Introduction
A patient with paroxysmal atrial fibrillation, implanted with a subcutaneous loop recorder (Reveal LINQ; Medtronic Inc, Minneapolis, MN), was seen at the outpatient clinic 6 months after pulmonary vein isolation. He reported recurrence of arrhythmia, albeit different from the palpitations he experienced prior to pulmonary vein isolation.
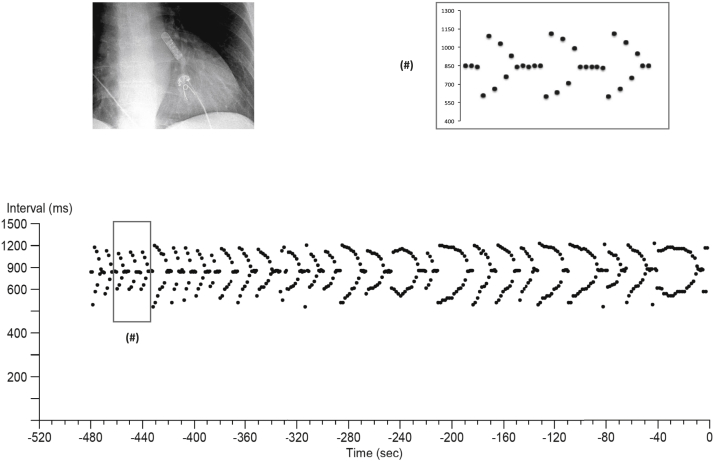
The tachogram (R-R interval plot) recorded by the Reveal LINQ at the time of symptoms showed an intriguing fishbone pattern (Figure 1). The repetitive nature of sequences causing this pattern suggests a specific underlying mechanism rather than sheer coincidence. What could be the underlying mechanism?
Figure 1.
Tachogram (R-R interval plot) retrieved from a subcutaneous loop recorder during an episode of arrhythmia.
Case report
Tachogram
At closer inspection of the tachogram (Figure 1), one recognizes that each sequence encompasses a series of regular intervals (with an interval corresponding to a rate of ≈70 beats/min), followed by a group of variable intervals alternating between shorter and longer cycle length (Figure 1, inset). Although at this stage T-wave sensing or noise cannot be excluded, one suspects that the shorter intervals are compatible with the coupling intervals (CI) of premature beats and the longer intervals with the subsequent compensatory phase (CP). Of interest, however, within each group of variable intervals there is a consistent pattern of progressive lengthening of the CI with concomitant shortening of the CP, converging toward the regular interval sequences and creating the image of a fishbone. Because the sum of the CI and the CP is twice the regular R-R interval (compatible with a fully compensatory pause), there is perfect symmetry with respect to the rate of the regular intervals. Of note, each group of premature beats seems to stop when the longest CI nears the regular interval and restarts after approximately 5 regular intervals. What mechanism explains a recurrent pattern of progressive lengthening of CIs?
Electrocardiogram
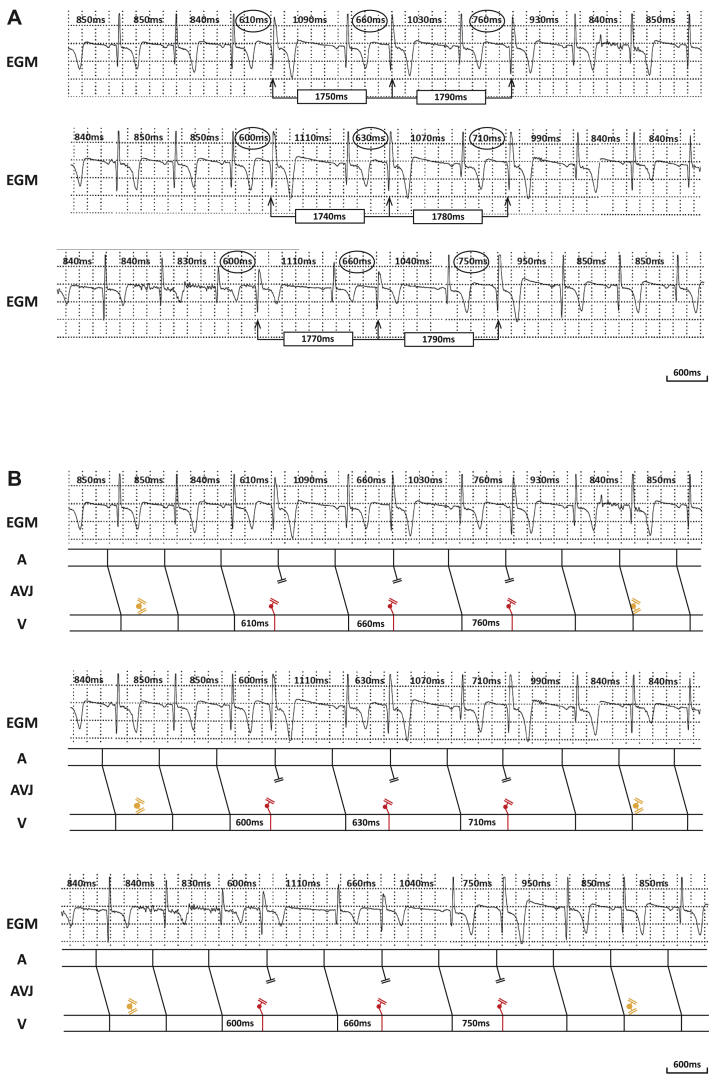
In Figure 2A the surface electrocardiogram (ECG) and R-R intervals (measured with a 10 ms resolution) provided by the Reveal LINQ corresponding to section (#) of the tachogram is plotted. The surface ECG confirms sinus rhythm with an intermittent pattern of progressively later coupled premature beats in bigeminy. The premature beats most likely originate from the atrioventricular junction, given the narrow width of the QRS and absence of a preceding P wave. There is no evident pattern of fusion (also not in the entire surface ECG strip). Each sequence starts with 5 sinus beats followed by 3 premature beats. Analysis of the R-R intervals confirms the relatively constant rate of sinus rhythm (≈840 ms), the progressive lengthening of the CI, and the progressive shortening of the subsequent CP. In contrast to the variable CIs, there is a nearly fixed interectopic interval (1740–1790 ms). Throughout the entire surface ECG strip (8-minute recording, 165 premature beats), the median number of sinus beats in between groups was 5 (interquartile range 5–5) and the confidence intervals ranged from 520 to 810 ms, whereas the interectopic interval was centered around a median of 1750 ms (with an interquartile range of 30 ms). The presence of progressive lengthening of the coupling intervals together with a fixed interectopic interval is the basis for the fishbone image but also the clue to understanding this arrhythmia.
