Abstract
Background
Methamphetamine dependence is a significant global health concern for which there are no approved medications. The cysteine prodrug, N-acetylcysteine (NAC), has been found to ameliorate glutamate dysregulation in addiction, and to reduce craving for methamphetamine and other drugs. We evaluated the efficacy and safety of NAC as a pharmacotherapy for methamphetamine dependence.
Methods
A parallel double-blind randomised placebo-controlled trial of people dependent on methamphetamine recruited from Geelong, Melbourne and Wollongong, Australia, between July 2018 and December 2019. Participants were randomised to receive either 12 weeks of oral NAC (2400 mg/day) or matched placebo, delivered as a take-home medication. The primary outcome was methamphetamine use, measured in two ways: (a) change in days of use in the past 4 weeks from baseline to weeks 4, 8 and 12, assessed using the Timeline Followback; and (b) methamphetamine-positive oral fluid samples taken weekly. Analyses were intention-to-treat and based on imputed data. Secondary outcomes were craving, severity of dependence, withdrawal severity and psychiatric symptoms (depression, suicidality, hostility and psychotic symptoms). Significance levels were p < 0.025 for primary outcomes and p < 0.01 for secondary outcomes. Adverse events were compared between groups by system organ class. The study was prospectively registered, ACTRN12618000366257.
Results
Participants (N = 153; 59% male, mean [SD] age 38 [8]) were randomised to placebo (n = 77) or NAC (n = 76). Both groups had a median (IQR) of 24 (15–28) days of methamphetamine use in the 4 weeks prior to baseline. Both groups significantly reduced methamphetamine use (mean [SE] reduction of 7.3 [1.2]) days for placebo, 6.8 [1.2] for NAC) but NAC did not reduce days of methamphetamine use more than placebo (group difference of 0.5 days, 97.5% CI -3.4–4.3). There was no significant effect of NAC on methamphetamine-positive oral fluid samples (placebo 79%, NAC 76%; mean difference -2.6, 97.5% CI -12.6–7.4). NAC did not significantly reduce craving, severity of dependence, withdrawal, suicidality, depression, hostility or psychotic symptoms relative to placebo. Adverse events did not differ significantly between placebo and NAC groups.
Interpretation
These findings suggest that take-home oral NAC has no significant effect on methamphetamine use or most clinically related outcomes amongst people who are dependent on the drug.
Keywords: Substance-related disorders, Methamphetamine, N-Acetylcysteine, Randomised controlled trials, Glutamate, Mental disorders, Neuroscience, Psychiatry
Research in context.
Evidence before this study
We searched PubMed from database inception until January 6 2021 for systematic reviews on the pharmacological treatment of methamphetamine dependence using the search string: (((amphetamine[MeSH Terms]) OR (methamphetamine[MeSH Terms])) OR (amphetamine*)) OR (methamphetamine*) Filters: Meta-Analysis, Systematic Review, in the last 5 years. From the 6 systematic reviews identified, no effective pharmacotherapies for methamphetamine dependence were found. Two trials of N-Acetylcysteine (NAC) were found. One trial found no difference between a combination of naltrexone and NAC compared to placebo on methamphetamine craving or use but was inconclusive due to the small sample size (N = 31) and high dropout (9 retained in the active group, 8 in placebo). A second small cross-over trial (N = 23) found reductions in methamphetamine craving relative to placebo, but outcomes for methamphetamine use were not reported.
Added value of this study
This is the first robust trial to evaluate the effect of NAC on methamphetamine use and related clinical outcomes. We demonstrated that NAC has no significant effect on either methamphetamine use, craving, withdrawal, or severity of dependence. Nor did we find compelling evidence of reductions in depression, suicidality, hostility or psychotic symptoms.
Implications of all the available evidence
Our trial suggests that NAC is unlikely to be of benefit as a take-home medication for people dependent on methamphetamine who are engaged in daily or near daily methamphetamine use. Our findings do not preclude potential benefits in other settings (e.g., to reduce craving during abstinence).
Alt-text: Unlabelled box
1. Introduction
Dependence on the illicit stimulant methamphetamine is a major global health concern [1,2] with an estimated 7.4 million people dependent on the drug worldwide in 2017 [3]. It is associated with elevated mortality, increased incidence of HIV and hepatitis C infection, poor mental health (suicidality, psychosis, depression, and violence), and an increased risk of cardiovascular events [3,4]. Existing treatment relies on psychosocial interventions [5]. With the exception of contingency management, these interventions have limited evidence to support their efficacy [3,[6], [7], [8]]. They are also resource intensive [9] and have limited reach [10]. Pharmacotherapy provides a potentially scalable cost-effective treatment option that could dramatically increase treatment coverage [5]. To date, no effective medications for methamphetamine dependence have been found [3,11,12].
Recent insights into glutamate dysregulation in addiction have provided new promise for medications to treat methamphetamine dependence [13]. Specifically, maladaptive neuroplastic changes in astrocyte function during addiction cause aberrant potentiation of glutamate transmission in the projections from the prefrontal cortex to the nucleus accumbens [13]. These changes are thought to be central to drug seeking and relapse [13]. This glutamate dysregulation can be normalised by boosting cystine levels via administration of N-acetylcysteine (NAC) [14,15], which blocks drug seeking and the reinstatement of drug-taking in animal models. Hence, NAC has become a popular candidate drug for the treatment of addiction [16].
NAC has been found to reduce craving for various drugs in humans [17], with one small randomised cross-over trial finding reductions in craving for methamphetamine [17,18]. However, this effect on methamphetamine craving has not yet been replicated, and no trial has explored whether such potential reductions in craving for methamphetamine lead to reductions in methamphetamine use per se. The seemingly generic effect of NAC on craving across drug classes [17] suggests that it may also be helpful in managing polysubstance dependence, which is prevalent amongst people dependent on methamphetamine [19]. Additionally, the antioxidant effects of NAC may protect against free radical induced toxicity. This is one of the key mechanisms that underpins NAC's growing use in neuropsychiatric conditions [20]. Hence, NAC may have additional benefits in reducing methamphetamine-related neuropsychiatric sequelae [21,22].
NAC is an appealing pharmacotherapy option because it is a generic (off-patent) medication with no known dependence liability, and a well-established safety profile (known adverse reactions are generally mild [23]). The ability to provide a take-home medication to treat methamphetamine dependence would allow rapid treatment scale-up. It could dramatically reduce service burden and improve treatment coverage, particularly in contexts where the availability of specialist alcohol and other drug services are limited.
The aim of the current trial was to test whether take-home oral NAC (2400 mg daily for 12 weeks) could reduce methamphetamine use and improve clinically related outcomes in people dependent on the drug. The primary hypothesis was that, relative to placebo, NAC would reduce methamphetamine use, indexed by (i) days of use and (ii) positive oral fluid samples. Secondary hypotheses were that NAC would reduce methamphetamine craving, severity of dependence, withdrawal severity, psychiatric symptoms (depression, suicidality, hostility and psychotic symptoms), not increase other substance use, and have an acceptable adverse event profile.
2. Methods
2.1. Study design
We conducted a randomised, double-blind, placebo-controlled, parallel trial across three Australian sites (Melbourne, Geelong and Wollongong). The detailed study protocol is published elsewhere [24]. The protocol was registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12618000366257) on 9 March, 2018. The protocol was subsequently revised to replace the Montgomery Asberg Depression Rating Scale [25] with the Brief Psychiatric Rating Scale [26] items of depression and suicidality. This revision was made prior to initiating recruitment. The coordinating sponsor was also changed from Curtin University to the University of New South Wales during the trial. The trial was overseen by an independent Data and Safety Monitoring Board and is reported here in accordance with the CONsolidate Standards Of Reporting Trials (appendix p 5). The first participant was enrolled on July 9th 2018 and the final participant was enrolled on December 6th 2019, with the final assessment conducted on March 30 2020. All participants provided written informed consent prior to participation and were reimbursed $30 per assessment. Details of ethics approvals and protocol modifications can be found in the study protocol which is available online (http://www.anzctr.org.au/).
