Abstract
Liver fibrosis induces intrahepatic microcirculation disorder and hypoxic stress. Hypoxic stress has the potential for an increase in the possibility of more liver fibrosis and carcinogenesis. Liver biopsy is a standard method that evaluates of intrahepatic hypoxia, however, is invasive and has a risk of bleeding as a complication. Here, we investigated the hypoxia reactive gene expressions in peripheral blood mononuclear cells (PBMC) from chronic liver disease patients to evaluate intrahepatic hypoxia in a non-invasive manner. The subjects enrolled for this study were composed of 20 healthy volunteers (HV) and 48 patients with chronic liver disease (CLD). CLD patients contained 24 patients with chronic hepatitis(CH)and 24 patients with liver cirrhosis (LC). PBMC were isolated from heparinized peripheral blood samples. We measured the transcriptional expression of hypoxia reactive genes and inflammatory cytokines by quantitative RT-PCR. mRNA expression of adrenomedullin (AM), vascular endothelial growth factor A (VEGFA) superoxide dismutase (SOD), glutathione peroxidase (GPx) (p < 0.05), Interleukin-6 (IL-6), transforming growth factor-beta (TGF-β) and heme oxygenase-1 (HO-1) in CLD group were significantly higher than HV. AM mRNA expression is correlated with serum lactate dehydrogenase (LDH), serum albumin (Alb), IL6, and SOD mRNA expression. The hypoxia reactive gene expression in PBMCs from CLD patients was more upregulated than HV. Especially, angiogenic genes were notably upregulated and correlated with liver fibrosis. Here, we suggest that mRNA expression of AM in PBMCs could be the biomarker of intrahepatic hypoxia.
Keywords: Adrenomedullin, Chronic liver disease, Intrahepatic hypoxia, Peripheral blood mononuclear cells
Abbreviations: HIF, hypoxia inducible factor; CLD, chronic liver disease; HCV, hepatitis C virus; ROS, reactive oxygen species; PT, prothrombin time; PBMC, Peripheral blood mononuclear cells; VEGF, vascular endothelial growth factor; HV, healthy volunteers; CH, chronic hepatitis; LC, liver cirrhosis; HCC, hepatocellular carcinoma; LDH, lactate dehydrogenase; AM, Adrenomedullin; VEGFA, vascular endothelial growth factor A; ANGPTL4, Angiopoietin-like 4; HO-1, heme oxygenase -1; VEGFR2, vascular endothelial growth factor receptor 2; SOD, Superoxide dismutase; GPx, glutathione peroxidase; IL-6, Interleukin-6; TNF-α, Tumor Necrosis Factor-α; MCP-1, Monocyte chemoattractant protein-1; TGF-β, transforming growth factor-beta
Highlights
-
•
The hypoxia reactive genes in PBMC were elevated in patients with chronic liver disease.
•Angiogenic genes were upregulated and correlated with liver fibrosis in patients with chronic liver disease.
•Adrenomedullin mRNA expression in PBMC was correlated with liver function.
•mRNA expression of adrenomedullin in PBMC could be the biomarker of intrahepatic hypoxia.
1. Introduction
Without an appropriate regulation of persistent inflammation, chronic hepatitis progresses to liver cirrhosis that is featured with advanced fibrosis and nodular regeneration. Such histological dysregulation induces the microcirculatory disturbance, leading to parenchymal hypoxia in cirrhotic liver [1,2].
It is well known that a group of genes, such as hypoxia inducible factor (HIF), is activated to adapt to the hypoxic circumstance via the alteration of cellular metabolism and the promotion of angiogenesis [3,4]. HIF activation through hypoxia-dependent and hypoxia-independent signals have been reported in liver disease of diverse etiologies, from acute liver injury to chronic liver diseases [3]. HIF plays an important role in the protection against hypoxia, however, hepatic stellate cells can be also activated in hypoxic conditions by the HIF-dependent mechanism [5] and hypoxia occurs in the development and progression of liver diseases [4].
Thus, intrahepatic hypoxia in chronic liver disease (CLD) may work as a stimulator for further fibrogenesis. Also, hypoxia may be involved in hepatic carcinogenesis. Not only the viral factors, such as oxidative stress by hepatitis C virus (HCV) core protein or chromosomal integration of hepatitis B virus X protein but also hepatocyte damage and persistent regeneration of liver cells are thought to promote DNA instability [[6], [7], [8]]. Moreover, oxygen insufficiency in mitochondrial respiration over-produces reactive oxygen species (ROS) [9], which is known to damage cellular DNA [10]. Hypoxia in cirrhotic liver may potentiate hepatocyte regeneration and DNA damage, which promote the process of hepatic carcinogenesis.
