Highlights
-
•
Discriminatory experiences are associated with thinner cortex across time.
-
•
Age, gender, and race/ethnicity significantly moderate these associations.
-
•
Results persist in healthy subjects and those at clinical high risk for psychosis.
-
•
Cortical thickness partially mediates links between discrimination and anxiety.
Keywords: Discrimination, Social adversity, Cortical thickness, Neurodevelopmental trajectory, Clinical high risk for psychosis, Anxiety
Abstract
Individuals face discrimination based on characteristics including race/ethnicity, gender, age, and disability. Discriminatory experiences (DE) are associated with poor psychological health in the general population and with worse outcomes among individuals at clinical high risk for psychosis (CHR). Though the brain is sensitive to stress, and brain structural change is a well-documented precursor to psychosis, potential relationships between DE and brain structure among CHR or healthy individuals are not known. This report assessed whether lifetime DE are associated with cortical thinning and clinical outcomes across time, after controlling for discrimination-related demographic factors among CHR individuals who ultimately do (N = 57) and do not convert to psychosis (N = 451), and healthy comparison (N = 208) participants in the North American Prodrome Longitudinal Study 2. Results indicate that DE are associated with thinner cortex across time in several cortical areas. Thickness in several right hemisphere regions partially mediates associations between DE and subsequent anxiety symptoms, but not attenuated positive symptoms of psychosis. This report provides the first evidence to date of an association between DE and brain structure in both CHR and healthy comparison individuals. Results also suggest that thinner cortex across time in areas linked with DE may partially explain associations between DE and cross-diagnostic indicators of psychological distress.
1. Introduction
Discrimination constitutes unfair or unjust treatment on the basis of a personal characteristic that is stigmatized, including race/ethnicity, gender, sexual orientation, appearance, religion, and disability (Brown et al., 2000, Carr and Friedman, 2005, Hatch and Dohrenwend, 2007, Kessler et al., 1999, LaVeist et al., 2003, Lee et al., 2019, Pérez et al., 2008, Thoits, 2010). Discrimination may act as an uncontrollable and unpredictable stressor that strains psychological resources and coping mechanisms, thereby compromising psychological well-being (Grollman, 2012, Pascoe and Richman, 2009, Sanders-Phillips et al., 2009, Williams and Mohammed, 2009) and contributing to worse physical and psychological health (Brody et al., 2012, Everett et al., 2016, Kessler et al., 1999, Pascoe and Richman, 2009, Pearce et al., 2019, Schmitt et al., 2014, Williams et al., 2003, Williams and Mohammed, 2009). Furthermore, discrimination appears to remain associated with adverse mental health outcomes after considering other life stressors (Thoits, 2010, Williams et al., 2003).
Prior work indicates that discriminatory experiences (DE), often called perceived discrimination,a are associated with the severity and/or frequency of psychotic and psychotic-like experiences (Berg et al., 2011, Pearce et al., 2019, Shaikh et al., 2016). Among clinical high risk (CHR) participants in the North American Prodrome Longitudinal Study 2 (NAPLS2), prior work has identified associations between DE and negative schemas about oneself and others (Saleem et al., 2014), and between DE and attenuated positive symptoms (APS; Stowkowy et al., 2016). Though CHR participants reported significantly more DE, bullying, and other forms of trauma compared with healthy comparison (HC) participants, only DE predicted later conversion to psychosis (Stowkowy et al., 2016). A well-documented diagnostic bias towards racial and ethnic minorities (Schwartz and Blankenship, 2014) may partially account for associations between DE and psychotic-like symptoms, though these associations are also present in studies involving participants from the same racial/ethnic background (Combs et al., 2006, Oh et al., 2014), and in studies assessing DE based on factors aside from race/ethnicity (Gevonden et al., 2015, Rippy and Newman, 2006). Furthermore, although CHR participants may self-report more DE as a result of clinically-elevated paranoia, prior work indicates that self-reported DE are more strongly associated with non-clinical paranoia compared with clinically-significant paranoid symptoms (Combs et al., 2006, Rippy and Newman, 2006).
Subthreshold psychotic experiences are typically transitory and only develop into a psychotic disorder in a small proportion of cases, and thus may be best conceptualized as a continuum of attenuated positive, negative, and affective symptoms (e.g. depression, anxiety) (Stefanis et al., 2002, van Os et al., 2009, Wigman et al., 2012). Social adversity and/or genetic vulnerability can cause symptoms to become abnormally persistent and impairing and lead to a poor prognosis in the form of a psychotic disorder or non-remitting subclinical symptoms (Van Os et al., 2009). Within the domain of affective symptoms, anxiety disorders are prominent among CHR individuals (Addington et al., 2011, Woods et al., 2009), and 51% of CHR participants in the NAPLS2 study met criteria for at least one anxiety disorder (McAusland et al., 2017). Though not associated with increased conversion risk, anxiety symptoms often cause more subjective distress than subthreshold psychotic symptoms (Fusar-Poli et al., 2014, Haroun et al., 2006, Hartley et al., 2013, Huppert and Smith, 2005, Lim et al., 2015, McAusland et al., 2017, Schlosser et al., 2012). DE have been linked with transdiagnostic psychiatric symptoms including anxiety, low mood, and low self-esteem (Kessler et al., 1999, Pascoe and Richman, 2009, Williams and Mohammed, 2009). Therefore, investigating links among DE and both anxiety and APS is important for understanding outcomes among CHR individuals, given that anxiety may be understood as a general risk indicator of persistent or worsening psychopathology, whereas APS are specific risk indicators of persistent or worsening core psychotic symptoms.
An important question remains regarding how neural mechanisms may link DE to illness outcomes. Brain structure is sensitive to environmental inputs including stress due to social adversity (McEwen, 2012, McEwen and Gianaros, 2010), and contributes to cognitive and psychological functioning in psychotic illness (Antonova et al., 2004) and other psychiatric conditions (Blakemore and Choudhury, 2006, Goodkind et al., 2015). Many studies to date have examined cortical volume as an index of brain structure, though its component parts—cortical thickness and surface area—are genetically, developmentally, and evolutionarily distinct (Panizzon et al., 2009, Rakic, 1995, Raznahan et al., 2011, Wierenga et al., 2014). Specifically, cortical thickness is a commonly used anatomical measure shown to index neuronal density, cytoarchitecture, and hierarchical organization of the cortex (Cahalane et al., 2012, Valk et al., 2020, Wagstyl et al., 2015). Developmental trajectories of neuroanatomical change are often a better marker of maturation or pathology than measures of brain structure at any one time point (Giedd and Rapoport, 2010, Gogtay et al., 2011). Of relevance to this report, aberrant neuromaturational changes during adolescence and young adulthood may play a role in psychotic illness onset (Borgwardt et al., 2008, Cannon et al., 2015, Pantelis et al., 2003, Sun et al., 2009, Takahashi et al., 2009a, Takahashi et al., 2009b, Ziermans et al., 2012). These prior studies have identified gray matter loss in widespread areas of the temporal, frontal, and parietal cortex associated with conversion to psychosis. In particular, progressive cortical volume decrease in the right superior frontal, middle frontal, and medial orbitofrontal cortex predicts conversion among participants in the NAPLS2 cohort (Cannon et al., 2015). Thus, DE could contribute to risk for conversion or other outcomes by acting as a catalyst for brain structural changes that contribute to psychotic and/or other psychiatric illnesses.
DE may influence brain structure via stress-related signaling cascades. Social defeat theory (Björkqvist, 2001) describes how being an outsider in one’s social environment (e.g. experiencing stigma) induces chronic stress and prolonged threat, which in turn cause hypothalamic-pituitary-adrenocortical (HPA) axis activation (McEwen, 2012). Stress exposure triggers glucocorticoid secretion (cortisol in humans), which governs the responsiveness of the HPA axis to stress (Lupien et al., 2009). Glucocorticoids are key for dendritic and axonal remodeling (Meyer, 1983), and they significantly alter neuronal maturation through impacting neuronal structure, synapse formation, and myelination, and delaying neurogenesis (Seckl, 2008). These same processes are hypothesized to be involved in determining cortical thickness in humans (Cahalane et al., 2012, Valk et al., 2020, Wagstyl et al., 2015). Stress sensitivity and stress-induced HPA axis activity is heightened during adolescence and young adulthood (Lupien et al., 2009, Perlman et al., 2007), providing a potential pathway through which DE could impact brain structure during this developmental period. However, given that DE remain uniquely associated with psychological health outcomes after controlling for other stressors (Thoits, 2010, Williams et al., 2003), it remains an open question whether associations between DE and brain structure would follow patterns observed in studies of other types of stress.
This report studied NAPLS2 CHR and HC participants who provided information on lifetime DE and completed at least one magnetic resonance imaging (MRI) scan. A first objective was to determine if DE predict change over time in cortical thickness, and if demographic and clinical factors associated with DE (e.g. gender, race/ethnicity, life stress, clinical group, paranoia) and/or neuroanatomical change (e.g. linear and nonlinear effects of age) moderate this relationship. Given prior associations between social stress and brain structure among clinical and non-clinical populations (Combs et al., 2006, Rippy and Newman, 2006), we hypothesized that both CHR and HC participants who experienced more DE would show steeper rates of cortical thinning across time, even after accounting for participant characteristics that covary with discrimination. A second objective was to determine if cortical thickness in DE-sensitive brain areas partially mediated associations between DE and anxiety and/or APS, and how these relationships are impacted by relevant participant characteristics. We hypothesized that brain structure would partially mediate associations between DE and the severity of both types of symptoms, and that mediation effects would be stronger among stigmatized groups.
2. Materials and methods
2.1. Subjects
Participants in NAPLS2 were evaluated at eight data collection sites and provided consent/parental assent to participate in accordance with Institutional Review Board-approved guidelines at each site. CHR participants were help-seeking and either self-referred or were referred through medical providers, educators, or social service agencies. All CHR participants met the Criteria of Psychosis-Risk Syndromes (COPS; McGlashan et al., 2010), assessed by the Structured Interview for Psychosis-Risk Syndromes (SIPS). General exclusion criteria included a lifetime history of meeting DSV-IV criteria for a psychotic disorder, neurological disorder, substance dependence, or a full-scale IQ < 70. HC participants were additionally excluded if they had a first-degree relative with a current or past psychotic disorder. Participant recruitment and clinical assessments are described in detail elsewhere (Addington et al., 2015).