2.2. Participants
The target population was people dependent on methamphetamine who were seeking to reduce their methamphetamine use but not currently receiving treatment for a substance use disorder. Participants were recruited via collaboration with local service providers (e.g., needle and syringe programs), media, Facebook advertisements, a dedicated website, flyers, and word-of-mouth. Phone screening was used to identify likely eligible participants who subsequently underwent a face-to-face consent, eligibility assessment and medical screening. This assessment also collected data on drug use history, demographics, and detailed contact information for follow-up. Participants met the following inclusion criteria: aged 18–60 years, DSM-IV criteria for methamphetamine dependence, seeking to reduce methamphetamine use, and able to provide informed consent and comply with the trial protocol. People were excluded from participation if they were undergoing other substance use treatment at the time of enrolling in the trial (including pharmacotherapy for substance use disorders), had a primary psychotic disorder, were in need of acute care for psychiatric or other major medical conditions, had a positive pregnancy screen at baseline (or were not able to avoid pregnancy during the trial), had a systemic medical disorder or other medical condition that may increase the risk of adverse reactions to NAC (e.g., history of seizures, gastrointestinal ulcers or renal stones, atopy), were taking NAC from other sources, reported previous hypersensitivity to NAC, or were taking medications contraindicated for NAC (e.g. carbamazepine, nitroglycerin). DSM-IV methamphetamine dependence was confirmed using the Composite International Diagnostic Interview Version 3.0 [27]. Participants were screened for a primary psychotic disorder using the Mini International Neuropsychiatric Interview (MINI) [28] for Schizophrenia and Psychotic Disorders Studies, English Version 7.0.1.
2.3. Randomisation and study blinding
Eligible participants were randomly assigned (1:1) by the trial researchers to receive either NAC (2400 mg/day for 12 weeks) or equivalent placebo according to a computer-generated block randomisation sequence (program written by WL) which was stratified by site, sex, and injecting vs. non-injecting methamphetamine use. The final randomisation schedule was generated by the DSMB statistician (MS), who provided the schedule directly to pharmacy. All trial investigators, research staff and participants were blind to group allocation. Unblinding during the trial was done via a password-protected portal (developed by LN). Trial medication (supplied by Pharmaceutical Packaging Professionals Pty Ltd) was encapsulated in white size 00 capsules (600 mg NAC or placebo),and bottled in identical plastic bottles fitted with eCAP™ lids (Med-ic eCAP™, Information Mediary Corp). Participants were asked what group they thought they were in at weeks 4, 8 and 12 (don't know, placebo, NAC).
2.4. Procedures
Following confirmation of eligibility, the baseline assessment was conducted, where medication was provided along with referral information for local health services and a self-help booklet (On Ice; National Drug and Alcohol Research Centre, UNSW). Participants were instructed to take two capsules of the trial medication each morning and evening. They were free to engage with other treatments and support services throughout the trial. Weekly assessments were conducted thereafter (follow-ups 1-12) either at the trial site or at a location convenient to the participant (e.g., cafes, malls). Replacement medication bottles were provided at weeks 3, 6 and 9. Oral fluid samples were taken at each assessment using a Quantisal Oral Fluid Collection DeviceTM and analysed for methamphetamine and amphetamine content by the Victorian Institute of Forensic Medicine. Medication adherence data were collected using eCAPTM bottle lids which record the exact time and date of each bottle opening. eCAPTM data were uploaded to a secure online data repository at each assessment via a near-field communication-enabled smart phone, and on bottle return using a CertiScan RFID desktop Reader (Med-ic Certiscan Version 2.5.1; Information Mediary Corp 2014-2018). De-identified trial data were entered onto a centralised secure online electronic database via REDCap (Research Electronic Data Capture [29]). All assessments were done one-on-one and face-to-face (or by phone where face-to-face interviews were not practical) by researchers who were trained in all assessment procedures. Weekly meetings were held across sites to review data collection. Primary endpoint data were double entered. Further details on procedures and measures can be found in the study protocol [30].
2.5. Measures
The primary outcome was methamphetamine use, which was measured in two ways: (1) change in days of methamphetamine use, and (2) number of methamphetamine-positive oral fluid samples (≥ 25 mg/L) during the medication phase. Days of methamphetamine use was assessed for the past 4 weeks prior to baseline and prior to weeks 4, 8, and 12, using the Timeline Followback (TLFB), with self-reported use updated at each weekly assessment [31].
We considered both of these primary outcomes as equally important but qualitatively distinct. Biological markers of abstinence are the accepted outcome for illicit substance use trials [32]. However, there is increasing recognition that achieving abstinence is an unrealistic treatment goal and that this outcome is insensitive to change [32]. Days of use assessed using the TLFB has been recommended as a more sensitive outcome for stimulant use trials [33].
Secondary outcomes were assessed for the past week at baseline and at each follow-up assessment. These included severity of methamphetamine dependence (Severity of Dependence Scale, SDS) [34], craving for methamphetamine (Craving Experience Questionnaire, CEQ) [35], withdrawal symptoms (Amphetamine Withdrawal Questionnaire, AWQ) [36], and psychiatric symptoms (Brief Psychiatric Rating Scale [BPRS, 26] hostility score of 4+, depression score of 4+, suicidality score of 3+, and psychotic symptoms were a score of 3+ on any of the items of unusual thought content, suspiciousness or hallucinations). Inter-rater reliability on 161 audiotaped BPRS ratings gave an inter-rater agreement of 83% (kappa 0.51) for psychotic symptoms, and inter-rater agreement of > 90% (kappa 0.82–0.87) for other BPRS measures. The Cronbach's alpha for the SDS, CEQ and AWQ at baseline was 0.77, 0.88 and 0.79, respectively.
Polysubstance use was defined as the total days use for other drug classes (tobacco, alcohol, cannabis, cocaine, ecstasy, hallucinogens, inhalants, and heroin) in the 4 weeks prior to baseline and prior to weeks 4, 8, and 12. Medication adherence was assessed as the percentage of non-missed medication doses according eCAPTM data. Concomitant substance use treatments and psychoactive medication use were recorded at baseline and updated at each assessment. Concomitant medications were categorized according to MIMS Class [37]. Tolerability was assessed using the Treatment Satisfaction Questionnaire for Medication version II (TSQM-II) [38]. Adverse events were coded by System Organ Class according to the Medical Dictionary for Regulatory Activities (MedDRA) [39].
2.6. Data analysis
Our planned sample size was 180, which was designed to detect a group difference of at least 3.5 days of methamphetamine use at follow-up [30] (appendix p 14). We undertook a post-hoc power analysis to determine what group difference we could confidently detect given the final sample size (N = 153) and the distribution of our primary outcome measure (appendix p 59). Based on this post-hoc calculation, we could detect an incidence-rate ratio of 0.78 with 80% power (two-sided test with p set at 0.025), which corresponds to a difference of 5.3 days of methamphetamine use between groups, across all follow-ups. For positive oral fluid samples, we could detect a 6% difference between groups (rate ratio of 0.92) with 80% power (two-sided test with p set at 0.025). Reductions in the frequency of stimulant use are associated with improvements in function, although the magnitude of change required to produce clinically meaningful benefits is unclear [32].
