Key Points
Question
What are the outcomes associated with autologous hematopoietic cell transplant based on conditioning regimen used in patients with primary central nervous system lymphoma (PCNSL)?
Findings
In this cohort study of registry data from 603 adult patients with PCNSL undergoing autologous hematopoietic cell transplant, the thiotepa-containing conditioning regimens were associated with higher survival rates compared with carmustine/etoposide/cytarabine/melphalan. Although thiotepa-containing conditioning regimens were associated with a lower relapse risk compared with thiotepa/carmustine, there was comparable survival owing to a higher nonrelapse mortality risk.
Meaning
In this study, thiotepa-based conditioning regimens were associated with favorable outcomes, suggesting that the use of carmustine/etoposide/cytarabine/melphalan should be avoided in patients with PCNSL.
Abstract
Importance
Primary central nervous system lymphoma (PCNSL) requires induction and consolidation to achieve potential cure. High-dose therapy and autologous hematopoietic cell transplant (AHCT) is an accepted and effective consolidation strategy for PCNSL, but no consensus exists on the optimal conditioning regimens.
Objective
To assess the outcomes in patients with PCNSL undergoing AHCT with the 3 most commonly used conditioning regimens: thiotepa/busulfan/cyclophosphamide (TBC), thiotepa/carmustine (TT-BCNU), and carmustine/etoposide/cytarabine/melphalan (BEAM).
Design, Setting, and Participants
This observational cohort study used registry data from the Center for International Blood and Marrow Transplant Research registry. The Center is a working group of more than 380 transplantation centers worldwide that contributed detailed data on HCT to a statistical center at the Medical College of Wisconsin, Milwaukee. The participant data were from 603 adult patients with PCNSL who underwent AHCT as initial, or subsequent, consolidation between January 2010 and December 2018. Patients were excluded if they had a non-Hodgkin lymphoma subtype other than diffuse large B-cell lymphoma, systemic non-Hodgkin lymphoma, or HIV; received an uncommon conditioning regimen; or were not in partial remission or complete remission prior to AHCT. Statistical analysis was performed from July 5, 2020, to March 1, 2021.
Interventions
Patients received 1 of 3 conditioning regimens: TBC (n = 263), TT-BCNU (n = 275), and BEAM (n = 65).
Main Outcomes and Measures
The primary outcome was progression-free survival. Secondary outcomes included hematopoietic recovery, incidence of relapse, nonrelapse mortality, and overall survival.
Results
Of 603 patients, the mean age was 57 (range, 19-77) years and 318 (53%) were male. The 3-year adjusted progression-free survival rates were higher in the TBC cohort (75%) and TT-BCNU cohort (76%) compared with the BEAM cohort (58%) (P = .03) owing to a higher relapse risk in the BEAM cohort (hazard ratio [HR], 4.34; 95% CI, 2.45-7.70; P < .001). In a multivariable regression analysis, compared with the TBC cohort, patients who received TT-BCNU had a higher relapse risk (HR, 1.79; 95% CI, 1.07-2.98; P = .03), lower risk of nonrelapse mortality (NRM) (HR, 0.50; 95% CI, 0.29-0.87; P = .01), and similar risk of all-cause mortality more than 6 months after HCT (HR, 1.54; 95% CI, 0.93-2.55; P = .10). Age of 60 years or older, Karnofsky performance status less than 90, and an HCT-comorbidity index greater than or equal to 3 were associated with lower rates of survival across all 3 cohorts. Subgroup analyses demonstrated that patients aged 60 years and older had considerably higher NRM with TBC.
Conclusions and Relevance
In this cohort study, thiotepa-based conditioning regimen was associated with higher rates of survival compared with BEAM, despite higher rates of early toxic effects and NRM; these findings may assist clinicians in choosing between TBC or TT-BCNU based on patient and disease characteristics.
This cohort study used registry data to assess outcomes in patients with primary central nervous system lymphoma undergoing autologous hematopoietic cell transplant with commonly used conditioning regimens.
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare extranodal subtype of non-Hodgkin lymphoma (NHL) associated with a high relapse risk without high-dose methotrexate-based induction and subsequent consolidation therapy.1 Commonly used consolidation strategies in patients with PCNSL include whole-brain radiotherapy, nonmyeloablative chemotherapy, and high-dose therapy with autologous hematopoietic cell transplant (AHCT), the latter of which abrogates the risk of chronic neurocognitive toxic effects associated with high-dose whole-brain radiotherapy.2,3,4,5,6,7 Extrapolating from experience with systemic lymphomas, early AHCT studies for PCNSL used carmustine/etoposide/cytarabine/melphalan (BEAM) conditioning with disappointing disease control and survival results.8,9,10 More recently, single-arm phase 2 studies demonstrated durable disease control and survival with central nervous system penetrant conditioning regimens, such as thiotepa/busulfan/cyclophosphamide (TBC) and thiotepa/carmustine (TT-BCNU).4,11,12,13,14,15,16,17
To our knowledge, no prospective studies have compared conditioning regimens for AHCT in PCNSL. A 2018 meta-analysis18 comparing mostly single-center studies suggested that TBC conditioning was associated with superior progression-free survival (PFS) and overall survival (OS) compared with TT-BCNU and other regimens despite the potential for a higher incidence of nonrelapse mortality (NRM) and regimen-related toxicities.4,19 Two pivotal randomized clinical trials, IELSG32 and PRECIS, investigated TT-BCNU- and TBC-conditioned AHCT, respectively, compared with whole-brain radiotherapy as up-front consolidation.5,7 Although both trials confirmed the efficacy of AHCT, there are limited data comparing thiotepa-based conditioning regimens with BEAM.
We used the Center for International Blood and Marrow Transplant Research (CIBMTR) registry to compare the outcomes of patients with PCNSL undergoing AHCT with the 3 most commonly used conditioning regimens: TBC, TT-BCNU, and BEAM. We hypothesized that patients who received TBC or TT-BCNU, owing to central nervous system bioavailability of thiotepa, would have superior outcomes compared with those who received BEAM.
