Abstract
Background
while the increased risk of major depressive disorder (MDD) in offspring of depressed parents is one of the best-replicated findings in psychiatry, their long-term outcomes are less well known. The clinical outcomes of biological offspring of depressed (high-risk) and not depressed (low-risk) parents who have been directly interviewed over the years are presented.
Methods
a longitudinal retrospective cohort study began in 1982, and 276 biological offspring of moderately-to-severely depressed or non-depressed parents from the same community were followed up to 38 years. Rates of psychiatric disorders for offspring were collected by clinically trained interviewers. Final diagnoses were made by M.D. or Ph.D. clinicians. Mortality and cause of death were obtained from relatives and registries.
Findings
high- compared to low-risk offspring continue to have about a three-fold increased risk of MDD, increased rates of anxiety disorder, substance dependence, and poorer functioning over the life course. Adolescence and early adulthood remain prime age of first onsets. Within high-risk group only, the death rate due to unnatural causes, suicides and overdose was 4·97/100 in the offspring and 5·36/100 in their parents. This subsample of White, lower-educated, often unemployed persons, who died by unnatural causes are similar demographically to those described as having a recent increase in ‘deaths of despair’.
Interpretation
family history of MDD continues to be a powerful predictor of clinical course and mortality and should be probed in clinical visits, especially in youth when depression usually first appears.
Research in context.
Evidence before this study
While the increased risk of major depression (MDD) in the offspring of depressed parents is one of the best replicated finding in psychiatry, long-term outcomes are less well known.
Added value of this study
A long term detailed clinical follow up of 276 biological offspring of depressed (high risk) vs. not depressed (low risk) was carried out up to 38 years (1982–2020). The final diagnoses were made by clinically trained psychiatrists or psychologists based on an all-available information (interviews and narrative summaries) blind to the parents’ clinical status. The high as compared to low-risk offspring had a threefold increased risk of MDD, an increased risk of anxiety disorder, substance dependence, and poor functioning over their life course, as well as increased rates of unnatural deaths (suicide, overdoses).
Implications of all the available evidence
Family history of MDD is a powerful predictor of clinical course of psychiatric illness in the offspring and mortality and should be probed in clinical care. Depressed patients with a family history of depression should be actively treated and followed.
Alt-text: Unlabelled box
1. Introduction
In 1980, we began planning a study of the offspring of depressed and non-depressed parents. The rationale was several-fold. The National Institute of Mental Health (NIMH), a few years before, held a conference to critique the concept of childhood depression. While clinicians reported depression in children and adolescents, and even treated them with tricyclic antidepressants, many argued that depressive symptoms in youth were “merely transitory developmental phenomena that dissipated as a function of time” [1]. They concluded that there were no relevant epidemiologic research, valid methods for assessing diagnoses, or longitudinal studies. For youth, “depression is based largely on surmise” [1]. Around the same time, the first population studies using clinical diagnoses were being completed [2]. These studies clearly showed that the age of first onset for depression was frequently in adolescence, but also occurred before puberty. Pilot work leading to these studies showed that the first onset of depression in menopause, which had previously been thought to be the prime onset age, was uncommon [3]. It was also becoming clear that depression was highly familial, although the mechanism of transmission was not, and still is not, fully understood [4]. We were interested in learning about the early signs of depression and its long-term clinical course and morbidity. It was against this background; we designed a study of the offspring of depressed parents.
This paper reports the most recent clinical outcomes of the offspring followed up to 38 years. There are now numerous excellent studies showing the risk of offspring with parental depression. Some studies are cross-sectional at a specific age; [[5], [6], [7]] others have large samples with diagnosis retrieved from birth and national registers, [8], or focus on mortality, and medical conditions [9]. Other studies are longitudinal, but for shorter periods [[10], [11], [12], [13]] or are even longer, but do not focus on parental depression [14] or study on mothers with prenatal depression [15]. The increased risk of depression and other problems in the offspring of depressed parents is one of the best-replicated findings in clinical psychiatry [16]. This paper adds to the literature by providing data on the course, morbidity, and mortality from the longest study of a well-characterized and directly interviewed sample of both high and low-risk offspring and their parents.
2. Methods
The methods for this follow-up have been fully described in the previous follow-ups. [[17], [18], [19], [20], [21], [22]]. Probands (generation 1 = G1) with moderate to severe major depressive disorder were selected from outpatient specialty settings. Non-depressed probands were selected from an epidemiologic sample from the same community. The procedures were kept similar across the waves, with few exceptions, to avoid introducing method bias. The subjects were all European Caucasian to reduce heterogeneity for genetic studies, as was the custom then.
The study began in 1982, and the latest interviews were completed in 2020. There were seven waves of interviews: at baseline and 2, 10, 20, 25, 30, and 38 years. The study after 20 years had smaller samples as the purpose was recruitment for MRI and EEG studies. In this paper, we included all 276 offspring from 91 families who entered the study. All interview waves were approved by the institutional review board at New York State Psychiatric Institute/Columbia University. Written informed consent was obtained from adults, and from parents with assent from minors. All co-authors had access to the data.
