Abstract
Background/Aims
Unlike other gastrointestinal tract cancers, there are relatively few reports on the clinical significance of circulating tumor cells (CTCs) and TWIST, a marker of epithelial-mesenchymal transition, in patients with esophageal squamous cell carcinoma (ESCC). This study aimed to evaluate the clinical significance of TWIST expression in CTCs in patients with ESCC.
Methods
Peripheral blood samples for CTC analyses were prospectively obtained from 52 patients with ESCC prior to treatment between September 2017 and September 2019. CTCs were detected using a centrifugal microfluidic system based on a fluid-assisted separation technique, and CTCs positive for TWIST on immunostaining were defined as TWIST (+) CTCs.
Results
Of the 52 patients with ESCC, CTCs and TWIST (+) CTCs were detected in 44 patients (84.6%) and 39 patients (75.0%), respectively. The CTC and TWIST (+) CTC counts were significantly higher in patients aged >65 years and those who had a large tumor (>3 cm) than in those aged ≤65 years and those who had a small tumor (≤3 cm), respectively. There were no differences in CTC and TWIST (+) CTC counts according to tumor location, histologic grade, or TNM stage. TWIST (+) CTCs were significantly associated with histologic grade; a proportion of TWIST (+) CTCs ≥0.5 was significantly associated with advanced histologic grade. Other clinicopathologic characteristics such as sex, age, tumor location, tumor size, and TNM stages were not significantly associated with TWIST (+) CTCs.
Conclusions
Our study showed that TWIST (+) CTCs were frequently detected in patients with ESCC, and a high proportion of TWIST (+) CTCs was associated with poor differentiation.
Keywords: Circulating tumor cells, Esophageal neoplasms, Squamous cell carcinoma, TWIST
INTRODUCTION
Esophageal cancer is the 11th most common malignant tumor and overall survival of patients has remained poor.1 In East Asian countries including Japan, China, and Korea, the proportion of squamous cell carcinoma (SCC) is more than 90% of all esophageal cancer cases, and the mortality rate is very high.1,2 Circulating tumor cells (CTCs) are defined as tumor cells originating from either primary or metastasis sites and circulating freely in the peripheral blood of patients; they are extremely rarely found in healthy persons.3 Some studies showed that CTCs could be a promising biomarker for early diagnosis and predicting prognosis and treatment efficiency in esophageal cancer.4-11 However, compared to other gastrointestinal tract cancers such as colorectal or gastric cancer, there have been a few studies on the clinical significance of CTCs in esophageal cancer.12-15
Epithelial-mesenchymal transition (EMT) is a potential factor in tumor progression, metastasis, and recurrence.16 In EMT processing of tumor cells, the expression of epithelial markers, such as epithelial cell adhesion molecule and cytokeratins (CKs), is downregulated, while the expression of mesenchymal markers, such as TWIST and vimentin, is upregulated.16 Among the mesenchymal markers, TWIST is identified as a development gene with a key role in EMT induction. Loss of TWIST expression hinders metastatic tumor cells from intravasation into the blood circulation.17 Although some studies already suggested that TWIST is an independent prognostic marker and is related to metastasis in esophageal cancer,18-20 studies on the role of TWIST in esophageal cancer are still limited.
Currently, the isolation of CTCs is possible using the immunoaffinity method, cell size-based filtration method, density method, dielectrophoresis method, and inertia-sorting method.21 Among these methods, the cell size-based filtration method filters CTCs quickly by size difference using a microfluidic chip structure or membrane with controlled pore size and shapes. Further, unlike other cell size-based filtration methods, centrifugal microfluidic system based on a fluid-assisted separation technique (FAST) separates CTCs within a centrifugal microfluidic device at a liquid-liquid interface, and provides uniform and efficient filtration without clogging under a low pressure drop without prior sample manipulation.22,23
In our previous study, CTCs detected using FAST were useful for the early diagnosis of esophageal SCC (ESCC), and CTC count was related to TNM stage, particularly lymph node metastasis.24 However, CTCs were also found in ESCC patients without clinical metastasis. This indicates that most CTCs do not have the metastatic potential, and only a small portion with EMT or stem cell-like properties can migrate to other organs to form secondary tumors.25 Therefore, we aimed to investigate the clinical significance of TWIST expression in CTCs in ESCC patients.
MATERIALS AND METHODS
1. Patients
This was a prospective study of patients who were histopathologically diagnosed with ESCC at Pusan National University Hospital (Busan, Korea). Between September 2017 and September 2019, 52 consecutive patients with ESCC who had not been diagnosed with other past or current malignancies were enrolled. The study protocol was reviewed and approved by the Institutional Review Board of Pusan National University Hospital (IRB number: H–1412–011–024), and written informed consent was procured from all patients prior to blood sampling.
