Highlights
-
•
Green tiger shrimp shell, a waste, is a good source of astaxanthin.
-
•
Astaxanthin extraction and properties depend on the solvent and method of extraction.
-
•
Polar/nonpolar combinations help to improve the extraction efficiency.
-
•
Ultrasound extraction (UAE) was optimized for astaxathin extraction using RSM.
-
•
UAE is an effective green extraction method for astaxanthin from shrimp shells.
Keywords: Shrimp shells, Astaxanthin, Ultrasound Extraction, Optimization, Antioxidant, Efficiency
Abstract
This study was aimed at optimizing the astaxanthin extraction efficiency from shrimp shell (green tiger, Penaeus semisulcatus). Astaxanthin was extracted using selected nonpolar/polar solvents (petroleum ether, n-hexane, ethanol, acetone) individually and in ternary mixtures of petroleum ether, acetone, and water in ratios of 15:50:35, 50:45:5, and 15:75:10 for different times (2,4 and 6 h). The results showed that solvents with higher polarity were more suitable for the extraction of astaxanthin, and increasing the extraction time from 2 to 6 h improved the extraction yield. The conditions of extraction of astaxanthin with the desirable solvent were then optimized with the ultrasonic method using the Box-Behnken design [variables included: extraction temperature (25 to 45 °C), extraction time (5 to 15 min), and ultrasound amplitude (20 to 100%)]. Optimal extraction conditions were determined as the ultrasonic amplitude of 23.6%, extraction time of 13.9 min, and extraction temperature of 26.3 °C. Under this optimum condition, the amount of astaxanthin, ferric reducing antioxidant power, and free radical scavenging capacity of the extract were obtained as 51.5%, 1705 μmol of Fe2+/g, and 73.9%, respectively. Extraction and analysis of the extract at the optimum point were used to validate the results.
1. Introduction
Astaxanthin (3,3′-dihydroxy-β,β-carotene-4,4′-dione) is a red keto-carotenoid (from the xanthophylls group) that has been identified in plants, animals, bacteria, and fungi. It has been shown that this pigment has unusual and potent antioxidant activity and prevents Alzheimer's and Parkinson's diseases, stroke, high cholesterol, eye disease, and cancer [1], [2].
Based on the source, astaxanthin market is segmented into synthetic and natural. Synthetic astaxanthin is produced from petrochemical sources, which accounts for>95% of the market share due to its lower cost (about $ 1,000 per kilogram). But because of issues related to food safety (toxicity potential in the final product) and pollution [3], to date, the extracted astaxanthin has only been used as an additive to feed the livestock, poultry and aquaculture for the purpose of improving biological performance (such as preventing the oxidation of unsaturated essential fatty acids) [4] and increasing nutritional value (such as producing vitamin A, improving growth, improving reproductive behaviors, and protecting effects of UV light) [5]. Natural astaxanthin is extracted from some algae, salmon, shrimp shells, etc. and can be used in the food industry (beverages, ice creams, desserts, candy, and meat products) in addition to feeding aquatic animals and house pets [6].
Penaeidae shrimps are a branch of the crustaceans that has two super families, the Penaeoidea and the Sergestoidea. The genus Penaeus is one of the most important genera of the Penaeoide family. The green tiger (Penaeus semisulcatus) shrimp is one of the most important species in the genus of Penaeus shrimps. In the waters of the Persian Gulf (Bushehr and Khuzestan provinces), the green tiger shrimp is most abundant and of great economic importance in the provinces' fishing. Published information on carotenoid and astaxanthin contents of wild marine shrimps is limited. Gopakumar and Nair [7] found a general average of 13.3 mg/kg total carotenoid content in four penaeid species (Metapenaeus affinis, M. dopsoni, Penaeus indicus, Parapenaeopsis stylifera) and 4.2 mg/kg in Metapenaeus monoceros from brackish water. Yanar et al. [8] reported mean carotenoid contents of Penaeus semisulcatus and Metapenaeus monoceros to be 14.1 ± 0.45 and 16.9 ± 0.26 mg/kg, respectively. These values are considerably higher than found in other seafood. Hooshmand et al. [9] reported extraction of carotenoids from by-products of blue crab (Portunus pelagicus) and shrimp (Penaeus semisulcatus) using hexane, isopropyl alcohol or acetone individually and the mixture of hexane and acetone (1:1 v/v) and hexane and isopropyl alcohol (1:1 v/v) and reported the highest yield (6.63 and 61.32 µg/g for blue crab and shrimp, respectively) with acetone. They also extracted carotenoids from by-products of blue crab (Portunus pelagicus) and shrimp (Penaeus semisulcatus) using different edible oils (sesame, sunflower, soybean and rice bran) with sunflower oil resulting in the highest yield (0.21 and 4.03 µg/g for blue crab and shrimp, respectively). but much lower than obtained from solvents.
Different methods have been used for extraction of bioactive compounds and their extraction efficiency and product quality differ considerably. Carotenoids, which are hydrophobic have limited solubility in water and are generally extracted using organic solvents [10]. Different solvents such as n-hexane-isopropyl alcohol (6:4 v/v) [11], acetone and n-hexane: isopropyl alcohol (1:1 v/v) [12], acetone and petroleum ether, acetone, and water (15:75:10) [13] and edible oils (such as sunflower oil, soybean oil, etc.) [14], [9] have been used to extract astaxanthin from shrimp and crab wastes. However, the choice of appropriate solvents (low viscosity and higher solubility with adequate stability) and extraction method are important factors for the efficient extraction of astaxanthin from various sources with high purity and bioactivity [15].
In recent years, novel methods such as ultrasonic-assisted extraction (UAE) have been developed for the extraction of active components from plants. UAE allows the addition of an auxiliary extractor and increases the polarity of the liquid phase [16]. However, there are contradictory reports on the efficiency UAE for the extraction of astaxanthin [17], [18], [19] and it uncertain whether UAE could increase the extraction efficiency of astaxanthin from green tiger shrimp shells. UAE process is influenced by several factors such as liquid-to-solid ratio, extraction temperature, extraction time, frequency, sonication power, ultrasonic wave distribution etc. [20]. In order to evaluate the influence of variables affecting UAE, response surface methodology (RSM) and optimization approaches have been widely used.
Therefore, the aim of the this study was to first select an appropriate solvent from polar and nonpolar choices, and to optimize the UAE procedure with appropriate solvent for maximizing astaxanthin extraction and optimum retention of antioxidant activity from green tiger shrimp shell.
2. Materials and methods
2.1. Preparation of raw material
Green tiger shrimp (Penaeus semisulcatus) shells were obtained from shrimp processing centers in Bushehr province (Iran) and transported to the laboratory in an insulated box mixed with ice. Then additional appendages are removed and the waste was packaged in polyethylene bags and stored −20 °C. Before the extraction, the shell was finely milled (Mulinex, Depose-Brevete S.G.C.G., France) and sieved using a sieve mesh #40 (425 µm).
