Abstract
Background/aim
Since the outbreak of the COVID-19, numerous therapies to counteract this severe disease have emerged. The benefits of Tocilizumab for severely infected COVID-19 patients and the methodologies of ongoing clinical trials are explored.
Materials and methods
A systematic search adhering to PRISMA guidelines was conducted in PubMed, Cochrane Central, medRxiv, and bioRxiv using the following keywords: “Tocilizumab,” “Actemra,” “COVID-19.” An additional subsearch was conducted on Clinicaltrials.gov to locate ongoing tocilizumab trials.
Results
A total of 13 studies were included in the meta-analysis comprising 2120 patients. The treatment group had lower mortality compared to the control group (OR = 0.42, 95% CI = 0.26 to 0.69, P = 0.0005, I2 = 55%). A descriptive analysis of 50 registered trials was conducted.
Conclusion
This review meta-analyzed the therapeutic benefits of tocilizumab in COVID-19 patients with severe disease for mortality, mechanical ventilation, and the characteristics of COVID-19 registered trials.
Keywords: Tocilizumab, actemra, coronavirus, COVID-19, clinical trial, cytokine storm syndrome
1. Introduction
Since the outbreak of the novel coronavirus disease 2019 in December 2019, throughout the Hubei province of China, several clinical trials have been conducted to assess the benefits of certain therapies. Tocilizumab, also known as atlizumab, is an immunosuppressive drug, mainly for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis, a severe form of arthritis in children. It is a humanized monoclonal antibody against the interleukin-6 receptor. Although every observational study of COVID 19 so far has hinted at the benefits of drugs that may block inflammatory cytokines, trials of interleukin 6 inhibitors, such as sarilumab, have yet to show any viable benefits [1]. As of 13th October 2020, 1.08 million individuals have died due to coronavirus disease 2019 (COVID-19), with 37.6 million cases documented worldwide. While the death toll reaching over 1 million individuals worldwide, the results of randomized, double-blind, placebo-controlled trials have raised questions about the benefits or tocilizumab in patients with COVID-19 [2].
The World Health Organization (WHO) estimates that the mortality rate of disease caused by COVID-19 is 3.7%, which is 10 times higher than that seen in influenza [3]. Afflicted patients may have an overwhelming immune reaction causing the cytokine storm syndrome with elements of the Macrophage Activation Syndrome (MAS), Cytokine-Release Syndrome (CRS), leading to Acute Respiratory Distress Syndrome (ARDS). SARS-CoV-2 leads to the production of inflammatory cytokines including Interleukin-6, which then contributes to cytokine storm syndromes damaging the lungs and other organs, ultimately leading to death. IL-6 is a pleiotropic proinflammatory cytokine produced by many cell types including fibroblasts, monocytes, and lymphocytes. The SARS-CoV-2 infection leads to a dose-based production of IL-6 from bronchial cells [4]. Interleukin inhibitors may help ameliorate severe damage to the lung tissue caused by cytokine release in patients infected with severe COVID-19 disease.
As of September 2020, there are 280 COVID-19 clinical trials registered for the treatment of COVID-19 in clinicaltrials.gov. The clinical course and mortality outcomes have baffled the population and healthcare organizations due to the heterogeneity in clinical presentations with some being asymptomatic to others acquiring severe pneumonia with respiratory failure leading to mechanical ventilation or death [5].
While the National Institute of Health guidelines proposed that insufficient data were present to support the use of interleukin-6 inhibitors other than for COVID-19 clinical trials, until more concrete evidence is available, healthcare providers must exercise caution in prescribing immune-modulating therapies [6]. This meta-analysis reviews the benefits of Tocilizumab for severe COVID-19 patients and critiques the methodologies of ongoing clinical trials.
2. Materials and methods
2.1. Search strategy
All potential studies were identified by conducting a systematic search using PRISMA guidelines. Two databases were searched to include observational studies including PubMed (MEDLINE) and Cochrane Central. Additional studies were located using medRxiv and bioRxiv, in addition to grey literature sources, such as the WHO-COVID database and Google Scholar. A combination of keywords was used including “Tocilizumab,” “Actemra”, and “COVID-19.” An additional search was conducted on Clinicaltrials.gov to locate all ongoing tocilizumab trials.