Full details of the statistical analysis plan (September 2, 2020) can be found in the appendix (p 7). The analysis was conducted in Stata Version 16.0 (©Statacorp LLC, College Station, TX). All tests were two-sided with adjustment of the p value for multiple comparisons, with significance set at p < 0.025 for the two primary outcomes, p < 0.01 for the secondary outcomes, and p < 0.05 for all other tests (with 97.5%, 99% and 95% CIs reported, respectively).
Analyses of the primary and secondary outcomes were conducted blind to group assignment on both the imputed intention-to-treat dataset (N = 153) and on the unimputed modified intention-to-treat dataset, which included all participants who received at least one dose of medication (n = 149). Missing data were imputed using multiple chained equations. Imputation models included data from the eligibility assessment (available for all participants) on days of methamphetamine use in the past four weeks and baseline variables that correlated with missing outcome data (see appendix pp 28–30 for correlates of missing data and p 53 for imputation models). For the primary outcomes, we specified the imputed intention-to-treat analysis as the main analysis. For secondary outcomes, we specified the unimputed analysis as the main analysis. This was done because secondary outcomes were not assessed at eligibility and therefore could only be included in the imputation model for participants who were not lost to follow-up.
The effect of NAC on methamphetamine use days was tested with a group (NAC vs. placebo) by time contrast (baseline [0], week 4 [1], week 8 [1] and week 12 [1]) interaction. A negative binomial generalised linear mixed model was used to model this effect, where the outcome was days of methamphetamine use in the past 4 weeks at each time point. An exposure (offset) term was included in the model for length of each follow-up period (28 days at baseline; median [IQR] days at 4 weeks: 28 [26–29], 8 weeks: 28 [25–30], and 12 weeks: 28 [27–31]). The effect of NAC on days of other substance use was similarly tested with a group (NAC vs. placebo) by time contrast (baseline [0], week 4 [1], week 8 [1] and week 12 [1]) interaction using a negative binomial generalised linear mixed model with an offset term for the length of each follow-up period. The effect of NAC on methamphetamine-positive oral fluid samples was tested using a main effect of group (NAC vs. placebo) using a Poisson mixed model. A Poisson model was used because of the small number of negative oral fluid samples. The outcome in this model was a time-varying variable for whether or not the participant had a methamphetamine-positive oral fluid sample [1 vs. 0] at each of the follow-ups (weeks 1–12). The effect of NAC on each secondary outcome was tested with a group by time (baseline [0] vs. weeks 1–12 [1]) interaction using a mixed model. A linear model was used for continuous outcomes, a logit model was used for depression, psychotic symptoms and hostility, and a Poisson model was used for suicidality due to the low number of participants reporting suicidality. All models used an unstructured covariance model and included a random intercept term for participant identifier to account for clustering of data on repeated measures. Mean group differences and their confidence limits were based on marginal effects extracted from these models (see appendix pp 55–104).
Time trends were examined by replicating the above analyses using a factorial time contrast that divided the 12-week medication period into 4-week blocks (i.e., baseline [0] vs. weeks 1–4 [1], weeks 5–8 [2], and weeks 9–12 [3]). Sensitivity analyses were conducted which adjusted for variables that differed between the placebo and the NAC group at the eligibility assessment, and which also adjusted for the number of other substance use treatment episodes that the participant initiated during the study. Per-protocol analyses used unimputed data and included participants who took at least one dose of the study medication, who were not lost to follow-up, and who were not withdrawn from the study for reasons unrelated to the study medication (Fig. 1).
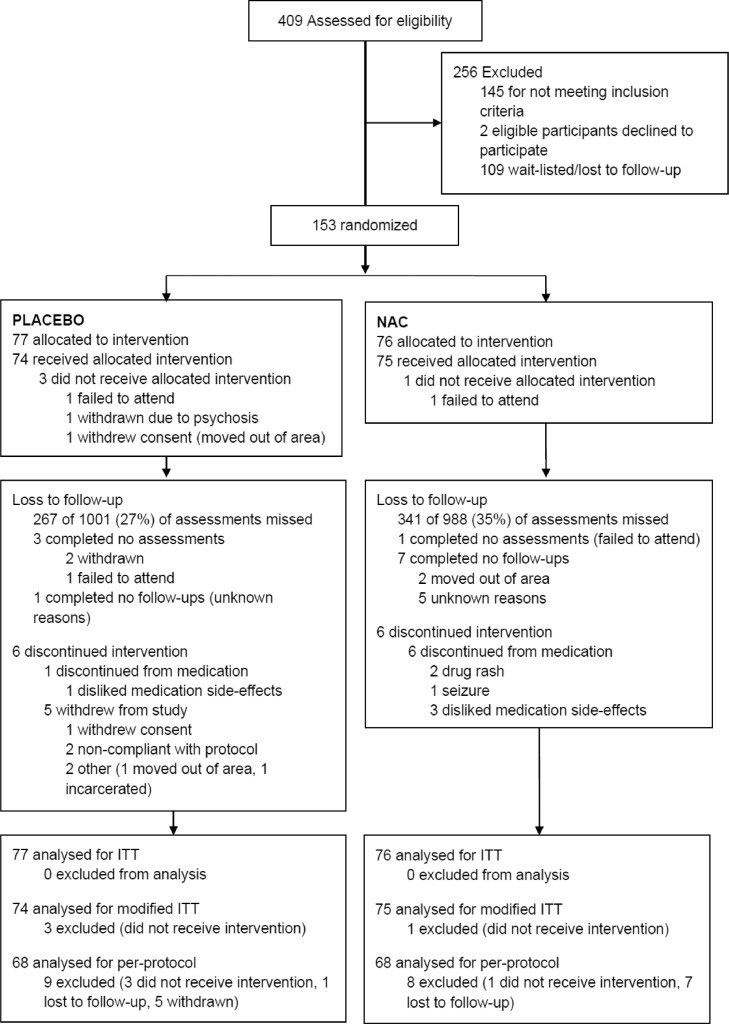
Fig. 1.
Trial profile (Notes. N-Acetylcysteine (NAC), intention to treat (ITT)).
Safety analyses compared the number and percentage of participants reporting adverse events in each treatment group by System Organ Class [39]. Groups were compared using chi-square tests. Other descriptive group comparisons were made using chi-square tests for categorical data, t-tests for normally distributed data, and median comparison tests for skewed continuous data, where interquartile ranges are presented and confidence limits were bootstrapped [40]. Spearman correlations were used.
2.7. Role of the funding source
The funding source, the Australian National Health and Medical Research Council (NHMRC Project Grant No. 1128147) played no role in the design of the study or the collection, analysis, and interpretation of data, or in writing the manuscripts from the study.
3. Results
3.1. Participants
Of the 409 participants screened for the study 153 were enrolled: 77 were randomised to placebo, 76 to NAC (Fig. 1). Participants had a mean (SD) age of 38 (8) years, and 59% were male. Both groups had a median (IQR) of 24 (15–28) days of methamphetamine use in the 4 weeks prior to baseline. Other characteristics were similar between groups at baseline except that the NAC group were less likely to be unemployed or on a low income (Table 1). There was no significant difference between groups in whether participants received other treatment for substance use disorders during the trial (Table 1). Concomitant medications taken during the trial differed only for hyperacidity, reflux and ulcers (placebo 3%, NAC 12%, p = 0.027). Psychotropic medication use was relatively common (antidepressants 35%, antianxiety agents 18%, antipsychotic agents 12%) but did not differ between groups (Table S5, appendix p 51). Other substance use consisted primarily of tobacco, alcohol and cannabis use, with no group difference in the total number of days that other substances were used (Table 1) or the use of specific substances (Table S7, appendix p161).