2.2. Chemicals and reagents
Ethanol 96%, petroleum ether, acetone, hexane, methanol, hydrochloric acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH) reagent, 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ) and Folin–Ciocalteu reagent were purchased from Sigma-Aldrich and Merck companies.
2.3. Solvent extraction
Different organic solvents such as petroleum ether, n-hexane, ethanol, acetone (individually), and a ternary mixture consisting of petroleum ether, acetone, and water with ratios of 15:75:10, 50:45:5, and 15:50:35 were used. Extraction was carried out in each of the solvents for different durations (2, 4 and 6 h) at boiling point of the solvent with a constant sample to solvent ratio (1 to 4 w/v) using the Soxhlet apparatus. When the extraction process was completed, the mixture was filtered, and the solvent was concentrated in vacuum rotary evaporator (Laborota 4000 efficient, Germany). The concentrated extracts were then dried in a freeze drier (Operon- Korea) (−55◦C, 0.15 mmHg) for 48 h. Finally, the dried samples were kept in the dark at −18◦C for further analysis.
2.4. Ultrasound-assisted extraction (UAE)
The ultrasound-assisted extraction (UAE) procedure standardized earlier by Sharayei et al. [21] was used with some modifications. The UAE unit was a closed rectangular ultrasonic processor (Heilscher, Germany Ultrasonic Electronic Equipment Co. Ltd.) with a maximum power of 400 W at the frequency of 20 KHz. For extraction, 10 g sample of milled shrimp shell was placed in the 100 ml flask, to which 40 ml of the preselected solvent was added (1:4 w/v), and the mixture was subjected to UAE treatment for different times (5, 10 and 15 min), amplitudes (20, 60 and 100%) and temperatures (25, 35 and 45 °C) based on response surface methodology (RSM) experimental design (Table 1). After the treatment, the mixture was transferred to the Soxhlet apparatus for solvent extraction for 6 h. When the extraction process was completed, the mixture was filtered, and the solvent was concentrated in vacuum rotary evaporator (Laborota 4000 efficient, Germany). The concentrate was then freeze dried (Operon-Korea) for 48 h (−55◦C, 0.15 mm Hg). Finally, the dried samples were kept in the dark at −18◦C for further analysis.
Table 1.
Box-Behnken design of UAE (variables and levels) with the resulting quality response parameters of green tiger shrimp shell extract.
| Exp.no | Extraction conditions |
Analytical results a |
||||
|---|---|---|---|---|---|---|
| Ultrasound amplitude (A) (℃) |
Exposure time (B) (min) |
Sonication temperature (C) (%) |
Astaxanthin content (µg/g) |
FRAP (µmol Fe2+/g) |
DPPHsc (%) |
|
| 1 | 100 (+1) | 10(0) | 45(+1) | 12.02 ± 0.21 | 630.56 ± 9.85 | 25.45 ± 0.45 |
| 2 | 60 (0) | 10(0) | 35(0) | 29.52 ± 0.45 | 1245.38 ± 14.21 | 43.74 ± 0.38 |
| 3 | 60 (0) | 10(0) | 35(0) | 28.45 ± 0.13 | 1085.35 ± 11.78 | 38.89 ± 0.27 |
| 4 | 60 (0) | 5 (−1) | 25(−1) | 25.37 ± 0.17 | 920.78 ± 6.98 | 42.48 ± 0.14 |
| 5 | 100 (+1) | 10(0) | 25(−1) | 17.02 ± 0.71 | 740.89 ± 13.44 | 20.03 ± 0.46 |
| 6 | 60 (0) | 15(+1) | 45(+1) | 19.01 ± 0.65 | 900.56 ± 16.05 | 21.37 ± 0.71 |
| 7 | 60 (0) | 10(0) | 35(0) | 31.80 ± 0.43 | 1180.23 ± 18.86 | 37.89 ± 0.24 |
| 8 | 20 (−1) | 5 (−1) | 35(0) | 42.05 ± 0.11 | 1410.89 ± 17.48 | 65.98 ± 0.18 |
| 9 | 20 (−1) | 10(0) | 45(+1) | 25.12 ± 0.19 | 1091.87 ± 11.52 | 40.12 ± 0.51 |
| 10 | 20 (−1) | 10(0) | 25(−1) | 47.23 ± 0.77 | 1580.25 ± 10.67 | 70.35 ± 0.37 |
| 11 | 60 (0) | 5 (−1) | 45(+1) | 20.41 ± 0.48 | 900.86 ± 9.74 | 40.18 ± 0.74 |
| 12 | 100 (+1) | 15(+1) | 35(0) | 25.79 ± 0.52 | 1065.89 ± 14.69 | 49.90 ± 0.26 |
| 13 | 100 (+1) | 5 (−1) | 35(0) | 21.58 ± 0.14 | 952.99 ± 12.04 | 37.89 ± 0.24 |
| 14 | 20 (−1) | 15(+1) | 35(0) | 47.80 ± 0.85 | 1590.78 ± 11.74 | 65.10 ± 0.47 |
| 15 | 60 (0) | 15(+1) | 25(−1) | 38.12 ± 0.33 | 1390.09 ± 10.64 | 55.52 ± 0.64 |
FRAP: Ferric Reducing Antioxidant Power.
DPPHsc: Scavenging activity of DPPH.
2.5. Shrimp shell analysis
Selected physicochemical properties were evaluated according to AOAC [22] standard methods. Moisture content was determined by drying in an electric oven (Memmert oven, model UL 40, Schwabach, Germany) at 105 ± 1 °C. Total ash was determined using an electric furnace (Ex.1200.2l, Excition Co., Iran) at 600 °C. Protein was measured using Kjeldahl (Gerhardt, German) method using a conversion factor of 6.25. Fat was determined by the Soxhlet extraction. The amount of total carbohydrates was calculated by subtracting the percentage total of moisture, protein, ash and fat from 100. All variables were examined in triplicate.
2.6. Measuring the astaxanthin content
The extracted astaxanthin was first dissolved in the hexane solvent (in 3 ml) and its absorption was read at 470 nm, and then the amount of astaxanthin was calculated using the Eq. (1):
| (1) |
AST: Astaxanthin concentration in µg/g, A: Absorbance, D: Extract volume in hexane, 106: Dilution multiple, G: Sample weight in g, d: Cuvette width, E: Extinction co-efficient, which is 2100 [14].
2.7. Antioxidant activity
The antioxidant activities were estimated by DPPH and FRAP assays. DPPH radical-scavenging assay was carried out according to Ramadan et al. [23] method. The radical-scavenging activity (DPPHsc) was calculated as a percentage of DPPH discoloration using the Eq. (2):
| DPPHsc% = [(ADPPH - AS)/ADPPH] × 100(2) |
where AS is the absorbance of the solution when the sample is added at a particular level and ADPPH is the absorbance of the DPPH solution.