2.2. Inclusion and exclusion criteria
All studies comparing the clinical benefits and all-cause mortality outcomes of tocilizumab were included in the study. The comparators for the treatment for COVID-19 named as controls in our study were receiving standard treatment of care or no treatment. Articles published after January 1st, 2020, were included with no language restrictions. We excluded 1) case studies, 2) case series, 3) letter to editors, 4) single-arm studies, and 5) two-arm studies that did not report any outcomes of interest.
6. National Institutes of Health (2020). COVID-19 Treatment Guidelines Panel. Therapeutic Options Under Investigation, Coronavirus Disease COVID-19 [online]. Website https://www.covid19treatmentguidelines.nih.gov/ [accessed 20 September 2020].
Two early-to-mid level researchers (ZS and AS) searched the articles independently. All discrepancies were resolved by the third author (MA). Data were systematically collected for the primary outcome, which was mortality in both treatment and control group, and the secondary outcome, mechanical ventilation using a shared spreadsheet for the meta-analysis. For all ongoing clinical trial registrations, findings were tabulated as 1) clinical trial identifier, 2) study design, 3) estimated enrollment, 4) conditions, 5) phase of the study, 6) interventions (experimental vs. comparator), 7) primary outcome measure and 8) recruitment status.
2.3. Objectives
The primary objective was to determine whether tocilizumab reduced the risk of mortality in the treatment groups. The secondary objective was to identify if the risk of mechanical ventilation was higher in either group.
2.4. Data analysis
All analyses were carried using Review Manager V5.4. The Mantel–Haenszel random-effects model was used with 95% confidence intervals. A test of P ≤ 0.05 was considered significant. A presentation of the Unadjusted Odds ratios (ORs) was given for dichotomous variables (I. mortality and II. mechanical ventilation). The I2 index was identified to assess heterogeneity among the included studies. A funnel plot was generated for visual inspection if more than 10 studies were included in either analysis. The study was not registered in an online register due to the time-sensitivity of the topic.
3. Results
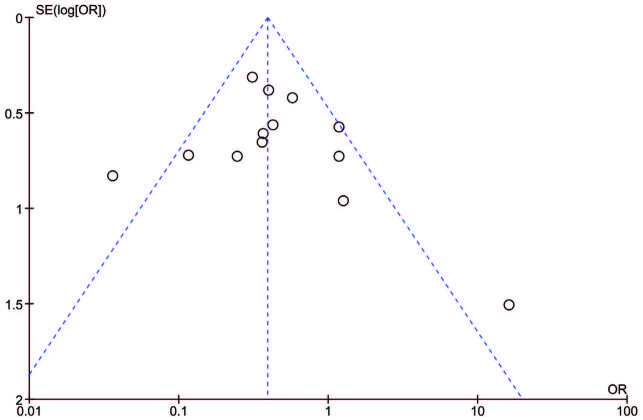
We included 13 studies in the meta-analysis [5,7–17]. There were a total of 2120 patients, with 674 (31.8%) patients in the tocilizumab group and 1446 (68.2%) patients in the control group. Our results indicate that patients treated with tocilizumab had lower risks of mortality as compared to those who received no treatment or standard COVID-19 therapies (OR = 0.42, 95% CI = 0.26 to 0.69, P = 0.0005). There was moderate heterogeneity in included studies (I2 = 55%) (Figure 1), whereas, the risk of mechanical ventilation was inconclusive in those who obtained tocilizumab therapy (OR = 0.95, 95% CI = 0.53 to 1.72, P = 0.88, I2 = 61%) (Figure 2). The included studies provide clear evidence that the treatment group has a lower risk of death in severe COVID-19 patients, requiring further proof in the form of randomized controlled trials. However, the findings do not identify a clear demarcation of risks of mechanical ventilation between the treatment and control groups. Publication bias was noted within acceptable limits and high to moderate level methodological studies were included in our analysis (Figure 3).
Figure 1.
Mortality associations between the tocilizumab group and the control group.
Figure 2.
Odds of mechanical ventilation between the tocilizumab group and the control group.