Evaluation of intrahepatic hypoxia may be helpful to predict the potential of fibrogenesis and carcinogenesis in the liver with CLD, and liver biopsy is a standard method that evaluates intrahepatic hypoxia. However, liver biopsy has a risk of bleeding as a complication [11] in CLD patients who are hemorrhagic because of prolonged prothrombin time (PT) and decreased platelet count in association with disease progression, and non-invasive methods to evaluate the levels of intrahepatic hypoxia in CLD patients are required.
Peripheral blood mononuclear cells (PBMC) consist of immune cells, such as monocytes and lymphocytes, and change their character in response to local or systemic circumstances [12,13]. It has been reported that the gene expression profile of PBMC is associated with the disease activity or treatment response in several diseases including diabetes mellitus, rheumatoid arthritis, and viral hepatitis [[14], [15], [16]]. Hypoxia can affect the production of cytokines in human PBMC [17]. Previous studies have demonstrated that hypoxia induces T helper 17 cell upregulation and IL-I7 expression in PBMC from severe cerebral infarction [18]. It has been also reported that the hypoxic synovial environment regulates expression of vascular endothelial growth factor (VEGF)in paired synovial fluid monocytic cells compared to matched PBMC from juvenile idiopathic arthritis patients. PBMC has been reported as a useful method that evaluates tissue hypoxia in some organs other than the liver. These features of PBMC prompted us to evaluate the mRNA expression levels of hypoxia reactive genes in PBMC isolated from the patients with CLD patients.
No previous studies have been reported that the expression profiles of hypoxia reactive genes in PBMC isolated from patients with liver disease.
In this study, we evaluated the expression levels of genes involved in angiogenesis, protection against oxidative stress, and inflammatory cytokines of PBMC, and estimated the correlations between those and the levels of hepatic fibrosis.
2. Materials and methods
2.1. Patients and samples
48 patients with chronic liver disease (CLD) and 20 healthy volunteers (HV) were enrolled for this study. Written consent was obtained from all of the patients and we excluded chronic heart failure, chronic renal failure, and chronic respiratory failure. 24 patients were chronic hepatitis (CH) and 24 patients were liver cirrhosis (LC). Chronic hepatitis was defined as abnormal serum ALT levels (>35 IU/l) for more than 6 months. Liver cirrhosis was diagnosed with ultrasound, Computed Tomography, or Magnetic Resonance Imaging. 13 patients in LC group have hepatocellular carcinoma (HCC). Characteristics of the enrolled subjects, including age, gender, lactate dehydrogenase (LDH), platelet count, and FIB4 index were documented (Table 1). FIB4 index is well known to be useful for evaluating hepatic fibrosis. FIB4 index was assessed as: age (yr) × AST (IU/L)/(platelet count (109/L) × √ALT (IU/L)). This study was approved by Kyushu University Hospital Ethics Committee.
Table 1.
Clinical characteristics of the patients.
| HV | CLD | p value | CH | LC | p value | |
|---|---|---|---|---|---|---|
| Number | 20 | 48 | 24 | 24 | ||
| Age(years) | 35 (33.25–48.25) | 63 (56–71) | p<0.0001 | 62 (55.75–67.25) | 68 (59.5–76.5) | p = 0.059 |
| Gender(M/F) | 13/7 | 28/20 | 10/14 | 18/6 | ||
| Etiology (HCV/HBV/NASH/others) | 25/11/5/7 | 7/8/4/4 | 18/3/1/3 | |||
| With HCC | 0 | 13 | 0 | 13 | ||
| T.Bil(mg/dl) | 0.9 (0.8–1.1) | 0.7 (0.5–1.0) | p = 0.247 | 0.6 (0.4–1.0) | 0.7 (0.5–1.25) | p = 0.099 |
| ALT(IU/l) | 19 (11.5–40) | 23 (14.25–48) | p = 0.200 | 23 (12.75–30) | 43 (17–48.5) | p = 0.476 |
| LDH(IU/l) | 191 (118–219) | 205 (178.75–236.5) | p = 0.059 | 187.5 (127.5–215) | 218 (186.5–247.5) | p = 0.044 |
| T.chol(mg/dl) | 192 (150–214) | 168 (149–194) | p = 0.227 | 181 (157–212) | 159 (125.5–177.5) | p = 0.028 |
| Albumin(g/dl) | 4.7 (4.4–4.8) | 4.05 (3.33–4.28) | p = 0.001 | 4.15 (3.88–4.35) | 3.7 (3.2–4.05) | p = 0.005 |
| Platelet count(X104/μl) | 22.95 (21.93–26.23) | 12.4 (9.68–17.15) | p = 0.003 | 17.1 (14.58–21.83) | 10.5 (7.05–12.3) | p<0.0001 |
| FIB4 index | 1.27 (0.72–1.88) | 4.09 (2.02–6.46) | p = 0.007 | 1.99 (1.49–3.12) | 5.53 (4.77–7.57) | p<0.0001 |
2.2. PBMC
PBMC were isolated from heparinized peripheral blood samples (7 ml) obtained from CLD patients and HV by Ficoll-Paque density gradient centrifugation and lymphocyte separation solution (Nacalai tesque, Kyoto, Japan).
