Abstract
BACKGROUND
Evidence has been published on the successful applications of the anti-tumor necrosis factor alpha antibody infliximab, such as induction therapy, salvage treatment for acute cellular rejection, and treatment for chronic ulcerative inflammation, in intestinal transplant recipients. However, the optimal protocol for the effective use of infliximab remains largely undetermined due to scarcity of available clinical data. We report a continuative application of infliximab as maintenance therapy for recurrent chronic ulcerative ileitis in a recipient of isolated intestinal transplantation (ITx).
CASE SUMMARY
The patient was a 11-year-old boy with intestinal motility disorder classified as a hypogenic type of intestinal dysganglionosis. The patient underwent living-donor related intestinal transplant. His immunosuppression regimen consisted of daclizumab, tacrolimus, and steroids. Although he did not show rejection while on tacrolimus monotherapy, routine screening endoscopy showed several ulcerative lesions in the distal end of the graft 2 years after the intestinal transplant. Endoscopic work up to evaluate the progression of anemia revealed stenosis with ulcerative inflammatory changes and multiple longitudinal ulcers in the graft. Since the endoscopic findings suggested ulcerative lesions in Crohn’s disease, infliximab treatment was considered. Treatment with infliximab and a small dose of oral prednisolone afforded successful withdrawal of total parenteral nutrition and maintenance of a well-functioning graft without infectious complications for 5 years since the administration of the first dose of infliximab.
CONCLUSION
Infliximab is effective as maintenance therapy for recurrent chronic ulcerative ileitis in an isolated ITx patient.
Keywords: Intestinal transplantation, Chronic ulcer, Infliximab, Crohn’s disease, Tumor necrosis factor alpha, Case report
Core Tip: Infliximab binds to soluble and transmembrane forms of human tumor necrosis factor alpha (TNF-α). Ulcerative inflammatory changes in the graft under intestinal transplantation (ITx) is an often-encountered finding. However, it does not meet the criteria for so-called rejection and is close to the pathology of Crohn’s disease. Studies in Crohn’s disease patients revealed that anti-TNF-α therapy provides better outcomes when combined with immunomodulatory agents and that therapeutic drug monitoring might help optimize dosing. Infliximab may be effective as a treatment for ulcerative inflammation in the intestinal graft that does not meet the criteria for acute cellular rejection not improved by immunosuppressant conditioning. The optimal management for recurrent ulcerative inflammation under ITx settings by using anti-TNF-α therapy needs further elucidation.
INTRODUCTION
Tumor necrosis factor alpha (TNF-α) is one of the central cytokines in the pathogenesis of mucosal inflammation in inflammatory bowel disease (IBD) and has been the primary target of biologic therapies. Although TNF is mainly produced by monocytes, macrophages, and T lymphocytes, it is also produced by mast cells, granulocytes, fibroblasts, and several other cell types[1]. TNF is a highly pro-inflammatory cytokine that is involved in key processes in inflammation, including the activation of coagulation and fibrinolytic responses, promotion of the development of the neutrophil-endothelial adhesion necessary for recruitment to inflammation sites[2-4], and promotion of granulomatous inflammation through its role in the recruitment of T lymphocytes, monocytes, and macrophages[5-7].
Infliximab is a chimeric immunoglobulin G1 monoclonal antibody that binds to soluble and transmembrane forms of human TNF-α. It was approved by the United States Food and Drug Administration in 1998 for Crohn’s disease and in 1999 for rheumatoid arthritis. Later, the approval was broadened for the treatment of various autoimmune diseases including diseases in pediatric patients. The advent of anti-TNF-α antibodies has resulted in a paradigm shift in the treatment of IBD. Anti-TNF-α antibodies are thought to have multiple mechanisms of action, including neutralization of TNF-α, reverse signaling, apoptosis, and cytotoxicity[8], and have a predilection and efficiency for distribution into inflamed tissue[9]. Anti-TNF-α antibodies also induce the apoptosis of activated lamina propria T lymphocytes[10], which is contradictory to a proposed pathological mechanism in Crohn’s disease, where mucosal T cell proliferation exceeds T cell apoptosis[11]. In addition, anti-TNF-α therapies are capable of inducing antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity[9].
Advances in treatment for IBD provide an insight into the potential mechanism to control immune responses in intestinal transplantation (ITx). The first case report about the use of infliximab in the ITx setting dates back to 2003[12]. The report describes two adult patients who were successfully treated for cellular rejection refractory to anti-CD3 monoclonal antibody (OKT3) treatment. Since then, 22 published cases related to “intestinal transplant and infliximab” have been found in PubMed. Studies in rodents suggested promising complementary effects of infliximab addition to conventional immunosuppressive regimens not only in terms of alleviation of ischemic reperfusion injury but also attenuation of acute cellular rejection (ACR)[13,14]. However, despite some promising clinical evidence available from both animal and human studies, little is known about the optimal protocol and safety of infliximab use in the ITx setting. Herein, we report the detailed clinical course with serial serum TNF-α levels of an intestinal transplant recipient for a prolonged period and discuss future prospects of infliximab treatment based on a literature review.
CASE PRESENTATION
Chief complaints
Routine screening endoscopy showed several ulcerative lesions in the distal end of the graft 2 years after ITx. The patient developed anorexia, showed weight loss, and experienced intermittent abdominal pain.