Ferric reducing-antioxidant power (FRAP) was measured using 2,4,6-tripyridyl-s-triazine (TPTZ) method according to Benzie and Strain [24]. The results were expressed in µmol Fe2+per g at 595 nm, against the control solution.
2.8. Statistical analysis
The effects of solvent type and extraction duration on astaxanthin content and antioxidative properties were carried out in triplicate and compared based on the factorial experiment in a completely randomized design. Analysis of variance (ANOVA) was carried out according to General Linear Model of SPSS (Version 16.0, 2007). Significant differences between means were determined by Duncan’s multiple range tests with p values<0.05 were considered statistically significant.
To investigate the UAE effects on astaxanthin content and antioxidative properties, the Box-Behnken design was used. The design consisted of three variables at three levels and with three replicates (Table 1). The software Design Expert version 10.0.7 (Minneapolis, USA) was used to evaluate regression and produce trace plots of 3D surface and Cox response. Means were compared with MstatC software.
3. Results and discussion
3.1. Physico-chemical properties of shrimp shell powder
Selected physico-chemical properties evaluated for Green tiger shrimp (Penaeus semisulcatus) shell are presented in Table 2. As evident from the table, shrimp shells contained significant amounts of ash and protein.
Table 2.
Physico-chemical analysis of dried shrimp shell powder.
| Parameters | Mean values ± SD |
|---|---|
| Moisture (g/100 g) | 15.88 ± 0.12a |
| Total ash (g/100 g) | 29.40 ± 1.83 |
| Proteins (g/100 g) | 31.16 ± 2.10 |
| Lipids (g/100 g) | 3.40 ± 1.45 |
| Total carbohydrates (g/100 g) | 20.16 ± 1.34 |
: Mean ± standard deviation of triplicate determinations from experiments
3.2. Effects of solvent extraction conditions on astaxanthin content
Table 3 shows data on the effect of solvent type and extraction time on the extracted astaxanthin yield. The extraction depended largely on the type of solvent used (p < 0.05). Many solvents (water, short-chain alcohols, halogenated solvents, ketones, ethyl acetate, diethyl ether, petroleum ether, hexane and toluene) have been used as solvents for different bioactive compounds [25]. During extraction, the solvent diffuses into the material and dissolve compounds with similar polarity, and this solubility varies greatly with changes in the polarity of the solvent. The polarity of the solvents used in this study for petroleum ether, hexane, ethanol, acetone, petroleum ether/acetone/water mixture with a ratio of 5:45:50, 15:50:35 and 15:75:10, were 2.8, 2, 5.0, 5.1, 4.14, 6.15 and 5.14, respectively. As can be seen in Table 3, polar solvents were more suitable for the extraction of astaxanthin. This phenomenon is probably due to the fact that the diffusion of nonpolar solvents through the hydrophilic layer that surrounds the pigment is more difficult [26].
Table 3.
Effect of solvent type and extraction time on astaxanthin content and antioxidant properties of shrimp shell extract.
| Solvent type | Extraction time | Astaxanthin content | DPPHsc | FRAP |
|---|---|---|---|---|
| (h) | (µg/g) | (%) | (µmol Fe2+/g) | |
| Petroleum ether | 2 | 10.40 m* | 23.73 h | 959.60f |
| 4 | 12.32 l | 26.42 g | 985.15f | |
| 6 | 14.24 k | 29.41f | 1038.61e | |
| Hexane | 2 | 18.95 h | 18.88 k | 1122.95d |
| 4 | 24.32 g | 26.53 g | 1230.47c | |
| 6 | 31.89 d | 38.90 e | 1291.05c | |
| Ethanol | 2 | 16.48 i | 19.17 k | 1058.80e |
| 4 | 20.86 h | 21.33 i | 1070.09e | |
| 6 | 26.96f | 29.67f | 1087.21e | |
| Acetone | 2 | 28.16 e | 43.62 d | 1175.82d |
| 4 | 35.76c | 48.64c | 1263.14c | |
| 6 | 45.79b | 64.88b | 1424.11b | |
| Petroleum ether/acetone/water (5:45:50) | 2 | 5.18p | 15.32 l | 915.65f |
| 4 | 6.13 op | 18.46 k | 956.04f | |
| 6 | 6.70o | 21.47 i | 1022.57e | |
| Petroleum ether/acetone/water (15:50:35) | 2 | 5.91p | 15.00 l | 906.14f |
| 4 | 6.11 op | 22.56 i | 947.13f | |
| 6 | 9.18n | 29.62f | 1011.28e | |
| Petroleum ether/acetone/water (15:75:10) | 2 | 32.14 d | 44.69 d | 1166.91d |
| 4 | 36.27c | 48.64c | 1295.21c | |
| 6 | 48.47 a | 67.64 a | 1504.90a |
*: Means within a column with the same lowercase letters are not significantly different at p < 0.05.
FRAP: Ferric Reducing Antioxidant Power
DPPHsc: scavenging activity of DPPH
Also, the influence of different ternary mixture of petroleum ether/acetone/water solvents on astaxanthin extraction was different (Table 3). The amount extracted in petroleum ether/acetone/water mixtures with a ratio of 15:75:10 and polarity of 5.14 was much higher than the other two ratios for this mixture and other solvents. It has been recognized that extraction of carotenoid compounds from low-moisture products change with slight changes in the polarity of solvents and complete extraction of carotenoids from plant tissues could be achieved by use of a mixture of slightly polar plus non-polar solvents [27]. Sachindra et al. [28] also observed that the extraction of astaxanthin in the mixture of isopropyl alcohol and hexane (50:50) was higher than that of acetone alone due to the inclusion of a non-polar solvent in the extraction medium.
The extent of astaxanthin extraction increased with increasing the extraction time from 2 to 6 h (p < 0.05). During extraction, the solvent diffuses into the plant material and dissolves compounds with similar polarity, and therefore it is natural that the extraction yield will increase with time, but eventually could reach a plateau at some point of time. Determining the end time of extraction is also an important factor in the type of component being extracated because with increasing extraction time can lead to increased decomposition of pigment and/or conversion of cis isomers to trans forms and vice versa [29]. Therefore, considering the economic costs and isomerization of the extract, increasing the extraction time to beyond 6 h was not considered practical.