2.1. Assessments
The diagnostic interview across all waves was the Schedule for Affective Disorders and Schizophrenia-Lifetime Version (SADS-L) [23] for adults and the child version Schedule for Affective Disorders and Schizophrenia for School-Age Children (Kiddie-SADS-E) [24]. DSM diagnoses were updated as criteria were modified. The assessments were administered by trained doctoral- or master's-level mental health professionals who were blind to the parents’ clinical status and to previous history information. Treatment data at each wave were collected, and a global functioning score was assigned at each wave and a mean score obtained across all waves using the Global Assessment Scale (GAS) [25]. Consistent with the literature and prior reports [21,22] individuals with an average GAS score ≤70 across all assessments were defined as having impairment. Final diagnoses of all generations were made using the best-estimate procedure by an M.D. or Ph.D. clinician not involved in the interview and who were blind to the offspring risk [26].
Information on mortality was obtained by reports from one or more relatives and confirmed by the Social Security Death Master File (https://www.ssdmf.com) and/or an online obituary search engine (tributes.com, genealogybank.com, and/or legacy.com). Cause of death was obtained primarily from multiple family members, and to confirm from multiple other sources, including death certificates, newspaper articles, and national death index. Cause of death could not be obtained from any source on four parents and two offspring, all in the high-risk group. These were classified as death by natural causes. Information on whether alive or not could not be found on 15 (12 high-risk and 3 low-risk offspring) of the 276 offspring; these were excluded from the mortality analyses. Due to the sample size, these were considered exploratory.
2.2. Statistical analysis
Comparisons of offspring characteristics by parental depression status were made by testing for differences in mean values for continuous outcomes by means of t-tests, ordinal outcomes by Kruskal Wallis tests, and categorical outcomes by chi-square tests. The effect of parental depression on offspring outcomes was estimated using linear regression when outcomes were continuous and logistic regression for binary outcomes. Age and sex of offspring were considered a priori to be confounding variables and were included in all models. These analyses were conducted within the framework of generalized estimating equations [27] using the GENMOD procedure in the SAS software package [28] to estimate parameters while adjusting for potential non-independence of outcomes in members of the same families. Survival analysis techniques were used to estimate (1) the age-specific incidence rates of psychiatric disorder in 10-year intervals as well as cumulative lifetime rates and failure curves of psychiatric disorder using life-table methods. Survival curves were compared across gender and by parental depression status using the log-rank test. Cox proportional hazards regression models [29] were used to estimate relative risk of disorder by parental depression status, while controlling for potential confounding variables. The analysis was adjusted to take into account the clustered nature of the data due to potential non-independence of outcomes of offspring in the same family. This approach uses a robust sandwich covariance matrix estimate [30] to account for intra-cluster dependence available in the PHREG procedure.
2.3. Role of the funding source
The funding source had no role in the design, data collection, analysis, or interpretation, and did not have to approve publication.
3. Results
3.1. Characteristics of offspring
At their last assessment, the offspring of depressed (high risk) and non-depressed (low risk) probands (G1) did not differ by gender (53·6% females), mean age at first interview (19·6 years, SD=7·6), mean age at last interview (43·2 years, SD=14·1), mean number of interviews (4·3, SD=1·8), or median number of years in the study (29 years, SD=12·7).
3.2. Cumulative and age-specific rates
Offspring of depressed vs. non-depressed parents had nearly a three-fold increased risk of MDD (Table 1). When we added impairment criteria, the absolute rates of MDD were lower in both groups; however, the relative risks between the high- and low-risk groups remained consistent and robust (Table S1). Bipolar disorder, anxiety disorders (notably panic disorder and GAD), and alcohol dependence were also increased in the high-risk group. There was over a four-fold increase of drug dependence.
Table 1.
Cumulative Rates of Psychiatric Disorders in 276 Offspring (G2) by Parental (G1) Depression Status.
| CUMULATIVE RATES | |||||||
|---|---|---|---|---|---|---|---|
| Diagnosis in Offspring | Offspring Having One or More Parents with MDD (N = 193) | Offspring Having No Parent with MDD (N = 83) | Analysis | ||||
| Lifetime | N | % | N | % | Relative Riska | 95% CI | p-valuea |
| Any mood disorder | 144 | 74.6 | 37 | 44.6 | 2.55 | 1.77– 3.67 | < 0.001 |
| MDD | 125 | 64.8 | 27 | 32.5 | 2.96 | 1.79 – 4.88 | < 0.001 |
| Bipolar I or II | 34 | 17.6 | 7 | 8.4 | 2.33 | 1.03 – 5.11 | 0.034 |
| Dysthymia | 62 | 32.1 | 20 | 24.1 | 1.49 | 0.9 –2.47 | 0.116 |
| Any anxiety disorder | 102 | 52.8 | 23 | 27.7 | 2.35 | 1.49–3.77 | < 0.001 |
| Phobia (social and/or specific) | 56 | 29.0 | 16 | 19.3 | 1.65 | 0.92 – 2.96 | 0.094 |
| Panic disorder | 34 | 17.6 | 4 | 4.8 | 4.38 | 1.38– 13.88 | 0.012 |
| OCD | 13 | 6.7 | 1 | 1.2 | 6.14 | 0.83 – 45.57 | 0.076 |
| GAD | 29 | 15.0 | 5 | 6.0 | 2.78 | 1.07 – 6.62 | 0.021 |
| Any substance abuse | 50 | 25.9 | 18 | 21.7 | 1.29 | 0.79 - 2.13 | 0.308 |
| Any substance dependence | 46 | 23.8 | 10 | 12.0 | 2.32 | 1.33 - 4.03 | 0.003 |
| Alcohol dependence | 34 | 17.6 | 7 | 8.4 | 2.35 | 1.12 – 4.92 | 0.024 |
| Drug dependence | 28 | 14.5 | 3 | 3.6 | 4.59 | 1.53 – 13.8 | 0.007 |
| Schizophrenia | 2 | 1.0 | 0 | 0.0 | – | – | – |
| Any of the Above Disorders | 162 | 83.9 | 51 | 61.4 | 2.06 | 1.52 - 2.79 | < 0.001 |
Adjusted for gender, correlations between family members and age at the last interview of the offspring using Cox proportional hazards regression models.