Methods
This study used data from the CIBMTR registry, a working group of more than 380 transplantation centers worldwide that contributed detailed HCT data to a statistical center at the Medical College of Wisconsin, Milwaukee. Participating centers are required to report all HCTs consecutively, and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensured data integrity. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Patients provided written informed consent for research. The institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
The CIBMTR collects data at 2 levels: Transplant Essential Data and Comprehensive Report Form data. Transplant Essential Data include disease type, age, sex, pre-HCT disease stage and chemotherapy responsiveness, date of diagnosis, graft type, conditioning regimen, post-HCT disease progression and survival, development of a new malignant neoplasm, and cause of death. All CIBMTR centers contribute Transplant Essential Data. The CIBMTR uses a weighted randomization scheme to select a subset of patients for Comprehensive Report Form reporting with more details about disease and pre-HCT and post-HCT clinical information. Both Transplant Essential Data and Comprehensive Report Form–level data are collected before HCT, at 100 days and 6 months after HCT, and annually thereafter or until death. Data for the current analysis were retrieved from CIBMTR Transplant (Transplant Essential Data and Comprehensive Report Form) report forms.
Patients
This retrospective analysis included adults aged 18 years or older who underwent their first AHCT with peripheral-blood mobilized autografts for chemotherapy-sensitive PCNSL as initial, or subsequent, consolidation between January 2010 and December 2018. Patients were excluded if they had an NHL subtype other than diffuse large B-cell lymphoma, systemic NHL, or HIV; received an uncommon conditioning regimen; or were not in partial remission or complete remission prior to AHCT. The study population was divided into 3 conditioning regimen cohorts: TBC, TT-BCNU, and BEAM (eTable 4 in the Supplement).
Study End Points and Definitions
Disease response prior to AHCT was assessed using standard criteria.20 The primary outcome was PFS, with treatment failure considered at lymphoma relapse, progression, or death from any cause. Secondary outcomes included hematopoietic recovery, relapse or progression, NRM, and OS. Nonrelapse mortality was defined as death without evidence of relapse or progression, with relapse considered a competing risk. Relapse or progression was defined as progressive lymphoma after AHCT, or recurrence after a complete remission, with NRM considered a competing risk. Patients who survived were censored at the date of last follow-up.
Neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count of greater than or equal to 500/μL (to convert neutrophils to ×109/L, multiply by 0.001) after AHCT nadir. Platelet recovery was defined as the first of 3 consecutive days with a platelet count of greater than or equal to 20 000/μL (to convert platelet count to ×109/L, multiply by 1.0) without platelet transfusion for 7 consecutive days. For neutrophil and platelet recovery, death without the event was considered a competing risk. The PFS and OS probabilities were calculated using the Kaplan-Meier estimates.
Statistical Analyses
Baseline patient and HCT characteristics were compared using the Pearson χ2 test for discrete variables and the Kruskal-Wallis test for continuous variables. Cumulative incidences of hematopoietic recovery, relapse, and NRM were calculated to accommodate for competing risks. Cox proportional hazards regression analysis for PFS and OS and the proportional cause-specific hazards model for relapse and NRM were used to identify prognostic factors via forward stepwise selection. The proportional hazard assumption for each variable was examined by testing whether its coefficient was constant over time. Time-varying effects were considered via piecewise proportional hazards models for the variables that violated the proportional hazards assumption.21 The interaction between the conditioning regimen and significant covariates was examined. Center effect was tested using the score test of homogeneity.22 Adjusted PFS and OS were calculated based on the final Cox model. Using the variables in the final cause-specific hazards model, cumulative incidences were calculated based on the Fine-Gray model.23,24 Covariates with a 2-sided P < .05 were considered statistically significant. The variables considered in the multivariable regression analysis are shown in eTable 1 of the Supplement. We also conducted sensitivity analyses to confirm the regression analysis and results using the inverse probability weighting method based on propensity score and the censoring distribution. The bootstrap method was used to evaluate the uncertainty of parameter estimates for the sensitivity analyses. Statistical analysis was performed from July 5, 2020, to March 1, 2021. All statistical analyses were performed using SAS, version 9.4 (SAS Institute) and R, version 4.0.3 (R Foundation for Statistical Computing).
Results
Baseline Characteristics
The study included 603 patients (mean age, 57 [range, 19-77] years; 318 [53%] were male and 285 [47%] were female. Patients received TBC (n = 263), TT-BCNU (n = 275), or BEAM (n = 65) conditioning (Table 1). The cohorts were comparable with respect to age, sex, race, HCT-comorbidity index (HCT-CI), and remission status at HCT. More patients with a Karnofsky performance status (KPS) of 90 to 100 received TBC (n = 147; 56%) compared with TT-BCNU (n = 121; 44%) and BEAM (n = 27; 42%) (P = .02). Median time from diagnosis to HCT in months was longer in the BEAM cohort (11 months; range, 3-122 months) compared with the TBC cohort (8, range 2-192 months) and TT-BCNU cohort (7, range 3-139 months) (P < .001). The conditioning regimen included rituximab more frequently in the BEAM cohort (22%) compared with the TBC cohort (12%) and the TT-BCNU cohort (8%) (P = .004). The median total dose of thiotepa was 18 mg/kg (range, 16-19 mg/kg) in the TBC cohort and 20 mg/kg (range, 10-20 mg/kg) in the TT-BCNU cohort. A higher percentage of patients in the BEAM cohort (55%) underwent AHCT between 2010 and 2014 compared with 2015 and 2018, whereas a higher percentage of patients in the TBC (65%) and TT-BCNU (77%) cohorts underwent AHCT between 2015 and 2018 (P < .001). Median follow-up time of survivors for TBC was 36 months (range, 1-120 months), for TT-BCNU was 24 months (range, 5-98 months), and for BEAM was 48 months (range, 6-97 months).
Table 1. Patient and HCT Characteristics.