Table 1.
Participant characteristics by group allocation.
| Placebo (n = 77) | NAC (n = 76) | P value | Total sample (N = 153) | |
|---|---|---|---|---|
| Age, mean (SD) | 37.9 (7.9) | 37.5 (8.4) | 0.75 | 37.7 (8.1) |
| Male, n (%) | 46 (60) | 45 (59) | 0.95 | 91 (59) |
| Immigrant, n (%) | 8 (10) | 2 (3) | 0.052 | 10 (7) |
| Married/de-facto, n (%) | 24 (31) | 19 (25) | 0.40 | 43 (28) |
| Unemployed, n (%) | 36 (47) | 49 (64) | 0.027 | 85 (56) |
| Net income in past fortnight, n (%) | ||||
| < $400 | 15 (19) | 6 (8) | 0.037 | 21 (14) |
| $400-799 | 29 (38) | 43 (57) | 0.019 | 72 (47) |
| $800-1199 | 14 (18) | 9 (12) | 0.27 | 23 (15) |
| $1200+ | 19 (25) | 18 (24) | 0.89 | 37 (24) |
| Years of schooling, median (IQR) | 10 (10–12) | 11 (10–12) | 0.91 | 11 (10–12) |
| Qualifications, n (%) | ||||
| No tertiary education | 24 (31) | 20 (26) | 0.51 | 44 (29) |
| Trade or technical | 44 (57) | 49 (64) | 0.35 | 93 (61) |
| University | 9 (12) | 7 (9) | 0.62 | 16 (10) |
| Prison history, n (%) | 19 (25) | 22 (29) | 0.55 | 41 (27) |
| Methamphetamine use | ||||
| Treatment history, n (%) | 43 (56) | 42 (55) | 0.94 | 85 (56) |
| Injecting, n (%) | 27 (35) | 27 (36) | 0.95 | 54 (35) |
| Duration of use, median (IQR) years | 14 (8–22) | 14 (9–24) | 0.94 | 14 (8–22) |
| Days of use in past 4 weeks, median (IQR) | 25 (17–28) | 24 (17–28) | 0.94 | 25 (17–28) |
| Days of other substance use,a median (IQR) | 30 (28–43) | 33 (28–50) | 0.38 | 31 (28–47) |
| Drug treatment during the trial, n (%) | ||||
| Received any other treatment, n (%) | 18 (23) | 16 (21) | 0.73 | 34 (22) |
| Number episodes (if received treatment), median (IQR) | 2 (1-3) | 2.5 (1–3.5) | 0.33 | 2 (1–3) |
Interquartile range (IQR).
Summed across all drug types in the past 4 weeks.
Four participants (3 on placebo, 1 on NAC) did not receive the trial medication and did not complete any assessments, five were withdrawn (all on placebo), and seven discontinued medication (6 on NAC, 1 on placebo) (Fig. 1). Two participants in the NAC group were unblinded due to serious adverse events, one in week 5, and one in week 10. In total, 69% of assessments were completed (73% placebo vs. 65% for NAC χ2 df = 1 = 14.4, p < 0.001). Data on days of methamphetamine use were available for 88% of timepoints (placebo 90%, NAC 86%, χ2 df = 1 = 1.7, p < 0.194). Oral fluid samples (n = 1,150) were available for more participants in the placebo group than the NAC group (67% vs. 58% χ2 df = 1 = 15.1 p < 0.001). See appendix p 23,24 for detailed data on follow-up. Medication adherence was 66% (IQR 52–79%; placebo 69% vs. NAC 62% χ2 df = 1 = 1.2, p = 0.268; based on 447 of 596 dispensed bottles from 139 participants). Satisfaction with the medication (TSQM-II scales) did not differ between groups (Table S3, appendix p 43). Similar proportions in each group believed they were receiving NAC, although there was a trend toward participants on NAC being more likely to say that they did not know their group allocation rather than believing that they were on placebo (these differences were not statistically significant at either 4, 8 or 12 weeks; Table S8, appendix p162).
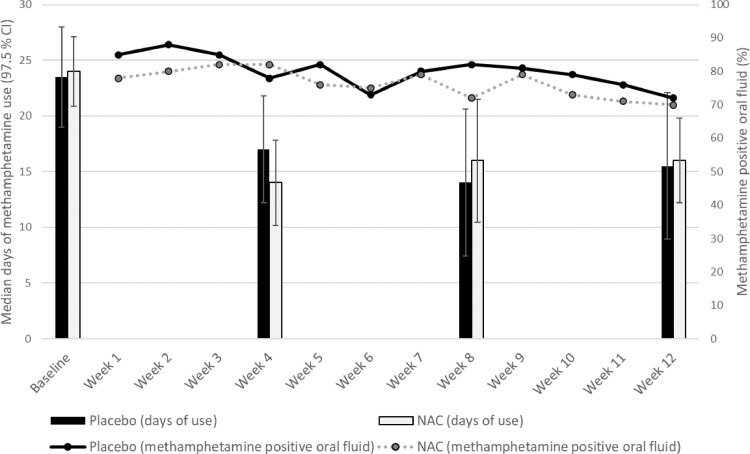
Methamphetamine use outcomes are shown in Fig. 2 and treatment effects are shown in Table 2 (see appendix p 39 for data at each follow-up, and p 60 for full models). There was a significant reduction in methamphetamine use days during the 12-week medication period compared to baseline, but NAC did not reduce methamphetamine use days more than placebo (Table 2). There was also no effect of NAC on days of methamphetamine use at any specific follow-up (week 4, week 8 and week 12) relative to baseline week 4: b(SE) = -0.01 (0.09) p = 0.890; week 8: b(SE) = 0.08 (0.09) p = 0.406; week 12 b(SE) = 0.08 (0.09) p = 0.374 (appendix p 142). Seventy-nine per cent (903 of 1150) of oral fluid samples tested positive for methamphetamine, with no significant difference between the NAC and placebo groups (placebo 80%, NAC 76%; Table 2). Nor was there any evidence of a time trend effect for NAC on methamphetamine oral fluid samples (appendix pp 143). Results did not change after adjusting for unemployment, income or other substance use treatment received during the study (appendix pp 103, 104) and equivalent results were obtained in the unimputed dataset (appendix p 83) and the per protocol dataset (appendix pp 132–133). Post-hoc analyses confirmed that results did not change when including stratification variables in the model (i.e., site, sex and injecting vs. non-injecting methamphetamine use). There was no significant change in the use of other substances during the 12-week trial period compared to baseline (appendix pp 81, 101), nor did NAC significantly change other substance use at follow-up relative to placebo (Table 2).
Fig. 2.
Methamphetamine use outcomes (median days of use in the past 4 weeks [97.5% CIs]; % methamphetamine positive oral fluid tests) by group.
Table 2.