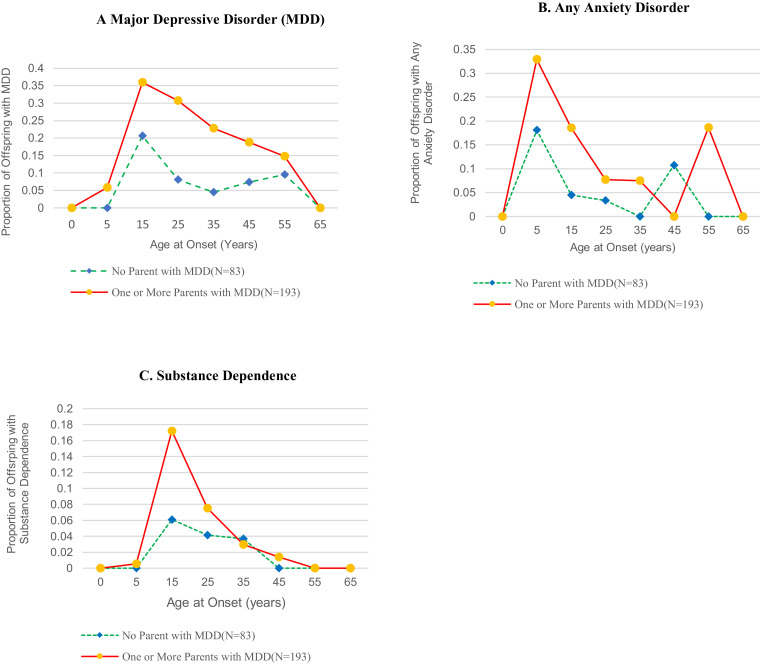
Fig. 1a shows that the peak incidence for MDD in both the high- and low-risk groups remained in adolescence and early adulthood (age 15–25years) and gradually declined after that. A slight increase was detected in the onset of major depression in women at around age 45–55-years in the low-risk group. The rates of first onsets between the low and high risk begin to converge because there are few unaffected in the high-risk group since most of their onsets were early. Due to the small numbers, we did not test for significance.
Fig. 1.
Age-Specific Rates of Onset of Psychiatric disorder Over 38 Years in Offspring (N = 276) of Depressed and Nondepressed Parents.
We compared risk groups on depression onsets between 20– and 40 years and 40–60 yrs. In each analysis, we excluded offspring who had already had an onset of depression before the age range tested. Fig. S2 shows a five-fold increase in the cumulative risk of onset between ages 20– and 40 years in the high-risk offspring (50%) compared to low-risk offspring (10%), p = 0·0001. The cumulative risk of onset between ages 40– and 60 years is 30% in the high-risk group while the cumulative risk of onset increases to 20% in the low-risk group, thus narrowing the risk between the two groups. The likelihood of onset in the high-risk group is now only 1·5 times the cumulative risk of onset in the low-risk group and is no longer statistically significant (p = 0·162). These patterns are also reflected in the age-specific rates shown in Fig. 1a.
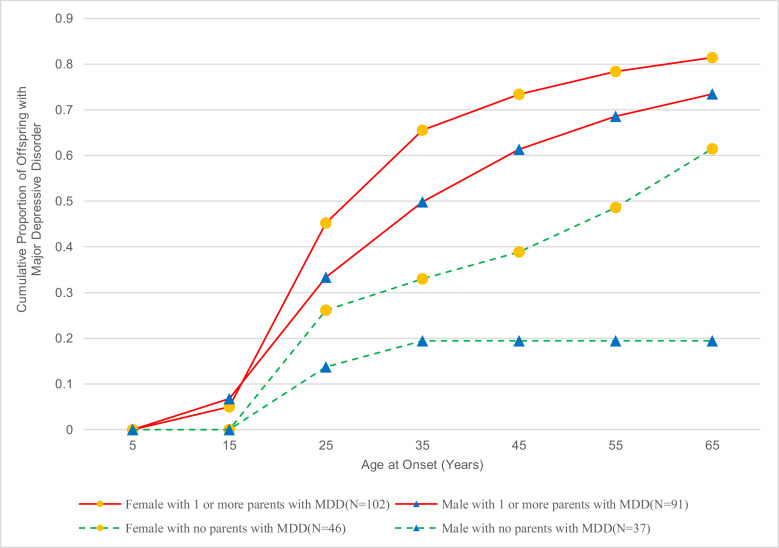
The cumulative rates of MDD by the age of onset and gender continue to be significantly higher in women than men in both risk groups (log-rank chi-square = 5·78, df =1, p = 0·016) (Fig. 2). However, among the high risk, the difference between cumulative rates of depression among women compared to men was a trend (log-rank chi-square =3·0795, df =1, p = 0·079). Among the low-risk offspring, the cumulative rates of depression were significantly greater in females than males, with a greater increase among women by age 65 (log-rank chi-square =4·697, df =1, p = 0·0302). There are several factors that may account for this. The rates of MDD in low-risk males are low. There were no first onsets after age 35 years. The death rate is higher in men in both risk groups. The other groups have onsets across their life span, although most occur by age 35. There is a slight increase in first onsets of major depression in low-risk women at a later age. As found in the 20 and 30-year follow-ups, there was an early age of onset of anxiety, mainly before puberty and in early adolescence and predominantly in the high-risk group offspring (Fig. 1b). There was no evidence of a peak of anxiety disorder onsets later, after age 25 in the high-risk group until age 55 and an increase in the low-risk group after age 45. Most of the participants who reported later first onset of anxiety disorders had evidence of mild anxiety symptoms such as social or simple phobia or separation anxiety as children or adolescents, which did not meet diagnostic criteria until interviews in adulthood. The peak onset of substance dependence was in adolescence and young adulthood, higher in the high-risk group at that period and (not shown), largely in males 32% as compared to females 18% (X2=7·05, df =1, p = 0·008) (Fig. 1c).