Figure 3.
Funnel plot showing publication bias of included studies. Each dot represents a single study. The X axis shows the result of the study expressed as an odds ratio for mortality in tocilizumab and control groups. The Y axis is the standard error of the effect estimate. The shape is asymmetrical as it does not resemble that of an inverted funnel or a pyramid representing publication bias. Given that the I2 value was 55%, study heterogeneity may have led to an asymmetrical funnel.
Using the keywords “COVID-19” and “tocilizumab,” a total of 67 studies were located on Clinicaltrials.gov, of which we present a total of 50 registered trails. Of all, we included 4(8%) nonrandomized, open-label studies, 7(14%) randomized, double-masked (participant and investigator) studies, 30(60%) randomized, open-label studies, 1(2%) randomized, quadruple masking (participant, care provider, investigator, outcomes assessor) study, 1(2%) randomized, single masking (investigator) study, and 7(14%) single group, open-label studies (Table).
Table.
Characteristics of registered tocilizumab trials.
| No | Clinical trialidentifier | Study designs | EstimatedEnrollment | Current phase(Updated 9/14/2020) | Interventions | Primary outcome measures | Recruitment status |
|---|---|---|---|---|---|---|---|
| 1 | NCT04331795 | Nonrandomized, open label | 32 | Phase 2 | Tocilizumab | Clinical response | Completed |
| 2 | NCT04339712 | Nonrandomized, open label | 40 | Phase 2 | Anakinra, Tocilizumab | Change of baseline total sequential organ failure assessment (SOFA) score| | Recruiting |
| 3 | NCT04492501 | Nonrandomized, open label | 600 | Not Applicable | Therapeutic plasma exchange, convalescent plasma, tocilizumab, remdesivir, Mesenchymal stem cell therapy | Survival | Completed |
| 4 | NCT04423042 | Non-Randomized, open label | 30 | Phase 3 | Tocilizumab | All-cause mortality | Not yet recruiting |
| 5 | NCT04438980 | Randomized, double masking between participant and investigator | 72 | Phase 3 | Methylprednisolone vs. Placebo | Proportion of patients developing treatment failure and Mortality at day 28 | Recruiting |
| 6 | NCT04412772 | Randomized, double masking between participant and investigator | 300 | Phase 3 | Tocilizumab vs. Placebo | Clinical status (on a 7-point ordinal scale) at day 28 | Recruiting |
| 7 | NCT04356937 | Randomized, double masking between participant and investigator | 243 | Phase 3 | Tocilizumab vs. Placebo | The time from administration of the investigational agent (or placebo) to requiring mechanical ventilation and intubation, or death for subjects who die prior to intubation| | Active, not recruiting |
| 8 | NCT04372186 | Randomized, double masking between participant and investigator | 379 | Phase 3 | Tocilizumab vs. Placebo | Cumulative Proportion of ParticipantsRequiring Mechanical Ventilation by Day 28 | Active, not recruiting |
| 9 | NCT04320615 | Randomized, double masking between participant and investigator | 450 | Phase 3 | Tocilizumab vs. Placebo | Clinical Status Assessed Using a 7-Category Ordinal Scale | Completed |
| 10 | NCT04409262 | Randomized, double masking between participant and investigator | 450 | Phase 3 | Remdesivir, Tocilizumab vs. Placebo | Clinical Status as Assessed by the Investigator Using a 7-Category Ordinal Scale of Clinical Status on Day 28 | Recruiting |
| 11 | NCT04380519 | Randomized, double masking between participant and investigator | 372 | Phase 2|Phase 3 | RPH-104 80 mg, Olokizumab 64 mg; vs. Placebo | Proportion of patients, responded to the study therapy, in each of the treatment groups | Recruiting |
| 12 | NCT04479358 | Randomized, open label | 332 | Phase 2 | Tocilizumab vs. Standard of Care | Time to Recovery, Achievement of Recovery and Overall Survival | Not yet recruiting |
| 13 | NCT04345445 | Randomized, open label | 310 | Phase 3 | Tocilizumab, Methylprednisolone | The proportion of patients requiring mechanical ventilation | Not yet recruiting |