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| TBC (n = 263) | TT-BCNU (n = 275) | BEAM (n = 65) | ||
| Age, y | ||||
| Median (range) | 59 (23-76) | 60 (20-78) | 62 (23-74) | .16 |
| 50-59 | 93 (35) | 74 (27) | 22 (34) | |
| 60-69 | 104 (40) | 115 (42) | 28 (43) | |
| ≥70 | 12 (4) | 29 (10) | 6 (9) | |
| Female sex | 129 (49) | 125 (46) | 31 (48) | .70 |
| Race | ||||
| White | 219 (84) | 231 (84) | 54 (83) | .10 |
| African American | 4 (1) | 12 (4) | 3 (5) | |
| Asian | 25 (9) | 15 (6) | 4 (6) | |
| Othera | 2 (1) | 5 (2) | 3 (5) | |
| Missing | 13 (5) | 12 (4) | 1 (1) | |
| KPS | ||||
| 90-100 | 147 (56) | 121 (44) | 27 (42) | .02 |
| <90 | 111 (42) | 148 (53) | 38 (58) | |
| Missing | 7 (3) | 9 (3) | 0 | |
| HCT-CI | ||||
| 0 | 76 (29) | 59 (21) | 18 (28) | .26 |
| 1-2 | 79 (30) | 91 (33) | 16 (25) | |
| ≥3 | 108 (41) | 125 (46) | 31 (48) | |
| Remission status at HCT | ||||
| CR1 | 143 (54) | 166 (60) | 32 (49) | .18 |
| CR2+ | 58 (22) | 41 (15) | 15 (23) | |
| Any PR | 62 (24) | 68 (25) | 18 (28) | |
| Time from diagnosis to HCT, mo | ||||
| Median (range) | 8 (2-192) | 7 (3-139) | 11 (3-122) | <.001 |
| <6 | 84 (32) | 106 (39) | 14 (22) | .03 |
| ≥6 | 175 (67) | 169 (61) | 51 (78) | |
| Missing | 4 (1) | 0 | 0 | |
| Remission status and time from diagnosis to HCT | ||||
| CR1 and ≤6 mo | 69 (26) | 83 (30) | 9 (14) | .09 |
| CR1 and ≥6 mo | 71 (27) | 83 (30) | 23 (35) | |
| CR2+ and >6 mo | 58 (22) | 41 (15) | 15 (23) | |
| PR and ≤6 mo | 16 (6) | 23 (8) | 5 (8) | |
| PR and ≥6 mo | 46 (17) | 45 (17) | 13 (20) | |
| Missing | 3 (1) | 0 | 0 | |
| Rituximab used in conditioning | ||||
| Yes | 32 (12) | 21 (8) | 14 (22) | .004 |
| No | 231 (88) | 254 (92) | 51 (78) | |
| Year of HCT | ||||
| 2010-2014 | 93 (35) | 62 (23) | 35 (54) | <.001 |
| 2015-2018 | 170 (65) | 213 (77) | 30 (46) | |
| Follow-up of survivors, median (range), mo | 36 (1-120) | 24 (5-98) | 48 (6-97) | |
Abbreviations: BEAM, carmustine, etoposide, cytarabine, melphalan; CR1, first complete remission; CR2+, second or later complete remission; HCT, hematopoietic cell transplant; HCT-CI, hematopoietic cell transplant–comorbidity index; KPS, Karnofsky performance status; PR, partial remission; TBC, thiotepa, busulfan, cyclophosphamide; TT-BCNU, thiotepa, carmustine.
Other includes other race, for TBC (n = 2): Native Hawaiian/Pacific Islander (n = 1); American Indian/Alaska Native (n = 1); for TT-BCNU (n = 5): Native Hawaiian/Pacific Islander (n = 2); more than one race (n = 3); and for BEAM (n = 3): American Indian/Alaska Native (n = 1); more than one race (n = 2).
Hematopoietic Recovery
The 1-month cumulative incidence of neutrophil recovery for the TBC cohort was 96% (95% CI, 94%-98%), for the TT-BCNU cohort was 100% (95% CI, 98%-100%), and for the BEAM cohort was 100% (95% CI, 0%-100%) (P = .03). The day 100 cumulative incidence of platelet recovery for the TBC cohort was 92% (95% CI, 88%-95%), for the TT-BCNU cohort was 98% (95% CI, 96%-100%), and for the BEAM cohort was 100% (95% CI, 0%-100%) (P = .002) (Table 2). The median number of days to neutrophil engraftment for the TBC cohort was 9 (range, 7-91 days), for the TT-BCNU cohort was 10 (range, 8-24 days), and for the BEAM cohort was 10 (range, 8-24 days) (P < .001). The median number of days to platelet engraftment for the TBC cohort was 17 (range, 10-180 days), for the TT-BCNU cohort was 16 (range, 10-64 days), and for the BEAM cohort was 16 (range, 10-50 days) (P = .61).
Table 2. Unadjusted and Adjusted Probability of Outcomes in Patients Undergoing AHCT for PCNSL.
| Outcome | TBC (n = 263) | TT-BCNU (n = 275) | BEAM (n = 65) | P value | |||
|---|---|---|---|---|---|---|---|
| No. evaluated | Probability, % (95% CI) | No. evaluated | Probability, % (95% CI) | No. evaluated | Probability, % (95% CI) | ||
| Neutrophil recovery | 261 | 271 | 63 | <.001 | |||
| At 30 d | 96 (94-98) | 100 (98-100) | 100 (0-100) | .03 | |||
| Platelet recovery | 253 | 266 | 63 | .02 | |||
| At 100 d | 92 (88-95) | 98 (96-100) | 100 (0-100) | .002 | |||
| Relapse | 263 | 273 | 63 | <.001 | |||
| At 1 y | 6 (3-9) | 10 (7-14) | 22 (13-34) | .01 | |||
| At 3 y | 11 (7-16) | 15 (10-20) | 36 (24-49) | .001 | |||
| Adjusted NRM | 263 | 273 | 63 | ||||
| At 100 d | 7 (4-10) | 2 (0.2-3) | 0a | <.001 | |||
| At 1 y | 11 (7-15) | 4 (2-6) | 4 (0-9) | .01 | |||
| At 3 y | 14 (9-19) | 9 (5-14) | 6 (0.5-12) | .14 | |||
| Adjusted PFS | 263 | 273 | 63 | ||||
| 1 y | 83 (79-88) | 86 (82-90) | 72 (62-83) | .05 | |||
| 3 y | 75 (69-81) | 76 (70-82) | 58 (46-70) | .03 | |||
| Adjusted OS | 263 | 275 | 65 | ||||
| 1 y | 87 (83-91) | 92 (88-95) | 90 (83-97) | .28 | |||
| 3 y | 81 (75-86) | 78 (72-85) | 69 (58-80) | .17 | |||
Abbreviations: AHCT, autologous hematopoietic cell transplant; BEAM, carmustine, etoposide, cytarabine, melphalan; NRM, nonrelapse mortality; OS, overall survival; PCNSL, primary central nervous system lymphoma; PFS, progression-free survival; TBC, thiotepa, busulfan, cyclophosphamide; TT-BCNU, thiotepa, carmustine.