Fig. 2.
Cumulative Rates of Major Depressive Disorder (MDD) in Female and Male Offspring (N = 276) of Depressed and Nondepressed Parents.
3.3. Offspring sociodemographic, treatment, and impairment
Offspring of depressed parents were more likely to be divorced or separated and have fewer children (Table 2). No differences were detected in education, employment status, or income. The high-risk group had significantly more and longer treatment duration for emotional problems and more continuous treatment. They also have been functioning more poorly over their lifetime, as measured by clinicians on the Global Adjustment Scale (p < 0·0001) [3].
Table 2.
Demographic Characteristics, Mental Health Treatment, and Overall Functioning of 276 Offspring (G2) at Last Interview by Parental (G1) Depression Status.
| CUMULATIVE RATES | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Offspring Having One or More Parents with MDD (N = 193) | Offspring Having No Parent with MDD (N = 83) | Group Comparison | ||||
| N | % | N | % | x2 | df | P | |
| Demographic characteristica | |||||||
| Marital status | 156 | 72 | 12.93 | 2 | 0.002 | ||
| Ever separated or divorced | 42 | 26.9 | 12 | 16.7 | |||
| Currently married or widowed | 76 | 48.7 | 53 | 73.6 | |||
| Never married | 38 | 24.4 | 7 | 9.7 | |||
| Mean | SD | Mean | SD | x2 | df | P | |
| Number of children | 1.4 | 1.29 | 1.8 | 1.43 | 4.70b | 1 | 0.030 |
| N | % | N | % | x2 | df | P | |
| Education | 146 | 70 | 1.06 | 3 | 0.786 | ||
| High school diploma or less | 54 | 37.0 | 28 | 40.0 | |||
| Less than 4 years of college | 33 | 22.6 | 16 | 22.9 | |||
| Undergraduate degree | 38 | 26.0 | 14 | 20.0 | |||
| Graduate degree | 21 | 14.4 | 12 | 17.1 | |||
| Employment status | 155 | 71 | 2.39 | 1 | 0.122 | ||
| Full-time employment | 102 | 65.8 | 54 | 76.1 | |||
| Part-time or no employment | 53 | 34.2 | 17 | 23.9 | |||
| Mean | SD | Mean | SD | x2 | df | P | |
| Income, $ | |||||||
| Individual | 48,379.3 | 28,339 | 52,769.2 | 25,465 | 1.462 | 1 | 0.228 |
| Household | 70,251.8 | 25,540 | 73,939.4 | 21,988 | 0.202 | 1 | 0.651 |
| N | % | N | % | x2 | df | P | |
| Mental health treatment | |||||||
| Outpatient | 22.422 | 1 | <0.001 | ||||
| None | 53 | 27.5 | 42 | 50.6 | |||
| Brief Period | 40 | 20.7 | 20 | 24.1 | |||
| Continuous for at least 6 mos. | 26 | 13.5 | 12 | 14.5 | |||
| Continuous for several years | 74 | 38.3 | 9 | 10.8 | |||
| Any outpatient treatment | 140 | 72.5 | 41 | 49.4 | 13.77 | 1 | <0.001 |
| Any hospitalization | 23 | 11.9 | 3 | 3.6 | 4.69 | 1 | 0.030 |
| Any psychotropic drug use | 103 | 53.4 | 32 | 38.6 | 5.10 | 1 | 0.024 |
| Any of the above | 147 | 76.2 | 49 | 59.0 | 8.27 | 1 | 0.004 |
| Mean | SD | Mean | SD | F | df | p | |
|
Overall functioning |
|||||||
| Global Assessment Scale score (GAS)c | 73.53 | 12.7 | 81.33 | 8.9 | 25.84 | 1, 274 | <0.001 |
All demographic characteristics are strictly current except for marital status, whose first category (“ever separated or divorced”) includes historical information.
Data were determined using the Kruskal-Wallis test.
Mean of subjects’ average GAS scores; each subject's average GAS score was calculated as the mean of all GAS scores given by the clinical best-estimator to the subject at each wave in which the subject was interviewed; higher scores denote better overall functioning.
3.4. Medical conditions
As in the 30-year follow-up, the rates of medical conditions did not differ by risk group (data not shown). At the 20-year follow-up, we had reported higher rates of cardiovascular and neuromuscular disorder in the high-risk group [20]. The lack of difference in rates of medical conditions at both later follow ups is likely due to the low-risk group developing more illnesses as they aged.