Participants included in this report completed one (N = 312), two (N = 234), or three (N = 170) MRI scans, which were typically completed at baseline, 12-months, and 24-months. If a participant converted to psychosis, as assessed by a treatment provider or a member of the study team at a scheduled visit, they completed a final MRI scan at the time of conversion. Exclusion criteria for this report are described in Supplementary Methods. 716 participants met inclusion criteria, including 57 who ultimately converted to psychosis (CHR-C), 451 who did not convert (CHR-NC), and 208 HC participants. Of these, 615 who completed repeat assessments of the Scale of Psychosis-Risk Symptoms (SOPS) and the Self-Rating Anxiety Scale (SAS) at least one month after their initial imaging visit were included in mediation analyses (N = 45 CHR-C, 383 CHR-NC, and 187 HC). See Supplementary Table 1 for participant demographics.
2.2. Procedures
2.2.1. Demographic information
Participants self-reported their age, gender, race, and ethnicity. All participants reported their gender as either male or female. Participants reported their ethnicity as Hispanic or non-Hispanic and reported their racial background from one of 10 categories: First Nations, East Asian, Southeast Asian, South Asian, Black, Central/South American, West/Central Asia and Middle East, White, Native Hawaiian/Pacific Islander, or Multiracial. Based on the sample sizes within each group, participant race/ethnic was categorized as either non-Hispanic white (N = 371) or as a racial minority and/or Hispanic (N = 345) for the purposes of this report. Detailed information on the number of participants of each race and ethnicity is provided in Supplementary Table 2.
2.2.2. Rating scales
DE were assessed at baseline using a modified self-report measure (Janssen et al., 2003) in which participants reported lifetime discrimination due to skin color, ethnicity, gender, age, appearance, disability, sexual orientation, religion, or other characteristics. Total DE scores were calculated as the number of types of discrimination endorsed, with possible scores ranging 0–9. The DE score distribution was right skewed (Supplementary Fig. 1) and therefore scores were compressed to a 0–6 scale, with scores 7, 8, and 9 rescored to 6, to avoid heteroskedasticity in regression analyses. Supplementary Table 3 shows the proportion of participants who endorsed each type of discrimination.
The SAS is a self-report scale measuring anxiety that manifests as motor, autonomic, cognitive, and central nervous system symptoms (Zung, 1971). The SOPS is a 19-item scale embedded within the SIPS (McGlashan et al., 2010) that assesses four domains of attenuated psychotic symptoms—Positive, Negative, Disorganization, and General Symptoms. Due to well-documented associations with DE, APS are considered as outcome measures (P1-unusual thought content/delusional ideas, P2-suspiciousness/persecutory ideas, P3-grandiose ideas, P4-perceptual abnormalities, P5-disorganized communication).
Cumulative life events stress (LES) was considered as a moderator in all analyses. LES scores were calculated by summing ratings of subjective stress across a modified list of events on the Life Events Scale (Dohrenwend et al., 1978).
2.2.3. MRI processing and quality
MRI acquisition parameters, inter-scanner reliability estimates, and quality control procedures are published in detail elsewhere (Cannon et al., 2015). Briefly, in terms of reliability, eight healthy subjects (4 males, 4 females) were scanned twice on successive days at each of the eight sites. Between-site and test–retest reliabilities were calculated using intraclass correlations and reliability estimates were excellent across scanners (Cannon et al., 2015). As part of NAPLS2 study design, two high resolution MRI scans were obtained at each assessment timepoint for each subject; the better of the two images was selected through visual inspection and underwent visual quality control to assess artifact due to motion, skull strip errors, segmentation or intensity normalization failures, white and pial surface misplacements, and topological defects.
T1 structural MR images were processed with FreeSurfer v5.3.b This process involves automatic whole-brain segmentation and surface-based cortical reconstruction in which surface-based thickness measures were extracted from each scan by calculating the shortest distance from each point on the gray/white matter boundary to the pial surface of each cortical vertex (Fischl et al., 2002, Fischl and Dale, 2000). T1 images were further processed using FreeSurfer’s longitudinal pipeline (Reuter et al., 2012). This pipeline registers each MR image to an unbiased within-subject template utilizing robust, inverse consistent registration (Reuter et al., 2010), which increases statistical power for detecting subtle changes in brain morphology over time (Reuter et al., 2012). Participants’ thickness maps were resampled from native subject space to a common space (fsaverage5) containing 10,242 vertices per hemisphere and 15 mm full-width half-maximum (FWHM) smoothing was applied, a level previously reported for surface-based analyses (Reuter et al., 2015).
2.3. Statistical analyses
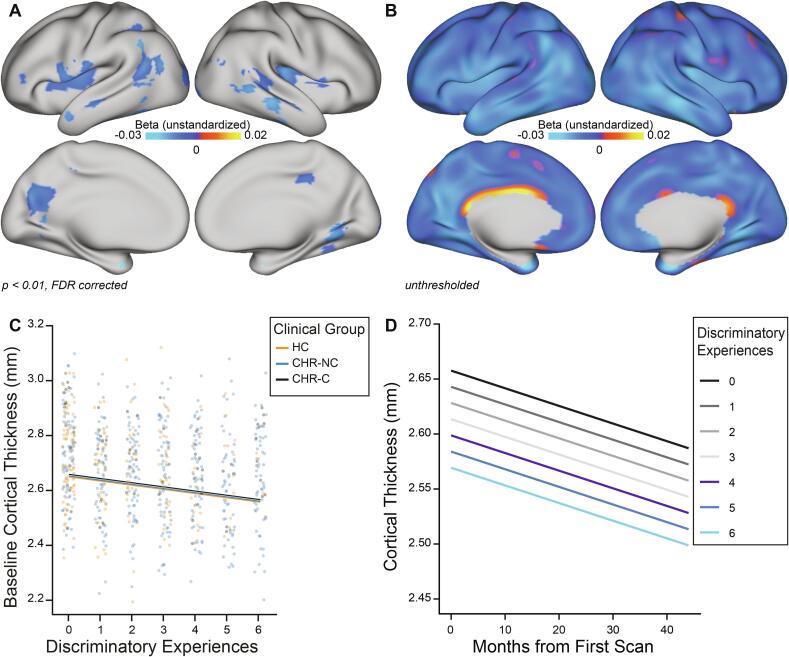
Vertex-wise cortical thickness was analyzed using a FreeSurfer MATLAB toolbox designed for spatiotemporal linear mixed effects (LME) modeling (Bernal-Rusiel et al., 2013a, Bernal-Rusiel et al., 2013b). Initial discovery analyses were conducted to determine the relationship between DE at baseline and cortical thickness change over time. Briefly, a likelihood ratio test was applied to compare a model with one random effect (random subject-specific intercept) to one with two random effects (random subject-specific intercept and random subject-specific slope). DE, time in months from first scan, and scanner were included as fixed effect predictors. The null hypothesis of no interaction between DE and time was tested, and vertex-wise FDR correction was applied using a built-in function in the FreeSurfer toolbox to correct for multiple comparisons across vertices and hemispheres. After determining that there is not a significant interaction between DE and time, relationships between DE and cortical thickness were further visualized at baseline and across time. This was accomplished by plotting baseline cortical thickness (averaged across all vertices significantly associated with DE) by the number of discriminatory experiences, with a separate best fit line for each clinical group, and by plotting cortical thickness across time, with a separate best fit line for each number of DE (0–6).
Further analyses examined the effects of variables hypothesized to moderate associations between DE and cortical thickness using a region of interest (ROI) from each hemisphere comprised of vertices associated with DE. These regions are hereafter referred to as the left ROI and right ROI for simplicity. A separate region was evaluated per hemisphere, given that FreeSurfer statistical analyses are performed separately by hemisphere and there are well-documented functional differences between hemispheres (Esteves et al., 2021, Toga and Thompson, 2003). A ROI approach was used for moderation and mediation analyses rather than a vertex-wise approach to permit complex model fitting and comparison methods optimized for LME models. However, to establish if the spatial pattern of effects differs when moderator variables known to covary with DE and brain structure were considered, vertex-level LME models including age, gender, race/ethnicity, and clinical group were conducted in a supplementary analysis.
Separate LME analyses were conducted in R using the lme4 package (Bates et al., 2014) to assess whether the relationship between DE and thickness in the left and right ROIs remained significant after accounting for each of the following seven moderators: 1) age at first scan, 2) age2, 3) gender, 4) race/ethnicity (coded as non-Hispanic white vs. racial minority and/or Hispanic), 5) clinical group (HC, CHR-NC, CHR-C), 6) baseline LES, and 7) baseline paranoid thinking (P2 SOPS score). Age, age2, gender, race/ethnicity, and clinical group were included as moderators based on well-documented relationships with DE and/or cortical thickness. Given that the typical developmental trajectory of cortical thickness is nonlinear within the age range examined in this study (12–36 years) (Giedd et al., 1999, Tamnes et al., 2017), non-linear age effects were assessed by adding a term for age at baseline squared. To assess whether DE and cortical thickness are associated after accounting for other forms of stress, LES was included as a moderator. Finally, baseline P2 SOPS scores were included as an additional moderator due to the potential for a bidirectional relationship between DE and paranoia. All models included time from baseline and MRI scanner as covariates. A final, composite model including DE and all significant moderators was constructed based on the results of likelihood ratio tests, and FDR correction was applied to account for comparisons across the left and right ROI. Though income level is an additional psychosocial stressor and socioeconomic status is associated with DE (Watson et al., 2002, Wickham et al., 2014, Williams et al., 2009), income was not considered as a primary moderator due to a high rate of missing data (140 participants included in this report, many of whom were minors, did not report household income). Model comparison procedures are described in detail in Supplementary Methods and final models with and without income are compared in Supplementary Table 8.