2.3. RT-PCR
Total RNA was extracted from cells using the Qiagen RNeasy Plus mini kit (Qiagen, Hilden, Germany) according to the manufacturers' instructions. mRNA was reverse transcribed into cDNA using the Prime Script TM Reverse Transcriptase (Takara Bio Inc., Shiga, Japan). Real time PCR was performed using light Cycled-fast start DNA master SYBR green 1 (Roche, Basel, Switzerland) according to the manufacturers' instruction. The PCR reaction was carried out with a denaturation step at 95 °C for 30 s, then 40 cycles at 95 °C for 5 s, and finally at 60 °C for 34 s. To control for variations in the reactions, all data were normalized to GAPDH expression. Relative expression was presented using the 2-ΔΔCq method. The results of CLD patients are expressed as the fold change relative to the HV levels. The primer sequences were listed in Table 2.
Table 2.
Primer sequences.
| Gene | Primer sequences(5'~3′) |
|---|---|
| GAPDH | F:ATCACCATCTTCCAGGAGCGA |
| R:TTCTCCATGGTGGTGAAGACG | |
| AM | F:AGTCGTGGGAAGAGGGAACT |
| R:CCCTGGAAGTTGTTCATGCT | |
| VEGFA | F:CCTTGCTGCTCTACCTCCAC |
| R:CACACAGGATGGCTTGAAGA | |
| ANGPTL4 | F:CACCATGAGCGGTGCTCCGACGGC |
| R:GGAGGCTGCCTCTGCTGCCA | |
| Tie2 | F:TACTAATGAAGAAATGACCCTGG |
| R:GGAGTGTGTAATGTTGGAAATCT | |
| HIF-1α | F:ACCATGCCCCAGATTCAGGA |
| R:ATCAGTGGTGGCAGTGGTAGTGGT | |
| HO-1 | F:ATG ACA CCA AGG ACC AGA GC |
| R:GTG TAA GGA CCC ATC GGA GA | |
| VEGFR2 | F:GTGACCAACATGGAGTCGTG |
| R:TGCTTCACAGAAGACCATGC | |
| SOD | F:AGGGCATCATCAATTTCGAG |
| R:ACATTGCCCAAGTCTCCAAC | |
| Catalase | F:CTGGAGAAGTGCGGAGATTC |
| R:AGTCAGGGTGGACCTCAGTG | |
| GPx | F:GTAGTGCTGGACAGTGACAACC |
| R:ATATTTGGAGGCAGTGGGAGATG | |
| IL-6 | F:GACAGCCACTCACCTCTTCA |
| R:TTCACCAGGCAAGTCCCTC | |
| TGF-β | F:TGAACCGCCTTTCCTGCTTCTCATG |
| R:GCGGAAGTCAATGTACAGCTGCCGC | |
| TNF-α | F:CCCAGGCAGTCAGATCATCTT |
| R:TCTCAGCTCCACGCCATT | |
| MCP-1 | F:GACAAGCAAACCCAAACTCC |
| R:GCAATTTCCCCAAGTCTCTG |
AM, Adrenomedullin; VEGFA, Vascular endothelial growth factor A; ANGPTL4, Angiopoietin-like 4; HIF-1α, Hypoxia inducible factor 1α; HO-1, Heme oxygenase -1; VEGFR2, vascular endothelial growth factor receptor 2; SOD, Superoxide dismutase; GPx, glutathione peroxidase; IL-6, Interleukin-6; TGF-β, transforming growth factor beta; TNF-α, Tumor Necrosis Factor-α; MCP-1, Monocyte chemoattractant protein-1.
2.4. Statistical analyses
Data were analyzed using the JMP Pro Version11 statistical software (SAS Institute, Inc. Cary, NC). The results were expressed as Median and inter-Quartile range in subjects characteristics and were expressed as the means of the standard deviation (SD) in the expression levels of genes. Correlations between the groups were calculated by the Pearsonʼs correlation coefficient. Significant differences between groups were assessed using the Mann-Whitney U test. A p-value < 0.05 was taken to indicate statistical significance. We have compared all other genes between the LC and CH groups. We have described only genes with differences.
3. Result
3.1. Subjects characteristics
We studied 20 healthy volunteers (HV) and 48 patients with chronic liver disease (CLD). CLD patients are composed of 24 patients with chronic hepatitis(CH)and 24 patients with liver cirrhosis (LC). 48 CLD patients included 25 HCV patients, 11 HBV patients, 5 NASH patients, and 7 other patients. 13 patients in LC group have hepatocellular carcinoma (HCC). CLD patients were significantly older than HV (p < 0.01). In CLD group, LC patients were older than CH patients (p < 0.05). Serum Albumin and platelet counts were significantly lower and FIB4 index was markedly higher in CLD group compared to HV group (p < 0.01). FIB4 index (p < 0.01) and serum LDH (p < 0.05) in LC group were significantly higher than CH group. Platelet counts (p < 0.05), serum albumin (p < 0.01), and serum total cholesterol (T. chol) (p < 0.05) in LC group were decreased than CH group (Table 1).