Primary and secondary endpoints at baseline and follow-up by group with treatment effects.
| Baseline | 12-week trial period | Treatment effectsa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unimputed | Imputed |
|||||||||
| Mean (SE) / Mean reduction (SE)b | Group difference / Group difference in reduction, mean (CI)b | b (SE) | P value | Predicted mean / Predicted mean reduction (SE)b | Predicted group difference Predicted/ group difference in reduction, mean (CI)b | b (SE) | P value | |||
| Methamphetamine-positive oral fluid, n (%) | ||||||||||
| Placebo | 497 (80) | 0.80 (0.04) | 0.79 (0.03) | |||||||
| NAC | 406 (76) | 0.77 (0.04) | -3.8 (-16.5–8.8) | -0.05 (0.07) | 0.50 | 0.76 (0.03) | -2.6 (-12.6–7.4) | -0.03 (0.06) | 0.56 | |
| Methamphetamine use days in past 4 weeks, median (IQR) | ||||||||||
| Placebo | 24 (15–28) | 16 (7–23) | -7.3 (1.3) | -7.3 (1.2) | ||||||
| NAC | 24 (15–28) | 16 (8–23) | -6.9 (1.3) | 0.40 (-3.70–4.50) | 0.04 (0.08) | 0.63 | -6.8 (1.2) | 0.46 (-3.36–4.29) | 0.05 (0.08) | 0.55 |
| Craving, mean (SD) CEQ score | ||||||||||
| Placebo | 5.0 (2.4) | 3.3 (2.3) | -1.6 (0.2) | -1.6 (0.2) | ||||||
| NAC | 5.2 (2.3) | 3.3 (2.2) | -1.9 (0.2) | -0.27 (-0.92–0.38) | -0.27 (0.25) | 0.28 | -1.8 (0.2) | -0.15 (-0.91– 0.60) | -0.15 (0.29) | 0.60 |
| Severity of dependence, mean (SD) SDS score | ||||||||||
| Placebo | 8.0 (4.1) | 5.6 (4.0) | -2.3 (0.3) | -2.1 (0.3) | ||||||
| NAC | 8.1 (3.4) | 5.3 (3.7) | -2.7 (0.3) | -0.34 (-1.43–0.76) | -0.34 (0.42) | 0.43 | -2.3 (0.3) | -0.19 (-1.40–1.03) | -0.19 (0.47) | 0.69 |
| Withdrawal, mean (SD) AWQ score | ||||||||||
| Placebo | 20.0 (7.6) | 16.0 (8.2) | -3.5 (0.6) | -3.7 (0.7) | ||||||
| NAC | 19.9 (7.3) | 15.4 (7.7) | -4.2 (0.6) | -0.70 (-2.96–1.55) | -0.70 (0.88) | 0.42 | -3.9 (0.7) | -0.21 (-2.78–2.37) | -0.21 (1.01) | 0.84 |
| Psychotic symptoms, n (%) | ||||||||||
| Placebo | 30 (41) | 172 (26) | -14.6 (4.9) | -14.4 (5.3) | ||||||
| NAC | 26 (35) | 134 (24) | -11.2 (4.8) | 3.5 (-14.3–21.2) | 0.19 (0.46) | 0.67 | -10.2 (5.1) | 4.2 (-14.8–23.2) | 0.22 (0.42) | 0.60 |
| Hostility, n (%) | ||||||||||
| Placebo | 30 (41) | 187 (29) | -11.1 (5.0) | -13.1 (5.4) | ||||||
| NAC | 34 (45) | 94 (17) | -25.5 (5.2) | -14.4 (-33.0–4.3) | -1.0 (0.45) | 0.021 | -24.6 (5.3) | -11.6 (-31.2–8.1) | -0.72 (0.41) | 0.080 |
| Depression, n (%) | ||||||||||
| Placebo | 31 (42) | 201 (31) | -10.9 (4.8) | -10.9 (4.9) | ||||||
| NAC | 31 (41) | 162 (29) | -12.3 (4.9) | -1.3 (-18.9–16.3) | -0.11 (0.48) | 0.81 | -11.4 (4.9) | -0.6 (-19.6–18.5) | -0.04 (0.42) | 0.92 |
| Suicidality, n (%) | ||||||||||
| Placebo | 16 (22) | 97 (15) | -6.8 (9.0) | -5.9 (4.0) | ||||||
| NAC | 13 (17) | 59 (10) | -9.2 (7.2) | -2.4 (-32.8–27.9) | -0.30 (0.42) | 0.48 | -5.7 (3.9) | 0.2 (-14.2–14.6) | 0.06 (0.56) | 0.91 |
| Days of other substance use in past 4 weeks,c median (IQR) | ||||||||||
| Placebo | 29 (8–46) | 29 (14–44) | 0.6 (1.1) | 0.1 (1.1) | ||||||
| NAC | 33 (28–52) | 33 (25–51) | 2.0 (1.6) | 1.29 (-2.47–5.06) | -0.02 (0.03) | 0.36 | 0.9 (1.4) | 0.80 (-2.73–4.32) | 0.02 (0.04) | 0.72 |
Standard error (SE), standard deviation (SD), interquartile range (IQR), confidence interval (CI), N-Acetylcysteine (NAC), Craving Experience Questionnaire (CEQ), Amphetamine Withdrawal Questionnaire (AWQ), Severity of Dependence Scale (SDS).
Treatment effects for oral fluid samples were based on a group difference over all follow-ups; for all other outcomes, treatment effects were based on a time (baseline vs all follow-up time points) by group allocation (placebo vs. NAC) interaction. Model coefficients were derived from mixed Poisson regression (oral fluid samples), mixed negative binomial regression (methamphetamine use days), mixed logistic regression (depression, psychotic symptoms, hostility and depression), and mixed linear regression (CEQ, AWQ, SDS).
Marginal effects for baseline vs. 12 week medication period are estimated from models. Confidence Intervals (CIs) are 97.5% for the two primary methamphetamine use outcomes, 95% for days of other substance use and 99% for remaining outcomes.
Includes tobacco, alcohol, cannabis, ecstasy, cocaine, heroin, inhalants, other hallucinogens.
There was a significant reduction in craving, severity of dependence and withdrawal during the 12-week medication period compared to baseline (Table 2; appendix pp 87–91). However, NAC did not reduce any of these outcomes more than placebo in either the imputed or the non-imputed analysis (Table 2), or in the per-protocol analysis (appendix pp 134–136). There were also no significant effects (p < 0.01) at any of the specific 4-week intervals (appendix pp 152–154).
NAC did not significantly reduce psychotic symptoms, depression, or suicidality more than placebo during the 12-week medication phase (Table 2; see appendix pp 137–140 for per-protocol analysis). Nor were any significant effects found at any of the specific 4-week intervals during the medication phase (appendix p155–158). There was a non-significant trend toward NAC reducing hostility more than placebo (Table 2), which persisted after adjustment for employment, income and exposure to other substance use treatment during the trial (b(SE) = -1.05 (0.44) p = 0.019) but was reduced when missing data were imputed (-0.72 (0.41) p = 0.080). There was no evidence of a time trend for the effect of NAC on hostility (appendix pp 156). Detailed data on secondary outcomes at each follow-up can be found in the appendix (p 40).
There was no difference between the NAC and placebo groups in the percentage of participants reporting adverse events by System Organ Class (Table S6, appendix p 160). Eleven serious adverse events occurred (affecting 10 participants: 6 on NAC, 4 on placebo). These were related to surgery after injuries (n = 3), psychosis (n = 2), drug overdose (n = 1), seizures (n = 1), meningoencephalitis (n = 1), non-fatal strangulation (n = 1), gastroenteritis (n = 1), and hospitalisation for detoxification (n = 1).
4. Discussion
We found that take-home NAC did not produce a clinically meaningful reduction in methamphetamine use amongst people dependent on the drug when compared to placebo. We also failed to confirm putative benefits of NAC on methamphetamine craving [17,18], and we did not find evidence of improvements in other indicators of methamphetamine use (severity of dependence or withdrawal). We also did not find any clear benefit of NAC in reducing comorbid psychiatric symptoms (psychotic symptoms, hostility depression, or suicidality) amongst people dependent on methamphetamine.