Mediation analyses explored the hypothesis that cortical thickness in the left and/or right ROIs (assessed at each participant’s first scan) partially mediates associations between lifetime DE and APS or anxiety symptoms assessed close to one year from baseline (mean 10.6 months for APS, mean 10.7 months for anxiety). Mediation analyses were conducted in R using the mediation package (Tingley et al., 2014). Significant moderators of the relationship between DE and thickness at first scan were determined using likelihood ratio testing (as described above) and were included in the best fit equation for the mediator (left ROI or right ROI). Significant moderators of the relationship between DE and each outcome measure (anxiety, APS) were included in the best fit equation for that outcome variable (indirect and direct paths). A different set of moderators for the mediator and outcome equations was considered, given the assumption that different moderators may impact each variable (Pearl, 2014). Significance of mediation effects was assessed using 95% confidence intervals based on 10,000 Monte Carlo simulations drawn from a quasi-Bayesian approximation as an alternative to bootstrapping (see Supplementary Methods). FDR correction was applied to mediation results to account for multiple comparisons across hemispheres and outcome measures.
After determining that the right ROI significantly mediates the association between DE and anxiety symptoms, post-hoc analyses were conducted to determine which particular region(s) within the right ROI may primarily contribute to this association. Since the right ROI encompasses functionally and structurally distinct elements of cortex, a parcellation that divided each cortical hemisphere into 34 gyral-based regions of interest by making use of prior knowledge of structure–function relationships was applied to the right hemisphere (i.e. Desikan-Killiany parcellation; Desikan et al., 2006). The number of vertices in the right ROI was calculated for each Desikan-Killiany region, and the top five regions (i.e. regions encompassing the greatest number of vertices from the right ROI) were further analyzed by testing if mean thickness in each region significantly mediated the association between DE and anxiety, following the mediation procedures described above. Post-hoc mediation analyses assessing change from baseline to follow-up in outcome measures were conducted in supplementary analyses, to clarify whether DE and cortical thickness relate to symptom change as well as individual differences in symptom measures.
A final objective was to determine how each moderator variable of interest affects the strength of the mediation relationship between DE, right ROI thickness, and anxiety. Mediation effects were further analyzed by setting each moderator of interest to a different value (e.g. comparing the mediation model with gender set to female vs. gender set to male) using the mediation package in R. In these supplementary analyses, all seven moderator variables of interest (listed above) were included in the mediation model, in order to assess how each moderator variable impacted direct and indirect effects. Moderated mediation was completed for each moderator variable separately and is described in further detail in Supplementary Methods.
3. Results
3.1. DE are associated with demographic indicators and clinical outcomes
Demographic characteristics of healthy control (HC), and CHR participants who ultimately converted to psychosis (CHR-C) and did not convert (CHR-NC) are presented in Supplementary Table 1. Groups did not differ significantly by gender, race/ethnicity, or income. The average number of MRI scans completed varied by clinical group (HC > CHR-NC > CHR-C). On average, participants in the CHR-NC and CHR-C groups were older than HC participants and experienced greater LES, APS, and anxiety. Relationships between DE and demographic and clinical outcomes were assessed to determine how DE relate to stigmatized identities (Table 1). Female, racial/ethnic minority, and CHR converter and nonconverter participants reported experiencing more DE. Age and LES were both positively associated with DE, whereas higher income was associated with lower DE.
Table 1.
Associations between DE and demographic and clinical characteristics. All statistics indicate the relationship between discriminatory experiences (DE) and the characteristic listed (t-test, F-test, or Spearman’s correlation). DE scores ranged from 0 to 6. Baseline (BL) measures were calculated for all 716 study participants; follow-up measures were calculated for participants who completed at least one assessment following their first imaging visit (N = 615). P-value terms: ns p > 0.05; * p < 0.05; ** p < 0.01; *** p < 0.001.
| Characteristic | Statistic |
|---|---|
| Gender | Mean male = 2.1, mean female = 2.9, T = -5.0, *** |
| Race/Ethnicity | Mean non-Hispanic white = 2.1, mean racial minority and/or Hispanic = 2.7, T = -6.2, *** |
| Clinical Group (HC, CHR-NC, CHR-C) | Mean HC = 1.7, Mean CHR-NC = 2.7, Mean CHR-C = 2.9, F = 21.1, *** |
| Spearman’s rho (r) | |
| Age at First Scan | r = 0.21, *** |
| Life Events Stress | r = 0.46, *** |
| Income | r = -0.21, *** |
| Anxiety (BL) | r = 0.35, *** |
| Anxiety (Follow-up) | r = 0.35, *** |
| Positive Symptoms (BL) | r = 0.29, *** |
| Positive Symptoms (Follow-up) | r = 0.29, *** |
| P1 (BL) | r = 0.21, *** |
| P2 (BL) | r = 0.28, *** |
| P3 (BL) | r = 0.13, *** |
| P4 (BL) | r = 0.20, *** |
| P5 (BL) | r = 0.24, *** |
APS assessed at baseline and follow-up were moderately positively associated with DE. Though prior theoretical and empirical work has suggested a bidirectional relationship between self-reported DE and paranoia (i.e. P2 on the SOPS) (Pearce et al., 2019), this relationship at baseline was not significantly stronger than the association between DE and other positive symptoms (Fisher’s Z >=1.56, p > 0.11) except for grandiose ideas (i.e. P3 on the SOPS; Fisher’s Z = 2.9, p = 0.003). Results indicated that DE and anxiety symptoms at baseline and follow-up were also moderately positively correlated. Relationships between DE and symptom measures remained significant after accounting for all moderators of interest (age, age2, gender, race/ethnicity, clinical group, LES, and baseline P2 SOPS scores; Anxiety: T = 6.50, p < 0.001 at baseline, T = 6.04, p < 0.001 at follow-up; APS: T = 3.44, p < 0.001 at baseline,c T = 4.68, p < 0.001 at follow-up).
3.2. DE are associated with thinner cortex across time
Likelihood ratio tests indicated that a random subject-specific intercept model fit the data significantly better than a model including a random intercept and random slope. Therefore, vertex-level linear mixed effects (LME) analyses of cortical thickness change consisted of a random subject-specific intercept, with DE, months from first scan, and scanner as fixed effects. Model fit did not improve when the interaction of DE and time from first scan was included, indicating that the rate of cortical thickness change is not significantly associated with DE. After applying a strict threshold (p < 0.01, FDR corrected), DE were found to be associated with thinner cortex across time in the bilateral insula, superior, and middle temporal cortex, as well as aspects of the left hemisphere inferior frontal gyrus, inferior and superior parietal cortex and precuneus, and right hemisphere lingual, fusiform, and posterior cingulate cortex (Fig. 1a). Vertex-level maps including age, gender, race/ethnicity, and clinical group are provided in Supplementary Fig. 2 and are visually similar to those presented in Fig. 1a. Whole-brain, unthresholded statistical maps indicated a pattern of lower cortical thickness associated with more DE across time in almost all cortical areas (Fig. 1b). Relationships between more DE and lower baseline cortical thickness were consistent across clinical groups (Fig. 1c). DE remained associated with lower cortical thickness at each timepoint but was not associated with the rate of cortical thickness change (Fig. 1d), as indicated by a null interaction term between DE and time.
Fig. 1.
Associations between DE and longitudinal cortical thickness. A) False discovery rate (FDR)-corrected maps indicate that discriminatory experiences (DE) are associated with lower cortical thickness across time in aspects of both brain hemispheres, described in detail in the text. B) Unthresholded maps indicate that DE are associated with lower cortical thickness across widespread areas of cortex. Note: unstandardized betas equal the change in cortical thickness (in mm) per one unit increase in DE. C) At baseline, across vertices identified as being significantly associated with DE (part A), more DE are associated with lower cortical thickness and this pattern remains stable across clinical groups (HC, CHR-NC, and CHR-C). D) More DE are associated with lower cortical thickness at each timepoint and the rate of cortical thinning remains consistent across each number of DE.
3.3. Demographic indicators moderate the relationship between DE and cortical thickness
Additional LME analyses assessed how demographic and symptom measures moderated the relationship between DE and the mean thickness across all vertices significantly associated with DE in the left and right hemisphere (left and right ROIs). Testing individual moderator variables through likelihood ratio tests indicated that the best fit model included the fixed effects described in Table 2. In both ROIs, age, age2, gender, and race/ethnicity significantly moderated the association between DE and thickness. Higher age, female gender, and racial/ethnic minority status were each associated with lower cortical thickness across time in the brain areas linked with DE. Importantly, the relationship between more DE and lower cortical thickness in both ROIs remained significant after accounting for these variables. Overall, cortical thickness decreased with time from baseline. Examining the moderating effects of age and age2 indicated that thickness decreased more steeply in the left and right ROIs from approximately ages 12–22 and then gradually began to slow (Supplementary Fig. 3). LES, clinical group, and baseline paranoia (P2 SOPS scores) did not relate to thickness in right or left ROI and excluding them from the model did not significantly impact model fit. Among the subset of participants with data available on household income, including income as a moderator did not improve model fit (Supplementary Table 8). The best-fit model presented in Table 2 was further assessed among only HC participants (N = 208), given that diagnostic group did not significantly moderate the association between DE and left or right ROI thickness. DE were significantly associated with lower thickness over time in the left ROI (unstandardized estimate (β) = -0.01, p < 0.05) but not the right ROI (β = -0.005, p = 0.14) among HC participants.
Table 2.