3.2. Up-regulation of angiogenetic genes in CLD patients
Hypoxia induces angiogenic factors that induce activation and proliferation of endothelial cells. We examined mRNA expression of the angiogenic factors adrenomedullin (AM), vascular endothelial growth factor A (VEGFA), angiopoietin-like 4 (ANGPTL4), Tie 2, hypoxia inducible factor-1 alpha (HIF-1α), heme oxygenase -1 (HO-1), and vascular endothelial growth factor receptor 2 (VEGFR2) in PBMC. AM and VEGFA, ANGPTL4, Tie2 and HO-1 mRNA expression in CLD group were higher than HV group (p < 0.01) (Fig. 1A). Especially, AM mRNA expression in LC group was upregulated compared with CH group (p < 0.01) (Fig. 1B). VEGFA gene in CH group was elevated than HV group (p < 0.05), but was coordinate with LC group (Fig. 1C). The expression of AM and VEGFA was not affected by the presence of HCC (data was not shown).
Fig. 1.
mRNA expression levels of AM, VEGFA, ANGPTL4, Tie2, HIF-1α、HO-1 and VEGFR2 genes in CLD and HV (A). RT-PCR analyses of the expression levels of AM gene in HV, CH and LC (B). RT-PCR analyses of the expression levels of VEGFA gene in HV, CH and LC (C). *p < 0.05 compared with HV, **p < 0.01 compared with HV, ♯ p < 0.05 compared with CH and ♯♯ p < 0.01 compared with CH.
HV, healthy volunteers; CH, chronic hepatitis; LC, liver cirrhosis; AM, Adrenomedullin; VEGFA, Vascular endothelial growth factor A; ANGPTL4, Angiopoietin-like 4; HIF-1α, Hypoxia inducible factor 1α; HO-1, Heme oxygenase -1; VEGFR2, vascular endothelial growth factor receptor 2.
3.3. Up-regulation of oxidative stress genes in CLD patients
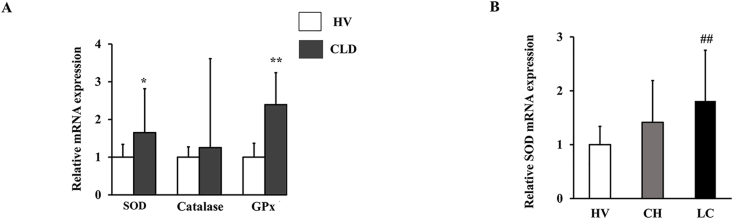
Oxidative stress caused by hypoxia induces endothelial cell injury and mitochondrial dysfunction, which exacerbates tissue hypoxia. On the other hand, an organism has an antioxidant system, and superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) were known to be antioxidant enzymes. SOD and GPx mRNA expressions in CLD group were higher than HV group (p < 0.05). Catalase transcription in CLD group was elevated compared with HV group, though the difference did not reach statistical significance (Fig. 2A). SOD mRNA expression in LC group was upregulated compared with CH group (p < 0.05) (Fig. 2B). The expression of SOD was not significantly different with or without HCC (data was not shown).
Fig. 2.
mRNA expression levels of SOD, Catalase and GPx genes in CLD and HV (A). RT-PCR analyses of the expression levels of SOD gene in HV, CH and LC (B). *p < 0.05 compared with HV, **p < 0.01 compared with HV, ♯ p < 0.05 compared with CH and ♯♯ p < 0.01 compared with CH·
HV, healthy volunteers; CH, chronic hepatitis; LC, liver cirrhosis; SOD, Superoxide dismutase; GPx, glutathione peroxidase.
3.4. Up-regulation of inflammatory cytokines in CLD patients
Inflammation causes liver sinusoidal endothelial cell (LSEC) damage by macrophage and neutrophils and disrupts microcirculation related to intrahepatic hypoxia. In hypoxic conditions, inflammatory, proinflammatory cytokines and chemokine including interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1) are elevated in the tissues. It is also known that transforming growth factor-beta (TGF-β) promotes the production of extracellular matrix in the mechanism of fibrosis caused by hypoxia. IL-6 and TGF-β mRNA expressions in CLD group were significantly higher than HV group (p < 0.05) (Fig. 3A). Furthermore, IL-6 mRNA expression in LC group was upregulated compared with CH group (p < 0.05) (Fig. 3B). TNF-α, and MCP-1 transcriptions in CLD group were not significantly elevated compared with HV group (Fig. 3A). The coexistence of HCC did not have any impact on the expression of the genes (data was not shown).