3.5. Mortality
Detailed data by case are not presented in order to preserve confidentiality. Due to small number of deaths, these findings are considered exploratory. We first explored the mortality rate in the original parent generation (Table 3). There were no differences by age at death: high-risk parents: 73·1 years; low-risk parents: 72·6 years. The mortality rate was significantly higher in the high-risk group, 58·9%, compared to the low-risk group, 34·2%. The major differences were in the cause of death: 53·5% high-risk and 34·2% low risk died of natural causes, whereas 5·36% at the high-risk and none at low-risk parents died of unnatural causes. The unnatural deaths were suicide and drug overdose. One death in the high-risk parents was due to COVID-19, which we classified as natural.
Table 3.
Mortality Rates, Natural and Unnatural Causes of Death by Risk Status in the Parent (G1) and Offspring (G2).
| PARENT (G1) | ||||||
|---|---|---|---|---|---|---|
| High Risk (N = 56) | Low Risk (N = 35) | Group Comparison | ||||
| Mean | Std.Err | Mean | Std.Err | T | p-value | |
| Age at Death | 73.15 | 2.3 | 72.67 | 3.9 | 0.11 | 0.914 |
| N | % | N | % | X2 | p-value | |
| Overall Mortality Rate | 33 | 58.93 | 12 | 34.29 | 5.23 | 0.022 |
| Natural Causes | 30 | 53.57 | 12 | 34.29 | 3.22 | 0.073 |
| Unnatural Causes | 3 | 5.36 | 0 | 0 | 0.282a | |
| OFFSPRING (G2)b | ||||||
| High Risk (N = 181) |
Low Risk (N = 80) |
Group Comparison |
||||
|---|---|---|---|---|---|---|
| Mean | Std.Err | Mean | Std.Err | T | p-value | |
| Age at Death | 46.1 | 3.3 | 51.0 | 4.9 | 0.061 | 0.549 |
| N | % | N | % | X2 | p-value | |
| Overall Mortality Rate | 16 | 8.84 | 3 | 3.75 | 2.13 | 0.145 |
| Natural Causes | 7 | 3.87 | 3 | 3.75 | 0.002 | 0.964 |
| Unnatural Causes | 9 | 4.97 | 0 | 0 | 0.061a | |
Data were analyzed using Fisher's exact test.
Information on whether alive or dead could not be found on 12 high risk and 3 low risk offspring of the 276 offspring and have been excluded from the analysis.
The age of death was slightly younger (46·1 years vs. 51·0 years), and the overall mortality was higher, 8·8% in high-risk offspring and 3·75% in low risk but did not reach significance. However, 4·97% of the offspring in the high-risk group and none in the low-risk group had a death from unnatural causes. These included four suicides, four drug overdoses, where suicidal intent could not be ascertained, and one due to a car accident. While mortality rates in the high-risk group were consistently higher than in the low-risk group for both generations G1 and G2, the differences did not always reach statistical significance because of the sample size.
4. Discussion
This additional 8 years of follow-up continues to show that offspring of depressed parents are at significantly increased risk of MDD, regardless of how strictly or broadly depression is defined [22]. The risk continues through their lifetime and is associated with increased risk for other psychiatric disorders, poorer functioning, more psychiatric treatment. There was suggestion of an increase in first onset depression in the low-risk group after age 45; however, adolescence and early adulthood remain the prime period for first-onset depression. The early age of onset of MDD and the increased rates of depression in offspring with a family history have now been replicated in numerous epidemiologic and high-risk studies. What our study adds is the long-term continuity and the impairment in the high-risk group and the possible small increase in later first onsets in either group. Perhaps menopausal depression is not a myth but also not of high prevalence. The increase of first onset anxiety disorder after age 45 years in the low-risk group and age 55 years in the high-risk group requires follow-up in larger samples and could be a problem of detection. All but three participants with late onset anxiety had earlier reports before puberty of possible separation anxiety not meeting diagnostic criteria and had mild symptoms of anxiety as children.
The striking new finding is the increased death rate, due to unnatural causes, including suicide or overdose, in the offspring of depressed parents and their parents, only in the high-risk group. Early warnings are evident in over four-fold risk of drug dependence. These risks continue to increase as we followed the offspring over time. Between the 30th and 38th study year, the death rates from unnatural causes in the high-risk offspring nearly doubled. There were no cases of death from unnatural causes ever detected in the low-risk offspring [22]. There are two points for discussion: the deaths from unnatural causes and a use of “Big Data" to confirm our findings.
Case and Deaton, in landmark work [31] showed marked increased rates of all-cause mortality in middle-aged White non-Hispanic men and women in the United States between 1999 and 2017. This increase was largely accounted for by increasing death rates from drug and alcohol poisoning, suicide, and chronic liver diseases in midlife. Those with no college degree had the highest increase. This change in rates reversed decades of progress in mortality and was confined to non-Hispanic Whites. Blacks and Hispanics at midlife and those aged 65 and over from every racial and ethnic group continued to see mortality rates fall. They called this increase “Deaths of Despair”. They noted the increase may be tied to economic insecurity and inequality, as the growth in median earning was slowed for individuals without a college education. Our most alarming finding is the high rates of deaths due to unnatural causes, mainly suicide and overdose, in our subsample that matches Case and Deaton's demographically. They are all White non-Hispanic. While the demographics of this sample included a range of education and employment (Table 2), the participants who died of unnatural causes with one exception for education but not employment had high school education or less and were unemployed or partially employed. Further details are not provided to protect confidentiality.