Medical history, physical examination, complete blood count testing, blood chemistry, endoscopic ultrasonography, and imaging studies using chest and abdominal computed tomography (CT) and/or positron emission tomography with CT (PET-CT) for tumor staging were evaluated. Staging was assessed according to the American Joint Committee on Cancer TNM staging for esophageal cancer (the 8th edition).26
2. Treatment modalities
Treatment was individualized according to initial clinical TNM stage at the time of diagnosis. Of the 52 patients, 10 patients underwent neoadjuvant chemotherapy and additional surgery; 13, surgical resection; 20, palliative chemotherapy; and nine, endoscopic submucosal dissection (ESD). In properly selected superficial ESCC cases, ESD was performed under conscious sedation.24 In cases of resectable esophageal cancer that was improper for ESD, the Ivor Lewis esophagogastrectomy or 3-hole minimally invasive esophagectomy was performed.24 In cases of locally advanced or metastatic cancer, neoadjuvant or palliative chemotherapy with 5-fluorouracil and cisplatin was administered. Some patients received concurrent chemoradiation therapy comprising of two cycles of combined 5-fluorouracil and cisplatin with 50 Gy of radiation therapy in the same period.
3. Isolation and enumeration of CTCs
Peripheral blood samples were collected prior to treatment including surgery, ESD, chemotherapy, or chemoradiation. CTCs were isolated and enumerated according to the protocol described in our previous study.24 First, after discarding the first 2 mL of drawn blood samples to prevent contamination of epithelial cells, 3 to 5 mL of peripheral blood samples were collected in K2-EDTA tubes and then inverted 10 times immediately. The blood samples were analyzed within 8 hours to avoid cell damages. Second, FAST was performed to isolate CTCs from whole blood without pretreatments such as red blood cell lysis or dilution. Before CTC isolation, the disc surface was passivated with 1% bovine serum albumin and then washed phosphate-buffered saline (PBS). Thereafter, 3 mL whole blood was introduced to the disc, and CTCs were isolated on a track-etched polycarbonate membrane using a spinning disc device. The total filtration time was less than 1 minute.
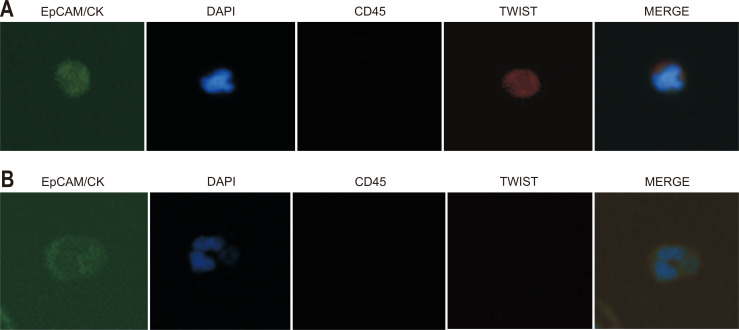
For identifying isolated CTCs from the blood sample, immunostaining of the disc was performed. First, captured cells were fixed with 4% formaldehyde for 20 minutes at room temperature. Fixed cells were then permeabilized with 0.1% Triton X-100 in PBS for 5 minutes and subsequently washed with PBS. Thereafter, samples were blocked with 20 μg/mL immunoglobulin G (Polyclonal Human IgG; R&D system, Minneapolis, MN, USA) for 20 minutes, followed by staining with the following antibodies. An anti-CD45 PE-Alexa Fluor (H130; Life Technologies, Carlsbad, CA, USA) was applied for 20 minutes to stain the white blood cells, and then samples were washed with 0.01% Tween 20 in PBS. For staining CTCs, a mixture of fluorescein isothiocyanate-conjugated anti-CK (CAM5.2; Becton, Dickinson and Company, Franklin Lakes, NJ, USA), Alexa Fluor 488-conjugated anti-pan CK (AE1/AE3; eBioscience, Inc., San Diego, CA, USA), fluorescein isothiocyanate-conjugated anti-epithelial cell adhesion molecule (9C4; BioLegend, San Diego, CA, USA), and TWIST (Twist2Cla; Bio Matrix Research, Chiba, Japan) was added to the membrane and incubated for 20 minutes. After washing with 0.01% Tween 20 in PBS, Alexa Fluor 647-conjugated secondary antibody (anti-Goat; Invitrogen, Carlsbad, CA, USA) for TWIST detection was incubated for 20 minutes and washed. Finally, the membrane was mounted on the glass slides with mounting medium containing fluorescent nucleic acid dye 4,6-diamidino-2-phenylindole (DAPI). For visualization of CTCs on the membrane, slides were scanned using the Eclipse Ti-E fluorescent microscope (Nikon, Tokyo, Japan) at 40× magnification. Captured cells were recognized as CTCs when they were CK+ or EpCAM+, CD45– and DAPI+, and their diameter was >8 μm. CTCs that were positive for TWIST immunostaining were defined as TWIST (+) CTCs (Fig. 1). Results were quantified as the number of CTCs per 7.5 mL of whole blood.