| 14 | NCT04435717 | Randomized, open label | 78 | Phase 2 | Tocilizumab 20 MG/ML Intravenous Solution;Drug: Tocilizumab 20 MG/ML Intravenous Solution (2 doses) | Change in IL-12 values in the 3 study groups from the start of treatment (Day 0) and on days 1 and 3 | Recruiting |
| 15 | NCT04377750 | Randomized, open label | 500 | Phase 4 | Tocilizumab | Survival | Recruiting |
| 16 | NCT04332094 | Randomized, open label | 276 | Phase 2 | Tocilizumab, Hydroxychloroquine, Azithromycin | In-hospital mortality | Recruiting |
| 17 | NCT04377659 | Randomized, open label | 40 | Phase 2 | Tocilizumab | Progression of respiratory failure or death | Recruiting |
| 18 | NCT04412291 | Randomized, open label | 120 | Phase 2 | Anakinra Prefilled Syringe, Tocilizumab Prefilled Syringe vs. Standard-of-care treatment | Time to recovery and Mortality | Recruiting |
| 19 | NCT04346355 | Randomized, open label | 126 | Phase 2 | Tocilizumab | Entry into Intensive Care with invasive mechanical ventilation or death from any cause or clinical aggravation | Terminated |
| 20 | NCT04377503 | Randomized, open label | 40 | Phase 2 | Tocilizumab 180 MG/ML, Methylprednisolone Sodium Succinate | Patient clinical status 15 days afterrandomization | Not yet recruiting |
| 21 | NCT04363736 | Randomized, open label | 100 | Phase 2 | Tociliuzumab | Serum Concentration of interleukin-6 (IL-6) Following Administration of 8 mg/kg IV TCZ | Completed |
| 22 | NCT04361032 | Randomized, open label | 260 | Phase 3 | Tocilizumab Injection, Deferoxamine | Mortality rate | Not yet recruiting |
| 23 | NCT04424056 | Randomized, open label | 216 | Phase 3 | Anakinra, Ruxolitinib vs. Standard of care | Ventilation free days at day 28 | Not yet recruiting |
| 24 | NCT04403685 | Randomized, open label | 129 | Phase 3 | Tocilizumab | Evaluation of clinical status and All-cause mortality | Terminated |
| 25 | NCT04335305 | Randomized, open label | 24 | Phase 2 | Tocilizumab, Pembrolizumab (MK-3475) | Percentage of patients with normalization of SpO2 on room air (measured without any respiratory support for at least 15 minutes) and Proportion of patients discharged from the emergency department and classified as low risk | Recruiting |
| 26 | NCT04333914 | Randomized, open label | 384 | Phase 2 | Chloroquine analog (GNS651), Nivolumab, Tocilizumab, Standard of care, Avdoralimab, Monalizumab | 28-day survival rate and Time to clinical improvement | Suspended |
| 27 | NCT04476979 | Randomized, open label | 120 | Phase 2 | Tocilizumab, Dexamethasone | Survival without needs of ventilator utilization at day 14 and WHO progression scale at day 7 and 14 | Recruiting |
| 28 | NCT04361552 | Randomized, open label | 0 | Phase 3 | Best Practice, Tocilizumab | 7-day length of invasive mechanical ventilation (MV) | Withdrawn |
| 29 | NCT04330638 | Randomized, open label | 342 | Phase 3 | Usual Care, Anakinra, Siltuximab, Tocilizumab | Time to Clinical Improvement| | Recruiting |
| 30 | NCT04331808 | Randomized, open label | 228 | Phase 2 | Tocilizumab | Survival without needs of ventilator utilization at day 14. Group 1 | Active, not recruiting |
| 31 | NCT04322773 | Randomized, open label | 200 | Phase 2 | RoActemra IV vs. Standard medical care | Time to independence from supplementary oxygen therapy | Recruiting |
| 32 | NCT04381936 | Randomized, open label | 15000 | Phase 2|Phase 3 | Lopinavir-Ritonavir, Corticosteroid, Hydroxychloroquine, Azithromycin, Convalescent plasma, Tocilizumab, Immunoglobulin | All-cause mortality and Duration of hospital stay | Recruiting |
| 33 | NCT04536363 | Randomized, open label | 284 | Phase 2 | Analogs, Prostaglandin vs. Standard therapeutic protocol | Mortality and Hypoxemia Resolution | Not yet recruiting |
| 34 | NCT04359095 | Randomized, open label | 1600 | Phase 2|Phase 3 | Hydroxychloroquine|Drug, Lopinavir / Ritonavir Pill, Azithromycin, vs. Standard treatment | Mortality | Not yet recruiting |
