Introduction
The estimated prevalence of arrhythmogenic right ventricular cardiomyopathy (ARVC) ranges from 1:2000 to 1:5000 in the general population, with a more common presentation between the second and fourth decade of life. Only 20% are diagnosed after age 50, although later diagnosis does not confer a benign prognosis. At birth, these patients have a structurally normal heart, progressively developing the pathologic phenotype. In the early stages of the disease, nonspecific symptoms such as palpitations, chest pain, and syncope may appear, with no apparent structural changes.1
Key Teaching Points.
-
•
Consultations for sports cardiology topics are increasing considerably owing to the escalation in physical activity and recreational athletics.
-
•
Cardiac magnetic resonance (CMR) imaging plays an important role in many diagnostic explorations in athletes with symptoms potentially referable to a cardiac arrhythmia.
-
•
This case proves the importance of CMR in diagnosing arrhythmogenic cardiomyopathy, particularly arrhythmogenic left ventricular cardiomyopathy, in the context of apparently healthy athletes.
Case report
A 54-year-old recreational male athlete (trail running; ∼5 hours per week) presented with palpitations occurring during the recent months, both at rest and during exercise. He had no chest pain, dyspnea, dizziness, syncope on exertion, or previous infection.
The electrocardiogram performed at his primary care center highlighted frequent premature ventricular contractions (PVCs), reportedly with a morphology of right bundle branch block and short bursts of nonsustained ventricular tachycardia (NSVT).
Vital signs and physical examination on admission included a heart rate of 66 beats/min and resting blood pressure of 124/81 mm Hg; weight: 80 kg; height: 183 cm; body mass index: 24. No heart murmur or signs of pulmonary congestion were present. He denied the use of doping and/or stimulants, including caffeine. He had never smoked, and other typical cardiovascular risk factors, such as diabetes and hypertension, were excluded. His mother died suddenly at the age of 72 (without autopsy), while his father died at age 60 owing to chronic illness. He had a sister with no known heart conditions, and he was the father of 2 healthy sons.
Owing to suspicion of several potential underlying medical conditions, and according to the algorithm recommended by D’Silva and Sharma,2 we performed tests to evaluate for hypertension, coronary artery disease, heart valve disease, hyperthyroidism, pericarditis, myocarditis, Wolff-Parkinson-White syndrome, atrial fibrillation, mitral valve prolapse, sarcoidosis, dilated or hypertrophic cardiomyopathy, Brugada syndrome, catecholaminergic ventricular tachycardia, ARVC, abnormal coronary arterial origin, or benign PVCs in response to stress and/or exercise. We also investigated alcohol consumption, sports performance–enhancing supplements or illicit drugs, “energy” drinks, and sympathomimetics in cold medicines; these were all absent.
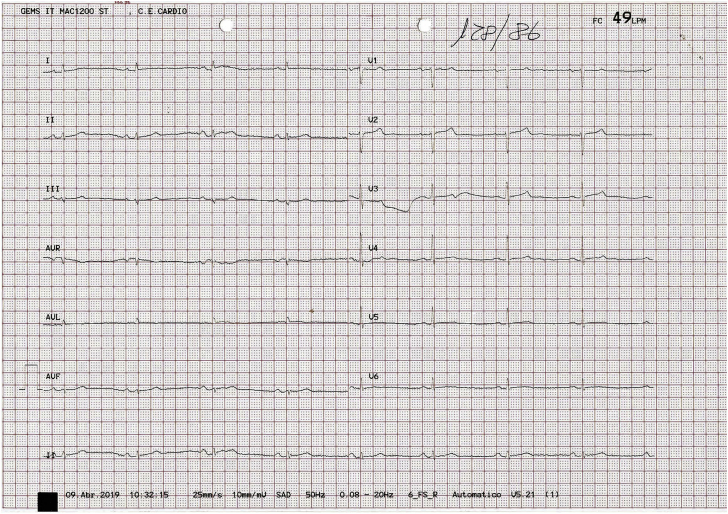
The physical examination was unremarkable and no recent infection was reported. Blood test results were within normal ranges, with no elevation of creatine kinase or troponin. The 12-lead electrocardiogram performed in our clinic showed sinus rhythm, normal atrioventricular conduction, normal intraventricular conduction, and generalized low QRS voltage (including <0.5 mV in the limb leads) (Figure 1).
Figure 1.
Baseline electrocardiogram recorded at rest.
The 2D Doppler echocardiogram showed no significant abnormality, including nondilated ventricular cavities with preserved systolic and diastolic function, nondilated atria, and no associated valvular disease (Figure 2).
Figure 2.
Apical 4-chamber 2-dimensional echocardiography image.
A Bruce protocol exercise stress test was performed. The patient reached 107% of his maximum heart rate (sixth stage), presenting isolated, infrequent polymorphic PVCs (Supplemental Figure S1) with morphologies suggesting both left and right ventricle (LV and RV) origins, 3 ventricular couplets, and a monomorphic triplet probably arising from the RV. No PVCs were observed during the recovery phase.