3.3. Effects of solvent extraction conditions on antioxidative activity
The effect of solvent type and extraction time on antioxidative properties of shrimp shell extract is also shown in Table 3. A direct relationship between the astaxanthin content and the antioxidant activity was observed, and extracts with ternary solvent of petroleum ether/acetone/water with ratio of 15:75:10 and acetone with 6 h duration had the highest amount of Ferric reducing (FRAP, 1505 and 1424 µmol/g) and DPPH radical-scavenging capacity (DPPHsc, 67.6 and 64.9%), respectively (p < 0.05). The antioxidant activity of astaxanthin has been evaluated in many studies. Due to its molecular structure, astaxanthin has very unique chemical properties and plays an important role in removing free radicals and heavy metals. The presence of hydroxyl and ketone moieties on the ion ring is responsible for the high antioxidant properties of this compound. Astaxanthin inhibits free radicals in both the conjugated unsaturated chain (polyene) and the ring terminal (c3 rings) [30], [31].
3.4. Optimization of UAE
3.4.1. Model fitting
Response surface methodology (RSM) was used to evaluate not only the effect of ultrasound amplitude (A, %), exposure time (B, min), and sonication temperature (C, ͦ C) on quality and quantity characteristics of shrimp shell extract which included astaxanthin content, DPPHsc and FRAP activities, but also to facilitate optimization. The solvent was first selected for UAE extraction which was petroleum ether/acetone/water solvent with a ratio of 15:75:10 as determined as the best combination from the solvent extraction step.
In order to determine the experimental model for predicting the response, polynomial equations including linear, two factorial (interactive), quadratic and cubic were fitted to the data obtained from the response surface methodology. Then, these models were statistically compared. The selected model should be statistically appropriate with Lack of fit test not significant and has the highest R2 and adjusted R2. The quadratic model was the best one and used for the construction of three dimensional response surface plots to assess the relationship between independent and dependent variables. Table 4 presents the ANOVA for response surface quadratic model.
Table 4.
Regression coefficients of predicted polynomial models for the investigated responses from shrimp shell extract.
| Astaxanthin content |
DPPHsc |
FRAP |
|||||
|---|---|---|---|---|---|---|---|
| source | (µg/g) |
(%) |
(µmol Fe2+/g) |
||||
| df | Coefficients of equations | Sum of squares | Coefficients of equations | Sum of squares | Coefficients of equations | Sum of squares | |
| Model | 9 | 29.92 | 182.05** | 40.17 | 357.88** | 1170.32 | 125669.3** |
| A: ultrasound amplitude | 1 | -10.72 | 919.99** | -13.54 | 1465.57** | -285.43 | 651773.7** |
| B: extraction time | 1 | 2.66 | 56.76** | 0.67 | 3.59 ns | 95.22 | 72542.41* |
| C: extraction temperature | 1 | -6.4 | 327.42** | -7.66 | 469.10 ** | -138.52 | 153502.3** |
| AB | 1 | -0.39 | 0.59 ns | 3.22 | 41.53ns | -16.75 | 1121.92 ns |
| AC | 1 | 4.28 | 73.19** | 8.91 | 317.73* | 94.51 | 35730.45 * |
| BC | 1 | -3.54 | 50.05** | -7.96 | 253.61* | -117.4 | 55133.39* |
| A2 | 1 | 2 | 14.78* | 6.82 | 171.84* | 33.82 | 4222.92ns |
| B2 | 1 | 2.38 | 20.92* | 7.72 | 220.17* | 50.92 | 9603.22ns |
| C2 | 1 | -6.58 | 159.70** | -8 | 236.78* | -193.25 | 137886** |
| Residual | 5 | 1.47 | 26.22 | 6092.14 | |||
| Lack of fit | 3 | 0.49 | 37.17 | 5836.19 | |||
| Pure error | 2 | 2.93 | 9.79 | 6476.06 | |||
| Cor total | 14 | ||||||
| R2 | 0.99 | 0.96 | 0.97 | ||||
| Adj R2 | 0.98 | 0.89 | 0.92 | ||||
| CV | 4.21 | 1.73 | 7.02 | ||||
Ns: Not significant (p > 0.05).
*Significant at (p < 0.05).
** Significant at (p < 0.01).
***Significant at (p < 0.001).
FRAP: Ferric Reducing Antioxidant Power.
DPPHsc: scavenging activity of DPPH.
3.4.2. Effects of extraction process conditions of UAE on astaxanthin content
Examination of the equation obtained for astaxanthin content showed that they had a high coefficient of determination (R2 = 0.99) and a high and significant adjusted coefficient of determination (adj-R2 = 0.98) for prediction. The lack of fit was not significant (p > 0.05) and coefficient of variation was low (CV = 4.21%), which indicated the suitability of the proposed model.
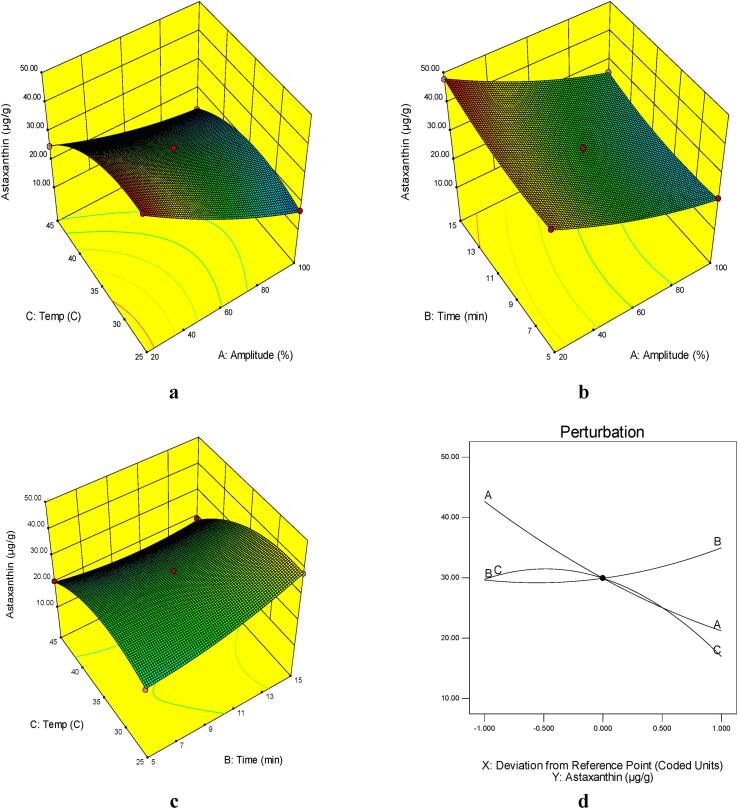
Three-dimensional (3D) plots for astaxanthin content udner different extraction conditions are shown in Fig. 1 (a-d). The astaxanthin extraction efficiency decreased with increasing ultrasound amplitude from 20 to 100%, while it increased with increasing time from 5 to 15 min [Fig. 1(a-d)]. Also, the extraction efficiency increased with temperature from 25 to 35 °C and then decreased sharply at 45 °C.
Fig. 1.