No patients with BEAM experienced either relapse or death by day 100. Thus, its estimate is 0.
Nonrelapse Mortality and Relapse
The adjusted cumulative incidence of NRM at day 100 for the TBC cohort was 7% (95% CI, 4%-10%), for the TT-BCNU cohort was 2% (95% CI, 0.2%-3%), and for the BEAM cohort was 0% (P < .001). The adjusted cumulative incidence of NRM at 1 year for the TBC cohort was 11% (95% CI, 7%-15%), for the TT-BCNU cohort was 4% (95% CI, 2%-6%), and for the BEAM cohort was 4% (95% CI, 0%-9%) (P = .01) (Table 2). In a multivariable regression analysis after adjusting for age and HCT-CI, the use of TT-BCNU (hazard ratio [HR], 0.5; 95% CI, 0.29-0.87; P = .01), but not BEAM (HR, 0.5; 95% CI, 0.2-1.28, P = .15) was associated with a reduced risk of NRM, compared with use of TBC (P = .03) (Table 3, Figure, A).
Table 3. Main Outcomes of Multivariable Regression Analysis.
| Variable | No. | HR (95% CI) | P value | Overall P value |
|---|---|---|---|---|
| Relapse | ||||
| Conditioning regimen | ||||
| TBC | 263 | 1 [Reference] | <.001 | |
| TT-BCNU | 275 | 1.79 (1.07-2.98) | .03 | |
| BEAM | 65 | 4.34 (2.45-7.70) | <.001 | |
| NRMa | ||||
| Conditioning regimen | ||||
| TBC | 263 | 1 [Reference] | .03 | |
| TT-BCNU | 275 | 0.50 (0.29-0.87) | .01 | |
| BEAM | 65 | 0.50 (0.20-1.28) | .15 | |
| PFSb | ||||
| Conditioning regimen | ||||
| TBC | 263 | 1 [Reference] | .04 | |
| TT-BCNU | 275 | 1.04 (0.72-1.50) | .86 | |
| BEAM | 65 | 1.74 (1.10-2.75) | .02 | |
| OSc | ||||
| Conditioning regimen (≤6 mo after HCT)a | ||||
| TBC | 263 | 1 [Reference] | .008 | |
| TT-BCNU | 275 | 0.35 (0.17-0.73) | .01 | |
| BEAM | 65 | 0.26 (0.06-1.12) | .07 | |
| Conditioning regimen (>6 mo after HCT)d | ||||
| TBC | 232 | 1 [Reference] | .002 | |
| TT-BCNU | 257 | 1.54 (0.93-2.55) | .10 | |
| BEAM | 62 | 2.73 (1.56-4.76) | <.001 | |
| Missing | 3 | 4.08 (0.53-31.55) | .18 | |
Abbreviations: BEAM, carmustine, etoposide, cytarabine, melphalan; HCT, hematopoietic cell transplant; HCT-CI, hematopoietic cell transplant comorbidity index; HR, hazard ratio; KPS, Karnofsky performance status; NRM, nonrelapse mortality; OS, overall survival; PFS, progression-free survival; TBC, thiotepa, busulfan, cyclophosphamide; TT-BCNU, thiotepa, carmustine.
NRM adjusted for significant covariates: age, HCT-CI.
PFS adjusted for significant covariates: age, KPS, disease status/time from diagnosis to HCT.
OS adjusted for significant covariates: age, HCT-CI, disease status/time from diagnosis to HCT.
The 6-month time frame was chosen as the cutoff OS based on the maximum likelihood value in the Cox model.
Figure. Autologous Hematopoietic Cell Transplant Outcomes for Patients With Primary Central Nervous System Lymphoma.
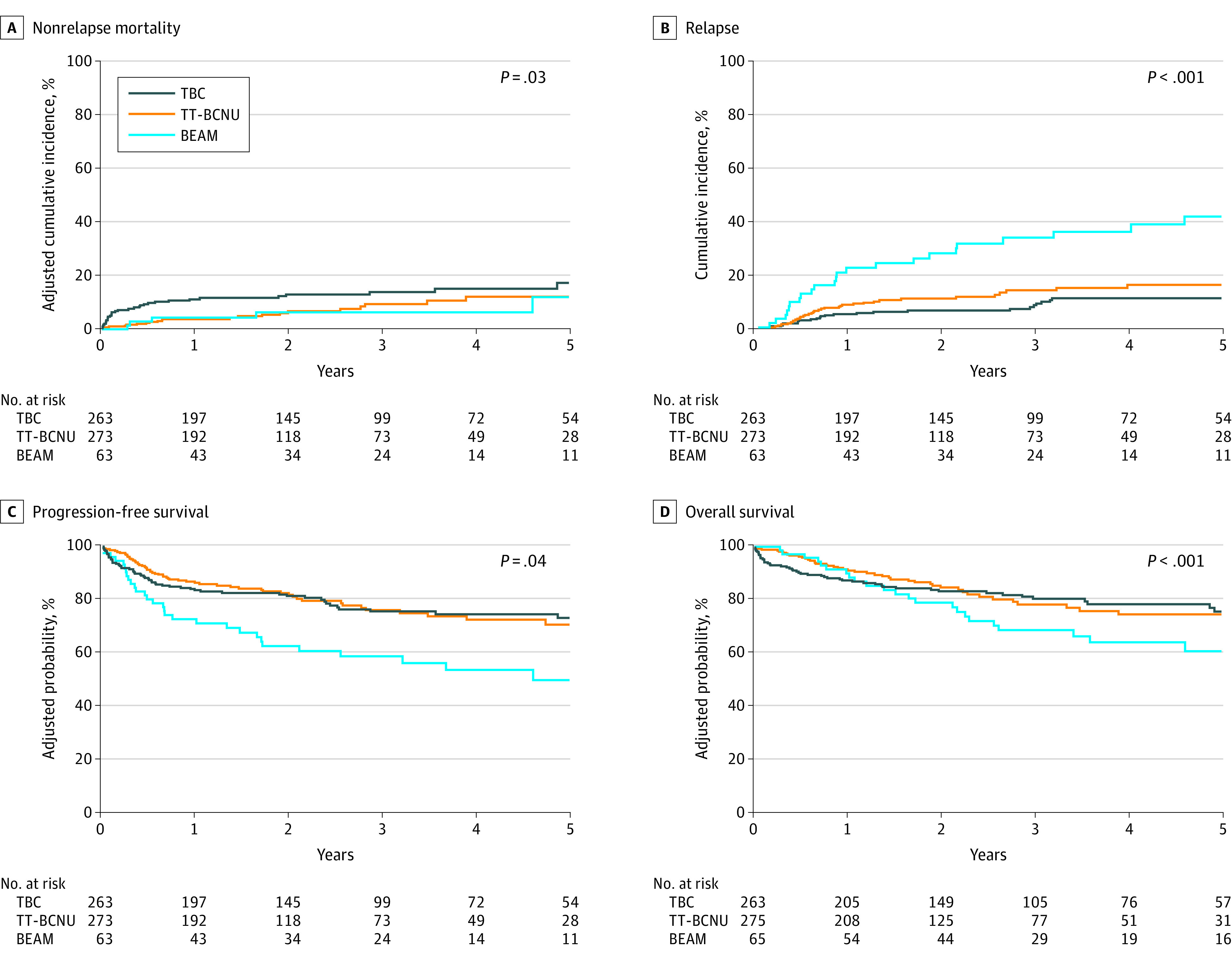
BEAM indicates carmustine, etoposide, cytarabine, melphalan; TBC, thiotepa, busulfan, cyclophosphamide; TT-BCNU, thiotepa, carmustine.
The cumulative incidence of relapse or progression at 3 years for the TBC cohort was 11% (95% CI, 7%-16%), for the TT-BCNU cohort was 15% (95% CI, 10%-20%), and for the BEAM cohort was 36% (95% CI, 24%-49%) (P = .001) (Table 2). In a multivariable regression analysis, use of TT-BCNU (HR, 1.79; 95% CI, 1.07-2.98; P = .03) and BEAM (HR, 4.34; 95% CI, 2.45-7.70; P < .001) were associated with an increased risk of relapse or progression, compared with use of TBC (P < .001) (Table 3, Figure, B). Compared with BEAM, use of TT-BCNU was associated with a lower risk of relapse or progression (HR, 0.41; 95% CI, 0.25-0.69; P < .001).