Other studies have reported on the risk of death by unnatural causes in the offspring of psychiatric parents. The Danish studies [9,32,33] used national population registers linked to psychiatric and death registers and samples of over 1 million, focusing on psychiatric inpatients and offspring. Webb et al. [32] found over five to ten-fold risk of death by unnatural causes (accidents, homicides, suicides) or in offspring up to age 25 of hospitalized psychiatrically ill parents. There was no evidence of a higher mortality rate from natural causes. The parents' specific diagnosis was not noted. A separate paper [32] reported mortality risk of offspring by parental diagnoses. They found higher mortality rates in older offspring of parents with alcohol-related disorders and neonates’ deaths in offspring of mothers with affective disorders, but not with schizophrenia. The most recent publication has over 2 million persons followed up to 30 years by 2012 and includes hospitalized parents with schizophrenia or affective disorders [9]. They found increased mortality rates in these offspring, mainly due to unnatural causes. The highest risk for all-cause mortality in offspring was associated with parental depression and not schizophrenia. Finally, a Taiwan-based study based on three nationwide datasets [34] compared 3000 offspring (< age 5) of parents with schizophrenia or affective disorders to over 25,000 children matched on maternal age, delivery date, sociodemographic and medical comorbidity. They found that the rise of overall mortality was 2·4 times higher in the offspring of psychiatrically ill parents and 8·35 times higher for unnatural deaths (accidents and homicides). Parental diagnoses were not differentiated.How do we put together these pieces on the effects of parental depression on offspring? The Taiwanese and Danish studies showed the association between serious parental psychiatric disorders and increased mortality due to unnatural causes in young offspring. Case and Deaton have shown the association between select sociodemographic characteristics (poor, less educated, White) and unnatural deaths. We have a sample of high- and low-risk parents and offspring with a subsample with similar demographic characteristics. However, we only found unnatural deaths in the offspring of depressed parents and their parents. While our sample size is modest and must be considered exploratory, the rates of unnatural deaths 5·36/100 in parents and 4·97/100 in offspring, should be viewed in the context of U.S. suicide rates 14·2/100,000 and overdose 20·7/100,000 [35]. The Case and Deaton article was written from an economist perspective. We may have identified an additional risk factor for Deaths of Despair, namely parental depression and personal history of depression in the less educated and economically challenged groups. This finding is worth exploring in a larger data set. Interestingly, the increase in Deaths of Despair were described as a U.S. problem related to economic conditions. However, recent articles from England, Wales, Scotland, and Canada describe similar trends. [[36], [37], [38]].
While our study was considered large when it was initiated, the sample is too small to be confident about several findings, as noted. Moreover, the inclusion of an all-White sample is no longer acceptable nor desirable. Except for a brief follow-up underway to determine the impact of COVID on these families, the clinical follow up of this generation has ended. There are now several large population-based datasets available for public use that include psychiatric diagnoses in racially and ethnically diverse samples. The large sample sizes limit direct interview of participants or their relatives. Since parental psychiatric diagnosis is a critical risk for the outcome in offspring, ways to determine the comparability of the history vs. the interview methods for obtaining parental diagnoses need to be considered [39].
There are other limitations to our data besides sample size, lack of sample diversity, and sample selection. There have been some changes in the components of the diagnostic criteria over time, e.g., the symptoms of hostility and irritability have had increasing emphasis in the diagnoses of depression. This addition may have increased the rates of depression in men. We tried to account for these changes using the Best Estimate procedures described. This is a limitation of a longitudinal study with intermittent follow-up. Finally, attrition is also a problem in a study of this length and is compounded by the funding of only subsamples over time. The detailed clinical interviews, independent diagnostic review by clinically trained persons, and the fact that the age of onset of most of these disorders is in youth partially resolves these issues. The major outcome was the difference between offspring at high and low risk for depression. There were no significant differences reported in confounding variables between these two groups of offspring.
A history of depression in a parent defines a group of offspring with a high probability of depression and other poor outcomes over the life course. Parental depression, should be part of standard screening tools in clinical practice, including pediatric care. Since most of the depression first occur in youth, a broader family intervention may be considered. Early identification and treatment of high-risk youth may reduce their adult impairment and perhaps save lives. This point was also noted in a recent National Academy of Medicine study [40].
Funding
This work is supported by NIMH grant R01MH-036,197 (P.I.s: Weissman, Posner).
Data sharing statement
The data reported in this paper come from a longitudinal family study with data collected at seven ‘waves’: at baseline (in calendar 1982, Year 0) and at Years 2, 10, 20, 25, 30, and 38 (calendar 2020). There were no repositories for data sharing until recently, so we could not ask for consent and initially exclude families who refused consent. Starting at Year 38, participants providing data were asked if they consented to have their study data put in a national data registry. A total of 106 offspring and 121 grandchildren gave informed consent for the data registry. This study is still in process, so numbers may vary. We do not know what data on grandchild will be available yet among the offspring who consented. Those whose data are included in this paper that is the offspring will have their de-identified data uploaded to the NIMH Data Archive (NDA), along with a data dictionary defining each field. The data will include the variables analyzed in this paper, including demographics, mental disorder diagnoses, the courses of these disorders, medical conditions, Global Assessment Scale (GAS) scores, and mortality information to the extent that the latter is judged to not compromise the deidentification of the data set. A data curation project overseen by the lead author of this paper is currently underway to harmonize the data from all seven waves of the longitudinal family study. Curation is a project to be completed sometime in 2022, after which the data described above will be in a suitable format to be uploaded to the NDA. The data stored at the NDA will be shared with researchers determined by NDA.