Figure 2.
A: Continuous surface electrocardiogram strips with R-R intervals (corresponding to section (#) of Figure 1). B: Lewis diagram illustrating the mechanism underlying the arrhythmia.
The fixed interectopic interval hints toward the diagnosis of a parasystolic pacemaker. In Figure 2B this hypothesis is transcribed to the Lewis diagrams below the corresponding surface ECGs. The parasystolic pacemaker, originating at the atrioventricular junction, has a rate of approximately 34 beats per minute (cycle length of ≈1750 ms). Because of the interplay between the sinus and parasystolic pacemaker rates, this ectopic focus can encounter refractory nodal tissue (leading to the series of regular sinus beats) or excitable tissue (causing premature depolarizations with progressive lengthening of the CIs). Of interest, toward the end of the fishbone tachogram, there are progressively more premature beats within a single group. This can be explained by both a shorter initial CI and a discrete but critical slowing of the sinus rate. The variation in shape and curvature is caused by subtle physiological sinus arrhythmia.
Parasystole was first described by Kaufmann and Rothberger1 in 1919 and is defined as a constant ectopic rhythm, protected by entry block, leading to constant ectopic beats whenever it can conduct in nonrefractory myocardium. The observation that the interectopic interval in between groups (in this case 5800 ms) is not an exact multiple of the parasystolic interval does not refute the hypothesis of parasystole. Several hypotheses have been put forward for this phenomenon: (1) intermittent parasystolic activity,2 (2) electrotonic modulation or true reset of the parasystolic focus by the sinus beats (which also explains discrete variation in the interectopic interval),3,4 or (3) misjudgment of the true rate of the parasystolic pacemaker. Schamroth5 suggested in 1962 that the true rate of the parasystolic focus could be underestimated in the presence of varying degrees of local exit block. It is therefore plausible that the true parasystolic rate in our case was not ≈1750 ms but rather ≈875 ms or ≈438 ms.
Discussion
This case illustrates how a parasystole can manifest itself on a tachogram. Tachograms are more and more available in diverse monitoring systems (insertable loop recorders, wearable devices with electrocardiography or photoplethysmography). Pattern recognition, visually or by means of artificial intelligence, can facilitate diagnosis of arrhythmia.
Key Teaching Points.
-
•
This case illustrates how a parasystole can manifest itself on tachograms, which are increasingly available in diverse rhythm monitoring systems.
-
•
Parasystole is defined as a constant ectopic rhythm, protected by entry block, leading to constant ectopic beats whenever it can conduct in nonrefractory myocardium.
-
•
Pattern recognition, visually or by means of artificial intelligence, can facilitate diagnosis of arrhythmia.
Footnotes
Funding and potential conflicts of interest: None are declared.
References
- 1.Kaufmann E., Rothberger C.J. Beiträge zur entstehungsweise extrasystolischer allorhythmien. Zeitschrift für die gesamte experimentelle Medizin. 1919;7:199–236. [Google Scholar]
- 2.Schamroth L., Marriott H.J. Intermittent ventricular parasystole with observations on its relationship to extrasystolic bigeminy. Am J Cardiol. 1961;7:799–809. doi: 10.1016/0002-9149(61)90398-8. [DOI] [PubMed] [Google Scholar]
- 3.Jalife J. Modulated parasystole: still relevant after all these years! Heart Rhythm. 2013;10:1441–1443. doi: 10.1016/j.hrthm.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Jalife J., Antzelevitch C., Moe G.K. The case for modulated parasystole. Pacing Clin Electrophysiol. 1982;5:911–926. doi: 10.1111/j.1540-8159.1982.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 5.Schamroth L. Ventricular parasystole with slow manifest ectopic discharge. Br Heart J. 1962;24:731–737. doi: 10.1136/hrt.24.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]