Response surface plots of the astaxanthin content (µg/g) of UAE as affected by: A) extraction temperature (°C) and amplitude (%), B) extraction time (min) and amplitude (%) C) extraction temperature (°C) and extraction time (min) and D) Perrturbation plot of astaxanthin content in UAE shrimp shell’s extract (A: amplitude(%), B: extraction time (min), C: extraction temperature (°C)).
The extraction efficiency of astaxanthin increased with increasing in ultrasonic amplitude up to 20%, but then decreased at further higher amplitudes. Similar trend has been reported in extractives from plants [32], [33]. Ultrasound represent mechanical waves with a frequency > 20 kHz consisting of a series of compression and rarefaction cycles that can be propagated through solid, liquid or gaseous media resulting in the displacement and dislodgement of the molecules from their original positions. At the high intensity, the negative pressure during rarefaction exceeds the attractive forces that bind the molecules and thus pulling them apart and creating cavitation bubbles. Acoustic cavitation is the main mechanism involved in the UAE. Collapsing cavitation bubbles and the sound waves may result in fragmentation, localised erosion, pore formation, shear force, increased absorption and swelling of the cellular matrix of the tissue. The collapsing cavitation bubbles generate shockwaves and accelerate inter-particle collision which can cause these effects. Rapid fragmentation leads to solubilisation of the bioactive components in the solvent due to decreased particle size, increased surface area and associated high mass transfer rates in the boundary layer of solid matrix [34], [35], [36]. Ultrasound also increases the swelling index of plant tissue matrix which helps in both desorption and diffusion of solutes resulting in increased extraction [37]. The increase in the extraction yield by UAE cannot be attributed to a single mechanism but to the combination of several of these activities associated with the sound. Nevertheless, astaxanthin content decreases when ultrasonic power is increased to much higher levels, probably due to overformation of bubbles which hampers the propagation of ultrasound waves [38], [39], or due to the heat produced by excessive ultrasonic power may not be completely dissipated in the short period of time, leading to the degradation of carotenoids [40].
Temperature is one of the effective factors in the ultrasound assisted extraction. In general, raising the temperature increases the extraction of effective compounds due to the increase in the breaking of the bonds in the composition, increasing the solubility of the compound, increasing the rate of diffusion and mass transfer, as well as reducing the viscosity and surface tension between the solvent and the compounds [41]. Also, the increase in temperature reduces cavitation owing to the decrease in surface tension and the increase in vapor pressure [42]. Hence, the increase in the astaxanthin extraction efficiency up to 35 °C in the present study, but the decrease at further higher temperatures is probably due to further degradation of the extracted compounds as well.
As evident from Fig. 1(d), by increasing extraction time from 5 to 15 min, the extraction efficiency of astaxanthin increased. Sahin and Samli [43], reported that the amount of effective compounds extracted by ultrasound, as a function of time, is carried out in two main stages. The first step, called washing, takes place within the first 10 to 20 min by dissolving the soluble components on the surface of the compound. At this stage, up to 90% of effective compounds maybe extracted. The second stage, known as slow extraction, is the transfer of mass from the surface of the compound to the solvent by the diffusion phenomenon. Dahmoune et al. [44] also reported that the extraction of bioactive compounds from P. Lentiscus plant leave intensifies after ten minutes. Wang et al. [45], in a study on the extraction of phenolic and flavonoid compounds using ultrasound from elaeagnus pungens fruit, reported that the highest efficiency of extraction of polyphenolic compounds was achieved at the 20 min mark. In an examination of the extraction of polyphenols from wheat bran by ultrasound, they also found that the extraction rate of these compounds increased significantly from 10 to 30 min but was almost constant from 30 to 50 min.
3.4.3. Effects of extraction process conditions of UAE on antioxidative activity
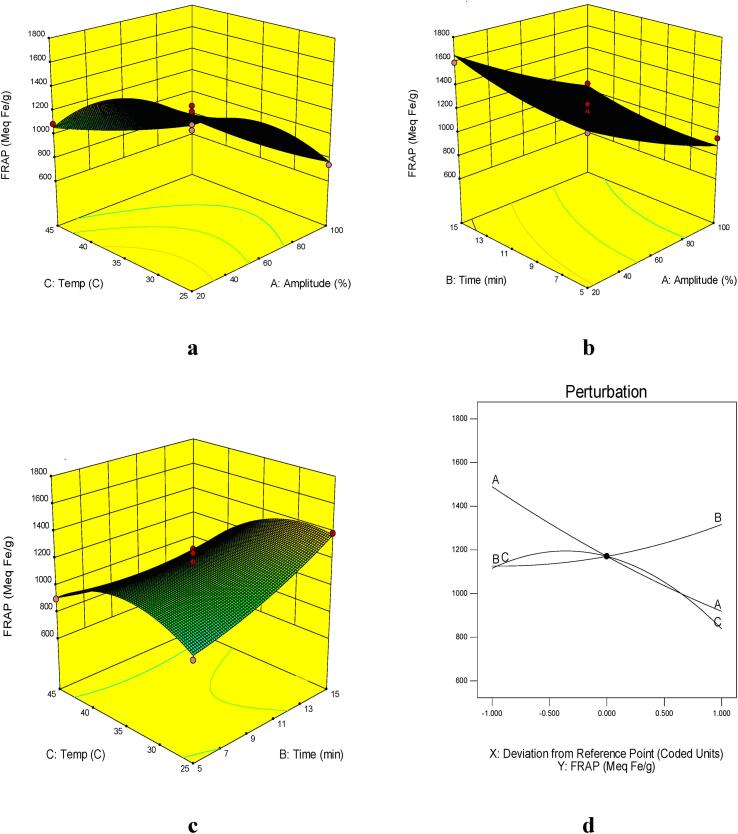
The results presented in Table 4 indicate that the linear effect of ultrasound amplitude, extraction temperature, extraction time, and quadratic term of extraction temperature, as well as the interaction of temperature with ultrasound amplitude and extraction time were significant (p ≤ 0.05) on FRAP. The coefficient of determination (R2) and of adjusted coefficient of determination (adj-R2) of the predicted models in FRAP were 0.97 and 0.92, respectively. Lack of fit test of the model was not significant (p > 0.05). These values would give a good fit to the mathematic model. Based on the sum of squares, ultrasound amplitude had the greatest effect on FRAP activity.