Significant moderators of the relationship between DE and longitudinal cortical thickness. Unstandardized parameter estimates along with standard error values are presented for the best fit model predicting left ROI and right ROI thickness from discriminatory experiences (DE), time from first scan, scanner, and significant moderators of interest. P-values for fixed effects are calculated using the Kenward-Roger method (Halekoh and Højsgaard, 2014) and are FDR corrected for multiple comparisons across hemispheres. In both hemispheres, DE, time (in months) from first scan, age at first scan, age2, gender, race/ethnicity, and scanner were significant predictors of cortical thickness. Likelihood ratio tests indicated that other moderators of interest (LES, clinical group, baseline P2 SOPS scores) did not improve model fit. P-value terms: ns p > 0.05; * p < 0.05; ** p < 0.01; *** p < 0.001.
| Fixed Effect |
Left ROI Estimate (SE), p-value |
Right ROI Estimate (SE), p-value |
|---|---|---|
| Discriminatory Experiences | −0.01 (0.00), *** | −0.01 (0.00), *** |
| Age at First Scan | −0.03 (0.01), *** | −0.03 (0.01), *** |
| Age2 | 0.00 (0.00), * | 0.00 (0.00), ** |
| Gender | −0.02 (0.01), * | −0.02 (0.01), ** |
| Race/Ethnicity | −0.03 (0.01), *** | −0.03 (0.01), *** |
| Time from first scan | −0.002 (0.00), *** | −0.002 (0.00), *** |
| Scanner |
Estimates vary by scanner F = 6.44, *** |
Estimates vary by scanner F = 3.91, *** |
3.4. Brain structural change partially mediates associations between DE and anxiety
Further analyses were conducted to determine if brain structure in areas associated with DE at first scan partially mediated relationships between DE and anxiety and/or APS at follow-up. Likelihood ratio tests indicated that participant age, age2, race/ethnicity, gender, and scanner significantly moderated associations between DE and right/left ROI thickness at first scan (Supplementary Table 4). Clinical group, baseline P2 SOPS scores, race/ethnicity, and age were significant moderators of the relationship between DE and anxiety (Supplementary Table 5). In a mediation model including scanner and these moderators, thickness in the right ROI partially mediated associations between DE and anxiety (Fig. 2). Specifically, more DE were associated with lower cortical thickness in the right ROI, which in turn was linked with higher anxiety. Moderated mediation analyses indicated that the value of any given moderator did not impact the strength of direct or indirect effects (Supplementary Table 6). Thickness in the left ROI did not significantly mediate associations between DE and anxiety (Supplementary Fig. 4), and therefore the effects of individual moderators were not assessed.
Fig. 2.
Cortical thickness in the right ROI partially mediates the association between DE and anxiety symptoms. Estimates indicate standardized beta coefficients. Lifetime discriminatory experiences (DE) negatively predicted cortical thickness in the right ROI at first scan, and right ROI thickness negatively predicted symptoms of anxiety at follow-up. Significant moderators of each component of the mediation model are listed in the text. P-values reflect FDR corrected estimates accounting for multiple comparisons across hemispheres and outcome measures. c = total effect; c′ = direct effect.
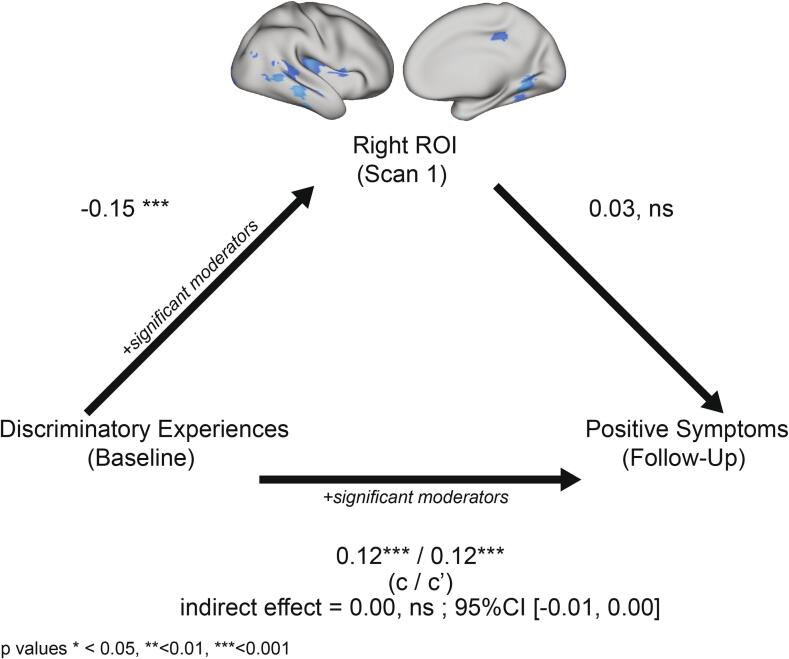
Thickness in neither the right ROI (Fig. 3) nor the left ROI (Supplementary Fig. 5) mediated associations between DE and APS at follow-up in a model including scanner and all moderator variables of interest. Likelihood ratio tests indicated that the relationship between DE and APS was moderated by clinical group and baseline P2 SOPS scores (Supplementary Table 7). Baseline and follow-up outcome measures were significantly correlated (Pearson’s R = 0.70, p < 0.001 for anxiety; Pearson’s R = 0.35, p < 0.001 for APS) and for both outcomes, the difference in scores across timepoints was not associated with left/right ROI thickness. More DE were associated with an increase in APS from baseline to follow-up, but DE were not associated with change in anxiety symptoms (Supplementary Table 9).
Fig. 3.
Cortical thickness in the right ROI does not mediate the association between DE and APS. Estimates indicate standardized beta coefficients. Lifetime discriminatory experiences (DE) negatively predicted cortical thickness in the right ROI at first scan, but right ROI thickness did not predict attenuated positive symptoms (APS) at follow-up. Significant moderators of each component of the mediation model are listed in the text. P-values reflect FDR corrected estimates accounting for multiple comparisons across hemispheres and outcome measures. c = total effect; c′ = direct effect.
Post-hoc analyses were conducted to further examine mediation effects in the right ROI with respect to anxiety. The five Desikan-Killiany (Desikan et al., 2006) regions containing the highest number of vertices from the right ROI were tested as mediators of the relationship between DE and anxiety (Table 3), including the same moderators determined to be significant in mediation analyses for the right ROI (see above and Supplementary Tables 3 and 4). The right middle temporal gyrus (MTG) significantly mediated the relationship between DE and anxiety symptoms, whereas thickness in the other four regions (right insula, lingual gyrus, superior temporal sulcus, and lateral occipital cortex) did not.
Table 3.
Post-hoc analyses testing thickness in Desikan-Killiany regions of interest as mediators of the relationship between DE and anxiety. Table shows the five Desikan-Killiany (Desikan et al., 2006) regions containing the highest number of vertices from the right ROI. Vertices in the right ROI comprised between 16 and 54 percent of the total vertices in each Desikan-Killiany region. Thinner cortex in the right middle temporal gyrus (MTG) was associated with higher anxiety scores at follow-up, whereas thickness in the right insula, lingual gyrus, banks of the superior temporal sulcus (STS) and lateral occipital cortex was not associated with anxiety. c = total effect; c′ = direct effect. P-value terms: ns p > 0.05; * p < 0.05; ** p < 0.01; *** p < 0.001.
| Desikan-Killiany Atlas Region (right hemisphere) | Number of Vertices from Right ROI | Number of Right ROI Vertices/Total Vertices in Region (%) | Mediation Statistics |
|---|---|---|---|
| Insula | 85 | 26.4% | c/c′ = 0.25***/0.25*** indirect effect: 0.00, ns CI: [-0.01, 0.01] |
| Middle temporal gyrus | 72 | 22.6% | c/c′ = 0.25***/0.24*** indirect effect: 0.01* CI: [0.00, 0.02] |
| Lingual gyrus | 70 | 28.2% | c/c′ = 0.25***/0.25*** indirect effect: 0.00, ns CI: [−0.01, 0.01] |
| Banks of the STS | 69 | 53.9% | c/c′ = 0.25***/0.25*** indirect effect: 0.01, ns CI: [0.00, 0.02] |
| Lateral occipital cortex | 59 | 16.0% | c/c′ = 0.25***/0.25*** indirect effect: 0.00, ns CI: [−0.01, 0.01] |
4. Discussion
The results of this study extend prior work demonstrating associations between DE and multiple demographic, stress-related, and symptom characteristics (Table 1) by illuminating a potential mechanistic contributor to these associations in the form of cortical thinning. Specifically, we found that DE are associated with lower thickness across time in bilateral cortical areas (Fig. 1). Relationships between DE and thinner cortex are moderated by several demographic variables (Table 2). This report also demonstrates that baseline thickness in the right hemisphere region associated with DE partially mediates the relationship between lifetime DE and anxiety symptoms—but not APS—assessed at follow-up (Fig. 2, Fig. 3) when considered in conjunction with relevant moderator variables. Furthermore, the value of individual moderators does not impact the strength of mediation effects. Post-hoc analyses suggest that the right MTG may preferentially contribute to the observed mediation between DE and anxiety (Table 3).
Relationships among DE and demographic and clinical characteristics replicate prior work indicating that female, racial/ethnic minority, and mentally ill individuals experience higher levels of stigma (Angermeyer and Dietrich, 2006, Cechnicki et al., 2011, Everett et al., 2016, Pavalko et al., 2003, Pearce et al., 2019, Schmitt et al., 2014). Associations between lower income and more DE are also consistent with past findings (Watson et al., 2002, Wickham et al., 2014, Williams et al., 2009). DE and LES were moderately correlated, in line with work suggesting that DE are social stressors that have unique properties and relationships with health (Thoits, 2010, Williams and Mohammed, 2009). Age was positively correlated with DE in this report. Given the age range of participants (12–36 years old), this finding is unlikely to reflect age-based discrimination found among older adults (Barnes et al., 2008), and instead likely reflects older participants having been exposed to potentially discriminatory events across a longer time period. This report replicates associations between DE and APS among NAPLS2 participants (Stowkowy et al., 2016) and extends findings of an association between DE and anxiety symptoms (Pascoe and Richman, 2009, Schmitt et al., 2014) to a sample of both CHR and HC participants.
Cortical thickness decreased across time in this report, and findings replicate well-documented associations between age and reductions in cortical thickness across adolescence and young adulthood (Gogtay et al., 2004). In line with prior work (Giedd et al., 1999, Tamnes et al., 2017), cortical thickness declined more steeply across adolescence compared with early adulthood, indicated by significant linear and non-linear age effects in linear mixed effects models. Of note, non-significant interactions between DE and time indicate that DE are not associated with a differentially steeper rate of cortical thinning. However, DE have stronger adverse effects on mental health in early life compared with adulthood (Schmitt et al., 2014) and the impacts of early life stress on brain structure and mental health outcomes vary across development (Gee and Casey, 2015, Lupien et al., 2009). Thus, it is possible that DE experienced earlier in life impact rates of cortical maturation outside the time span of this study.