Potential benefits of NAC previously found in other contexts (e.g., reducing relapse in people who have achieved abstinence from stimulants [41], reducing cannabis use in adolescents[42]) should not be discounted based on the current findings. However, our findings are consistent with two of the more recent larger placebo-controlled trials of NAC for substance use disorders (one for cocaine use [41] and one for cannabis use disorder [43]). Like the current study, both trials had high baseline levels of substance use and sub-optimal medication adherence. Although participants in our sample engaged in other substance use (particularly cannabis, tobacco and alcohol use), this is typical of people who use illicit drugs [44]. We found that NAC did not change other substance use, and nor did other substance use account for our null findings. We also do not think that the reduced final sample size significantly compromised our ability to detect a clinically meaningful effect of NAC on methamphetamine use. This is because we still had sufficient statistical power to detect 5 or more days difference in use between groups (over the previous 4 weeks), and because the observed group differences were negligible (< 1 day use in the past 4 weeks). However, we may have not had sufficient statistical power to detect small effects on our secondary outcomes at our nominated p value, and this may have accounted for our null finding on hostility, where we observed a 14% difference between groups in favour of NAC. Taken together with previous results, our findings cast doubt on the utility of take-home NAC as an agent that can reduce substance dependence in people who are actively engaged in heavy substance use.
The large post-treatment reduction in methamphetamine use seen in both the NAC and placebo groups is typical of clinical trial outcomes [45,46]. This so-called placebo responding is often considered troublesome in trials because it can reduce statistical power make it difficult to detect treatment effects [47]. In the context of this trial, these non-specific reductions in methamphetamine use are likely to reflect expectations about the efficacy of the trial medication [48,49] and the interaction with the trial staff (particularly the regular monitoring of substance use during the trial and provision of adjunctive support) [49]. Other factors, including natural remission [50] and demand bias seen with self-reported outcomes [45,50], may also play a role. These non-specific reductions in methamphetamine use have potential clinical relevance given the lack of effective medications to treat methamphetamine dependence. Although placebo interventions pose ethical issues, they have been actively used in clinical practice [51,52], and even have benefit over no treatment when they are labelled as placebo [48].
The main strength of our study was that it was powered to detect a clinically meaningful reduction in methamphetamine use, and we had relatively complete data on our primary endpoint of methamphetamine use days. The main limitation was sub-optimal medication adherence (with a median of 66% of doses taken). However, poor adherence is typical of stimulant treatment trials [3,41], and is probably at least as good as what could be expected if the medication was made available in routine clinical practice. It could be argued that 12 weeks was not sufficient to obtain clinically meaningful benefits, and this is likely to be the case for psychiatric symptoms, where benefits can take up to 4–6 months to emerge [53]. However, benefits of NAC on craving have been seen within days in experimental settings [54], and have developed within 4 weeks in clinical trial settings [18]. Hence, any benefits of NAC on methamphetamine dependence should have emerged within the 12-week timeframe. One possible explanation for the failure to demonstrate the previously observed benefits of NAC on methamphetamine use is that this oral formulation and dosage may not have been sufficient to normalise glutamate function in the context of ongoing methamphetamine use [55]. For example, although previous studies have found that NAC can reverse glutamate dysregulation during abstinence from stimulant use [15], this normalisation of glutamate function was not seen in a more recent trial of people who continued to use stimulants while on NAC [55].
In sum, we failed to replicate early promising results for NAC in managing methamphetamine dependence [18] and our findings are inconsistent with previous positive outcomes for NAC on substance dependence more generally [17,42]. Taken together with other recent findings [41,43,55], our results cast doubt on the utility of take-home NAC as an agent that can reduce methamphetamine dependence, and arguably dependence on other substances, in people who are actively engaged in daily or near daily substance use. Future exploration of NAC's potential in drug dependence should perhaps instead focus on its potential to reduce relapse amongst people who have already achieved abstinence, or who have less entrenched patterns of substance use.
Declaration of Competing Interest
OMD has received grant support from Lilly and ASBDD/Servier. She has also received in kind support from BioMedica Nutracuticals, NutritionCare and Bioceuticals. MB has received Grant/Research Support from the A2 milk company, Meat and Livestock Board, Woolworths, and Avant, has been a speaker for Abbott, Astra Zeneca, Janssen, Lundbeck, Merck, Otsuka, Milken Institute, Sandoz, Allori (for Eisai), Medplan Canada, Servier and Medisquire India, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Janssen, Lundbeck Merck, Pfizer and Servier, and has licences with Allen and Unwin and Cambridge University Press. MB has received patents for agents that modulate physiological processes and diseases of the central nervous system and related processes, including xanthone-rich plant extracts. DL has provided consultancy advice to Lundbeck and Indivior and has received travel support and speaker honoraria from Astra Zeneca, Camurus, Indivior, Janssen, Lundbeck, Servier and Shire. PD has received investigator-initiated funding from Gilead Sciences, an untied educational grant from Indivior and was an unpaid member of an Advisory Board for Mundipharma for work unrelated to this study. PH has received investigator-driven research funding from Gilead Sciences and Abbvie for work on hepatitis C unrelated to this study. GC has received speaker fees from Otsuka (Australia), Servier, Lundbeck and Janssen, all unrelated to the current study. SA has received speaker honoraria from Gilead, Janssen and Camurus for work unrelated to this study. Other researchers have no interests to declare.
Acknowledgments
Funding
This research was funded by the Australian NHMRC (Project Grant No. 1128147).
Authors' contributions
All authors contributed to writing and reviewing the final manuscript. RM drafted the original manuscript. RM OMD AT PK PH PD BQ DL GC AB MB BS and DR contributed to the conceptualisation, funding acquisition, methodology and supervision of the project. RM OMD AT PK BQ DL PD BS DR SH VM and SA contributed to project administration and supervision. HH FC and SA contributed to investigation. LS NtP DKR RB AW and TT contributed to project administration and data curation. PC and MM contributed to the formal analyses. All study investigators (RM OMD AT PK PH PD BQ DL GC) had to access the full dataset. RM, PC and MM accessed the full dataset for the purposes of analysing data for this paper.
Data sharing
Anonymised data will be made available upon reasonable request, which must include a protocol and statistical analysis plan, be consistent with our data sharing policy (available on request from RM), involve collaboration with one of our project investigators, and not conflict with our prespecified publication plan. Requests for data sharing will be considered by RM and the trial investigator team and will require relevant ethics approval.
Acknowledgments
RM was supported by a Curtin Senior Research Fellowship. OMD is supported by a R.D. Wright Biomedical NHMRC Career Development Fellowship (APP1145634). MB is supported by a NHMRC Senior Principal Research Fellowship (1059660 and 1156072). PD and AB are supported by NHMRC Research Fellowships (1136908, 1135901). DL is supported by a NHMRC Investigator grant (1196892). DKR was supported by an Australian Government Research Training Scholarship. Thanks go to members of our DSMB (Jason White, Matthew Spittal, Debra Kerr, Grant Sara, Juanita Koeijers), Wenbin Liang (WL) for development of the randomisation program, Long Nguyen (LN) for developing our online unblinding portal, Steven Shoptaw for advice on trial methods, staff at the Victorian Institute of Forensic Medicine (Linda Glowacki, Dimitri Gerostamoulos) for assisting with oral fluid collection protocols, pharmacy staff, trial staff (including Nicole Edwards, Margie Kent, Alcy Meehan, Davinia Rizzo, Bruno Agustini, Ellie Brown, Behrooz Maylie, Olalekan Ogunleye, and Vicky Phan), agencies and individuals who have assisted with recruitment efforts, and the trial participants.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101005.