A 24-hour Holter monitor was subsequently carried out. Sinus rhythm was recorded, with frequent PVCs (n = 419, or 0.5% of all beats) with frequent couplets and 1 NSVT of 5 beats. The predominant PVC morphology was negative for both Holter leads, although other morphologies were also registered. Couplets were mainly monomorphic. In contrast to the predominant PVC, the morphology of NSVT was positive in both leads.
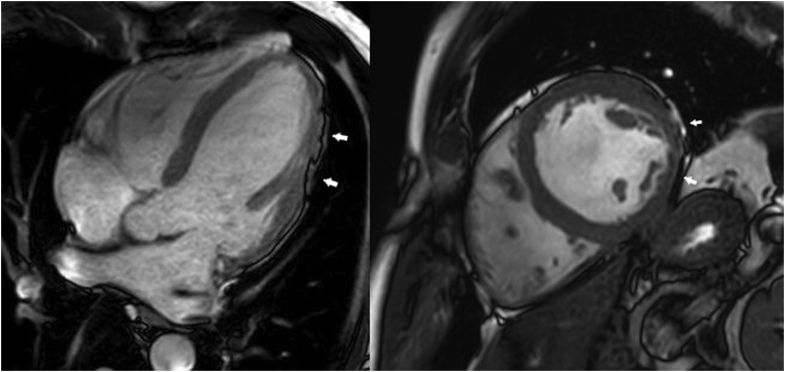
Given these results, and suspecting that the echocardiography could provide suboptimal structural measurements and functional assessment, cardiac magnetic resonance (CMR) imaging was performed. On CMR, the LV was dilated, showing moderately depressed systolic function (LV ejection fraction = 43%) and mild hypokinesia in the lateral wall (Supplemental Videos 1 and 2). Likewise, both atria were enlarged. In the film sequences, marked irregularities were observed in the epicardial contour, mainly on the lateral and inferior LV surfaces, suggesting subepicardial fatty infiltration (Figure 3). This finding has been recently described in patients with arrhythmogenic left ventricular cardiomyopathy (ALVC), termed the “rat-bite sign.”3 In the late gadolinium enhancement study, meso-subepicardial enhancement compatible with fibrosis was observed in the inferior and lateral aspects of the LV (Supplemental Figure S2). Thus, the patient’s CMR findings were highly suggestive of ALVC.
Figure 3.
Chamber and short-axis still cine images showing irregularities in the epicardial contour on the inferior and lateral walls (arrows) in relation to subepicardial foci of fatty infiltration.
Finally, genetic analysis of genes associated with arrhythmogenic cardiomyopathy (ACM) was also conducted, eliciting negative results. These tests included the sequencing and analysis of the genes related to ACM, specifically all the relevant desmosomal genes and other priority genes and secondary genes associated with the disease.
Following the evaluation above, the patient began treatment with 2.5 mg bisoprolol once daily. Considering that the patient had several risk factors for life-threatening ventricular arrhythmias in ARVC (younger age, male sex, PVC count, and number of leads with T-wave inversion),4 and given the high suspicion of ACM with moderate systolic dysfunction, the NSVT, the LV involvement, and the mother’s history of sudden cardiac death (SCD), we decided to implant a single-chamber implantable cardioverter-defibrillator (ICD) for primary prevention of SCD. The patient was also advised to abstain from intense/competitive physical exercise.
The patient was included in an ICD remote monitoring program. So far, no sustained ventricular arrhythmias or ICD therapies have occurred.
Discussion
This case demonstrates the importance of CMR imaging in the evaluation and diagnosis of ACM. Briefly, there are 3 types of ACM: (1) ARVC, which mainly affects the RV; (2) ALVC, which affects mostly the LV; and (3) biventricular arrhythmogenic cardiomyopathy, which affects both ventricles. It has been reported recently that low QRS voltage may be a common finding in athletes with no apparent structural cardiopathy, often associated with frequent ventricular ectopic activity.5 Our case is a call to action in athletes with low QRS voltage and frequent PVCs, advocating for in-depth study that includes early CMR.
Echocardiography typically is the first imaging technique employed when ACM is suspected. However, echocardiography may not provide diagnostic information in ALVC. Although genetic testing can be useful in ACM, specific mutations’ detection rate is only 40%–50%.6 CMR is the most important imaging technique used to diagnose this entity, giving us information on ventricular volumes, systolic function, and regional wall movement; characterization of myocardial tissue composition; and prognostic information.
An important and common CMR finding in ALVC patients, which was observed in this case, is the focal epicardial contour irregularities observed in 2-, 3-, and 4-chamber cine images. Feliu and colleagues3 described this pattern as “the rat-bite sign,” given its resemblance to a piece of cheese bitten by rats, with a special mention of mid-wall and/or subepicardial pattern of late gadolinium enhancement (91.9%), fatty epicardial infiltration (83.8%), and LV segmental contractility abnormalities (47.9%). At a mean follow-up of 3.74 years, approximately one-third of patients had a major adverse cardiovascular event.