Fig. 1.
Circulating tumor cells (CTCs) detected in whole blood from esophageal cancer patients. CTCs are defined as captured cells that are CK+ or EpCAM+, CD45– and DAPI+ and have a diameter >8 μm. (A) Representative images of CTCs positive for TWIST immunostaining. (B) Representative images of CTCs negative for TWIST immunostaining.
CK, cytokeratin; EpCAM, epithelial cell adhesion molecule; DAPI, 4,6-diamidino-2-phenylindole.
4. Statistical analysis
Data were expressed as median and ranges. Differences in total CTC and TWIST (+) CTC count according to clinicopathologic features were analyzed by using the Mann-Whitney test or Kruskal-Wallis test. Differences in clinicopathologic features according to presence of CTCs and TWIST (+) CTCs and the proportion of TWIST (+) CTCs were analyzed by using the chi-square test or Fisher exact test. All statistical analyses were performed using IBM SPSS version 22.0 for Windows (IBM Corp., Armonk, NY, USA). A p-value was <0.05 was considered statistically significant.
RESULTS
1. Baseline clinicopathologic characteristics of patients with ESCC
Clinicopathologic characteristics of the 52 ESCC patients are summarized in Table 1. The patients consisted of 49 men and three women, with a median age of 67 years (range, 50 to 84 years). Twelve tumors were located at the upper third of the esophagus, 18 tumors at the middle third, and 22 tumors at the lower third. The median tumor size was 4.0 cm (range, 0.8 to 21.6 cm). Histopathologically, eight tumors were well differentiated, 39 were moderately differentiated, and five were poorly differentiated. With regard to T and N stages, 15, 7, 17, and 13 tumors were T1, T2, T3, and T4, respectively, and 18, 9, 12, and 13 tumors were N0, N1, N2, and N3, respectively. Distant metastasis was observed in nine patients. For clinical TNM stage, 15 patients were diagnosed as stage I, seven as stage II, 10 as stage III, and 20 as stage IV.
Table 1.
Baseline Clinicopathologic Characteristics of the 52 Patients with Esophageal Squamous Cell Carcinoma
| Variable | Value |
|---|---|
| Median age, range, yr | 67 (50–84) |
| Sex | |
| Male | 49 |
| Female | 3 |
| Tumor location | |
| Upper third | 12 |
| Middle third | 18 |
| Lower third | 22 |
| Median tumor size, range, cm | 4.0 (0.8–21.6) |
| Histologic grade | |
| Well differentiated | 8 |
| Moderately differentiated | 39 |
| Poorly differentiated | 5 |
| T stage | |
| T1 | 15 |
| T2 | 7 |
| T3 | 17 |
| T4 | 13 |
| N stage | |
| N0 | 18 |
| N1 | 9 |
| N2 | 12 |
| N3 | 13 |
| M stage | |
| M0 | 43 |
| M1 | 9 |
| TNM stage | |
| I | 15 |
| II | 7 |
| III | 10 |
| IV | 20 |
2. Incidence of CTCs in patients with ESCC
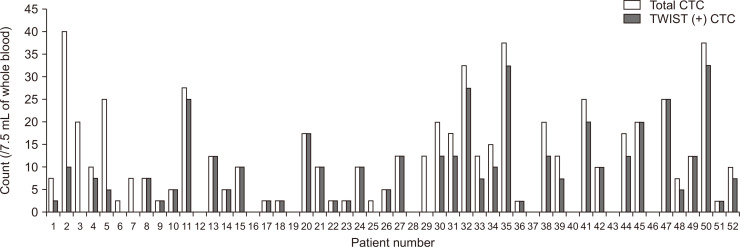
The CTC and TWIST (+) CTC counts of each patient with ESCC are shown in Fig. 2. CTCs were identified in 44 of the 52 patients (84.6%), and the median CTC count was 10 (range, 0 to 40) per 7.5 mL of blood. TWIST (+) CTCs were detected in 39 of the 52 patients (75.0%), and the median TWIST (+) CTC count was 7.5 (range, 0 to 32.5) per 7.5 mL of blood.
Fig. 2.
Circulating tumor cell (CTC) and TWIST (+) CTC counts in the 52 patients with esophageal squamous cell carcinoma.