Fig. 3.
mRNA expression levels of IL-6, TNF-β, TNFα and MCP-1 genes in CLD and HV(A). RT-PCR analyses of the expression levels of IL-6 gene in HV, CH and LC (B). *p < 0.05 compared with HV, **p < 0.01 compared with HV, ♯ p < 0.05 compared with CH and ♯♯ p < 0.01 compared with CH·
HV, healthy volunteers; CH, chronic hepatitis; LC, liver cirrhosis; IL-6, Interleukin-6; TGF-β, transforming growth factor beta; TNF-α, Tumor Necrosis Factor-α; MCP-1, Monocyte chemoattractant protein-1.
3.5. Adrenomedullin was correlated with serum LDH, serum Alb, IL-6 expression, and SOD expression
We analyzed the relations between hypoxic reactive gene mRNA expressions and the serum parameters. Lactate dehydrogenase (LDH) is involved in anaerobic glycolysis that catalyzes pyruvate to lactate and is also known to be activated in tissue hypoxia. Albumin is primarily synthesized by hepatocytes and is a sensitive marker of liver function, and lower albumin is a predictor of impaired liver synthesis and progressed hepatic fibrosis. We examined a relation between AM expression in PBMC and the serum paramaters. AM was positively correlated with serum LDH (r = 0.46 p < 0.01), IL-6 mRNA expression (r = 0.36 p < 0.01) and SOD mRNA expression (r = 0.6 p < 0.01), and inversely related with serum Alb (r = 0.48 p < 0.01) (Fig. 4).
Fig. 4.
Correlations between AM mRNA expression and other parameters; serum LDH (A), serum Alb (B), IL-6 mRNA expression (C) and SOD mRNA expression (D).AM, Adrenomedullin; LDH, lactate dehydrogenase; Alb, Albumin; IL-6, Interleukin-6; SOD, Superoxide dismutase.
4. Discussion
PBMCs are blood cells with a round-shaped nucleus, such as monocytes and lymphocytes, with the lymphocyte population comprised of T cells, B cells, and NK cells. These cells are a critical component of the immune system, playing an integral role in the body's defenses [11,20]. A previous study reported that the activation state of PBMC was correlated with histological liver damage [16]. Recently, it is widely accepted that hypoxia affects the immune system in PBMCs from severe cerebral infarction [18]. It has been reported that the hypoxic synovial environment regulates the expression of VEGF in paired synovial fluid monocytic cells compared to matched PBMC from juvenile idiopathic arthritis patients [19]. These observations suggest that analysis of mRNA expression in PBMC could be a novel approach to evaluate intrahepatic hypoxia. The expression profiles of hypoxia reactive genes in PBMC isolated from patients with liver disease have not been reported.
Hypoxic environment plays a role in various liver diseases and has an enormous influence on the progression of the disease [[21], [22], [23], [24], [25], [26]], and hypoxia exerts a huge influence on the inflammatory gene, angiogenetic, and oxidative stress gene [[27], [28], [29]].
Thus, we examined the hypoxia reactive genes mRNA expression in PBMC from liver disease patients. Angiogenetic and inflammatory genes were upregulated in CLD group compared with HV group in our study. Hypoxia is a major inducer of angiogenesis together with inflammation. Regardless of etiology, hepatic angiogenesis takes place in chronic liver disease that is characterized by inflammation and progressive fibrosis [30]. Angiogenesis is known to be involved during chronic liver injury. Progressive tissue hypoxia stimulates angiogenesis in many liver diseases. This pathway leads to more inflammation and fibrosis [31]. We analyzed the mRNA expressions of all hypoxia reactive genes between the LC and CH groups. Especially, AM mRNA expression in LC patients was upregulated and the expression in CLD patients was correlated with IL-6 expression. AM is the potent hypotensive peptide discovered in the human pheochromocytoma. It has been reported that hypoxia induces the production of endogenous vasoactive peptides in macrophages of cirrhotic patients [32]. AM is constitutively secreted by vascular endothelial cells and smooth muscle cells and is considered to cause potent vasodilation via the synthesis of NO in the vascular endothelial cells [33,34]. Several clinical studies have demonstrated that the circulating AM levels are increased along with the progression of the liver disease and correlate with hemodynamic parameters in cirrhosis [[35], [36], [37]]. Moreover, the gene expression of AM in the vascular tissue was enhanced in the cirrhotic rats than in the control [38,39]. These observations suggest that AM level of blood and tissue is upregulated in LC. Sinusoidal circulatory disturbance leads to intrahepatic hypoxia in LC state, although it is not completely demonstrated that AM mRNA expression in PBMC reflects intrahepatic condition.