Associations between DE and cortical thickness are also moderated by gender and race/ethnicity. Specifically, this report replicates findings of steeper rates of cortical thinning in females across adolescence in social brain areas including right temporal and left temporoparietal regions (Mutlu et al., 2013). Results also indicate that racial/ethnic minority status is associated with lower cortical thickness in brain areas associated with DE. Relationships between race/ethnicity and brain structure likely reflect, at least in part, epigenetic and environmental factors associated with the construct of race/ethnicity, including stress and reduced access to resources, which impact the underlying neuronal processes hypothesized to influence cortical thickness (Akdeniz et al., 2014, Galanter et al., 2017.; Williams et al., 2003, Williams and Sternthal, 2010). Nevertheless, this report is not poised to investigate nuanced relationships between race/ethnicity and brain structure and we caution against over-interpreting these results.
Cumulative life event stress (LES) did not significantly moderate associations between DE and cortical thickness, despite both theoretical (Thoits, 2010, Williams and Mohammed, 2009) and empirical (Table 1) overlap between DE and other stressors. Though the left ROI contains a small portion of dorsolateral prefrontal cortex, and both ROIs contain temporal and parietal areas previously associated with stress exposure (Brito and Noble, 2014, Hanson et al., 2013, Jednoróg et al., 2012, Noble et al., 2012), overall, regions associated with DE do not reflect those most typically associated with stressful life events. Brain areas associated with DE also do not overlap with aspects of cortex previously associated with conversion to psychosis in the NAPLS2 study sample (Cannon et al., 2015). Clinical group status and baseline paranoid thinking (e.g. P2 SOPS scores) do not moderate associations between DE and left or right ROI thickness, and thickness in these areas does not mediate associations between DE and APS. Weaker associations between DE and cortical thickness among HC participants are likely explained in part by the lower average number of DE experienced by this group. Taken together, these findings may suggest that DE are not associated with brain structure linked to core features of psychosis. However, it is important to note that DE are intercorrelated with the moderators examined in this report. Thus, cortical thickness in areas linked with conversion or core psychotic symptoms could be influenced by demographic and clinical variables that are interrelated with DE.
The overlap between DE and other psychosocial factors is an important theoretical and empirical consideration in this report. In addition to more discriminatory experiences, within the United States racial and ethnic minorities experience income, education, and other resource disparities (Williams et al., 2016), all of which have been demonstrated to impact brain structure as well as mental health (Brito and Noble, 2014, Kim et al., 2018). Women experience higher rates of discrimination, but also experience more life stress (Bale and Epperson, 2015, Hatch and Dohrenwend, 2007) and psychological distress (Rosenfield and Mouzon, 2013) which are also linked with brain structure and long-term mental health (Lupien et al., 2009, McEwen, 2012). Furthermore, participant identities (e.g. race/ethnicity, gender) intersect (Cho et al., 2013), impacting the number of types of DE experienced (Collins, 2002, Samuels and Ross-Sheriff, 2008) and the severity of adverse mental health outcomes (Grollman, 2012). In acknowledgement of the strong relationships between DE and other psychosocial factors, all analyses in this report tested if age, gender, race/ethnicity, LES, clinical group, and baseline paranoia moderated the relationship between brain structure and/or symptom outcomes. Statistically accounting for these moderating factors at the ROI level suggests that links among DE, cortical thickness, and symptom outcomes are not fully explained by other psychosocial factors, though the conceptual connections between DE and these variables remain important to consider. In vertex-level discovery analyses, including age, gender, clinical group, and race/ethnicity as moderators reduced—but did not eliminate—the significant relationship between DE and cortical thickness, but did not substantially alter the visual pattern of effects (Supplementary Fig. 2). This finding is likely due in part to the shared variance between DE and these moderators. Of note, moderated mediation analyses (Supplementary Table 6) indicate that mediation effects are not dependent on the value of any given moderator variable—for example, testing how the right ROI mediates associations among DE and anxiety among males vs. females, or non-Hispanic white participants vs. racial/ethnic minority participants, or HC vs. CHR participants does not yield statistically different patterns. These findings suggest that discrimination merits consideration as a separate predictor of neural and symptom outcomes, beyond its overlap with demographic and psychosocial factors.
Several brain areas associated with DE in this report have previously been linked with anxiety symptoms. Converging evidence from prior work indicates that anxiety disorders and subclinical symptoms of anxiety are associated with thinner cortex in dorsolateral and ventromedial prefrontal, rostral anterior cingulate, superior frontal, insula, precuneus, and temporal areas of cortex (Besteher et al., 2020, Feurer et al., 2021, Frick et al., 2013, Gold et al., 2017, Liu et al., 2019, Newman et al., 2016, Suffren et al., 2019, Zhang et al., 2020). In this report, the left and right ROIs both contain aspects of the insula, precuneus, and lateral temporal regions, and the left ROI contains a small portion of ventrolateral prefrontal cortex. Right hemisphere temporal areas (including the MTG, which partially mediates associations between DE and anxiety in this report), have also been preferentially linked with social cognitive abilities (Schurz et al., 2014). DE may contribute to social cognitive deficits by negatively affecting schemas about oneself and the world (Borders and Liang, 2011, Brondolo et al., 2018, Landau et al., 2010, Mendoza-Denton et al., 2002), as found previously among CHR individuals (Saleem et al., 2014). Schema activation likely involves activation and connectivity among multiple brain areas involved in detecting threat, memory, and social cognition, including aspects of the left and right ROI in this report (e.g. MTG, posterior STS) (Chen et al., 2017, Gilboa and Marlatte, 2017, Mar, 2011, McKenzie et al., 2014, Tse et al., 2011, van Kesteren et al., 2012). Social cognitive processes have been theorized as a mechanism through which DE impacts mental health (Brondolo et al., 2018), and are impaired in individuals with both anxiety and psychotic disorders (Alvi et al., 2020, Bora et al., 2009, Frith, 1992, Washburn et al., 2016). However, directly analyzing social cognitive abilities was beyond the scope of this study and future work is needed to study reltionships among DE, brain structure, and social cognition.
Of note, anxiety was highly consistent from baseline to follow-up, whereas APS were more variable. Additionally, neither DE nor cortical thickness were associated with change over time in anxiety scores once significant moderators were considered (Supplementary Table 9). These findings may suggest that relationships between DE, cortical thickness, and anxiety are relatively stable across time amidst fluctuations in psychotic-like symptoms. The clinical high-risk syndrome only develops into a psychotic disorder in a small percentage of individuals (11% of CHR participants in this report). However, individuals who do not convert to psychosis may still experience longstanding affective symptoms (e.g. anxiety, depression) (Van Os et al., 2009). Further work examining how DE relates to the duration of anxiety symptoms among CHR and non-CHR individuals would be useful in understanding the extent to which DE impacts long-term outcomes.
There are several limitations to the present study. First, DE were measured using retrospective self-report and did not consider the frequency or intensity of discriminatory events (Grollman, 2012, Paradies, 2006, Williams et al., 2003). Additionally, CHR participants may perceive more discrimination as a result of clinically-elevated paranoia, which has been linked to perceptions of social scrutiny and social threat (Kay et al., 1987, Pearce et al., 2019). However, self-reported DE were not more strongly associated with paranoia compared with other positive symptoms (Table 1), and baseline P2 SOPS scores were considered as a covariate in all analyses. Lastly, to clarify the extent to which the relationship between DE and anxiety identified in this report is unique to CHR individuals, future work will benefit from examining how observed relationships generalize in other samples.
5. Conclusions
Among CHR and HC adolescents and young adults, more lifetime discriminatory experiences are associated with thinner cortex across time, even after considering demographic and clinical factors associated with discrimination. Participant age, gender, and race/ethnicity—but not life stress, clinical group, or symptoms of paranoia—moderate associations between DE and cortical thickness over time. Thickness of right hemisphere cortical areas associated with DE partially mediates associations between DE and anxiety, but not attenuated positive symptoms, suggesting that brain structure associated with DE relates more closely with general risk indicators of psychological distress, as opposed to specific symptoms of psychotic disorders.
Acknowledgments
Acknowledgements:
This work was supported by the National Science Foundation (NSF) (No. DGE-1752134) to Ms. Collins, by National Institutes of Health (NIH) grants U01 MH081902 to Dr. Cannon, P50 MH066286 to Dr. Bearden, U01 MH081857 to Dr. Cornblatt, U01 MH82022 to Dr. Woods, U01 MH066134 to Dr. Addington, U01 MH081944 to Dr. Cadenhead, R01 U01 MH066069 to Dr. Perkins, R01 MH076989 to Dr. Mathalon, U01 MH081928 to Dr. Seidman, and U01 MH081988 to Dr. Walker.
Author contributions
M.A.C. and T.D.C conceptualized the study; J.A., C.E.B., K.S.C, B.A.C, D.H.M, T.H.M, D.O.P, L.J.S, M.T.T., E.F.W, S.W.W, and T.D.C designed and organized the whole NAPLS2 consortium and collected the data; Y.C. processed the MRI data; M.A.C. analyzed the data; and M.A.C. and T.D.C drafted the paper with comments from all authors.
Disclosures
Ms. Collins, Dr. Addington, Dr. Bearden, Dr. Cannon, Dr. Cadenhead, Dr. Cornblatt, Dr. Chung, Dr. McGlashan, Dr. Perkins, Dr. Seidman, Dr. Tsuang, and Dr. Walker have no biomedical financial interests or conflicts of interest to disclose. Dr. Mathalon is a consultant for Boehringer-Ingelheim, Cadent Therapeutics, Recognify, and Syndisi. Dr. Woods reports that during the last 36 months he has received sponsor-initiated research funding support from Teva, Boehringer-Ingelheim, Amarex, and SyneuRx. He has consulted to Boehringer-Ingelheim, New England Research Institute, and Takeda. He has been granted US patent no. 8492418 B2 for a method of treating prodromal schizophrenia with glycine agonists and has received royalties from Oxford University Press.