Table 2 summarizes the CTC and TWIST (+) CTC counts according to the clinicopathologic characteristics of the 52 ESCC patients. The CTC count in patients aged >65 years was higher than that in those aged ≤65 years (12.5 vs 7.5, p=0.034). The CTC count in patients with a large tumor (>3 cm) was higher than that in patients with a small tumor (≤3 cm) (12.5 vs 6.3, p=0.028). The TWIST (+) CTC count was also higher in patients aged >65 years than in those aged ≤65 years (8.8 vs 2.5, p=0.044) and in those who had a large tumor (>3 cm) than in those who had a small tumor (≤3 cm) (8.8 vs 2.5, p=0.039). There was no difference in CTC and TWIST (+) CTC counts according to tumor location, histologic grade, and TNM stage.
Table 2.
CTC and TWIST (+) CTC Counts According to Clinicopathologic Characteristics in the 52 Patients with Esophageal Squamous Cell Carcinoma
| Variable | CTC count (/7.5 mL of whole blood) | p-value* | TWIST (+) CTC count (/7.5 mL of whole blood) | p-value* |
|---|---|---|---|---|
| Sex | 0.683 | 0.912 | ||
| Male | 10 (0–40) | 7.5 (0–32.5) | ||
| Female | 2.5 (0–37.5) | 2.5 (0–32.5) | ||
| Age, yr | 0.034 | 0.044 | ||
| ≤65 | 7.5 (0–17.5) | 2.5 (0–17.5) | ||
| >65 | 12.5 (0–40) | 8.8 (0–32.5) | ||
| Tumor location | 0.087 | 0.256 | ||
| Upper third | 12.5 (0–37.5) | 8.8 (0–32.5) | ||
| Middle third | 11.3 (0–40) | 7.5 (0–32.5) | ||
| Lower third | 3.8 (0–25) | 2.5 (0–25) | ||
| Tumor size, cm | 0.028 | 0.039 | ||
| ≤3 | 6.3 (0–25) | 2.5 (0–25) | ||
| >3 | 12.5 (0–40) | 8.8 (0–32.5) | ||
| Histologic grade | 0.389 | 0.147 | ||
| Well differentiated | 6.3 (0–25) | 0 (0–20) | ||
| Moderately differentiated | 10 (0–40) | 5 (0–32.5) | ||
| Poorly differentiated | 12.5 (0–25) | 12.5 (2.5–12.5) | ||
| T stage | 0.108 | 0.235 | ||
| T1 | 10 (0–37.5) | 7.5 (0–32.5) | ||
| T2 | 2.5 (0–10) | 2.5 (0–10) | ||
| T3 | 12.5 (0–40) | 7.5 (0–27.5) | ||
| T4 | 5 (0–25) | 5 (0–25) | ||
| N stage | 0.519 | 0.603 | ||
| N0 | 10 (0–37.5) | 5 (0–32.5) | ||
| N1 | 12.5 (2.5–27.5) | 10 (0–25) | ||
| N2 | 13.8 (0–40) | 6.3 (0–27.5) | ||
| N3 | 5 (0–25) | 5 (0–25) | ||
| M stage | 0.568 | 0.721 | ||
| M0 | 10 (0–40) | 5 (0–32.5) | ||
| M1 | 12.5 (0–25) | 7.5 (0–25) | ||
| TNM stage | 0.552 | 0.895 | ||
| I | 10 (0–37.5) | 7.5 (0–32.5) | ||
| II | 10 (0–27.5) | 7.5 (0–25) | ||
| III | 17.5 (0–40) | 7.5 (0–27.5) | ||
| IV | 7.5 (0–25) | 5 (0–25) |
Data are presented as median (range).
CTC, circulating tumor cell.
*p-values were derived using the Mann-Whitney test or Kruskal-Wallis test.
3. Association between CTCs and clinicopathologic characteristics in patients with ESCC
Based on our previous study, two CTCs per 7.5 mL of blood was defined as the optimal cutoff of CTC count for differentiating ESCC patients from healthy controls.24 Thus, we classified patients according to this CTC cutoff, the presence of TWIST (+) CTCs, and the proportion of TWIST (+) CTCs and examined their association with clinicopathologic characteristics. The results are summarized in Table 3. Tumor size was larger in patients with ≥2 CTCs per 7.5 mL of blood than in those with <2 CTCs per 7.5 mL of blood, but the difference did not reach significant difference (p=0.108). Histologic grade was also associated with CTCs, but there was no significant difference (p=0.128). Other clinicopathologic characteristics such as sex, age, tumor location, and TNM stages were not associated with CTCs. TWIST (+) CTCs were significantly associated with histologic grade (p=0.018); the frequency of TWIST (+) CTCs increased as the histologic grade worsened. Other clinicopathologic characteristics such as sex, age, tumor location and size, and TNM stages were not significantly associated with TWIST (+) CTCs. The proportion of TWIST (+) CTCs (<0.5 vs ≥0.5) was significantly associated with the histologic grade (p=0.047), but not with other clinicopathologic characteristics.