Progression-Free Survival
The adjusted PFS rate at 1 year for the TBC cohort was 83% (95% CI, 79%-88%), for the TT-BCNU cohort was 86% (95% CI, 82%-90%), and for the BEAM cohort was 72% (95% CI, 62%-83%) (P = .05). At 3 years the adjusted PFS rate for the TBC cohort was 75% (95% CI, 69%-81%), for the TT-BCNU cohort was 76% (95% CI, 70%-82%), and for the BEAM cohort was 58% (95% CI, 46%-70%) (P = .03) (Table 2). On multivariable regression analysis after adjusting for age, KPS, and disease status/time from diagnosis to HCT, use of TT-BCNU was associated with similar PFS (HR, 1.04; 95% CI, 0.72-1.5; P = .86), whereas BEAM was associated with inferior PFS (HR, 1.74; 95% CI, 1.1-2.75; P = .02), compared with use of TBC (P = .04) (Table 3, Figure, C).
Overall Survival
The adjusted OS rate at 1 year for the TBC cohort was 87% (95% CI, 83%-91%), for the TT-BCNU cohort was 92% (95% CI, 88%-95%), and for the BEAM cohort was 90% (95% CI, 83%-97%) (P = .28). The adjusted OS rate at 3 years for the TBC cohort was 81% (95% CI, 75%-86%), for the TT-BCNU cohort was 78% (95% CI, 72%-85%), and for the BEAM cohort was 69% (95% CI, 58%-80%) (P = .17) (Table 2). On multivariable regression analysis, the proportional hazards assumption for Cox regression model for OS was violated. Thus, a piecewise proportional hazards model was built, wherein the best cutoff of 6 months after HCT was selected based on the maximum likelihood method. After adjusting for age, HCT-CI, and disease status or time from diagnosis to HCT, in 6 months or less after HCT, the use of TT-BCNU (HR, 0.35; 95% CI, 0.17-0.73; P = .01) was associated with a lower risk of all-cause mortality, compared with use of TBC, but after 6 months, use of TT-BCNU was not associated with a different mortality risk (HR, 1.54; 95% CI, 0.93-2.55; P = .10) compared with TBC. The use of BEAM (HR, 2.73; 95% CI, 1.56-4.76; P < .001) was associated with a higher risk of all-cause mortality compared with use of TBC after 6 months post-HCT (overall P = .002) (Figure, D).
Additional significant covariates in the multivariable analysis for NRM, relapse, PFS, and OS are detailed in eTable 2 in the Supplement. To address potential treatment selection biases, we performed an inverse probability weighting analysis using propensity score. To minimize biases associated with censoring mechanisms or time-dependent selection, we performed an inverse probability censoring weighted regression. The HRs in these analyses were directionally consistent with the regression analysis in Table 3 and are detailed in eTable 5 in the Supplement.
Subgroup Analyses
Among patients aged 59 years or younger who received TBC, the cumulative incidence of NRM was 6% (95% CI, 2%-10%), for those who received TT-BCNU, the cumulative incidence of NRM was 6% (95% CI, 2%-13%), and for those who received BEAM, the cumulative incidence of NRM was 3% (95% CI, 0%-12%) (P = .004). The cumulative incidence of relapse among the same group who received TBC was 8% (95% CI, 4%-14%), for those who received TT-BCNU was 15% (95% CI, 8%-22%), and for those who received BEAM was 43% (95% CI, 24%-62%) (P = .002). The PFS rate for those who received TBC was 86% (95% CI, 79%-92%), for those who received TT-BCNU was 79% (95% CI, 70%-87%), and for those who received BEAM was 54% (95% CI, 35%-72%) (P = .01). The OS rate for those who received TBC was 90% (95% CI, 84%-95%), for those who received TT-BCNU was 81% (95% CI, 71%-89%), and for those who received BEAM was 73% (95% CI, 55%-58%) (P = .05).