Appendix. Supplementary materials
References
- 1.United Nations Office on Drugs and Crime . United Nations; New York: 2017. World drug report 2017; pp. 13–20. [Google Scholar]
- 2.Degenhardt L., Baxter A.J., Lee Y.Y., Hall W., Sara G.E., Johns N., Flaxman A., Whiteford H.A., Vos T. The global epidemiology and burden of psychostimulant dependence: findings from the global burden of disease study 2010. Drug Alcohol Depend. 2014;137:36–47. doi: 10.1016/j.drugalcdep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Farrell M., Martin N.K., Stockings E., Borquez A., Cepeda J.A., Degenhardt L., Ali R., Tran L.T., Rehm J., Torrens M., Shoptaw S., McKetin R. Responding to global stimulant use: challenges and opportunities. Lancet. 2019;394:1652–1667. doi: 10.1016/S0140-6736(19)32230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKetin R., Leung J., Stockings E., Huo Y., Foulds J., Lappin J.M. Mental health outcomes associated with of the use of amphetamines: a systematic review and meta-analysis. EClinicalMedicine. 2019;16:81–97. doi: 10.1016/j.eclinm.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colfax G., Santos G.M., Chu P., Vittinghoff E., Pluddemann A., Kumar S., Hart C. Amphetamine-group substances and HIV. Lancet. 2010;376:458–474. doi: 10.1016/S0140-6736(10)60753-2. [DOI] [PubMed] [Google Scholar]
- 6.Ronsley C., Nolan S., Knight R., Hayashi K., Klimas J., Walley A. Treatment of stimulant use disorder: a systematic review of reviews. PLoS ONE. 2020;15(6):e0234809. doi: 10.1371/journal.pone.0234809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minozzi S., Saulle R., De Crescenzo F., Amato L. Psychosocial interventions for psychostimulant misuse. Cochrane Database Syst Rev. 2016;9(9):CD011866. doi: 10.1002/14651858.CD011866.pub2. CD011866-CD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Crescenzo F., Ciabattini M., D'Alò G.L., De Giorgi R., Del Giovane C., Cassar C., Janiri L., Clark N., Ostacher M.J., Cipriani A. Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: a systematic review and network meta-analysis. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roebuck M.C., French M.T., McLellan AT. DATStats: results from 85 studies using the drug abuse treatment cost analysis program. J Subst Abus Treat. 2003;25:51–57. doi: 10.1016/s0740-5472(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 10.Degenhardt L., Glantz M., Evans-Lacko S., Sadikova E., Sampson N., Thornicroft G., Aguilar-Gaxiola S., Al-Hamzawi A., Alonso J., Helena Andrade L., Bruffaerts R., Bunting B., Bromet E.J., Miguel Caldas de Almeida J., de Girolamo G., Florescu S., Gureje O., Maria Haro J., Huang Y., Karam A., Karam E.G., Kiejna A., Lee S., Lepine J.P., Levinson D., Elena Medina-Mora M., Nakamura Y., Navarro-Mateu F., Pennell B.E., Posada-Villa J., Scott K., Stein D.J., Ten Have M., Torres Y., Zarkov Z., Chatterji S., Kessler R.C., World Health Organization's World Mental Health Surveys collaborators Estimating treatment coverage for people with substance use disorders: an analysis of data from the World Mental Health Surveys. World Psychiatry Off J World Psychiatr Assoc (WPA) 2017;16:299–307. doi: 10.1002/wps.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoptaw S.J., Kao U., Ling W. Treatment for amphetamine psychosis.[update of Cochrane Database Syst Rev. 2008;(4):CD003026; PMID: 18843639] Cochrane Database Syst Rev. 2009:CD003026. doi: 10.1002/14651858.CD003026.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Shoptaw S.J., Kao U., Heinzerling K., Ling W. Treatment for amphetamine withdrawal. Cochrane Database Syst Rev. 2009:CD003021. doi: 10.1002/14651858.CD003021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scofield M.D., Kalivas PW. Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. The Neuroscientist. 2014;20:610–622. doi: 10.1177/1073858413520347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker D.A., McFarland K., Lake R.W., Shen H., Tang X.C., Toda S., Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 15.Schmaal L., Veltman D.J., Nederveen A., van den Brink W., Goudriaan AE. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2012;37:2143–2152. doi: 10.1038/npp.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKetin R., Dean O.M., Baker A.L., Carter G., Turner A., Kelly P.J., Berk M. A potential role for N-acetylcysteine in the management of methamphetamine dependence. Drug Alcohol Rev. 2017;36:153–159. doi: 10.1111/dar.12414. [DOI] [PubMed] [Google Scholar]
- 17.Duailibi M.S., Cordeiro Q., Brietzke E., Ribeiro M., LaRowe S., Berk M., Trevizol AP. N-acetylcysteine in the treatment of craving in substance use disorders: Systematic review and meta-analysis. Am J addict. 2017;26:660–666. doi: 10.1111/ajad.12620. [DOI] [PubMed] [Google Scholar]
- 18.Mousavi S.G., Sharbafchi M.R., Salehi M., Peykanpour M., Karimian Sichani N., Maracy M. The efficacy of N-acetylcysteine in the treatment of methamphetamine dependence: a double-blind controlled, crossover study. Arch Iran Med. 2015;18:28–33. [PubMed] [Google Scholar]
- 19.Kelly P.J., Robinson L.D., Baker A.L., Deane F.P., McKetin R., Hudson S., Keane C. Polysubstance use in treatment seekers who inject amphetamine: drug use profiles, injecting practices and quality of life. Addict Behav. 2017;71:25–30. doi: 10.1016/j.addbeh.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Berk M., Malhi G.S., Gray L.J., Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34:167–177. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Voce A., McKetin R., Calabria B., Burns R., Castle DJ. A systematic review of the symptom profile and course of methamphetamine associated psychosis. Subst Use Misuse. 2019;54(4):549–559. doi: 10.1080/10826084.2018.1521430. [DOI] [PubMed] [Google Scholar]
- 22.McKetin R., Dawe S., Burns R.A., Hides L., Kavanagh D.J., Teesson M., Mc D.Y.R., Voce A., Saunders JB. The profile of psychiatric symptoms exacerbated by methamphetamine use. Drug Alcohol Depend. 2016;161:104–109. doi: 10.1016/j.drugalcdep.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Cetylev (TM) (acetylcysteine) effervescent tablets for oral solution [Full prescribing information]. 2016.