Table 3.
Clinicopathologic Characteristics of the 52 Patients with Esophageal Squamous Cell Carcinoma According to the CTCs, TWIST (+) CTCs, and the Proportion of TWIST (+) CTCs
| Variable | CTCs | p-value* | TWIST (+) CTCs | p-value* | Proportion of TWIST (+) CTCs | p-value* | |||
|---|---|---|---|---|---|---|---|---|---|
| <2 (n=8) |
≥2 (n=44) |
Absent (n=13) |
Present (n=39) |
<0.5 (n=16) |
≥0.5 (n=36) |
||||
| Sex | 0.375 | 0.731 | 0.921 | ||||||
| Male | 7 | 42 | 12 | 37 | 15 | 34 | |||
| Female | 1 | 2 | 1 | 2 | 1 | 2 | |||
| Age, yr | 1.000 | 0.199 | 0.330 | ||||||
| ≤65 | 4 | 20 | 8 | 16 | 9 | 15 | |||
| >65 | 4 | 24 | 5 | 23 | 7 | 21 | |||
| Tumor location | 0.323 | 0.749 | 0.430 | ||||||
| Upper third | 2 | 10 | 2 | 10 | 2 | 10 | |||
| Middle third | 1 | 17 | 5 | 13 | 7 | 11 | |||
| Lower third | 5 | 17 | 6 | 16 | 7 | 15 | |||
| Tumor size, cm | 0.108 | 0.108 | 0.356 | ||||||
| ≤3 | 5 | 13 | 7 | 11 | 7 | 11 | |||
| >3 | 3 | 31 | 6 | 28 | 9 | 25 | |||
| Histologic grade | 0.128 | 0.018 | 0.047 | ||||||
| Well differentiated | 3 | 5 | 5 | 3 | 5 | 3 | |||
| Moderately differentiated | 5 | 34 | 8 | 31 | 11 | 28 | |||
| Poorly differentiated | 0 | 5 | 0 | 5 | 0 | 5 | |||
| T stage | 0.568 | 0.160 | 0.839 | ||||||
| T1 | 3 | 12 | 5 | 10 | 5 | 10 | |||
| T2 | 1 | 6 | 2 | 5 | 2 | 5 | |||
| T3 | 1 | 16 | 1 | 16 | 4 | 13 | |||
| T4 | 3 | 10 | 5 | 8 | 5 | 8 | |||
| N stage | 0.513 | 0.331 | 0.359 | ||||||
| N0 | 4 | 14 | 7 | 11 | 7 | 11 | |||
| N1 | 0 | 9 | 1 | 8 | 1 | 8 | |||
| N2 | 2 | 10 | 3 | 9 | 5 | 7 | |||
| N3 | 2 | 11 | 2 | 11 | 3 | 10 | |||
| M stage | 0.696 | 0.832 | 0.855 | ||||||
| M0 | 7 | 36 | 11 | 32 | 13 | 30 | |||
| M1 | 1 | 8 | 2 | 7 | 3 | 6 | |||
| TNM stage | 0.924 | 0.614 | 0.995 | ||||||
| I | 3 | 12 | 5 | 10 | 5 | 10 | |||
| II | 1 | 6 | 2 | 5 | 2 | 5 | |||
| III | 1 | 9 | 1 | 9 | 3 | 7 | |||
| IV | 3 | 17 | 5 | 15 | 6 | 14 | |||
CTCs, circulating tumor cells.
*p-values were derived using the chi-square test or Fisher exact test.
DISCUSSION
In the present study, CTCs and TWIST (+) CTCs were detected in 44 patients (84.6%) and 39 patients (75.0%), respectively, out of the 52 patients with ESCC. The proportion of TWIST (+) CTCs was ≥0.5 in 36 patients (69.2%). The presence of TWIST (+) CTCs and a high proportion of TWIST (+) CTCs were associated with poorly differentiated histology. To the best of our knowledge, this is the first study to detect TWIST (+) CTCs using FAST and to evaluate their correlation with clinicopathologic characteristics in patients with ESCC.