Among patients aged 60 years or older who received TBC, the cumulative incidence of NRM at 3 years was 21% (95% CI, 14%-29%), for those who received TT-BCNU, the cumulative incidence of NRM at 3 years was 13% (95% CI, 7%-22%), and for those who received BEAM, the cumulative incidence of NRM at 3 years was 10% (95% CI, 2%-24%) (P = .22). The cumulative incidence of relapse for those who received TBC was 14% (95% CI, 8%-23%), for those who received TT-BCNU was 15% (95% CI, 8%-22%), and for those who received BEAM was 30% (95% CI, 15%-47%) (P = .25). The PFS rate for those who received TBC was 65% (95% CI, 55%-74%), for those who received TT-BCNU was 72% (95% CI, 62%-81%), and for those who received BEAM was 60% (95% CI, 43%-77%) (P = .43). The OS rate for those who received TBC was 69% (95% CI, 59%-78%), for those who received TT-BCNU was 76% (95% CI, 66%-85%), and for those who received BEAM was 57% (95% CI, 39%-74%) (P = .15) (eTable 3 in the Supplement).
Causes of Death
The primary cause of death was relapsed or progressive PCNSL in 38% (20 of 53) of patients for TBC, 72% (33 of 46) of patients for TT-BCNU, and 76% (19 of 25) of patients for BEAM (Table 4). Organ failure in 21% (11 of 53) and infection in 15% (8 of 53) of patients were common causes of death in the TBC cohort. The 11 organ failure–related deaths were pulmonary (6 of 11 [55%]), hepatic (2 of 11 [18%]), cardiac (1 of 11 [9%]), neurologic (1 of 11 [9%]), and multiorgan (1 of 11 [9%]).
Table 4. Causes of Death.
| Characteristic | No. (%) | ||
|---|---|---|---|
| TBC (n = 263) | TT-BCNU (n = 275) | BEAM (n = 65) | |
| Overall survival | |||
| Alive | 210 (80) | 229 (83) | 40 (62) |
| Dead | 53 (20) | 46 (17) | 25 (38) |
| Cause of death | |||
| Primary disease | 20 (38) | 33 (72) | 19 (76) |
| Infection | 8 (15) | 3 (7) | 2 (8) |
| IPS | 0 | 1 (2) | 0 |
| ARDS | 0 | 1 (2) | 0 |
| Organ failure | 11 (21) | 2 (4) | 1 (4) |
| Secondary malignant neoplasm | 2 (4) | 1 (2) | 1 (4) |
| Hemorrhage | 1 (2) | 0 | 1 (4) |
| Vascular | 0 | 1 (2) | 0 |
| Other | 6 (11) | 2 (4) | 0 |
| Unknown | 5 (9) | 2 (4) | 1 (4) |
Abbreviations: ARDS, acute respiratory distress syndrome; BEAM, carmustine, etoposide, cytarabine, melphalan; IPS, idiopathic pneumonia syndrome; TBC, thiotepa, busulfan, cyclophosphamide; TT-BCNU, thiotepa, carmustine.
Discussion
To address the lack of comparative data regarding the conditioning regimen of choice in patients with PCNSL undergoing AHCT, we performed a registry analysis comparing outcomes of patients across 3 cohorts of the most commonly used conditioning regimens. Despite finding more toxic effects, lower early engraftment rates, and higher early NRM, we also found that use of the thiotepa-containing conditioning regimens, TBC and TT-BCNU, was associated with higher rates of survival compared with use of BEAM owing to protection from relapse and found that BEAM was associated with lower rates of survival and is likely not suitable in patients with PCNSL. We speculate that one potential explanation is the inadequate central nervous system penetration of BEAM.3,8,9,17,25 Patients who received TBC had a lower risk of relapse than those who received TT-BCNU, but at the cost of a higher risk of NRM, that ultimately was associated with similar long-term OS. The adjusted 3-year NRM rate of 14% for the TBC cohort is high but comparable to the variable rates seen in previous studies.3,18 However, patients aged 59 years or younger who received TBC conditioning had highly favorable outcomes with 3-year PFS and OS rates of 86% and 90%, respectively.