Declaration of Competing Interest
All authors declare that the paper was funded by an NIMH grant.
Authors AT, MJG, LP, JS, and PJW declare no competing interests.
JP- Reports research support from Takeda (formerly Shire) and Aevi Genomics, outside the submitted work, as well as consulting fees from Innovative Science.
MMW- Reports research grants in the last three years from NIMH, Brain and Behavior Foundation, Templeton Foundation and Yale. Book royalties from the Perseus Press, Oxford Press, and APA Publishing. MMW's salary is paid by NY State and endowed chair from Columbia University. MMW has also received royalties on the social adjustment scale from Multihealth Systems but receives no royalties from this study or any of her studies that includes this scale. Honorarium for giving grand rounds at Mass General Hospital and Hospital in White Plains. Travel expenses to give lectures by the World Psychiatric Association, Mass General Hospital and University of California-San Diego. MMW serves on scientific advisory boards (uncompensated) of Brain and Behavior, DBSA, and American Foundation for Suicide Prevention None of these represent a conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101000.
Appendix. Supplementary materials
References
- 1.Lefkowitz M.M., Burton N. Childhood depression: a critique of the concept. Psychol Bull. 1978;85(4):716–726. [PubMed] [Google Scholar]
- 2.Weissman M.M. Big data begin in psychiatry. JAMA Psychiatry. 2020;77(9):967–973. doi: 10.1001/jamapsychiatry.2020.0954. [DOI] [PubMed] [Google Scholar]
- 3.Weissman M.M. The myth of involutional melancholia. JAMA. 1979;242(8):742–744. [PubMed] [Google Scholar]
- 4.Kendler K.S., Ohlsson H., Sundquist J., Sundquist K. An extended Swedish national adoption study of bipolar disorder illness and cross-generational familial association with schizophrenia and major depression. JAMA Psychiatry. 2020;77(8):814–822. doi: 10.1001/jamapsychiatry.2020.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein D.N., Lewinsohn P.M., Seeley J.R., Rohde P. A family study of major depressive disorder in a community sample of adolescents. Arch Gen Psychiatry. 2001;58(1):13–20. doi: 10.1001/archpsyc.58.1.13. [DOI] [PubMed] [Google Scholar]
- 6.Garber J., Cole D. Intergenerational transmission of depression: a launch and grow model of change across adolescence. Dev Psychopathol. 2010;22(4):819–830. doi: 10.1017/S0954579410000489. [DOI] [PubMed] [Google Scholar]
- 7.Pettit J.W., Olino T.M., Roberts R.E., Seeley J.R., Lewinsohn P.M. Intergenerational transmission of internalizing problems: effects of parental and grandparental major depressive disorder on child behavior. J Clin Child Adolesc Psychol. 2008;37(3):640–650. doi: 10.1080/15374410802148129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Josefsson A., Vikström J., Bladh M., Sydsjö G. Major depressive disorder in women and risk for future generations: population-based three-generation study. BJ Psych Open. 2019;5(1):1–8. doi: 10.1192/bjo.2018.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranning A., Benros M.E., Thorup A.A., Davidsen K.A., Hjorthøj C., Nordentoft M. Morbidity and mortality in the children and young adult offspring of parents with schizophrenia or affective disorders-a nationwide register-based cohort study in 2 million individuals. Schizophr Bull. 2020;46(1):130–139. doi: 10.1093/schbul/sbz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman S.H., Rouse M.H., Connell A.M., Broth M.R., Hall C.M., Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev. 2011;14(1):1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- 11.Lieb R., Isensee B., Höfler M., Pfister H., Wittchen H.U. Parental major depression and the risk of depression and other mental disorders in offspring. Arch Gen Psychiatry. 2002;59(4):365–374. doi: 10.1001/archpsyc.59.4.365. [DOI] [PubMed] [Google Scholar]
- 12.Murray L., Arteche A., Fearon P., Halligan S., Goodyer I., Cooper P. Maternal postnatal depression and the development of depression in offspring up to 16 years of age. J Am Acad Child Adolesc Psychiatry. 2011;50(5):460–470. doi: 10.1016/j.jaac.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Hammen C., Hazel N.A., Brennan P.A., Najman J. Intergenerational transmission and continuity of stress and depression: depressed women and their offspring in 20 years of follow-up. Psychol Med. 2012;42(5):931–942. doi: 10.1017/S0033291711001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caspi A., Houts R.M., Ambler A., Danese A., Elliott M.L., Hariri A., Harrington H., Hogan S., Poulton R., Ramrakha S., Rasmussen L.J. Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the Dunedin Birth Cohort Study. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.3221. -e203221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tirumalaraju V., Suchting R., Evans J., Goetzl L., Refuerzo J., Neumann A. Risk of depression in the adolescent and adult offspring of mothers with perinatal depression: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammen C. Risk factors for depression: an autobiographical review. Annu Rev Clin Psychol. 2018;14:1–28. doi: 10.1146/annurev-clinpsy-050817-084811. [DOI] [PubMed] [Google Scholar]
