
Giovanna Petrangolini https://orcid.org/0000-0001-6681-7329
References
- 1.Price CT, Koval KJ, Langford JR. Silicon: a review of its potential role in the prevention and treatment of postmenopausal osteosporosis. Int J Endocrinol 2013; 2013:6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jugdaohsingh R. Silicon and bone health. J Nutr Health Aging 2007;99–110 [PMC free article] [PubMed] [Google Scholar]
- 3.Carlisle EM. Silicon: a possible factor in bone calcification. Science 1970; 167:279–80 [DOI] [PubMed] [Google Scholar]
- 4.Carlisle EM. Silicon: an essential element for the chick. Science 1972; 178:619–21 [DOI] [PubMed] [Google Scholar]
- 5.Rodella LF, Bonazza V, Labanca M, Lonati C, Rezzani R. A review of the effects of dietary silicon intake on bone homeostasis and regeneration. J Nutr Health Aging 2014; 18:820–6 [DOI] [PubMed] [Google Scholar]
- 6.Jugdaohsingh R, Anderson SHC, Tucker KL, Elliott H, Kiel DP, Thompson RPH, Powell JJ. Dietary silicon intake and absorption. Am J Clin Nutr 2002; 75:887–93 [DOI] [PubMed] [Google Scholar]
- 7.Food and Nutrition Board. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium. Washington, D.C.: National Academies Press, 2001 [PubMed] [Google Scholar]
- 8.Pennington JAT. Silicon in foods and diets. Food Addit Contam 1991; 8:97–118 [DOI] [PubMed] [Google Scholar]
- 9.Powell JJ, McNaughton SA, Jugdaohsingh R, Anderson SHC, Dear J, Khot F, Mowatt L, Gleason KL, Sykes M, Thompson RPH, Bolton-Smith C, Hodson MJ. A provisional database for the silicon content of foods in the United Kingdom. Br J Nutr 2005; 94:804–12 [DOI] [PubMed] [Google Scholar]
- 10.Götz W, Tobiasch E, Witzleben S, Schulze M. Effects of silicon compounds on biomineralization, osteogenesis, and hard tissue formation. Pharmaceutics 2019; 11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robberecht H, Van Cauwenbergh R, Van Vlaslaer V, Hermans N. Dietary silicon intake in Belgium: sources, availability from foods, and human serum levels. Sci Total Environ 2009; 407:4777–82 [DOI] [PubMed] [Google Scholar]
- 12.Choi MK, Kim MH. Dietary silicon intake of Korean young adult males and its relation to their bone status. Biol Trace Elem Res 2017; 176:89–104 [DOI] [PubMed] [Google Scholar]
- 13.McNaughton SA, Bolton-Smith C, Mishra GD, Jugdaohsingh R, Powell JJ. Dietary silicon intake in post-menopausal women. Br J Nutr 2005; 94:813–7 [DOI] [PubMed] [Google Scholar]
- 14.Nielsen FH. Update on the possible nutritional importance of silicon. J Trace Elem Med Biol 2014; 28:379–82 [DOI] [PubMed] [Google Scholar]
- 15.European Food Safety Authority. Tolerable upper intake levels for vitamins and minerals. Scientific Committee on Food; Scientific Panel on Dietetic Products, Nutrition and Allergies, 2006
- 16.Martin KR. The chemistry of silica and its potential health benefits. J Nutr Health Aging 2007; 11:94–8 [PubMed] [Google Scholar]
- 17.Tatara MR, Krupski W, Szpetnar M, Dąbrowski A, Bury P, Szabelska A, Charuta A, Boguszewska-Czubara A, Maciejewski R, Wallner G. Effects of total gastrectomy on plasma silicon and amino acid concentrations in men. Exp Biol Med 2015; 240:1557–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reffitt DM, Ogston N, Jugdaohsingh R, Cheung HFJ, Evans BAJ, Thompson RPH, Powell JJ, Hampson GN. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003; 32:127–35 [DOI] [PubMed] [Google Scholar]
- 19.Kim EJ, Bu SY, Sung MK, Choi MK. Effects of silicon on osteoblast activity and bone mineralization of MC3T3-E1 cells. Biol Trace Elem Res 2013; 152:105–12 [DOI] [PubMed] [Google Scholar]
- 20.Merkley J, Miller E. The effect of sodium fluoride and sodium silicate on growth and bone strength of broilers. Poult Sci 1983; 62:798–804 [DOI] [PubMed] [Google Scholar]
- 21.Maehira F, Iinuma Y, Eguchi Y, Miyagi I, Teruya S. Effects of soluble silicon compound and deep-sea water on biochemical and mechanical properties of bone and the related gene expression in mice. J Bone Miner Metab 2008; 26:446–55 [DOI] [PubMed] [Google Scholar]
- 22.Kim M-H, Bae Y-J, Choi M-K, Chung Y-S. Silicon supplementation improves the bone mineral density of calcium-deficient ovariectomized rats by reducing bone resorption. Biol Trace Elem Res 2009; 128:239–47 [DOI] [PubMed] [Google Scholar]