Footnotes
To avoid inadvertently communicating a dichotomy between perceived and “actual” discrimination, authors of this report prefer the term discriminatory experiences to perceived discrimination, while acknowledging that the latter term is widely used and accepted in the literature cited.
FreeSurfer can be accessed at: https://surfer.nmr.mgh.harvard.edu.
Baseline P2 SOPS scores are a component of the baseline APS calculation and therefore were not included as a moderator in the relationship between DE and baseline APS.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102757.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Addington J., Cornblatt B.A., Cadenhead K.S., Cannon T.D., McGlashan T.H., Perkins D.O., Seidman L.J., Tsuang M.T., Walker E.F., Woods S.W., Heinssen R. At clinical high risk for psychosis: outcome for nonconverters. Am. J. Psychiatry. 2011;168(8):800–805. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J., Liu L., Buchy L., Cadenhead K.S., Cannon T.D., Cornblatt B.A., Perkins D.O., Seidman L.J., Tsuang M.T., Walker E.F., Woods S.W., Bearden C.E., Mathalon D.H., McGlashan T.H. North American Prodrome Longitudinal Study (NAPLS 2): the prodromal symptoms. J. Nerv. Ment. Dis. 2015;203:328–335. doi: 10.1097/NMD.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdeniz C., Tost H., Streit F., Haddad L., Wüst S., Schäfer A., Schneider M., Rietschel M., Kirsch P., Meyer-Lindenberg A. Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA Psychiatry. 2014;71:672–680. doi: 10.1001/jamapsychiatry.2014.35. [DOI] [PubMed] [Google Scholar]
- Alvi T., Kouros C.D., Lee J., Fulford D., Tabak B.A. Social anxiety is negatively associated with theory of mind and empathic accuracy. J. Abnorm. Psychol. 2020;129:108–113. doi: 10.1037/abn0000493. [DOI] [PubMed] [Google Scholar]
- Angermeyer M.C., Dietrich S. Public beliefs about and attitudes towards people with mental illness: a review of population studies. Acta Psychiatr. Scand. 2006;113(3):163–179. doi: 10.1111/acp.2006.113.issue-310.1111/j.1600-0447.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- Antonova E., Sharma T., Morris R., Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr. Res. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Bale T.L., Epperson C.N. Sex differences and stress across the lifespan. Nat. Neurosci. 2015;18(10):1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes L.L., de Leon C.F.M., Lewis T.T., Bienias J.L., Wilson R.S., Evans D.A. Perceived Discrimination and Mortality in a Population-Based Study of Older Adults. Am. J. Public Health. 2008;98(7):1241–1247. doi: 10.2105/AJPH.2007.114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, D., Maechler, M., Bolker, B., Walker, S., 2014. lme4: Linear mixed-effects models using Eigen and S4. R package version 1. pp, 1–7.
- Berg A.O., Melle I., Rossberg J.I., Romm K.L., Larsson S., Lagerberg T.V., Andreassen O.A., Hauff E. Perceived discrimination is associated with severity of positive and depression/anxiety symptoms in immigrants with psychosis: a cross-sectional study. BMC Psychiatry. 2011;11:77. doi: 10.1186/1471-244X-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Rusiel J.L., Greve D.N., Reuter M., Fischl B., Sabuncu M.R., Initiative A.D.N. Statistical analysis of longitudinal neuroimage data with linear mixed effects models. Neuroimage. 2013;66:249–260. doi: 10.1016/j.neuroimage.2012.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Rusiel J.L., Reuter M., Greve D.N., Fischl B., Sabuncu M.R. Spatiotemporal linear mixed effects modeling for the mass-univariate analysis of longitudinal neuroimage data. NeuroImage. 2013;81:358–370. doi: 10.1016/j.neuroimage.2013.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besteher B., Gaser C., Nenadić I. Brain structure and subclinical symptoms: a dimensional perspective of psychopathology in the depression and anxiety spectrum. Neuropsychobiology. 2020;79(4-5):270–283. doi: 10.1159/000501024. [DOI] [PubMed] [Google Scholar]
- Björkqvist K. Social defeat as a stressor in humans. Physiol. Behav., Social Stress: Acute and Long-term Effects on. Physiol. Behav. 2001;73(3):435–442. doi: 10.1016/S0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J. Child Psychol. Psychiatry. 2006;47(3-4):296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Bora E., Yucel M., Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr. Res. 2009;109(1-3):1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Borders A., Liang C.T.H. Rumination partially mediates the associations between perceived ethnic discrimination, emotional distress, and aggression. Cultur. Divers. Ethnic Minor. Psychol. 2011;17(2):125–133. doi: 10.1037/a0023357. [DOI] [PubMed] [Google Scholar]
- Borgwardt S.J., McGuire P.K., Aston J., Gschwandtner U., Pflüger M.O., Stieglitz R.-D., Radue E.-W., Riecher-Rössler A. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr. Res. 2008;106(2-3):108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Brito N.H., Noble K.G. Socioeconomic status and structural brain development. Front. Neurosci. 2014;8 doi: 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G.H., Kogan S.M., Chen Y.-f. Perceived discrimination and longitudinal increases in adolescent substance use: gender differences and mediational pathways. Am. J. Public Health. 2012;102(5):1006–1011. doi: 10.2105/AJPH.2011.300588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondolo, E., Blair, I.V., Kaur, A., 2018. Biopsychosocial mechanisms linking discrimination to health: A focus on social cognition.
- Brown T.N., Williams D.R., Jackson J.S., Neighbors H.W., Torres M., Sellers S.L., Brown K.T. “Being black and feeling blue”: the mental health consequences of racial discrimination. Race Soc. 2000;2(2):117–131. [Google Scholar]
- Cahalane D.J., Charvet C.J., Finlay B.L. Systematic, balancing gradients in neuron density and number across the primate isocortex. Front. Neuroanat. 2012;6:28. doi: 10.3389/fnana.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon T.D., Chung Y., He G., Sun D., Jacobson A., van Erp T.G.M., McEwen S., Addington J., Bearden C.E., Cadenhead K., Cornblatt B., Mathalon D.H., McGlashan T., Perkins D., Jeffries C., Seidman L.J., Tsuang M., Walker E., Woods S.W., Heinssen R. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol. Psychiatry, Schizophrenia and Neurodevelopment. 2015;77(2):147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D., Friedman M.A. Is obesity stigmatizing? Body weight, perceived discrimination, and psychological well-being in the United States. J. Health Soc. Behav. 2005;46(3):244–259. doi: 10.1177/002214650504600303. [DOI] [PubMed] [Google Scholar]
- Cechnicki A., Angermeyer M.C., Bielańska A. Anticipated and experienced stigma among people with schizophrenia: its nature and correlates. Soc. Psychiatry Psychiatr. Epidemiol. 2011;46(7):643–650. doi: 10.1007/s00127-010-0230-2. [DOI] [PubMed] [Google Scholar]
- Chen J., Leong Y.C., Honey C.J., Yong C.H., Norman K.A., Hasson U. Shared memories reveal shared structure in neural activity across individuals. Nat. Neurosci. 2017;20(1):115–125. doi: 10.1038/nn.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S., Crenshaw K.W., McCall L. Toward a field of intersectionality studies: theory, applications, and praxis. Signs J. Women Cult. Soc. 2013;38(4):785–810. doi: 10.1086/669608. [DOI] [Google Scholar]
- Collins P.H. Routledge; 2002. Black Feminist Thought: Knowledge, Consciousness, and the Politics of Empowerment. [Google Scholar]
- Combs D.R., Penn D.L., Cassisi J., Michael C., Wood T., Wanner J., Adams S. Perceived racism as a predictor of paranoia among African Americans. J. Black Psychol. 2006;32(1):87–104. [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dohrenwend B.S., Askenasy A.R., Krasnoff L., Dohrenwend B.P. Exemplification of a method for scaling life events: The PERI Life Events Scale. J. Health Soc. Behav. 1978;19(2):205. doi: 10.2307/2136536. [DOI] [PubMed] [Google Scholar]
- Esteves M., Ganz E., Sousa N., Leite-Almeida H. Asymmetrical Brain Plasticity: Physiology and Pathology. Neurosci., Lifestyle Brain Metaplasticity. 2021;454:3–14. doi: 10.1016/j.neuroscience.2020.01.022. [DOI] [PubMed] [Google Scholar]
- Everett B.G., Onge J.S., Mollborn S. Effects of minority status and perceived discrimination on mental health. Popul. Res. Policy Rev. 2016;35(4):445–469. doi: 10.1007/s11113-016-9391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurer C., Suor J.H., Jimmy J., Klumpp H., Monk C.S., Phan K.L., Burkhouse K.L. Differences in cortical thinning across development among individuals with and without anxiety disorders. Depress. Anxiety. 2021;38(3):372–381. doi: 10.1002/da.v38.310.1002/da.23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Frick A., Howner K., Fischer H., Eskildsen S.F., Kristiansson M., Furmark T. Cortical thickness alterations in social anxiety disorder. Neurosci. Lett. 2013;536:52–55. doi: 10.1016/j.neulet.2012.12.060. [DOI] [PubMed] [Google Scholar]
- Frith C.D. Psychology press; 1992. The Cognitive Neuropsychology of Schizophrenia. [Google Scholar]