| 35 | NCT02735707 | Randomized, open label | 7100 | Phase 4 | Fixed-duration Hydrocortisone, Shock-dependent hydrocortisone, Ceftriaxone, Moxifloxacin or Levofloxacin, Piperacillin-tazobactam, Ceftaroline, Amoxicillin-clavulanate, Macrolide administered for 3-5 days or 14 days, 5/10-days oseltamivir, Lopinavir/ritonavir, Hydroxychloroquine Hydroxychloroquine + lopinavir/ritonavir|Drug: Interferon-1a Anakinra, Fixed-duration higher dose Hydrocortisone, Tocilizumab, Sarilumab, Vitamin C, Therapeutic anticoagulation, Simvastatin, Convalescent plasm,Protocolised mechanical ventilation strategy | All-cause mortality | Recruiting |
| 36 | NCT04374539 | Randomized, open label | 116 | Phase 2 | Plasma exchange vs. Standard medical treatment | Impact of plasma exchange | Recruiting |
| 37 | NCT04366245 | Randomized, open label | 72 | Phase 1|Phase 2 | Hyperimmune plasma vs Standard of care for SARS-CoV-2 infection | Safety: Incidence of Adverse Events and Serious Adverse Events grade 3 and 4, related to the product under investigation or the administration procedure, graduated according to the common toxicity criteria scale (CTCAE) | Recruiting |
| 38 | NCT04346693 | Randomized, open label | 320 | Phase 3 | Standard therapy recommended by the Ministry of Health of the Russian Federation and Dalargin intramuscular injection | The change of viral load in patients with SARS-COVID-19 | Active, not recruiting |
| 39 | NCT04401410 | Randomized, open label | 58 | Phase 1 | Dose Finding Phase (MTD), Partially HLA-matched SARS-CoVSTs, Routine care | Graft versus Host Disease (GvHD) and Cytokine Release Syndrome (CRS) | Not yet recruiting |
| 40 | NCT04392414 | Randomized, open label | 60 | Phase 2 | COVID-19 convalescent hyperimmune plasma, Non-convalescent fresh frozen plasma (Standard plasma) | 30-day mortality rate | Recruiting |
| 41 | NCT04414631 | Randomized, open label | 120 | Phase 2 | Conestat alfa | Disease severity and Time to clinical improvement | Recruiting |
| 42 | NCT04335071 | Randomized, quadruple masking among participant, care provider, investigator, and outcomes assessor | 100 | Phase 2 | Tocilizumab, Placebo | Number of patients with ICU admission, intubation and death | Recruiting |
| 43 | NCT04349410 | Randomized, singlemasking for investigator | 500 | Phase 2|Phase 3 | Hydroxychloroquine, Azithromycin/ Doxycycline/ Clindamycin/ Primaquine - low dose, Clindamycin,Primaquine - high dose, Remdesivir, Tocilizumab, Methylprednisolone, Interferon-Alpha2B, Losartan, Convalescent Serum | Improvement in FMTVDM Measurement with nuclear imaging, Ventilator status, and survival status | Enrolling by invitation |
| 44 | NCT04445272 | Single group, open label | 500 | Phase 2 | Tocilizumab | Mortality rate and metrics of respiratory function | Recruiting |
| 45 | NCT04317092 | Single Group, open label | 400 | Phase 2 | Tocilizumab Injection | One-month mortality rate | Recruiting |
| 46 | NCT04363853 | Single Group, open label | 200 | Phase 2 | Tocilizumab | Hematic biometry and Blood chemistry | Recruiting |
| 47 | NCT04370834 | Single group, open label | 217 | Phase 2 | Tocilizumab | Clinical outcome as evaluated by the 7-category Clinical Status Ordinal Scale | Suspended |
| 48 | NCT04315480 | Single group, open label | 38 | Phase 2 | Tocilizumab | arrest in deterioration of pulmonary function and death | Active, not recruiting |
| 49 | NCT04386239 | Single Group, open label | 40 | Early Phase 1 | Sarilumab Prefilled Syringe | Proportion of patients who show an improvement of the respiratory function | Not yet recruiting |
| 50 | NCT04335123 | Single Group, open label | 50 | Phase 1 | Losartan | Number of participants with treatment-related adverse events as assessed by protocol definition of AE | Recruiting |
4. Discussion