Although this study cannot definitively determine the optimal thiotepa-containing regimen, it may inform a clinician’s choice based on key patient and disease characteristics. Across all 3 cohorts, age (≥60 years), lower KPS (<90), and high HCT-CI (≥3) were associated with worse survival. More chemotherapy-resistant disease (eg, second or later complete remission, partial remission) and greater time from PCNSL diagnosis to HCT were associated with higher risks of relapse and mortality. Although the TBC cohort had a higher proportion of patients with KPS greater than 90, early NRM was higher than that of the TT-BCNU cohort, leading to similar PFS and OS owing to an increased risk of relapse in patients who received TT-BCNU. In subgroup analyses, patients aged 59 years or younger who received TBC vs TT-BCNU, respectively, had a lower risk of relapse (8% vs 15%), better PFS (86% vs 79%) and OS (90% vs 81%), and comparable NRM (6% vs 6%) at 3 years. For patients aged 60 years or older who received TBC vs TT-BCNU, respectively, despite similar incidences of relapse (14% vs 15%), there was worse 3-year PFS (65% vs 72%) and OS (69% vs 76%), likely reflecting higher NRM (21% vs 13%). This finding suggests that TBC may be more suited to patients who are younger and fitter, particularly those with more advanced disease beyond complete remission 1 given the better protection from relapse, and that TT-BCNU may be more suited to patients who are older with more comorbid conditions.7,19,26
Limitations
This study has limitations. Limitations include potential patient-selection and center-specific practice biases in choosing the conditioning regimens. This point may be evidenced by greater use of TBC and TT-BCNU in recent years compared with BEAM. Notably, the TT-BCNU cohort had a shorter median follow-up time compared with the TBC cohort (the BEAM cohort had the longest follow-up), although it is uncertain whether relapse and survival rates would be similar with equivalent follow-up. Important data elements were unavailable, including the types of methotrexate-based induction used, whether or when patients received whole-brain radiotherapy, whether pharmacokinetically targeted busulfan was used, if additional consolidation was used after HCT, and which therapies were used after relapse, such as immunotherapies and Bruton tyrosine kinase inhibitors.27,28 There were insufficient data to evaluate the number of lines of prior therapy and to estimate a PCNSL-specific disease-risk score, thereby limiting a thorough understanding of each cohort’s inherent disease course.29 We attempted to account for this limitation by evaluating best response prior to HCT and time from diagnosis to HCT as surrogates and noted worse OS in patients whose time from diagnosis to HCT was greater than 6 months despite achieving complete remission or partial remission prior to AHCT, suggesting that patients who have longer time from diagnosis to AHCT may have more chemotherapy-resistant disease. Other conditioning regimens that have been studied in PCNSL, such as TT-busulfan and thiotepa-free regimens, are not yet well represented in the registry for adequate comparison.30,31,32 Our results do not apply to patients with secondary CNSL who were excluded from this study. However, given that thiotepa-containing regimens have been successfully used for secondary CNSL, a separate analysis would be of interest.19,33,34,35,36
Conclusions
To our knowledge, this is the largest retrospective analysis comparing outcomes of patients with PCNSL undergoing consolidative AHCT. Our findings suggest that thiotepa-containing regimens should be considered the standard in patients with PCNSL wherein AHCT is determined to be the consolidation of choice.18 Given the similar OS seen with TBC and TT-BCNU, a prospective randomized clinical trial would be required to determine the preferred alkylating agent partners with thiotepa that balance the goals of maximal disease control with minimal risk of NRM, although this type of study may be difficult to power. Randomized clinical trials should include comparative toxic effect evaluations and health care resource use data that may better define regimen tolerability and allow for more personalized decision-making for patients. Moreover, we strongly support ongoing efforts to refine the TBC and TT-BCNU conditioning regimens, define optimal chemotherapy dosing, and develop novel approaches for reducing early toxicities to help extend this potentially curative therapy to patients who are older with more comorbid conditions with PCNSL.37
eTable 1. Variables Considered in the Multivariable Analysis
eTable 2. Multivariable Regression Analysis of Outcomes
eTable 3. Univariable Subgroup Analysis of Outcomes by Age Group
eTable 4. Conditioning Chemotherapy Regimen Details
eTable 5. Multivariate Regression – Inverse Probability Weighted Estimator (IPWE-PS) Based on Propensity Score and Inverse Probability Censoring Weighted Estimator (IPCW)
References
- 1.Grommes C, DeAngelis LM. Primary CNS Lymphoma. J Clin Oncol. 2017;35(21):2410-2418. doi: 10.1200/JCO.2017.72.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31(25):3061-3068. doi: 10.1200/JCO.2012.46.9957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreri AJM, Illerhaus G. The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood. 2016;127(13):1642-1649. doi: 10.1182/blood-2015-10-636340 [DOI] [PubMed] [Google Scholar]
- 4.Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403-1410. doi: 10.1182/blood-2014-10-604561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreri AJM, Cwynarski K, Pulczynski E, et al. ; International Extranodal Lymphoma Study Group (IELSG) . Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017;4(11):e510-e523. doi: 10.1016/S2352-3026(17)30174-6 [DOI] [PubMed] [Google Scholar]
- 6.Birsen R, Willems L, Pallud J, et al. Efficacy and safety of high-dose etoposide cytarabine as consolidation following rituximab methotrexate temozolomide induction in newly diagnosed primary central nervous system lymphoma in immunocompetent patients. Haematologica. 2018;103(7):e296-e299. doi: 10.3324/haematol.2017.185843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houillier C, Taillandier L, Dureau S, et al. ; Intergroupe GOELAMS–ANOCEF and the LOC Network for CNS Lymphoma . Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J Clin Oncol. 2019;37(10):823-833. doi: 10.1200/JCO.18.00306 [DOI] [PubMed] [Google Scholar]
- 8.Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21(22):4151-4156. doi: 10.1200/JCO.2003.05.024 [DOI] [PubMed] [Google Scholar]
- 9.Colombat P, Lemevel A, Bertrand P, et al. High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: a multicenter phase II study of the GOELAMS group. Bone Marrow Transplant. 2006;38(6):417-420. doi: 10.1038/sj.bmt.1705452 [DOI] [PubMed] [Google Scholar]
- 10.Chen YB, Lane AA, Logan B, et al. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(6):1046-1053. doi: 10.1016/j.bbmt.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Illerhaus G, Marks R, Ihorst G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24(24):3865-3870. doi: 10.1200/JCO.2006.06.2117 [DOI] [PubMed] [Google Scholar]