Antioxidant enzyme mRNA expressions including SOD and GPx were elevated in CLD group. ROS plays a crucial role in the development of numerous chronic liver diseases and stimulates their progression. Oxidative stress participates in the liver fibrogenic response [40]. It is possible that antioxidant enzymes mRNA expression was elevated in response to intrahepatic hypoxia caused by liver fibrosis.
After ischemia reperfusion, HIF-1α is upregulated promptly and then produced intense oxidative stress [21,22]. HIF-1α is originally found as a critical mediator for inducible expression of the erythropoietin gene by hypoxia and has been shown to activate transcription of many genes including tyrosine hydroxylase, inducible nitric oxide synthase, and various glycolytic enzymes in a hypoxia-dependent manner. It is also reported that HIF-1α is induced by hypoxic conditions; intermittent hypoxia in sleep apnea syndrome with the overweight patient or non-alcoholic fatty liver [23], while HIF-1α expression in CLD group was not significantly elevated in this study. HIF-1α affects transcriptional activity at the level of protein expression, so there might be no difference in HIF-1α mRNA expression and it is known that HIF-1α mRNA remained unchanged at a much lower level and this gene expression is almost undetectable because of HIF gene's function as a transcription factor and instability [41].
AM expression in CLD patients was correlated with serum LDH, serum Alb, IL-6 expression, and SOD expression. HIF-1α is known to regulate AM and LDH expression simultaneously. AM expression was induced in the anaerobic environment including hypoxia, and correlated with serum LDH in CLD patients [42]. Albumin decreases with hepatic fibrosis progression, which triggers tissue ischemia accompanied with the progression to LC, suggesting that AM expression was well correlated with serum Alb concentration [43]. IL-6 and SOD are reported to be elevated in hypoxic conditions, and these expressions were consequently upregulated with AM expression, which reflected tissue hypoxia [40]. Thus, the progression of intrahepatic hypoxia and liver fibrosis affected and elevated AM expression.
The limitation of this study is that we did not gather HV that was age-matched to CLD group. Age-related changes might affect mRNA expression of hypoxia reactive genes in PBMC; however, the lack of correlation between age and expression of hypoxia-related genes suggested that the expressions were influenced by tissue hypoxia (data not shown).
These findings suggest that mRNA expression of hypoxia reactive genes in PBMC might reflect intrahepatic hypoxia. We couldn't analyze the correlation between mRNA expressions of PBMC and those of liver in this study, but the evaluation of profile in PBMC is very useful to assess the pathology of the liver, especially hypoxia without the risk of liver biopsy.
5. Conclusion
The hypoxia reactive genes including the angiogenetic gene in PBMC were elevated in patients with chronic liver disease. Especially, AM mRNA expression in PBMC was correlated with liver function, suggesting that it may be applied as a useful marker to reflect intrahepatic hypoxia.
Funding
This work was supported in part by Takeda Science Foundation.
Availability of data and materials
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
A. Kuwano, M. Kohjima, and M. Kato designed the study. A. Kuwano performed experiments. M. Kurokawa, H. Suzuki, S. Tashiro, K. Imoto and T.Goya assisted experiments and data analyses. A. Kuwano wrote the initial draft of the manuscript. M. Kohjima, M. Tanaka, M. Kato and Y. Ogawa contributed to analysis and interpretation of data. M. Kohjima, M. Tanaka, M. Kato and Y. Ogawa assisted in the preparation of the manuscript and critically reviewed the manuscript. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work.
Ethics approval and consent to participate
Ethics committee approval was obtained. The study was conducted in accordance with Declaration of Helsinki ethical principles.
Declaration of competing interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
We appreciate the technical assistance from The Research Support Center, Kyushu University Graduate School of Medical Sciences.