Recently, CTCs and EMT markers have been reported to be promising biomarkers for early diagnosis and prediction of treatment efficacy and prognosis in gastrointestinal cancers.3 Several studies have also reported the clinical significance of CTCs and TWIST in esophageal cancer. CTCs were associated with a higher T stage, lymph node metastasis and progression of esophageal cancers,4,5,7,10,11 and TWIST was associated with ESCC progression and had potential roles as the promoter of tumor invasion and metastasis.16,18 Furthermore, TWIST was proposed as an independent prognostic biomarker for predicting distant metastasis and survival rates of ESCC patients.19 In our previous study, CTCs were related to a higher N stage and TNM stage, suggesting that CTCs detected by FAST are useful for the diagnosis of ESCC.24 In the present study, CTC and TWIST (+) CTC counts were not associated with TNM stage. Our different results might be caused by the heterogeneity in the baseline clinicopathologic features (including a relatively small number of patients) and discrepancy between the clinical and pathological stages. TWIST (+) CTCs were detected even in T1 or N0 stage; furthermore, there was no difference in the proportion of TWIST (+) CTCs between early stage and advanced stage ESCC. This suggests that the EMT process occurs even in the early stage of ESCC. In general, most CTCs do not have a metastatic potential; only a small subset with EMT or stem cell-like properties can migrate to other organs to establish secondary tumors.25 However, the high proportion of TWIST (+) CTCs in T1 or N0 stage in our study could be one of the reasons for the higher frequency of lymph node and distant metastasis in early stage ESCC than that in other gastrointestinal cancers, in addition to the rich lymphatic networks in the muscularis mucosa and submucosa of the esophagus.
In the present study, the CTC and TWIST (+) CTC counts were higher in elderly patients (> 65 years) and those with a large tumor (>3 cm), and TWIST (+) CTCs was associated with histologic grade. These results indicate that a larger CTC count might be related to more extensive tumor burden, similarly to our previous study.24 Also, a larger TWIST (+) CTC count might reflect a larger CTC count in patients with a large tumor, or suggest an increased possibility of EMT in a large tumor. On the other hand, the CTC counts did not differ according to age in our previous study;24 therefore, higher CTC and TWIST (+) counts in elderly patients might be caused by the different baseline demographics in the included patients. In one report investigating TWIST messenger RNA in tumor tissue, TWIST messenger RNA expression was higher in poorly differentiated ESCC (0.765±0.234) than in well (0.532±0.183) or moderately (0.746±0.186) differentiated ESCC.18 Although there was no significant difference in TWIST expression according to tumor histology in this study (p=0.072), TWIST expression was higher in poorly differentiated ESCC. This was consistent with our result that the frequency of TWIST (+) CTCs increased in patients with poorly differentiated ESCC. This might explain the worse prognosis in patients with poorly differentiated ESCC than in patients with well-/moderately differentiated ESCC.27 Studies comparing TWIST expression in CTCs with that in the resected specimen will be needed to elucidate whether the presence of TWIST (+) CTCs might be a simple indicator of the tumor histopathology in the primary site or the result of EMT process occurring during cancer progression.
Compared with other gastrointestinal cancers, there have been limited reports on the TWIST expression in esophageal cancers to date. Further, most studies on the clinical significance of TWIST in esophageal cancer are based on immunohistochemical staining using the tissue, not blood sampling.18,20,28-30 However, it is difficult to apply the results of tissue biopsy for diagnosis and treatment of ESCC in the real world. In contrast, CTCs, as one of the promising liquid biopsy markers, have been proposed as a promising noninvasive biomarker for diagnosing and predicting tumor progression. However, there are still limitations in the application and interpretation of CTCs in ESCC owing to the variety of methods for CTC enumeration and difficulties of standardization of CTC isolation. The CellSearch system, the only US Food and Drug Administration-authorized and immune affinity-based method, also has the main limitations of incapability to capture and analyze cells deficient in epithelial marker expression, poorly differentiated cells, or tumor stem cells. Furthermore, epithelial marker-based methods such as the CellSearch system cannot detect mesenchymal tumor cells already or partially undergoing EMT.3,21,31 In contrast, FAST is a rapid, size-based method that has been reported to have several advantages compared with other CTC detection methods, including being user-friendly, cost-effective, and an efficient CTC capture technique.22,23 In addition, FAST enables further downstream molecular analysis for detection of TWIST (+) CTCs using immunostaining. Although more processes are needed for immunostaining using TWIST antibody, the detection time is not long (2 to 3 hours). By checking CTCs and TWIST expression in CTCs concurrently with a single blood sampling, FAST can be helpful not only for detecting CTC phenotype, but also for predicting prognosis and response to chemotherapy in cancer patients.