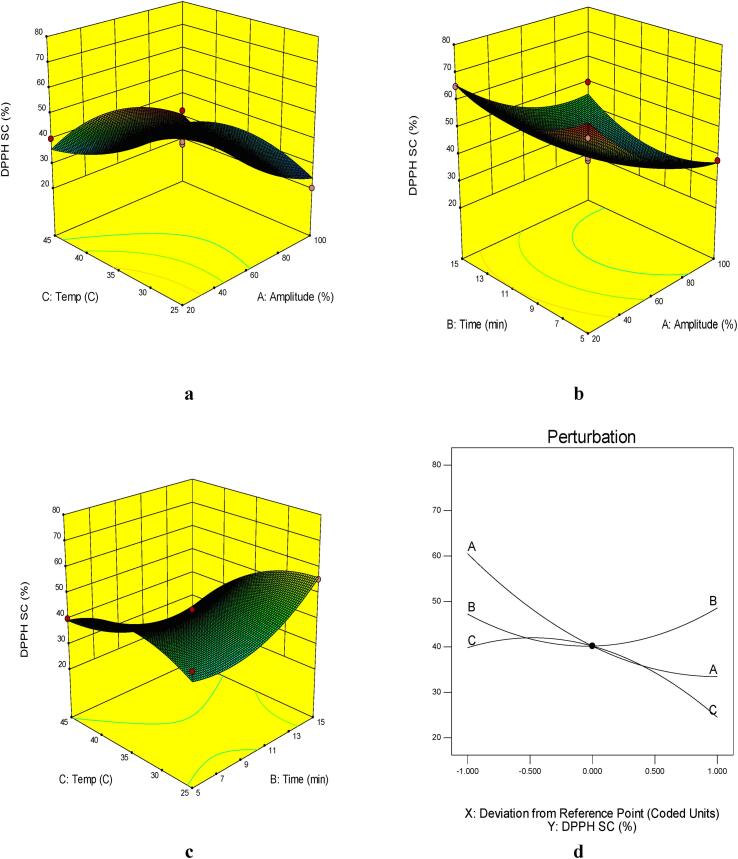
The results of this study also showed that the amplitude and temperature of sonication and the quadratic terms of amplitude, time, and temperature of sonication and their interactions had a significant effect on the DPPHsc (Table 4) with high R2 and adj-R2. The lack of fit of the model was not significant indicating a good model fit.
The trend of changes in antioxidant compounds was similar to astaxanthin content and a direct correlation could be observed between them. Antioxidative activity (FRAP and DPPHsc) increased significantly by increasing the extraction time up to 15 min and the extraction temperature up to about 35 °C (Fig. 2, Fig. 3). Astaxanthin, a reddish-orange compound, has stronger antioxidant activity than various other carotenoids such as lutein, lycopene, α-carotene, and β-carotene [46]. Astaxanthin's strong antioxidant activity is linked to the keto group, which stimulates the hydroxyl group and thus promotes the transfer of hydrogen to the peroxyl radical [47]. Antioxidants with higher ferric reducing antioxidant power have a greater ability to terminate the destructive reactions of radical chains [48].
Fig. 2.
Response surface plots of the FRAP activity (µmol Fe2+/g) of UAE as affected by: A) extraction temperature (°C) and amplitude (%), B) extraction time (min) and amplitude (%) C) extraction temperature (°C) and extraction time (min) and D) Perrturbation plot of astaxanthin content in UAE shrimp shell’s extract (A: amplitude(%), B: extraction time (min), C: extraction temperature(°C)).
Fig. 3.
Response surface plots of the DPPHsc activity (%) of UAE as affected by: A) extraction temperature (°C) and amplitude (%), B) extraction time (min) and amplitude (%) C) extraction temperature (°C) and extraction time (min) and D) Perrturbation plot of astaxanthin content in UAE shrimp shell’s extract (A: amplitude(%), B: extraction time (min), C: extraction temperature(°C)).
3.4.4. Optimization of UAE and verification
The optimal conditions for extraction of shrimp extract by the UAE on the responses were determined through numerical optimization and graphical optimization by Design Expert software. The target was to obtain maximum of astaxanthin content, FRAP and DPPHsc.
Under optimal conditions (ultrasound amplitude: 23.62%, extraction time: 13.9 min, and extraction temperature: 28.3 °C), with the ternary mixture of petroleum ether/acetone/water (with ratio of 15:75:10 and solid to solvent ratio 1 to 4) the astaxanthin content, FRAP and DPPHsc of the extract were obtained as 51.5 µg/g, 1705 μmol /g, and 73.9%, respectively. To ensure the validity of the conditions, the experiment was repeated under these optimal conditions and the responses obtained are given in Table 5. The absence of significant differences between predicted and observed values (p > 0.05) indicates a good correspondence between predicted and experimental data. UAE increased the astaxanthin extraction (up to 6.2%) and antioxidant activity (FRAP and DPPHsc up to 13.3 and 9.24%, respectively).
Table 5.
Predicted and experimental values of the responses at optimum conditions for ultrasound- assisted and conventional extraction methods.
| Extraction method | Astaxanthin content |
DPPHsc |
FRAP |
|---|---|---|---|
| (µg/g) | (%) | (µmol Fe2+/kg) | |
| Solvent extraction (petroleum ether/acetone/water Solvent; 15:75:10) | |||
| Experimental values1 | 48.47 ± 0.35b* | 67.64 ± 0.71b | 1504.90 ± 16.39b |
| Ultrasound-assisted extraction (UAE) | |||
| Optimized values2 | 51.47a | 73.89a | 1704.71a |
| Experimental values 1 | 50.78 ± 0.15a | 72.0 ± 0.48a | 1689.90 ± 17.85a |
The data within a column with the same letters are not significantly different at p < 0.05.
Mean ± standard deviation of triplicate determinations from experiments.
Predicted using response surface quadratic model.
There are differing opinions in the literature on the use of UAE for astaxanthin extraction. Tsiaka et al. [17] reported improvement with extraction efficiency of carotenoid compounds from shrimp (Aristeus antennatus) with UAE and identified the optimal extraction conditions as follows: ultrasound exposure time: 5 min, ultrasound power: 600 w; mixing ratio of 1: 20 ml/g with acetone solvent. Also, they reported that UAE method is a faster, easier and more reproducible than conventional extraction methods. On the other hand, Zhao et al. [19] observed a negative effect of this process on the extraction of astaxanthin, which caused the discoloration of astaxanthin.
Singh et al. [49] investigated the extraction of zeaxanthin and β-carotene from green microalgae. They reported results that under optimal extraction conditions (mixing ratio: 67.38 µl of acetone solvent per mg of sample, amplitude of sound: 27.82%, pulsation time: 19.7 s, extraction time:13.48 min) more zeaxanthin (11.2 mg/g) and beta-carotene (4.98 mg/g) were extracted. Jaeschke et al. [50] reported the highest carotenoid recycling efficiency (80%) from microalgae (Heterochlorella Luteoviridis) using UAE (amplitude of sound: 50%; 30° C with ethanol 75%). Also, they reported that with increasing sound intensity by up to 100%, the extraction efficiency decreases by about 59%.