The 2010 Task Force Criteria are currently the standard for diagnosis of ARVC, with a scoring system that combines the 6 categories of disease characteristics: RV structural alteration, tissue characterization, repolarization and depolarization abnormalities, arrhythmias, and family history. However, these criteria do not apply to the LV-dominant forms of ACM.7 In this case, the echocardiogram study performed was normal, as seen in previous studies, wherein the last echocardiogram was normal in approximately half of cases.
ICD implantation is crucial in patients at high risk for potentially fatal ventricular arrhythmias, as it is the only definitive therapy to prevent SCD. Miles and colleagues8 published a series of 202 deceased patients with the diagnosis of ACM, mostly men without previous cardiac symptoms, of whom 20% had participated in competitive sports. This series demonstrated that in ACM, the probability of dying while exercising is higher in men than in women, as well as in competitive athletes compared to non-athletes. While isolated RV involvement was found in 13%, LV histopathological involvement was present in a striking 87% of cases, including isolated LV involvement in 17% and biventricular involvement in 70%. Given this high rate, it is mandatory to rule out LV involvement, since it is associated with a higher probability of SCD, particularly in athletes.9 Noticeably, it is not currently advised to conduct an electrophysiological study when ACM is suspected, given the low predictive accuracy to identify patients at high risk of suffering SCD.10
Conclusion
ACM should be considered among the differential diagnoses in any endurance athlete with palpitations, arrhythmias, or presyncope, especially in the presence of low voltages and PVCs. If we had not performed a CMR in the present case, the patient likely would have been diagnosed with a mildly-to-moderately dilated cardiomyopathy or even as free of disease, since the echocardiogram was normal. This case exemplifies the importance of CMR in diagnosing ACM, particularly ALVC, in the context of apparently healthy athletes.
Footnotes
Funding: There is no funding to declare. Disclosures: None of the authors have any disclosures relevant to this article.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2021.03.026.
Appendix. Supplementary data
Four-chamber view cine sequence showing a dilated left ventricle, with a slightly depressed systolic function.
Four-chamber view cine sequence showing a dilated left ventricle with minor hypokinesia as well as multiple irregularities in the epicardial contour can be seen on the lateral wall (“rat-bite sign”).
Supplemental Figure S1.
12-lead ECG with clinical PVCs.
Supplemental Figure S2.
Chamber and short-axis late gadolinium enhancement images showing mid-wall subepicardial fibrosis on the inferior and lateral walls (arrows), coinciding with the location of epicardial contour irregularities, being suggestive of fibrofatty infiltration.
References
- 1.Leischik R., Dworrak B., Strauss M. Special Article - Exercise-induced right ventricular injury or arrhythmogenic cardiomyopathy (ACM): the bright side and the dark side of the moon. Prog Cardiovasc Dis. 2020;63:671–681. doi: 10.1016/j.pcad.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 2.D'Silva A., Sharma S. Management of young competitive athletes with cardiovascular conditions. Heart. 2017;103:463–473. doi: 10.1136/heartjnl-2016-309435. [DOI] [PubMed] [Google Scholar]
- 3.Feliu E., Moscicki R., Carrillo L. Importance of cardiac magnetic resonance findings in the diagnosis of left dominant arrhythmogenic cardiomyopathy. Rev Esp Cardiol (Engl Ed) 2020;73:885–892. doi: 10.1016/j.rec.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Cadrin-Tourigny J., Bosman L.P., Nozza A. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2019;40:1850–1858. doi: 10.1093/eurheartj/ehz103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mango F., Caselli S., Luchetti A., Pelliccia A. Low QRS voltages in Olympic athletes: Prevalence and clinical correlates. Eur J Prev Cardiol. 2020;27:1542–1548. doi: 10.1177/2047487320914758. [DOI] [PubMed] [Google Scholar]
- 6.Sen-Chowdhry S., Syrris P., McKenna W.J. Role of genetic analysis in the management of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2007;50:1813–1821. doi: 10.1016/j.jacc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Marcus F.I., McKenna W.J., Sherrill D. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miles C., Finocchiaro G., Papadakis M. Sudden death and left ventricular involvement in arrhythmogenic cardiomyopathy. Circulation. 2019;139:1786–1797. doi: 10.1161/CIRCULATIONAHA.118.037230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampert R., Olshansky B., Heidbuchel H. Safety of sports for athletes with implantable cardioverter-defibrillators: long-term results of a prospective multinational registry. Circulation. 2017;135:2310–2312. doi: 10.1161/CIRCULATIONAHA.117.027828. [DOI] [PubMed] [Google Scholar]
- 10.Towbin J.A., McKenna W.J., Abrams D.J. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy: executive summary. Heart Rhythm. 2019;16:e373–e407. doi: 10.1016/j.hrthm.2019.09.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Four-chamber view cine sequence showing a dilated left ventricle, with a slightly depressed systolic function.
Four-chamber view cine sequence showing a dilated left ventricle with minor hypokinesia as well as multiple irregularities in the epicardial contour can be seen on the lateral wall (“rat-bite sign”).