We thank Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
References
- 1.Llovet J.M., Bartolí R., March F. Translocated intestinal bacteria cause spontaneous bacterial peritonitis in cirrhotic rats: molecular epidemiologic evidence. J. Hepatol. 1998 Feb;28(2):307–313. doi: 10.1016/0168-8278(88)80018-7. [DOI] [PubMed] [Google Scholar]
- 2.Maksan S.M., Ryschich E., Ulger Z. Disturbance of hepatic and intestinal microcirculation in experimental liver cirrhosis. World J. Gastroenterol. 2005 Feb 14;11(6):846–849. doi: 10.3748/wjg.v11.i6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nath B., Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology. 2012;55:622–633. doi: 10.1002/hep.25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosmorduc O., Housset C. Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin. Liver Dis. 2010 Aug;30(3):258–270. doi: 10.1055/s-0030-1255355. [DOI] [PubMed] [Google Scholar]
- 5.Deng J., Huang Q., Wang Y. Hypoxia-inducible factor-1alpha regulates autophagy to activate hepatic stellate cells. Biochem. Biophys. Res. Commun. 2014 Nov 14;454(2):328–334. doi: 10.1016/j.bbrc.2014.10.076. [DOI] [PubMed] [Google Scholar]
- 6.Fujinaga H., Tsutsumi T., Yotsuyanagi H. Hepatocarcinogenesis in hepatitis C: HCV shrewdly exacerbates oxidative stress by modulating both production and scavenging of reactive oxygen species. Oncology. 2011;81(Suppl 1):11–17. doi: 10.1159/000333253. [DOI] [PubMed] [Google Scholar]
- 7.Liu S., Koh S.S., Lee C.G. Hepatitis B virus X protein and hepatocarcinogenesis. Int. J. Mol. Sci. 2016 Jun 14;(6):17. doi: 10.3390/ijms17060940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiss A., Lotz G., Kaposi N.P. Hepatitis viruses and hepatocarcinogenesis. Orv. Hetil. 2002 Jan 13;143(2):83–86. doi: 10.1016/s0928-4257(01)00057-2. [DOI] [PubMed] [Google Scholar]
- 9.Angelova P.R., Abramov A.Y. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic. Biol. Med. 2016 Jun 11 doi: 10.1016/j.freeradbiomed.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Cooke M.S., Evans M.D., Dizdaroglu M. Oxidative DNA damage: mechanisms, mutation, and disease. Faseb. J. 2003 Jul;17(10):1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 11.Williams M.A., Newland A.C., Kelsey S.M. The potential for monocyte-mediated immunotherapy during infection and malignancy: Part I. Apoptosis induction and cytotoxic mechanisms. Leuk. Lymphoma. 1999 Jun;34(1–2):1–23. doi: 10.3109/10428199909083376. [DOI] [PubMed] [Google Scholar]
- 12.Thampanitchawong Pornpen, Piratvisuth Teerha. Liver biopsy: complications and risk factors. World J. Gastroenterol. 1999 Aug 15;5(4):301–304. doi: 10.3748/wjg.v5.i4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller C., Zielinski C. Interleukin-6 production by peripheral blood monocytes in patients with chronic liver disease and acute viral hepatitis. J. Hepatol. 1992;15:372–377. doi: 10.1016/0168-8278(92)90071-v. [DOI] [PubMed] [Google Scholar]
- 14.Tateno M., Honda M., Kawamura T. Expression profiling of peripheral-blood mononuclear cells from patients with chronic hepatitis C undergoing interferon therapy. J. Infect. Dis. 2007 Jan 15;195(2):255–267. doi: 10.1086/509893. [DOI] [PubMed] [Google Scholar]
- 15.Sakai Y., Honda M., Fujinaga H. Common transcriptional signature of tumor-infiltrating mononuclear inflammatory cells and peripheral blood mononuclear cells in hepatocellular carcinoma patients. Canc. Res. 2008 Dec 15;68(24):10267–10279. doi: 10.1158/0008-5472.CAN-08-0911. [DOI] [PubMed] [Google Scholar]
- 16.Jabłońska J., Pawłowski T., Laskus T. The correlation between pretreatment cytokine expression patterns in peripheral blood mononuclear cells with chronic hepatitis C outcome. BMC Infect. Dis. 2015 Dec 4;15:556. doi: 10.1186/s12879-015-1305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naldini A., Carraro F., Silvestri S. Hypoxia affects cytokine production and proliferative responses by human peripheral mononuclear cells. J. Cell. Physiol. 1997;173 doi: 10.1002/(SICI)1097-4652(199712)173:3<335::AID-JCP5>3.0.CO;2-O. 335-42. [DOI] [PubMed] [Google Scholar]
- 18.Yin Y., Li G. Hypoxia induces T Helper 17 cell upregulation in cultured peripheral blood mononuclear cells from chronic stage patients of severe cerebral infarction. Microbiol. Immunol. 2011 Feb;55(2):130–134. doi: 10.1111/j.1348-0421.2010.00301.x. [DOI] [PubMed] [Google Scholar]
- 19.Bosco M.C., Delfino S., Ferlito F. The hypoxic synovial environment regulates expression of vascular endothelial growth factor and osteopontin in juvenile idiopathic arthritis. J. Rheumatol. 2009 Jun;36(6):1318–1329. doi: 10.3899/jrheum.080782. [DOI] [PubMed] [Google Scholar]
- 20.Burczynski M.E., Twine N.C., Dukart G. Transcriptional profiles in peripheral blood mononuclear cells prognostic of clinical outcomes in patients with advanced renal cell carcinoma. Clin. Canc. Res. 2005 Feb 1;11(3):1181–1189. [PubMed] [Google Scholar]