- 17.Weissman M.M., Gammon G.D., John K., Merikangas K.R., Warner V., Prusoff B.A. Children of depressed parents: increased psychopathology and early onset of major depression. Arch Gen Psychiatry. 1987;44(10):847–853. doi: 10.1001/archpsyc.1987.01800220009002. [DOI] [PubMed] [Google Scholar]
- 18.Weissman M.M., Fendrich M., Warner V., Wickramaratne P. Incidence of psychiatric disorder in offspring at high and low risk for depression. J Am Acad Child Adolesc Psychiatry. 1992;31(4):640–648. doi: 10.1097/00004583-199207000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Weissman M.M., Warner V., Wickramaratne P., Moreau D., Olfson M. Offspring of depressed parents: 10 years later. Arch Gen Psychiatry. 1997;54(10):932–940. doi: 10.1001/archpsyc.1997.01830220054009. [DOI] [PubMed] [Google Scholar]
- 20.Weissman M.M., Wickramaratne P., Nomura Y., Warner V., Verdeli H., Pilowsky D.J. Families at high and low risk for depression: a 3-generation study. Arch Gen Psychiatry. 2005;62(1):29–36. doi: 10.1001/archpsyc.62.1.29. [DOI] [PubMed] [Google Scholar]
- 21.Weissman M.M., Wickramaratne P., Nomura Y., Warner V., Pilowsky D., Verdeli H. Offspring of depressed parents: 20 years later. Am J Psychiatry. 2006;163(6):1001–1008. doi: 10.1176/ajp.2006.163.6.1001. [DOI] [PubMed] [Google Scholar]
- 22.Weissman M.M., Wickramaratne P., Gameroff M.J., Warner V., Pilowsky D., Kohad R.G. Offspring of depressed parents: 30 years later. Am J Psychiatry. 2016;173(10):1024–1032. doi: 10.1176/appi.ajp.2016.15101327. [DOI] [PubMed] [Google Scholar]
- 23.Mannuzza S., Fyer A.J., Klein D.F., Endicott J. Schedule for affective disorders and schizophrenia—Lifetime version modified for the study of anxiety disorders (SADS-LA): rationale and conceptual development. J Psychiatr Res. 1986;20(4):317–325. doi: 10.1016/0022-3956(86)90034-8. [DOI] [PubMed] [Google Scholar]
- 24.Orvaschel H., Puig-Antich J., Chambers W., Tabrizi M.A., Johnson R. Retrospective assessment of prepubertal major depression with the Kiddie-SADS-E. J Am Acad Child Psychiatry. 1982;21(4):392–397. doi: 10.1016/s0002-7138(09)60944-4. [DOI] [PubMed] [Google Scholar]
- 25.Endicott J., Spitzer R.L., Fleiss J.L., Cohen J. The global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 26.Leckman J.F., Sholomskas D., Thompson D., Belanger A., Weissman M.M. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39(8):879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 27.Liang K.Y., Zeger S.L. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 28.SAS Institute Inc.; 2010. SAS software 9.3. CARY, NC. [Google Scholar]
- 29.Cox D.R. Regression models and life-tables. J R Stat Soiety: Ser B Methodol. 1972;34(2):187–202. [Google Scholar]
- 30.Lin D.Y., Wei L.J. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–1078. [Google Scholar]
- 31.Case A., Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A. 2015;112(49):15078–15083. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webb R.T., Abel K.M., Pickles A.R., Appleby L., King-Hele S.A., Mortensen P.B. Mortality risk among offspring of psychiatric inpatients: a population-based follow-up to early adulthood. Am J Psychiatry. 2006;163(12):2170–2177. doi: 10.1176/appi.ajp.163.12.2170. [DOI] [PubMed] [Google Scholar]
- 33.Webb R.T., Pickles A.R., Appleby L., Mortensen P.B., Abel K.M. Death by unnatural causes during childhood and early adulthood in offspring of psychiatric inpatients. Arch Gen Psychiatry. 2007;64(3):345–352. doi: 10.1001/archpsyc.64.3.345. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y.H., Chiou H.Y., Tang C.H., Lin H.C. Risk of death by unnatural causes during early childhood in offspring of parents with mental illness. Am J Psychiatry. 2010;167(2):198–205. doi: 10.1176/appi.ajp.2009.09070979. [DOI] [PubMed] [Google Scholar]
- 35.Hedegaard H., Curtin S.C., Warner M. National Center for Health Statistics; 2020. Increase in suicide mortality in the United States, 1999–2018. NCHS Data Brief No 362. [Google Scholar]
- 36.Koltai J., Varchetta F.M., McKee M., Stuckler D. Deaths of despair and Brexit votes: cross-local authority statistical analysis in England and Wales. Am J Public Health. 2020;110(3):401–406. doi: 10.2105/AJPH.2019.305488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allik M., Brown D., Dundas R., Leyland A.H. Deaths of despair: cause-specific mortality and socioeconomic inequalities in cause-specific mortality among young men in Scotland. Int J Equity Health. 2020;19(1):1–10. doi: 10.1186/s12939-020-01329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Probst C., Rehm J. Alcohol use, opioid overdose and socioeconomic status in Canada: a threat to life expectancy? CMAJ. 2018;190(44):E1294–E1295. doi: 10.1503/cmaj.180806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Dijk M.T., Murphy E., Posner J.E., Talati A., Weissman M.M. Association of multigenerational family history of depression with lifetime depressive and other psychiatric disorders in children: Results from the Adolescent Brain Cognitive Development (ABCD) Study. JAMA Psychiatry. 2021 doi: 10.1001/jamapsychiatry.2021.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Academies of Sciences, Engineering, and Medicine . National Academies Press; 2019. Fostering healthy mental, emotional, and behavioral development in children and youth. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.