To the best of our knowledge, this is the first review that meta-analyses the benefits of tocilizumab in severe COVID-19 patients along with reviewing methodologies of ongoing clinical trials. Our findings must be read with caution due to the lack of strong evidence from randomized controlled trials. Tocilizumab is the humanized anti-IL-6 receptor monoclonal antibody that is approved specifically for cytokine release syndrome, systematic juvenile idiopathic arthritis, and rheumatic arthritis [18]. The results indicate that tocilizumab improves mortality outcomes in severely infected COVID-19 patients; however, the results do not elucidate clear demarcation of risks of mechanical ventilation between treatment and control groups.
The National Institute of Health published Interim guidelines suggesting that tocilizumab may be considered for COVID-19 patients meeting the following six criteria, who are otherwise ineligible for steroid therapy [19]. The patient 1) must be COVID-19 positive, 2) must have abnormal chest imaging as seen in coronavirus infections, worsening respiratory status over 1–2 days necessitating 4–6 L/min of oxygen, 3) must not have any systemic fungal or bacterial co-infection, 4) must be suspected for cytokine release syndrome support by an elevation of inflammatory markers (for example, D-dimer > 1 mg/L, Ferritin > 600 ug/mL, LDH > 250U/L), along with clinical decline, 5) must not have had a poor prognosis indicating an unlikely survival of over 48 h, and 6) must have received mechanical ventilation for 24 h or less.
Of the studies included in the meta-analysis, Somers et al. only tested the benefits of tocilizumab on severe COVID-19 patients obtaining mechanical ventilation. The duration of mechanical ventilation among the tocilizumab and control group was 13.8d (IQR: 7.1d, 27.5d) and 13d (IQR: 8.1d, 23.5d), respectively [12]. It is noted that the benefits of tocilizumab therapy on patients who have been mechanically ventilated patients for over 24 h may be low due to low chances of clinical improvement.
19. Centers for Disease Control and Prevention (2020). Information for Clinicians on Investigational Therapeutics for Patients with COVID-19 [online]. Website https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html [accessed 20 September 2020].
However, 56% of patients (n = 76) in the treatment group were discharged alive by the end of follow-up process as compared to 30% of patients (n = 76) in the control group suggesting that the clinical benefits must be assessed in the ongoing placebo-controlled trials. In the COVACTA trial, where 452 severe COVID-19 patients were randomized, improvements in clinical status at day 28 and mortality outcomes were not observed [20]. Both the safety and efficacy of tocilizumab must be assessed for patients with severe disease in the several randomized, double-blind, placebo-controlled phase 3 trials namely COVACTA, REMDACTA, and EMPACTA to corroborate the true benefits of the drug in acute care settings.
Our meta-analytical findings have limitations. Most of the included studies were retrospective cohorts; however, updated preprints of placebo-controlled trials solidified our findings. There was a lack of diagnostic criteria for severe COVID-19 between all studies that led to a difference in mechanical ventilation outcomes between the treatment and control groups. The inflammatory markers reduced ventilatory support requirements, and radiological improvement signs were not always presented in the included studies.
5. Recommendations
All placebo-controlled trials must ascertain the optimal dosage of tocilizumab and the potential utility of multiple dosages. IL-6 serum concentration tests must be made routinely available when tocilizumab response is to be assessed [12]. Drug administration in the acute care setting ought to be guided by strict institutional criteria, thus being completely standardized. In addition to the regular follow up period of 28 days, the full course of hospitalization ought to be determined to characterize long-term sequelae in treatment and control groups.