- 12.Illerhaus G, Müller F, Feuerhake F, Schäfer AO, Ostertag C, Finke J. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica. 2008;93(1):147-148. doi: 10.3324/haematol.11771 [DOI] [PubMed] [Google Scholar]
- 13.Cheng T, Forsyth P, Chaudhry A, et al. High-dose thiotepa, busulfan, cyclophosphamide and ASCT without whole-brain radiotherapy for poor prognosis primary CNS lymphoma. Bone Marrow Transplant. 2003;31(8):679-685. doi: 10.1038/sj.bmt.1703917 [DOI] [PubMed] [Google Scholar]
- 14.Soussain C, Hoang-Xuan K, Taillandier L, et al. ; Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire . Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol. 2008;26(15):2512-2518. doi: 10.1200/JCO.2007.13.5533 [DOI] [PubMed] [Google Scholar]
- 15.Cote GM, Hochberg EP, Muzikansky A, et al. Autologous stem cell transplantation with thiotepa, busulfan, and cyclophosphamide (TBC) conditioning in patients with CNS involvement by non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2012;18(1):76-83. doi: 10.1016/j.bbmt.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 16.Illerhaus G, Kasenda B, Ihorst G, et al. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol. 2016;3(8):e388-e397. doi: 10.1016/S2352-3026(16)30050-3 [DOI] [PubMed] [Google Scholar]
- 17.Kondo E, Ikeda T, Izutsu K, et al. ; Adult Lymphoma Working Group of the Japan Society for Hematopoietic Cell Transplantation . High-dose chemotherapy with autologous stem cell transplantation in primary central nervous system lymphoma: data from the Japan Society for Hematopoietic Cell Transplantation Registry. Biol Blood Marrow Transplant. 2019;25(5):899-905. doi: 10.1016/j.bbmt.2019.01.020 [DOI] [PubMed] [Google Scholar]
- 18.Alnahhas I, Jawish M, Alsawas M, et al. Autologous stem-cell transplantation for primary central nervous system lymphoma: systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2019;19(3):e129-e141. doi: 10.1016/j.clml.2018.11.018 [DOI] [PubMed] [Google Scholar]
- 19.Scordo M, Bhatt V, Hsu M, et al. A comprehensive assessment of toxicities in patients with central nervous system lymphoma undergoing autologous stem cell transplantation using thiotepa, busulfan, and cyclophosphamide conditioning. Biol Blood Marrow Transplant. 2017;23(1):38-43. doi: 10.1016/j.bbmt.2016.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheson BD, Fisher RI, Barrington SF, et al. ; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin’s Study Group; Japanese Lymphoma Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein J, Moeschberger M.. Survival Analysis: Techniques for Censored and Truncated Data. Springer Nature; 2003. doi: 10.1007/b97377 [DOI] [Google Scholar]
- 22.Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal. 1995;1(2):145-156. doi: 10.1007/BF00985764 [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95-101. doi: 10.1016/j.cmpb.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101(1):87-93. doi: 10.1016/j.cmpb.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiebe VJ, Smith BR, DeGregorio MW, Rappeport JM. Pharmacology of agents used in bone marrow transplant conditioning regimens. Crit Rev Oncol Hematol. 1992;13(3):241-270. doi: 10.1016/1040-8428(92)90092-5 [DOI] [PubMed] [Google Scholar]
- 26.Scordo M, Morjaria SM, Littmann ER, et al. Distinctive infectious complications in patients with central nervous system lymphoma undergoing thiotepa, busulfan, and cyclophosphamide-conditioned autologous stem cell transplantation. Biol Blood Marrow Transplant. 2018;24(9):1914-1919. doi: 10.1016/j.bbmt.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasenda B, Loeffler J, Illerhaus G, Ferreri AJM, Rubenstein J, Batchelor TT. The role of whole brain radiation in primary CNS lymphoma. Blood. 2016;128(1):32-36. doi: 10.1182/blood-2016-01-650101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreri AJM, Holdhoff M, Nayak L, Rubenstein JL. Evolving treatments for primary central nervous system lymphoma. Am Soc Clin Oncol Educ Book. 2019;39:454-466. doi: 10.1200/EDBK_242547 [DOI] [PubMed] [Google Scholar]
- 29.Ferreri AJM, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266-272. doi: 10.1200/JCO.2003.09.139 [DOI] [PubMed] [Google Scholar]
- 30.Yoon DH, Lee DH, Choi DR, et al. Feasibility of BU, CY and etoposide (BUCYE), and auto-SCT in patients with newly diagnosed primary CNS lymphoma: a single-center experience. Bone Marrow Transplant. 2011;46(1):105-109. doi: 10.1038/bmt.2010.71 [DOI] [PubMed] [Google Scholar]
- 31.Miyao K, Sakemura R, Imai K, et al. Upfront autologous stem-cell transplantation with melphalan, cyclophosphamide, etoposide, and dexamethasone (LEED) in patients with newly diagnosed primary central nervous system lymphoma. Int J Hematol. 2014;100(2):152-158. doi: 10.1007/s12185-014-1608-9 [DOI] [PubMed] [Google Scholar]
- 32.Sanders S, Chua N, Larouche JF, Owen C, Shafey M, Stewart DA. Outcomes of consecutively diagnosed primary central nervous system lymphoma patients using the Alberta Lymphoma Clinical Practice Guideline incorporating thiotepa-busulfan conditioning for transplantation-eligible patients. Biol Blood Marrow Transplant. 2019;25(8):1505-1510. doi: 10.1016/j.bbmt.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 33.Welch MR, Sauter CS, Matasar MJ, et al. Autologous stem cell transplant in recurrent or refractory primary or secondary central nervous system lymphoma using thiotepa, busulfan and cyclophosphamide. Leuk Lymphoma. 2015;56(2):361-367. doi: 10.3109/10428194.2014.916800 [DOI] [PubMed] [Google Scholar]
- 34.DeFilipp Z, Li S, El-Jawahri A, et al. High-dose chemotherapy with thiotepa, busulfan, and cyclophosphamide and autologous stem cell transplantation for patients with primary central nervous system lymphoma in first complete remission. Cancer. 2017;123(16):3073-3079. doi: 10.1002/cncr.30695 [DOI] [PubMed] [Google Scholar]
- 35.Qualls D, Sullivan A, Li S, et al. High-dose thiotepa, busulfan, cyclophosphamide, and autologous stem cell transplantation as upfront consolidation for systemic non-Hodgkin lymphoma with synchronous central nervous system involvement. Clin Lymphoma Myeloma Leuk. 2017;17(12):884-888. doi: 10.1016/j.clml.2017.08.100 [DOI] [PubMed] [Google Scholar]
- 36.Korfel A, Elter T, Thiel E, et al. Phase II study of central nervous system (CNS)-directed chemotherapy including high-dose chemotherapy with autologous stem cell transplantation for CNS relapse of aggressive lymphomas. Haematologica. 2013;98(3):364-370. doi: 10.3324/haematol.2012.077917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schorb E, Kasenda B, Ihorst G, et al. High-dose chemotherapy and autologous stem cell transplant in elderly patients with primary CNS lymphoma: a pilot study. Blood Adv. 2020;4(14):3378-3381. doi: 10.1182/bloodadvances.2020002064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Variables Considered in the Multivariable Analysis
eTable 2. Multivariable Regression Analysis of Outcomes
eTable 3. Univariable Subgroup Analysis of Outcomes by Age Group
eTable 4. Conditioning Chemotherapy Regimen Details
eTable 5. Multivariate Regression – Inverse Probability Weighted Estimator (IPWE-PS) Based on Propensity Score and Inverse Probability Censoring Weighted Estimator (IPCW)