4. Conclusions
The findings of this study revealed that a significant amount of the valuable pigment astaxanthin could be extracted from Green tiger shrimp shells, a waste resource. The extraction of astaxanthin and its antioxidant properties improved when the proper solvent with an appropriate polarity was used. The Box-Behnken design was an effective statistical method for optimizing astaxanthin extraction conditions from shrimp shell. The extraction rate of astaxanthin and its antioxidant activity were optimized by employing proper combinations of ultrasound amplitude, temperature, and extraction time using RSM. UAE can be effectively used as a green extraction method to extract astaxanthin pigment with higher antioxidant activity.
CRediT authorship contribution statement
Parvin Sharayei: Conceptualization, Methodology, Resources, Investigation, Formal analysis, Funding acquisition, Supervision, Project administration, Writing - original draft, Writing - review & editing. Elham Azarpazhooh: Data curation, Validation, Software, Supervision, Project administration, Writing - original draft, Writing - review & editing. Shahin Zomorodi: Methodology, Investigation, Formal analysis. Soodabeh Einafshar: Methodology, Investigation, Formal analysis. Hosahalli S. Ramaswamy: Visualization, Data curation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Iran National Scientific Foundation (INSF) and Agricultural Engineering Research Institute (AERI) for the financial support (first author).
Contributor Information
Parvin Sharayei, Email: p.sharayei@areeo.ac.ir.
Hosahalli S. Ramaswamy, Email: hosahalli.ramaswamy@mcgill.ca.
References
- 1.Abdel-Aal E.S.M., Akhtar H., Zaheer K., Ali R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients. 2013;5(4):1169–1185. doi: 10.3390/nu5041169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon S.O., Hee-Sook J. Role of bioactive food components in diabetes prevention: effects on beta-cell function and preservation. Nutrition Metabolic Insights. 2014;7:51–59. doi: 10.4137/NMI.S13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pérez-López P., González-García S., Jeffryes C., Agathos S.N., McHugh E., Walsh D., Moreira M.T. Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: from lab to pilot scale. J. Cleaner Prod. 2014;64:332–344. [Google Scholar]
- 4.Shimidzu N., Goto M., Miki W. Carotenoids as singlet oxygen quenchers in marine organisms. Fish. Sci. 1996;62:134–137. [Google Scholar]
- 5.Ahmadi M.R., Bazyar A.A., Safi S., Ytrestøyl T., Bjerkeng B. Effect of dietary astaxanthin supplementation on reproductive characteristics of rinbow trout (Oncorhynchus mykiss) J. Appl. Ichthyol. 2006;22:388–394. [Google Scholar]
- 6.Delgado-Vargas F., Paredes-Lopez O. FL, CRC Press; Boca Raton: 2003. Natural colorants for food and nutraceutical uses. [Google Scholar]
- 7.Gopakumar K., Nair M.R. Lipid composition of five species of Indian prawn. J. Sci. Food Agric. 1975;26:319–325. doi: 10.1002/jsfa.2740260312. [DOI] [PubMed] [Google Scholar]
- 8.Yanar Y., Çelik M., Yanar M. Seasonal changes in total carotenoid contents of wild marine shrimps (Penaeus semisulcatus and Metapenaeus monoceros) inhabiting the eastern Mediterranean. Food Chem. 2004;88(2):267–269. [Google Scholar]
- 9.Hooshmand H., Shabanpour B., Moosavi-Nasab M., Golmakani M.T. Optimization of carotenoids extraction from blue crab (Portunus pelagicus) and shrimp (Penaeus semisulcatus) wastes using organic solvents and vegetable oils. J. Food Process. Preserv. 2017:00:e13171. [Google Scholar]
- 10.Ishida B.K., Chapman M.H. Carotenoid extraction from plants using a novel, environmentally friendly solvent. J. Agric. Food Chem. 2008;57:1051–1059. doi: 10.1021/jf8026292. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Camargo A.P., Martinez-Correa H.A., Paviani L.C., Cabral F.A. Supercritical co2 extraction of lipids and astaxanthin from Brazilian redspotted shrimp waste. J. Supercrit. Fluids. 2011;56:164–173. [Google Scholar]
- 12.Gimeno M., Ramírez-Hernández J.Y., Mártinez-Ibarra C., Pacheco N., García-Arrazola R., Bárzana E., Shirai K. One-solvent extraction of astaxanthin from lactic acid fermented shrimp wastes. J. Agric. Food. Chem. 2007;55:10345–10350. doi: 10.1021/jf071469h. [DOI] [PubMed] [Google Scholar]
- 13.Meyers S.P., Bligh D. Characterization of astaxanthin pigments from heat-processed crawfish waste. J. Agric. Food. Chem. 1981;29:505–508. doi: 10.1021/jf00105a017. [DOI] [PubMed] [Google Scholar]
- 14.Sachindra N.M., Mahendrakar N.S. Process optimization for extraction of carotenoids from shrimp waste with vegetable oils. Bioresour. Technol. 2005;96:1195–1200. doi: 10.1016/j.biortech.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Brendler T., Williamson E.M. Astaxanthin how much is too much? a safety review. Phytother. Res. 2019;33:3090–3311. doi: 10.1002/ptr.6514. [DOI] [PubMed] [Google Scholar]
- 16.Vinatoru M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001;8:303–313. doi: 10.1016/s1350-4177(01)00071-2. [DOI] [PubMed] [Google Scholar]
- 17.Tsiaka T., Zoumpoulakis P., Sinanoglou V., Makris C., Heropoulos G., Calokerinos A. Response surface methodology toward the optimization of high-energy carotenoid extraction from Aristeus antennatus shrimp. Anal. Chim. Acta. 2015;877:100–110. doi: 10.1016/j.aca.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 18.Khoo K.S., Chew K.W., Yew G.Y., Manickam S., Ooi C.W., Show P.L. Integrated ultrasound-assisted liquid biphasic flotation for efficient extraction of astaxanthin from Haematococcus pluvialis. Ultrason. Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105052. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L., Zhao G., Chen F., Wang Z., Wu J., Hu X. Different effects of microwave and ultrasound on the stability of (all-E)-astaxanthin. J. Agric. Food Chem. 2006;54(21):8346–8351. doi: 10.1021/jf061876d. [DOI] [PubMed] [Google Scholar]
- 20.Chemat F., Rombaut N., Meullemiestre A., Turk M., Perino S., Fabiano-Tixier A.S., Abert-Vian M. Review of green food processing techniques.Preservation, transformation, and extraction. Innovative Food Sci. Emerg. Technol. 2017;41:357–377. [Google Scholar]
- 21.Sharayei P., Azarpazhooh E., Zomorodi S.h., Ramaswamy H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT- Food Science and Technology. 2019;101:342–352. [Google Scholar]
- 22.AOAC, Official Methods of Analysis. Association of Official Analytical Chemists, Washington. DC, (2006).