- 21.Cursio R., Miele C., Filippa N., Van Obberghen E. Liver HIF-1 alpha induction precedes apoptosis following normothermic ischemia-reperfusion in rats. Transplant. Proc. 2008 Jul-Aug;40(6):2042–2045. doi: 10.1016/j.transproceed.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Tacchini L., Fusar Poli D., Bernelli-Zazzera A. Transferrin receptor gene expression and transferrin-bound iron uptake are increased during postischemic rat liver reperfusion Hepatology. 2002 Jul;36(1):103–111. doi: 10.1053/jhep.2002.33997. [DOI] [PubMed] [Google Scholar]
- 23.Yuan G., Nanduri J., Khan S. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J. Cell. Physiol. 2008 Dec;217(3):674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bangoura G., Liu Z.S., Qian Q. Prognostic significance of HIF-2alpha/EPAS1 expression in hepatocellular carcinoma. World J. Gastroenterol. 2007 Jun 21;13(23):3176–3182. doi: 10.3748/wjg.v13.i23.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L., Chen S.H., Zhang Y. Is the hypoxia-inducible factor-1 alpha mRNA expression activated by ethanol-induced injury, the mechanism underlying alcoholic liver disease? Hepatobiliary Pancreat. Dis. Int. 2006 Nov;5(4):560–563. [PubMed] [Google Scholar]
- 26.Nath B., Levin I., Csak T. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011 May;53(5):1526–1537. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peyssonnaux C., Cejudo-Martin P., Doedens A. Cutting edge: essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J. Immunol. 2007 Jun 15;178(12):7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 28.Imtiyaz H.Z., Williams E.P., Hickey M.M. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Invest. 2010 Aug;120(8):2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majmundar A.J., Wong W.J., Simon M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010 Oct 22;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coulon S., Heindryckx F., Geerts A. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011;31:146–162. doi: 10.1111/j.1478-3231.2010.02369.x. [DOI] [PubMed] [Google Scholar]
- 31.Elpek G.Ö. Angiogenesis and liver fibrosis. World J. Hepatol. 2015 Mar 27;7(3):377–391. doi: 10.4254/wjh.v7.i3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cejudo-Martín P., Morales-Ruiz M., Ros J. Hypoxia is an inducer of vasodilator agents in peritoneal macrophages of cirrhotic patients. Hepatology. 2002 Nov;36(5):1172–1179. doi: 10.1053/jhep.2002.36371. [DOI] [PubMed] [Google Scholar]
- 33.Kitamura K., Kangawa K., Kawamoto M. Adrenomedullin a novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 34.Genesca J., Gonzalez A., Catalan R. Adrenomedullin, a vasodilator peptide implicated in hemodynamic alterations of liver cirrhosis: relationship to nitric oxide. Dig. Dis. Sci. 1999;44:372–376. doi: 10.1023/a:1026618904493. [DOI] [PubMed] [Google Scholar]
- 35.Kojima H., Tsujimoto T., Uemura M. Significance of increased plasma adrenomedullin concentration in patients with cirrhosis. J. Hepatol. 1998;28:840–846. doi: 10.1016/s0168-8278(98)80235-3. [DOI] [PubMed] [Google Scholar]
- 36.Guevara M., Ginès P., Jiménez W. Increased adrenomedullin levels in cirrhosis: relationship with hemodynamic abnormalities and vasoconstrictor systems. Gastroenterology. 1998;114:336–343. doi: 10.1016/s0016-5085(98)70486-x. [DOI] [PubMed] [Google Scholar]
- 37.Fernández-Rodriguez C.M., Prada I.R., Prieto J., Montuenga L.M. Circulating adrenomedullin in cirrhosis: relationship to hyperdynamic circulation. J. Hepatol. 1998;29:250–256. doi: 10.1016/s0168-8278(98)80010-x. [DOI] [PubMed] [Google Scholar]
- 38.Sakurai S., Kojima H., Uemura M. Local regulator adrenomedullin contributes to the circulatory disturbance in cirrhotic rats. World J. Gastroenterol. 2006 Apr 7;12(13):2095–2102. doi: 10.3748/wjg.v12.i13.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kojima H., Sakurai S., Uemura M. Adrenomedullin contributes to vascular hyporeactivity in cirrhotic rats with ascites via a release of nitric oxide. Scand. J. Gastroenterol. 2004;39:686–693. doi: 10.1080/00365520410005306. [DOI] [PubMed] [Google Scholar]
- 40.Cichoż-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014 Jul 7;20(25):8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ema M., Taya S., Yokotani N. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1a regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dehne N., Kerkweg U., Otto T. The HIF-1 response to simulated ischemia in mouse skeletal muscle cells neither enhances glycolysis nor prevents myotube cell death. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007 Oct;293(4):R1693–R1701. doi: 10.1152/ajpregu.00892.2006. [DOI] [PubMed] [Google Scholar]
- 43.Spinella R., Sawhney R., Jalan R. Albumin in chronic liver disease: structure, functions and therapeutic implications. Hepatol Int. 2016 Jan;10(1):124–132. doi: 10.1007/s12072-015-9665-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.