- 24.McKetin R., Dean O.M., Turner A., Kelly P.J., Quinn B., Lubman D.I., Dietze P., Carter G., Higgs P., Baker A.L., Sinclair B., Reid D., Manning V., te Pas N., Liang W., Thomas T., Bathish R., Kent M., Raftery D., Arunogiri S., Cordaro F., Hill H., Berk M. A study protocol for the N-ICE trial: A randomised double-blind placebo-controlled study of the safety and efficacy of N-acetyl-cysteine (NAC) as a pharmacotherapy for methamphetamine (“ice”) dependence. Trials. 2019;20:325. doi: 10.1186/s13063-019-3450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asberg M., Montgomery S.A., Perris C., Schalling D., Sedvall G. A comprehensive psychopathological rating scale. Acta Psychiatr Scand Suppl. 1978:5–27. doi: 10.1111/j.1600-0447.1978.tb02357.x. [DOI] [PubMed] [Google Scholar]
- 26.Lukoff D., Nuechterlein K.H., Ventura J. Manual for the expanded brief psychiatric rating scale. Schizophr Bull. 1986;12:594–602. [Google Scholar]
- 27.Andrews G., Peters L. The psychometric properties of the composite international diagnostic interview. Soc Psychiatry Psychiatr Epidemiol. 1998;33:80–88. doi: 10.1007/s001270050026. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar GC. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4-57. [PubMed] [Google Scholar]
- 29.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKetin R., Dean O.M., Turner A., Kelly P.J., Quinn B., Lubman D.I. A study protocol for the N-ICE trial: A randomised double-blind placebo-controlled study of the safety and efficacy of N-acetyl-cysteine (NAC) as a pharmacotherapy for methamphetamine (“ice”) dependence. Trials. 2019;20:325. doi: 10.1186/s13063-019-3450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobell L.C., Sobell MB. Addiction Research Foundation; Toronto: 1996. Alcohol timeline followback (TLFB) users’ manual. [Google Scholar]
- 32.Kiluk B.D., Carroll K.M., Duhig A., Falk D.E., Kampman K., Lai S., Litten R.Z., McCann D.J., Montoya I.D., Preston K.L., Skolnick P., Weisner C., Woody G., Chandler R., Detke M.J., Dunn K., Dworkin R.H., Fertig J., Gewandter J., Moeller F.G., Ramey T., Ryan M., Silverman K., Strain EC. Measures of outcome for stimulant trials: ACTTION recommendations and research agenda. Drug Alcohol Depend. 2016;158:1–7. doi: 10.1016/j.drugalcdep.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trivedi M.H., Greer T.L., Potter J.S., Grannemann B.D., Nunes E.V., Rethorst C., Warden D., Ring K.M., Somoza E. Determining the primary endpoint for a stimulant abuse trial: lessons learned from STRIDE (CTN 0037) Am J Drug Alcohol Abus. 2011;37:339–349. doi: 10.3109/00952990.2011.598589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gossop M., Darke S., Griffiths P., Hando J., Powis B., Hall W., Strang J. The severity of dependence scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction. 1995;90:607–614. doi: 10.1046/j.1360-0443.1995.9056072.x. [DOI] [PubMed] [Google Scholar]
- 35.May J., Andrade J., Kavanagh D.J., Feeney G.F.X., Gullo M.J., Statham D.J., Skorka-Brown J., Connolly J.M., Cassimatis M., Young R.M., Connor JP. The craving experience questionnaire: a brief, theory-based measure of consummatory desire and craving. Addiction. 2014;109:728–735. doi: 10.1111/add.12472. [DOI] [PubMed] [Google Scholar]
- 36.Srisurapanont M., Jarusuraisin N., Jittiwutikan J. Amphetamine withdrawal: I. Reliability, validity and factor structure of a measure. Aust N Z J Psychiatry. 1999;33:89–93. doi: 10.1046/j.1440-1614.1999.00517.x. [DOI] [PubMed] [Google Scholar]
- 37.MIMS Australia Pty Ltd . 2021 MIMS Australia; Australia: 2021. MIMSOnline.http://www.mimsonline.com.au [Google Scholar]
- 38.Atkinson M.J., Kumar R., Cappelleri J.C., Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM Version II) among outpatient pharmacy consumers. Value Health. 2005;8:S9–S24. doi: 10.1111/j.1524-4733.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 39.Medical Dictionary for Regulatory Activities (MedDRA®). Pharmacovigilance; 2006: 168-83.
- 40.Mooney C.Z., Duval RD. SAGE; Newbury Park, CA: 1993. Bootstrapping: a nonparametric approach to statistical inference. [Google Scholar]
- 41.LaRowe S.D., Kalivas P.W., Nicholas J.S., Randall P.K., Mardikian P.N., Malcolm RJ. A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence. Am J Addict. 2013;22:443–452. doi: 10.1111/j.1521-0391.2013.12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray K.M., Carpenter M.J., Baker N.L., DeSantis S.M., Kryway E., Hartwell K.J., McRae-Clark A.L., Brady KT. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents.[Erratum appears in Am J Psychiatry. 2012 Aug 1;169(8):869] Am J Psychiatry. 2012;169:805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gray K.M., Sonne S.C., McClure E.A., Ghitza U.E., Matthews A.G., McRae-Clark A.L., Carroll K.M., Potter J.S., Wiest K., Mooney L.J., Hasson A., Walsh S.L., Lofwall M.R., Babalonis S., Lindblad R.W., Sparenborg S., Wahle A., King J.S., Baker N.L., Tomko R.L., Haynes L.F., Vandrey R.G., Levin FR. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 2017;177:249–257. doi: 10.1016/j.drugalcdep.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connor J.P., Gullo M.J., White A., Kelly AB. Polysubstance use: diagnostic challenges, patterns of use and health. Curr Opin Psychiatry. 2014;27:269–275. doi: 10.1097/YCO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 45.Hróbjartsson A., Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010:CD003974. doi: 10.1002/14651858.CD003974.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutherford B.R., Roose SP. A model of placebo response in antidepressant clinical trials. Am J Psychiatry. 2013;170:723–733. doi: 10.1176/appi.ajp.2012.12040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burke M.J., Kaptchuk T.J., Pascual-Leone A. Challenges of differential placebo effects in contemporary medicine: the example of brain stimulation. Ann Neurol. 2019;85:12–20. doi: 10.1002/ana.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kam-Hansen S., Jakubowski M., Kelley J.M., Kirsch I., Hoaglin D.C., Kaptchuk T.J. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med. 2014;6:218ra5. doi: 10.1126/scitranslmed.3006175. this is correct - see the webpage: https://pubmed.ncbi.nlm.nih.gov/24401940/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hróbjartsson A. What are the main methodological problems in the estimation of placebo effects? J Clin Epidemiol. 2002;55:430–435. doi: 10.1016/s0895-4356(01)00496-6. [DOI] [PubMed] [Google Scholar]
- 50.Hróbjartsson A., Kaptchuk T.J., Miller FG. Placebo effect studies are susceptible to response bias and to other types of biases. J Clin Epidemiol. 2011;64:1223–1229. doi: 10.1016/j.jclinepi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linde K., Fässler M., Meissner K. Placebo interventions, placebo effects and clinical practice. Philos Trans R Soc Lond B Biol Sci. 2011;366:1905–1912. doi: 10.1098/rstb.2010.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hróbjartsson A., Norup M. The use of placebo interventions in medical practice—a national questionnaire survey of Danish clinicians. Eval Health Prof. 2003;26:153–165. doi: 10.1177/0163278703026002002. [DOI] [PubMed] [Google Scholar]
- 53.Berk M., Dean O.M., Cotton S.M., Jeavons S., Tanious M., Kohlmann K., Hewitt K., Moss K., Allwang C., Schapkaitz I., Robbins J., Cobb H., Ng F., Dodd S., Bush A.I., Malhi GS. The efficacy of adjunctive N-acetylcysteine in major depressive disorder: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2014;75:628–636. doi: 10.4088/JCP.13m08454. [DOI] [PubMed] [Google Scholar]
- 54.LaRowe S.D., Myrick H., Hedden S., Mardikian P., Saladin M., McRae A., Brady K., Kalivas P.W., Malcolm R. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 55.Schulte M., Goudriaan A.E., Kaag A.M., Kooi D.P., van den Brink W., Wiers R.W., Schmaal L. The effect of N-acetylcysteine on brain glutamate and gamma-aminobutyric acid concentrations and on smoking cessation: a randomized, double-blind, placebo-controlled trial. J Psychopharmacol. 2017;31:1377–1379. doi: 10.1177/0269881117730660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.