6. Conclusion
Anti-IL-6 drugs for COVID-19 are a cause of contention since the outbreak of the global pandemic. Tocilizumab, which has had mixed results in RCTs, is being utilized off-label and as an experimental therapy for patients with COVID-19 who are sick or deteriorating with a slight chance of recovery. The ongoing pandemic has created ethical challenges concerning the nonapproved use among patients and choosing the most appropriate patient to receive the experimental therapy in the setting of ongoing randomized controlled trials [21]. The review attempted to link the key tocilizumab studies, find benefits in case of mortality and the risk of mechanical ventilation by the end of treatment, to steer the narrative for ongoing registered trials, which will ultimately form the fate for the use of tocilizumab in COVID-19.
Funding
We obtained no funding for this study.
References
- Bryant F. COVACTA trial raises questions about tocilizumab’s benefit in. COVID-19. The Lancet Rheumatology . 2020;2:30313–1. doi: 10.1016/S2665-9913(20)30313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection is Suspected: Interim Guidance 2020.
- Rizk JG Kalantar-Zadeh K Mehra MR Lavie CJ Rizk Y Pharmaco-immunomodulatory therapy in COVID-19. Drugs . 2020;80:1267–1292. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G Meschiari M Cozzi-Lepri A Milic J Tonelli R Tocilizumab in patients with severe COVID-19: a retrospective cohort study. The Lancet Rheumatology . 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra R De Rossi N Mattioli F Romanelli G Scarpazza C Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. European Journal of Internal Medicine . 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götzinger F Santiago-García B Noguera-Julián A Lanaspa M Lancella L -19 in children and adolescents in Europe: a multinational, multicentre cohort study. The Lancet Child and Adolescent Health . 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumier M Paule R Vallée A Rohmer J Ballester M Tocilizumab for severe worsening COVID-19 pneumonia: a propensity score analysis. Journal of Clinical Immunology . 2020;10:1–12. doi: 10.1007/s10875-020-00911-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alattar R Ibrahim TBH Shaar SH Abdalla S Shukri K Tocilizumab for the treatment of severe coronavirus disease 2019. Journal of Medical Virology . 2020;92:2042–2049. doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro C Della-Torre E Cavalli G De Luca G Ripa M Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. European Journal of Internal Medicine . 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers EC Eschenauer GA Troost JP Golob JL Gandhi TN Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clinical Infectious Diseases . 2020;954 doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer J Vesterbacka J Svensson AK Stojanovic B Wagrell C Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID-19: a retrospective cohort study. Journal of Internal Medicine . 2020;10 doi: 10.1111/joim.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadud N Ahmed N Mannu Shergil M Khan M Krishna MG Improved survival outcome in SARs-CoV-2 (COVID-19) Acute respiratory distress syndrome patients with tocilizumab administration. medRxiv . 2020;10 [Google Scholar]
- Colaneri M Bogliolo L Valsecchi P Sacchi P Zuccaro V Tocilizumab for treatment of severe covid-19 patients: preliminary results from smatteo covid19 registry (smacore) Microorganisms . 2020;10 doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy M Mannam P Comer R Sinclair E McQuaid DB Off-label real world experience using tocilizumab for patients hospitalized with COVID-19 disease in a regional community health system: a case-control study. medRxiv . 2020;10 [Google Scholar]
- Quartuccio L Sonaglia A McGonagle D Fabris M Peghin M Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. Journal of Clinical Virology . 2020;129:104444–104444. doi: 10.1016/j.jcv.2020.104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R Wang L Kuo HCD Shannar A Peter R An update on current therapeutic drugs treating COVID-19. Current Pharmacology Reports . 2020;10:1–15. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas I Bräu N Waters M Go RC Hunter BD Tocilizumab in hospitalized patients with COVID-19 pneumonia. 2020;10 doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C Rajendram P Sacha G Calabrese L Practical aspects of targeting IL-6 in COVID-19 disease. Cleveland Clinic Journal of Medicine . 2020;10 doi: 10.3949/ccjm.87a.ccc018. [DOI] [PubMed] [Google Scholar]