- 23.Qi S, Zheng H. Combined effects of phytoestrogen genistein and silicon on ovariectomy-induced bone loss in rat. Biol Trace Elem Res 2017; 177:281–7 [DOI] [PubMed] [Google Scholar]
- 24.Bae Y-J, Kim J-Y, Choi M-K, Chung Y-S, Kim M-H. Short-term administration of water-soluble silicon improves mineral density of the femur and tibia in ovariectomized rats. Biol Trace Elem Res 2008; 124:157–63 [DOI] [PubMed] [Google Scholar]
- 25.Calomme M, Geusens P, Demeester N, Behets GJ, D’Haese P, Sindambiwe JB, Van Hoof V, Vanden Berghe D. Partial prevention of long-term femoral bone loss in aged ovariectomized rats supplemented with choline-stabilized orthosilicic acid. Calcif Tissue Int 2006; 78:227–32 [DOI] [PubMed] [Google Scholar]
- 26.Bu SY, Kim M-H, Choi M-K. Effect of silicon supplementation on bone status in ovariectomized rats under calcium-replete condition. Biol Trace Elem Res 2016; 171:138–44 [DOI] [PubMed] [Google Scholar]
- 27.Jugdaohsingh R, Calomme MR, Robinson K, Nielsen F, Anderson SHC, D’Haese P, Geusens P, Loveridge N, Thompson RPH, Powell JJ. Increased longitudinal growth in rats on a silicon-depleted diet. Bone 2008; 43:596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck GR, Ha S-W, Camalier CE, Yamaguchi M, Li Y, Lee J-K, Weitzmann MN. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomedicine 2012; 8:793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weitzmann MN, Ha SW, Vikulina T, Roser-Page S, Lee JK, Beck GR. Bioactive silica nanoparticles reverse age-associated bone loss in mice. Nanomed Nanotechnol Nanomed 2015; 11:959–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim BS, Yang SS, Yoon JH, Lee J. Enhanced bone regeneration by silicon-substituted hydroxyapatite derived from cuttlefish bone. Clin Oral Implants Res 2017; 28:49–56 [DOI] [PubMed] [Google Scholar]
- 31.Egger M, Dickersin K, Smith GD. Problems and limitations in conducting systematic reviews. In: Matthias E, George DS & Douglas GA (eds) Systematic reviews in health care. London, UK: BMJ Publishing Group, 2008, pp.43–68
- 32.Jugdaohsingh R, Tucker KL, Qiao N, Cupples LA, Kiel DP, Powell JJ. Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham offspring cohort. J Bone Miner Res 2004; 19:297–307 [DOI] [PubMed] [Google Scholar]
- 33.Macdonald H, Hardcastle A, Jugdaohsingh R, Reid D, Powell J. Dietary silicon intake is associated with bone mineral density in premenopausal women and postmenopausal women taking HRT. J Bone Miner Res 2005; 20:S393 [Google Scholar]
- 34.Macdonald HM, Hardcastle AC, Jugdaohsingh R, Fraser WD, Reid DM, Powell JJ. Dietary silicon interacts with oestrogen to influence bone health: evidence from the Aberdeen prospective osteoporosis screening study. Bone 2012; 50:681–7 [DOI] [PubMed] [Google Scholar]
- 35.Eisinger J, Clairet D. Effects of silicon, fluoride, etidronate and magnesium on bone mineral density: a retrospective study. Magnes Res 1993; 6:247–9 [PubMed] [Google Scholar]
- 36.Li Z, Karp H, Zerlin A, Lee TYA, Carpenter C, Heber D. Absorption of silicon from artesian aquifer water and its impact on bone health in postmenopausal women: a 12 week pilot study. Nutr J 2010; 9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spector TD, Calomme MR, Anderson SH, Clement G, Bevan L, Demeester N, Swaminathan R, Jugdaohsingh R, Berghe DAV, Powell JJ. Choline-stabilized orthosilicic acid supplementation as an adjunct to calcium/vitamin D3 stimulates markers of bone formation in osteopenic females: a randomized, placebo-controlled trial. BMC Musculoskelet Disord 2008; 9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spector T, Calomme M, Anderson S, Swaminathan R, Jugdaohsingh R, Powell J. Effect on bone turnover and BMD oflow dose oral silicon as an adjunct to calcium/vitamin D3 in a randomized, placebo-controlled trial. J Bone Miner Res 2005; 20:S172 [Google Scholar]
- 39.Jugdaohsingh R, Hui M, Anderson SHC, Kinrade SD, Powell JJ. The silicon supplement ‘monomethylsilanetriol’ is safe and increases the body Pool of silicon in healthy pre-menopausal women. Nutr Metab 2013; 10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]