- Fusar-Poli P., Nelson B., Valmaggia L., Yung A.R., McGuire P.K. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr. Bull. 2014;40(1):120–131. doi: 10.1093/schbul/sbs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanter J.M., Gignoux C.R., Oh S.S., Torgerson D., Pino-Yanes M., Thakur N., Eng C., Hu D., Huntsman S., Farber H.J., Avila P.C., Brigino-Buenaventura E., LeNoir M.A., Meade K., Serebrisky D., Rodríguez-Cintrón W., Kumar R., Rodríguez-Santana J.R., Seibold M.A., Borrell L.N., Burchard E.G., Zaitlen N. Differential methylation between ethnic sub-groups reflects the effect of genetic ancestry and environmental exposures. eLife. 2017;6 doi: 10.7554/eLife.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Casey B.J. The impact of developmental timing for stress and recovery. Neurobiol. Stress, Stress Resilience. 2015;1:184–194. doi: 10.1016/j.ynstr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevonden M.J., Myin-Germeys I., van den Brink W., van Os J., Selten J.P., Booij J. Psychotic reactions to daily life stress and dopamine function in people with severe hearing impairment. Psychol. Med. 2015;45(8):1665–1674. doi: 10.1017/S0033291714002797. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A., Marlatte H. Neurobiology of Schemas and Schema-Mediated Memory. Trends Cogn. Sci. 2017;21(8):618–631. doi: 10.1016/j.tics.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Vyas N.S., Testa R., Wood S.J., Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr. Bull. 2011;37(3):504–513. doi: 10.1093/schbul/sbr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold A.L., Steuber E.R., White L.K., Pacheco J., Sachs J.F., Pagliaccio D., Berman E., Leibenluft E., Pine D.S. Cortical thickness and subcortical gray matter volume in pediatric anxiety disorders. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017;42(12):2423–2433. doi: 10.1038/npp.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M., Eickhoff S.B., Oathes D.J., Jiang Y., Chang A., Jones-Hagata L.B., Ortega B.N., Zaiko Y.V., Roach E.L., Korgaonkar M.S., Grieve S.M., Galatzer-Levy I., Fox P.T., Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman E.A. Multiple forms of perceived discrimination and health among adolescents and young adults. J. Health Soc. Behav. 2012;53(2):199–214. doi: 10.1177/0022146512444289. [DOI] [PubMed] [Google Scholar]
- Halekoh U., Højsgaard S. A kenward-roger approximation and parametric bootstrap methods for tests in linear mixed models–the R package pbkrtest. J. Stat. Softw. 2014;59:1–30. [Google Scholar]
- Hanson J.L., Hair N., Shen D.G., Shi F., Gilmore J.H., Wolfe B.L., Pollak S.D., Baud O. Family poverty affects the rate of human infant brain growth. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0080954. e80954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroun, N., Dunn, L., Haroun, A., Cadenhead, K.S., 2006. Risk and protection in prodromal schizophrenia: ethical implications for clinical practice and future research. [DOI] [PMC free article] [PubMed]
- Hartley S., Barrowclough C., Haddock G. Anxiety and depression in psychosis: a systematic review of associations with positive psychotic symptoms. Acta Psychiatr. Scand. 2013;128(5):327–346. doi: 10.1111/acps.12080. [DOI] [PubMed] [Google Scholar]
- Hatch S.L., Dohrenwend B.P. Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES and age: a review of the research. Am. J. Community Psychol. 2007;40:313–332. doi: 10.1007/s10464-007-9134-z. [DOI] [PubMed] [Google Scholar]
- Huppert J.D., Smith T.E. Anxiety and schizophrenia: the interaction of subtypes of anxiety and psychotic symptoms. CNS Spectr. 2005;10(9):721–731. doi: 10.1017/s1092852900019714. [DOI] [PubMed] [Google Scholar]
- Janssen I., Hanssen M., Bak M., Bijl R.V., De Graaf R., Vollebergh W., McKenzie K., Van Os J. Discrimination and delusional ideation. Br. J. Psychiatry. 2003;182(1):71–76. doi: 10.1192/bjp.182.1.71. [DOI] [PubMed] [Google Scholar]
- Jednoróg K., Altarelli I., Monzalvo K., Fluss J., Dubois J., Billard C., Dehaene-Lambertz G., Ramus F., Frasch M.G. The influence of socioeconomic status on children’s brain structure. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0042486. e42486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Mickelson K.D., Williams D.R. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J. Health Soc. Behav. 1999;40(3):208. doi: 10.2307/2676349. [DOI] [PubMed] [Google Scholar]
- Kim P., Evans G.W., Chen E., Miller G., Seeman T. Handbook of Life Course Health Development. Springer International Publishing; Cham: 2018. How socioeconomic disadvantages get under the skin and into the brain to influence health development across the lifespan; pp. 463–497. [DOI] [PubMed] [Google Scholar]
- Landau M.J., Meier B.P., Keefer L.A. A metaphor-enriched social cognition. Psychol. Bull. 2010;136(6):1045–1067. doi: 10.1037/a0020970. [DOI] [PubMed] [Google Scholar]
- LaVeist T.A., Rolley N.C., Diala C. Prevalence and patterns of discrimination among US health care consumers. Int. J. Health Serv. 2003;33(2):331–344. doi: 10.2190/TCAC-P90F-ATM5-B5U0. [DOI] [PubMed] [Google Scholar]
- Lee R.T., Perez A.D., Boykin C.M., Mendoza-Denton R., Montazeri A. On the prevalence of racial discrimination in the United States. PLoS ONE. 2019;14(1) doi: 10.1371/journal.pone.0210698. e0210698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Rekhi G., Rapisarda A., Lam M., Kraus M., Keefe R.S.E., Lee J. Impact of psychiatric comorbidity in individuals at Ultra High Risk of psychosis—findings from the Longitudinal Youth at Risk Study (LYRIKS) Schizophr. Res. 2015;164(1-3):8–14. doi: 10.1016/j.schres.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Liu W., Gan J., Fan J., Zheng H., Li S., Chan R.C.K., Tan C., Zhu X. Associations of cortical thickness, surface area and subcortical volumes with insight in drug-naïve adults with obsessive-compulsive disorder. NeuroImage Clin. 2019;24:102037. doi: 10.1016/j.nicl.2019.102037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mar R.A. The neural bases of social cognition and story comprehension. Annu. Rev. Psychol. 2011;62(1):103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- McAusland L., Buchy L., Cadenhead K.S., Cannon T.D., Cornblatt B.A., Heinssen R., McGlashan T.H., Perkins D.O., Seidman L.J., Tsuang M.T., Walker E.F., Woods S.W., Bearden C.E., Mathalon D.H., Addington J. Anxiety in youth at clinical high risk for psychosis. Early Interv. Psychiatry. 2017;11(6):480–487. doi: 10.1111/eip.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Brain on stress: How the social environment gets under the skin. Proc. Natl. Acad. Sci. 2012;109(Supplement_2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan T., Walsh B., Woods S. Oxford University Press; 2010. The Psychosis-risk Syndrome: Handbook for Diagnosis and Follow-up. [Google Scholar]
- McKenzie S., Frank A., Kinsky N., Porter B., Rivière P., Eichenbaum H. Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron. 2014;83(1):202–215. doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Denton R., Downey G., Purdie V.J., Davis A., Pietrzak J. Sensitivity to status-based rejection: implications for African American students’ college experience. J. Pers. Soc. Psychol. 2002;83(4):896–918. doi: 10.1037//0022-3514.83.4.896. [DOI] [PubMed] [Google Scholar]
- Meyer J.S. Early adrenalectomy stimulates subsequent growth and development of the rat brain. Exp. Neurol. 1983;82(2):432–446. doi: 10.1016/0014-4886(83)90415-6. [DOI] [PubMed] [Google Scholar]
- Mutlu A.K., Schneider M., Debbané M., Badoud D., Eliez S., Schaer M. Sex differences in thickness, and folding developments throughout the cortex. NeuroImage. 2013;82:200–207. doi: 10.1016/j.neuroimage.2013.05.076. [DOI] [PubMed] [Google Scholar]
- Newman E., Thompson W.K., Bartsch H., Hagler D.J., Chen C.-H., Brown T.T., Kuperman J.M., McCabe C., Chung Y., Libiger O., Akshoomoff N., Bloss C.S., Casey B.J., Chang L., Ernst T.M., Frazier J.A., Gruen J.R., Kennedy D.N., Murray S.S., Sowell E.R., Schork N., Kenet T., Kaufmann W.E., Mostofsky S., Amaral D.G., Dale A.M., Jernigan T.L. Anxiety is related to indices of cortical maturation in typically developing children and adolescents. Brain Struct. Funct. 2016;221(6):3013–3025. doi: 10.1007/s00429-015-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Kan E., Sowell E.R. Neural correlates of socioeconomic status in the developing human brain. Dev. Sci. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., Yang L.H., Anglin D.M., DeVylder J.E. Perceived discrimination and psychotic experiences across multiple ethnic groups in the United States. Schizophr. Res. 2014;157(1-3):259–265. doi: 10.1016/j.schres.2014.04.036. [DOI] [PubMed] [Google Scholar]
- Panizzon M.S., Fennema-Notestine C., Eyler L.T., Jernigan T.L., Prom-Wormley E., Neale M., Jacobson K., Lyons M.J., Grant M.D., Franz C.E. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C., Velakoulis D., McGorry P.D., Wood S.J., Suckling J., Phillips L.J., Yung A.R., Bullmore E.T., Brewer W., Soulsby B., Desmond P., McGuire P.K. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. The Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Paradies Y. A systematic review of empirical research on self-reported racism and health. Int. J. Epidemiol. 2006;35:888–901. doi: 10.1093/ije/dyl056. [DOI] [PubMed] [Google Scholar]
- Pascoe E.A., Richman L.S. Perceived discrimination and health: a meta-analytic review. Psychol. Bull. 2009;135:531–554. doi: 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko E.K., Mossakowski K.N., Hamilton V.J. Does perceived discrimination affect health? Longitudinal relationships between work discrimination and women’s physical and emotional health. J. Health Soc. Behav. 2003;44:18–33. doi: 10.2307/1519813. [DOI] [PubMed] [Google Scholar]
- Pearce J., Rafiq S., Simpson J., Varese F. Perceived discrimination and psychosis: a systematic review of the literature. Soc. Psychiatry Psychiatr. Epidemiol. 2019;54(9):1023–1044. doi: 10.1007/s00127-019-01729-3. [DOI] [PubMed] [Google Scholar]
- Pearl J. Interpretation and identification of causal mediation. Psychol. Methods. 2014;19(4):459–481. doi: 10.1037/a0036434. [DOI] [PubMed] [Google Scholar]
- Pérez D.J., Fortuna L., Alegría M. Prevalence and correlates of everyday discrimination among US Latinos. J. Community Psychol. 2008;36(4):421–433. doi: 10.1002/jcop.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman W.R., Webster M.J., Herman M.M., Kleinman J.E., Weickert C.S. Age-related differences in glucocorticoid receptor mRNA levels in the human brain. Neurobiol. Aging. 2007;28(3):447–458. doi: 10.1016/j.neurobiolaging.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18(9):383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How Does Your Cortex Grow? J. Neurosci. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Rosas H.D., Fischl B. Highly accurate inverse consistent registration: a robust approach. NeuroImage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Tisdall M.D., Qureshi A., Buckner R.L., van der Kouwe A.J.W., Fischl B. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. NeuroImage. 2015;107:107–115. doi: 10.1016/j.neuroimage.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippy A.E., Newman E. Perceived religious discrimination and its relationship to anxiety and paranoia among muslim Americans. J. Muslim Ment. Health. 2006;1(1):5–20. doi: 10.1080/15564900600654351. [DOI] [Google Scholar]