Our study has several limitations. First, because we focused on the CTCs and TWIST (+) CTCs at the time of diagnosis, we did not evaluate long-term follow-up results, such as therapeutic efficacies, prognosis, and survival. Although not included in the present study, we found that serial CTC and TWIST (+) CTC counts decreased after treatment compared with before treatment in some patients. In the future, we are going to investigate the roles of CTCs and TWIST (+) CTCs in the prediction of long-term outcomes and response to chemotherapy or chemoradiotherapy in ESCC patients. Second, the number of cases included in the present study was small, and endoscopic or surgical resection was not performed in all patients; therefore, we assessed the TNM stage based on endoscopic ultrasonography, CT, and/or PET-CT in 30 patients who did not undergo resection. Accordingly, there might have been a discrepancy between clinical and pathological stages. However, as the combined use of endoscopic ultrasonography, CT, and PET-CT could increase the sensitivity and specificity for TNM stage to the acceptable level (~80%),32 this limitation might not have affected our results. Third, other mesenchymal markers such as vimentin or stem cell markers (e.g., CD44) were not studied along with TWIST in CTCs. Finally, CTCs detected in patients with ESCC are not organ specific. Even though we excluded other current malignancies via the aforementioned examinations, there is still be a possibility that undetected malignancies could be present in other organs which could be a source of CTCs.
In conclusion, TWIST (+) CTCs were detected in three-fourths of patients with ESCC, even in the early stage, and were associated with poorly differentiated histology. This could explain the poorer prognosis of patients with ESCC than those with other gastrointestinal tract cancer. The additional role of TWIST (+) CTCs as a predictive biomarker for prognosis and response to chemotherapy should be explored in large, prospective, long-term follow-up studies.
ACKNOWLEDGEMENTS
This study was supported by the Medical Research Center Program through the National Research Foundation of Korea grant funded by the Korea government (NRF-2015R1A5A2009656). The biospecimens and data used in this study were provided by the Biobank of Pusan National University Hospital, a member of the Korea Biobank network.
Footnotes
CONFLICTS OF INTEREST
G.H.K. is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Data analysis and writing - original draft: H.J.L., G.H.K., S.J.P. Study design: G.H.K. Writing - review and editing: C.H.K., M.W.L., B.E.L., D.H.B. Advice on the study design: H.I. Statistical support and data acquisition: H.J.L., G.H.K., M.W.L. All authors have read and approved the manuscript.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Jung HK. Epidemiology of and risk factors for esophageal cancer in Korea. Korean J Helicobacter Up Gastrointest Res. 2019;19:145–148. doi: 10.7704/kjhugr.2019.19.3.145. [DOI] [Google Scholar]
- 3.Wu T, Cheng B, Fu L. Clinical applications of circulating tumor cells in pharmacotherapy: challenges and perspectives. Mol Pharmacol. 2017;92:232–239. doi: 10.1124/mol.116.108142. [DOI] [PubMed] [Google Scholar]
- 4.Qiao Y, Li J, Shi C, et al. Prognostic value of circulating tumor cells in the peripheral blood of patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2017;10:1363–1373. doi: 10.2147/OTT.S129004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han L, Li YJ, Zhang WD, Song PP, Li H, Li S. Clinical significance of tumor cells in the peripheral blood of patients with esophageal squamous cell carcinoma. Medicine (Baltimore) 2019;98:e13921. doi: 10.1097/MD.0000000000013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Li Y, Yuan D, Peng Y, Qin J. Practical value of identifying circulating tumor cells to evaluate esophageal squamous cell carcinoma staging and treatment efficacy. Thorac Cancer. 2018;9:956–966. doi: 10.1111/1759-7714.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeh M, Effenberger KE, Koenig AM, et al. Circulating tumor cells as a biomarker for preoperative prognostic staging in patients with esophageal cancer. Ann Surg. 2015;261:1124–1130. doi: 10.1097/SLA.0000000000001130. [DOI] [PubMed] [Google Scholar]
- 8.Su PJ, Wu MH, Wang HM, et al. Circulating tumour cells as an independent prognostic factor in patients with advanced oesophageal squamous cell carcinoma undergoing chemoradiotherapy. Sci Rep. 2016;6:31423. doi: 10.1038/srep31423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaganoi J, Shimada Y, Kano M, Okumura T, Watanabe G, Imamura M. Detection of circulating oesophageal squamous cancer cells in peripheral blood and its impact on prognosis. Br J Surg. 2004;91:1055–1060. doi: 10.1002/bjs.4593. [DOI] [PubMed] [Google Scholar]