- 23.Ramadan M.F., Kroh L.W., Morsel J.T. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.), and niger (Guizotia abyssinica Cass.) crude seed oils and oil fractions. J. Agric. Food. Chem. 2003;51(24):6961–6969. doi: 10.1021/jf0346713. [DOI] [PubMed] [Google Scholar]
- 24.Benzie I.F., Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 25.Handa S.S., Singh Khanuja S.P., Longo G., Rakesh D.D. International center for science and high technology. ISC UNIDO. 2008. Extraction technologies for medicinal and aromatic plants; pp. 21–54. [Google Scholar]
- 26.Delgado-Vargas F., Jimenez A.R., Paredes-Lopez O. Natural pigments: Carotenoids, anthocyanins, and betalains - Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000;40(3):173–289. doi: 10.1080/10408690091189257. [DOI] [PubMed] [Google Scholar]
- 27.E. De Ritter, A.E. Purcell,.Carotenoid analytical methods. In carotenoids as colorants and vitamin a precursors; J. C . Bauern- feind, Ed.; Academic Press, London (1981) 815-923.
- 28.Sachindra N.M., Bhaskar N., Mahendrakar N.S. Recovery of carotenoids from shrimp waste in organic solvents. Waste Manage. 2006;26:1092–1098. doi: 10.1016/j.wasman.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Saini R.K., Keum Y.S. Carotenoid extraction methods: a review of recent developments. Food Chem. 2017;200:81–86. doi: 10.1016/j.foodchem.2017.07.099. [DOI] [PubMed] [Google Scholar]
- 30.Kishimoto Y., Tani M., Uto-Kondo H., Iizuka M., Saita E., Sone H., Kurata H., Kondo K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur. J. Nutr. 2010;49:119–126. doi: 10.1007/s00394-009-0056-4. [DOI] [PubMed] [Google Scholar]
- 31.Goto S., Kogure K., Abe K., Kimata Y., Kitahama K., Yamashita E., Terada H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. BBA. 2001;1512:251–258. doi: 10.1016/s0005-2736(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 32.Yan F., Fan K., He J., Gao M. Ultrasonic-assisted solvent extraction of carotenoids from rapeseed meal: optimization using response surface methodology. J. Food Qual. 2016;38:377–386. [Google Scholar]
- 33.Ye J., Feng L., Xiong J., Xiong Y. Ultrasound-assisted extraction of corn carotenoids in ethano. Int. J. Food Sci. Technol. 2011;46:2131–2136. [Google Scholar]
- 34.Roselló-Soto E., Koubaa M., Moubarik A., Lopes R.P., Saraiva J.A., Boussetta N., Barba F.J. Emerging opportunities for the effective valorization of wastes and byproducts generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015;45(2):296–310. [Google Scholar]
- 35.Kusters K.A., Pratsinis S.E., Thoma S.G., Smith D.M. Energy—size reduction laws for ultrasonic fragmentation. Powder Technol. 1994;80(3):253–263. [Google Scholar]
- 36.Xu D.P., Zhou Y., Zheng J., Li S., Li A.N., Li H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from the flower of Jatropha integerrima by response surface methodology. Molecules. 2016;21:18. doi: 10.3390/molecules21010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dezhkunov N.V., Leighton T.G. Study into correlation between the ultrasonic capillary effect and sonoluminescence. J. Eng. Phys. Thermophys. 2004;77(1):53–61. [Google Scholar]
- 38.Entezari M.H., Kruus P. Effect of frequency on sonochemical reactions II. Temperature and intensity effects. Ultrason. Sonochem. 1996;3:19–24. doi: 10.1016/s1350-4177(96)00016-8. [DOI] [PubMed] [Google Scholar]
- 39.Lou Z., Wang H., Zhang M., Wang Z. Improved extraction of oil from chickpea under ultrasound in a dynamic system. J. Food Eng. 2010;98:13–18. [Google Scholar]
- 40.Xu Y., Pan S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.) Ultrason. Sonochem. 2013;20(4):1026–1032. doi: 10.1016/j.ultsonch.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Hossain M.B., Brunton N.P., Patras A., Tiwari B., O’Donnell C., Martin-Diana A.B., Barry-Ryan C. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram (Origanum majorana L.) using response surface methodology. Ultrason. Sonochem. 2012;19(3):582–590. doi: 10.1016/j.ultsonch.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Carrió E., Vallès J. Ethnobotany of medicinal plants used in Eastern Mallorca (Balearic Islands, Mediteraneean Sea) J. Ethnopharmacol. 2012;141:1021–1040. doi: 10.1016/j.jep.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 43.Sahin S., Samlı R. Optimization of olive leaf extract obtained by ultrasound - assisted extraction with response surface methodology. Ultrason. Sonochem. 2013;20(1):595–602. doi: 10.1016/j.ultsonch.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 44.Dahmoune F., Remini H., Dairi S., Aoun O., Moussi K., Bouaoudia-Madi N., Adjeroud N., Kadri N., Lefsih Kh, Boughani L., Mouni L., Nayak B., Madani Kh. Ultrasound assisted extraction of phenolic compounds from P.lentiscus L. leaves: Comparative study of artificial neural network (ANN) versus degree of experiment for prediction ability of phenoliccompounds recovery. Ind. Crops Prod. 2015;77:251–261. [Google Scholar]
- 45.Wang L., Yang B., Du X., Yi C. Optimisation of supercritical fluid extraction of flavonoids from Pueraria lobata. Food Chem. 2008;108(2):737–741. doi: 10.1016/j.foodchem.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 46.Galasso C., Orefice I., Pellone P., Cirino P., Miele R., Ianora A., Brunet C., Sansone C. On the neuroprotective role of astaxanthin: new perspectives? Mar. Drugs. 2018;16:247. doi: 10.3390/md16080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X., Osawa T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys. Res. 2007;357:187–193. doi: 10.1016/j.bbrc.2007.03.120. [DOI] [PubMed] [Google Scholar]
- 48.Shahidi F., Wanasundara P.K. Methods for measuring oidative rancidity. In: Akoh C.C., Min D.B., editors. Food Lipids Chemistry, Nutrition and Biotechnology. 3nd ed. Marcel Dekker; New York: 2008. [Google Scholar]
- 49.Singh D., Barrow C.J., Mathur A.S., Tuli D.K., Puri M. Optimization of zeaxanthin and β-carotene extraction from Chlorella saccharophila isolated from New Zealand marine waters. Biocatalysis Agric. Biotechnol. 2015;4(2):166–173. [Google Scholar]
- 50.Jaeschke D.P., Rech R., Marczak L.D.F., Mercali G.D. Ultrasound as analternative technology to extract carotenoids and lipids from Heterochlorella luteoviridis. Bioresour. Technol. 2017;224:753–757. doi: 10.1016/j.biortech.2016.11.107. [DOI] [PubMed] [Google Scholar]