- Rosenfield S., Mouzon D. Handbook of the Sociology of Mental Health. Springer; 2013. Gender and mental health; pp. 277–296. [Google Scholar]
- Saleem M.M., Stowkowy J., Cadenhead K.S., Cannon T.D., Cornblatt B.A., McGlashan T.H., Perkins D.O., Seidman L.J., Tsuang M.T., Walker E.F., Woods S.W., Addington J. Perceived discrimination in those at clinical high risk for psychosis. Early Interv. Psychiatry. 2014;8(1):77–81. doi: 10.1111/eip.2014.8.issue-110.1111/eip.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels G.M., Ross-Sheriff F. Sage Publications Sage CA; Los Angeles, CA: 2008. Identity, Oppression, and Power: Feminisms and Intersectionality Theory. [Google Scholar]
- Sanders-Phillips K., Settles-Reaves B., Walker D., Brownlow J. Social inequality and racial discrimination: risk factors for health disparities in children of color. Pediatrics. 2009;124(Supplement 3):S176–S186. doi: 10.1542/peds.2009-1100E. [DOI] [PubMed] [Google Scholar]
- Schlosser D.A., Jacobson S., Chen Q., Sugar C.A., Niendam T.A., Li G., Bearden C.E., Cannon T.D. Recovery from an at-risk state: clinical and functional outcomes of putatively prodromal youth who do not develop psychosis. Schizophr. Bull. 2012;38(6):1225–1233. doi: 10.1093/schbul/sbr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M.T., Branscombe N.R., Postmes T., Garcia A. The consequences of perceived discrimination for psychological well-being: a meta-analytic review. Psychol. Bull. 2014;140:921–948. doi: 10.1037/a0035754. [DOI] [PubMed] [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Schwartz R.C., Blankenship D.M. Racial disparities in psychotic disorder diagnosis: a review of empirical literature. World J. Psychiatry. 2014;4:133–140. doi: 10.5498/wjp.v4.i4.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl J.R. Glucocorticoids, developmental “programming” and the risk of affective dysfunction. Prog. Brain Res. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- Shaikh M., Ellett L., Dutt A., Day F., Laing J., Kroll J., Petrella S., McGuire P., Valmaggia L.R. Perceived ethnic discrimination and persecutory paranoia in individuals at ultra-high risk for psychosis. Psychiatry Res. 2016;241:309–314. doi: 10.1016/j.psychres.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Stefanis N.C., Hanssen M., Smirnis N.K., Avramopoulos D.A., Evdokimidis I.K., Stefanis C.N., Verdoux H., Van Os J. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol. Med. 2002;32(2):347–358. doi: 10.1017/s0033291701005141. [DOI] [PubMed] [Google Scholar]
- Stowkowy J., Liu L.u., Cadenhead K.S., Cannon T.D., Cornblatt B.A., McGlashan T.H., Perkins D.O., Seidman L.J., Tsuang M.T., Walker E.F., Woods S.W., Bearden C.E., Mathalon D.H., Addington J. Early traumatic experiences, perceived discrimination and conversion to psychosis in those at clinical high risk for psychosis. Soc. Psychiatry Psychiatr. Epidemiol. 2016;51(4):497–503. doi: 10.1007/s00127-016-1182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffren S., Chauret M., Nassim M., Lepore F., Maheu F.S. On a continuum to anxiety disorders: adolescents at parental risk for anxiety show smaller rostral anterior cingulate cortex and insula thickness. J. Affect. Disord. 2019;248:34–41. doi: 10.1016/j.jad.2019.01.028. [DOI] [PubMed] [Google Scholar]
- Sun D., Phillips L., Velakoulis D., Yung A., McGorry P.D., Wood S.J., van Erp T.G.M., Thompson P.M., Toga A.W., Cannon T.D., Pantelis C. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr. Res. 2009;108(1-3):85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Wood S.J., Yung A.R., Phillips L.J., Soulsby B., McGorry P.D., Tanino R., Zhou S.-Y., Suzuki M., Velakoulis D., Pantelis C. Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr. Res. 2009;111(1-3):94–102. doi: 10.1016/j.schres.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Wood S.J., Yung A.R., Soulsby B., McGorry P.D., Suzuki M., Kawasaki Y., Phillips L.J., Velakoulis D., Pantelis C. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch. Gen. Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Herting M.M., Goddings A.-L., Meuwese R., Blakemore S.-J., Dahl R.E., Güroğlu B., Raznahan A., Sowell E.R., Crone E.A., Mills K.L. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J. Neurosci. 2017;37(12):3402–3412. doi: 10.1523/JNEUROSCI.3302-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoits P.A. Stress and Health: Major Findings and Policy Implications. J. Health Soc. Behav. 2010;51(1_suppl):S41–S53. doi: 10.1177/0022146510383499. [DOI] [PubMed] [Google Scholar]
- Tingley D., Yamamoto T., Hirose K., Keele L., Imai K. mediation : R package for causal mediation analysis. J. Stat. Softw. 2014;59 doi: 10.18637/jss.v059.i05. [DOI] [Google Scholar]
- Toga A.W., Thompson P.M. Mapping brain asymmetry. Nat. Rev. Neurosci. 2003;4(1):37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Tse D., Takeuchi T., Kakeyama M., Kajii Y., Okuno H., Tohyama C., Bito H., Morris R.G.M. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333(6044):891–895. doi: 10.1126/science:1205274. [DOI] [PubMed] [Google Scholar]
- Valk S.L., Xu T., Margulies D.S., Masouleh S.K., Paquola C., Goulas A., Kochunov P., Smallwood J., Yeo B.T.T., Bernhardt B.C., Eickhoff S.B. Shaping brain structure: genetic and phylogenetic axes of macroscale organization of cortical thickness. Sci. Adv. 2020;6(39):eabb3417. doi: 10.1126/sciadv.abb3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren M.T.R., Ruiter D.J., Fernández G., Henson R.N. How schema and novelty augment memory formation. Trends Neurosci. 2012;35(4):211–219. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- van Os J., Linscott R.J., Myin-Germeys I., Delespaul P., Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol. Med. 2009;39(2):179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- Wagstyl K., Ronan L., Goodyer I.M., Fletcher P.C. Cortical thickness gradients in structural hierarchies. Neuroimage. 2015;111:241–250. doi: 10.1016/j.neuroimage.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn D., Wilson G., Roes M., Rnic K., Harkness K.L. Theory of mind in social anxiety disorder, depression, and comorbid conditions. J. Anxiety Disord. 2016;37:71–77. doi: 10.1016/j.janxdis.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Watson J.M., Scarinci I.C., Klesges R.C., Slawson D., Beech B.M. Race, socioeconomic status, and perceived discrimination among healthy women. J. Womens Health Gend. Based Med. 2002;11(5):441–451. doi: 10.1089/15246090260137617. [DOI] [PubMed] [Google Scholar]
- Wickham S., Shryane N., Lyons M., Dickins T., Bentall R. Why does relative deprivation affect mental health? The role of justice, trust and social rank in psychological wellbeing and paranoid ideation. J. Public Ment. Health. 2014;13:114–126. doi: 10.1108/JPMH-06-2013-0049. [DOI] [Google Scholar]
- Wierenga L.M., Langen M., Oranje B., Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Wigman J.T., van Nierop M., Vollebergh W.A., Lieb R., Beesdo-Baum K., Wittchen H.-U., van Os J. Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity—implications for diagnosis and ultra–high risk research. Schizophr. Bull. 2012;38:247–257. doi: 10.1093/schbul/sbr196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.R., John D.A., Oyserman D., Sonnega J., Mohammed S.A., Jackson J.S. Research on Discrimination and Health: An Exploratory Study of Unresolved Conceptual and Measurement Issues. Am. J. Public Health. 2012;102:975–978. doi: 10.2105/AJPH.2012.300702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.R., Mohammed S.A. Discrimination and racial disparities in health: evidence and needed research. J. Behav. Med. 2009;32(1):20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.R., Neighbors H.W., Jackson J.S. Racial/ethnic discrimination and health: Findings from community studies. Am. J. Public Health. 2003;93(2):200–208. doi: 10.2105/ajph.93.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.R., Priest N., Anderson N.B. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol. 2016;35(4):407–411. doi: 10.1037/hea0000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.R., Sternthal M. Understanding racial-ethnic disparities in health: sociological contributions. J. Health Soc. Behav. 2010;51(1_suppl):S15–S27. doi: 10.1177/0022146510383838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S.W., Addington J., Cadenhead K.S., Cannon T.D., Cornblatt B.A., Heinssen R., Perkins D.O., Seidman L.J., Tsuang M.T., Walker E.F. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr. Bull. 2009;35:894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Luo Q., Wang S., Qiu L., Pan N., Kuang W., Lui S.u., Huang X., Yang X., Kemp G.J., Gong Q. Dissociations in cortical thickness and surface area in non-comorbid never-treated patients with social anxiety disorder. EBioMedicine. 2020;58:102910. doi: 10.1016/j.ebiom.2020.102910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermans T.B., Schothorst P.F., Schnack H.G., Koolschijn P.C.M.P., Kahn R.S., van Engeland H., Durston S. Progressive structural brain changes during development of psychosis. Schizophr. Bull. 2012;38:519–530. doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung W.W. A rating instrument for anxiety disorders. Psychosom. J. Consult. Liaison Psychiatry. 1971 doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.