- 10.Xu HT, Miao J, Liu JW, Zhang LG, Zhang QG. Prognostic value of circulating tumor cells in esophageal cancer. World J Gastroenterol. 2017;23:1310–1318. doi: 10.3748/wjg.v23.i7.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah AK, Saunders NA, Barbour AP, Hill MM. Early diagnostic biomarkers for esophageal adenocarcinoma: the current state of play. Cancer Epidemiol Biomarkers Prev. 2013;22:1185–1209. doi: 10.1158/1055-9965.EPI-12-1415. [DOI] [PubMed] [Google Scholar]
- 12.Jeon HK, Kim GH. Clinical significance of circulating tumor cells in gastric cancer. Korean J Helicobacter Up Gastrointest Res. 2018;18:162–167. doi: 10.7704/kjhugr.2018.18.3.162. [DOI] [Google Scholar]
- 13.Kang EA, Han YM, Park JM, Yoo IK, Hong SP, Hahm KB. Liquid biopsy: current status and future perspective in gastric cancer and Helicobacter infection. Korean J Helicobacter Up Gastrointest Res. 2018;18:150–156. doi: 10.7704/kjhugr.2018.18.3.150. [DOI] [Google Scholar]
- 14.Kang HM, Kim GH, Jeon HK, et al. Circulating tumor cells detected by lab-on-a-disc: role in early diagnosis of gastric cancer. PLoS One. 2017;12:e0180251. doi: 10.1371/journal.pone.0180251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MW, Kim GH, Jeon HK, Park SJ. Clinical application of circulating tumor cells in gastric cancer. Gut Liver. 2019;13:394–401. doi: 10.5009/gnl18484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han D, Chen K, Che J, Hang J, Li H. Detection of epithelial-mesenchymal transition status of circulating tumor cells in patients with esophageal squamous carcinoma. Biomed Res Int. 2018;2018:7610154. doi: 10.1155/2018/7610154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Xuan XY, Zhang HY, et al. Relationship between TWIST expression and epithelial-mesenchymal transition of oesophageal squamous cell carcinoma. Cell Biol Int. 2012;36:571–577. doi: 10.1042/CBI20100195. [DOI] [PubMed] [Google Scholar]
- 19.Xie F, Li K, Ouyang X. Twist, an independent prognostic marker for predicting distant metastasis and survival rates of esophageal squamous cell carcinoma patients. Clin Exp Metastasis. 2009;26:1025–1032. doi: 10.1007/s10585-009-9292-5. [DOI] [PubMed] [Google Scholar]
- 20.Yuen HF, Chan YP, Wong ML, et al. Upregulation of Twist in oesophageal squamous cell carcinoma is associated with neoplastic transformation and distant metastasis. J Clin Pathol. 2007;60:510–514. doi: 10.1136/jcp.2006.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim M, Cho YK. Current methods of circulating tumor cell detection. Korean J Helicobacter Up Gastrointest Res. 2018;18:157–161. doi: 10.7704/kjhugr.2018.18.3.157. [DOI] [Google Scholar]
- 22.Kim TH, Lim M, Park J, et al. FAST: size-selective, clog-free isolation of rare cancer cells from whole blood at a liquid-liquid interface. Anal Chem. 2017;89:1155–1162. doi: 10.1021/acs.analchem.6b03534. [DOI] [PubMed] [Google Scholar]
- 23.Lee A, Park J, Lim M, et al. All-in-one centrifugal microfluidic device for size-selective circulating tumor cell isolation with high purity. Anal Chem. 2014;86:11349–11356. doi: 10.1021/ac5035049. [DOI] [PubMed] [Google Scholar]
- 24.Choi MK, Kim GH, I H, et al. Circulating tumor cells detected using fluid-assisted separation technique in esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2019;34:552–560. doi: 10.1111/jgh.14543. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Zhang B, Zhang Z, et al. Stem cell-like circulating tumor cells indicate poor prognosis in gastric cancer. Biomed Res Int. 2014;2014:981261. doi: 10.1155/2014/981261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK. AJCC Cancer Staging Manual. 8th ed. Springer; New York: 2017. [DOI] [PubMed] [Google Scholar]
- 27.Blum Murphy M, Xiao L, Patel VR, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival: The University of Texas MD Anderson Cancer Center experience. Cancer. 2017;123:4106–4113. doi: 10.1002/cncr.30953. [DOI] [PubMed] [Google Scholar]
- 28.Gong T, Xue Z, Tang S, et al. Nuclear expression of Twist promotes lymphatic metastasis in esophageal squamous cell carcinoma. Cancer Biol Ther. 2012;13:606–613. doi: 10.4161/cbt.19851. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima TE, Yoshida H, Okamoto N, et al. Nucleostemin and TWIST as predictive markers for recurrence after neoadjuvant chemotherapy for esophageal carcinoma. Cancer Sci. 2012;103:233–238. doi: 10.1111/j.1349-7006.2011.02142.x. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki K, Natsugoe S, Ishigami S, et al. Significance of Twist expression and its association with E-cadherin in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2009;28:158. doi: 10.1186/1756-9966-28-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balakrishnan A, Koppaka D, Anand A, et al. Circulating tumor cell cluster phenotype allows monitoring response to treatment and predicts survival. Sci Rep. 2019;9:7933. doi: 10.1038/s41598-019-44404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98:547–557. doi: 10.1038/sj.bjc.6604200. [DOI] [PMC free article] [PubMed] [Google Scholar]