History of present illness
A patient was an 11-year-old boy with intestinal motility disorder classified as a hypogenic type of intestinal dysganglionosis. The patient underwent living-donor related ITx from his father because of a lack of vascular access and growth retardation due to malnutrition. The graft was 110 cm of donor ileum from a point 20 cm proximal to the Bauhin valve. His initial immunosuppression regimen consisted of daclizumab as induction therapy, tacrolimus, and steroids. Daclizumab was continued until 12 wk after ITx as the maintenance treatment. Tacrolimus was started on the day of surgery after reperfusion. It was initially administered in a continuous intravenous manner, before switching to oral administration 2 wk after the transplant. The target trough level was between 20 and 25 ng/mL for the first month, 15-20 ng/mL for the next 2 mo, 10-15 ng/mL for the next 3 mo, and 10 ng/mL thereafter. Despite two episodes of steroid-responsive mild ACR and cytomegalovirus (CMV) infection within the first year after ITx, he was free of rejection on tacrolimus monotherapy.
History of past illness
Hypogenic type of intestinal dysganglionosis.
Personal and family history
There was no relevant family history.
Physical examination
Routine screening endoscopy showed several ulcerative lesions in the distal end of the graft 2 years after ITx. Partial resection of the small intestine including the ulcerative lesions was performed when he underwent revision of the stoma (Bishop-Koop procedure) in preparation for a complete reversal of ileostomy at the age of 13 years. Histological analysis of the ulcerative lesion revealed non-specific inflammatory changes. However, the patient developed anorexia, showed weight loss, and experienced intermittent abdominal pain several months after the previous operation. These symptoms persisted on and off over several months.
Laboratory examinations
Regular blood tests revealed hypoalbuminemia, a low total cholesterol level, and anemia; the other findings were unremarkable, including C-reactive protein level, erythrocyte sedimentation rate, and white blood cell count.
Imaging examinations
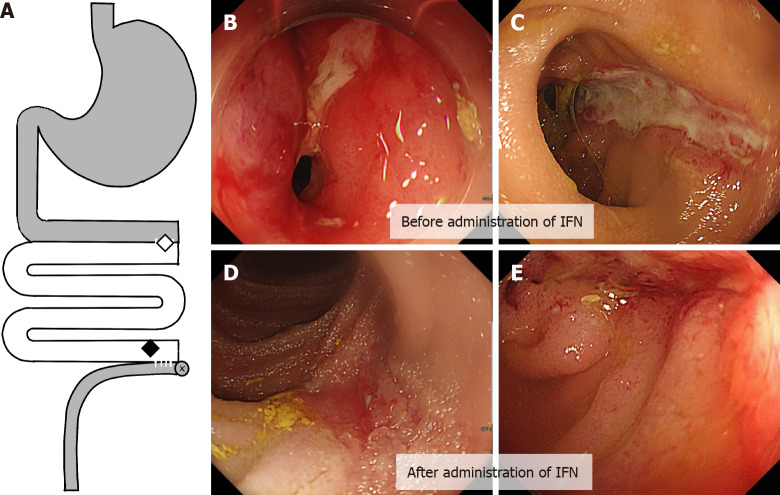
The endoscopic work up to evaluate the progression of anemia revealed stenosis with ulcerative inflammatory changes in the proximal part of the anastomosis and multiple longitudinal ulcers in the graft near the distal anastomosis site (Figure 1A and B). Computed tomography showed thickened intestinal walls with inflamed fat tissue.
Figure 1.
Ulcerative lesion in graft before and after administration infliximab. A: The location of the diseases in the graft; B and C: Stenosis with inflammation in the proximal anastomosis between the graft and the original intestine (B, indicated by a white diamond in A), and a longitudinal ulcerative lesion in the distal end of the graft (C, indicated by a black diamond in A); D and E: Mucosal healing was observed in the distal ulcerative lesion after the administration of two doses of infliximab. IFN: Infliximab.
Pathological examinations
Mucosal biopsy showed non-specific inflammation of the mucosa, which did not fulfill the criteria of ACR or chronic rejection (CR). C4d staining was negative; the anti-donor human leukocyte antigen antibody was absent in the patient’s serum. Polymerase chain reaction performed using the mucosal specimen was negative for CMV and Epstein-Barr virus (EBV).
FINAL DIAGNOSIS
The final diagnosis was suggested to be ulcerative lesions in Crohn’s disease.
TREATMENT
Several attempts to dilate the stenotic lesions with a balloon only provided transient relief. Anorexia, pain, and anemia were progressive. Since the endoscopic findings suggested ulcerative lesions in Crohn’s disease, infliximab treatment was considered. Before the initiation of infliximab treatment, screening tests for malignancy and latent infections, including tuberculosis and infections with EBV, CMV, and hepatitis B virus, were performed, all of which were negative.
The initial, second, third, and subsequent doses of infliximab were administered on day 0, day 14, day 42, and every 8 wk thereafter, respectively.
OUTCOME AND FOLLOW-UP
The patient’s oral intake was restored immediately after the first infusion of infliximab, and abdominal pain alleviated. Endoscopy, which was performed after the administration of the second dose of infliximab, showed mucosal healing of the ulcerative lesions (Figure 1C-E). However, the effect was only transient, the symptoms relapsed, and ulcerative inflammation recurred within several weeks. Furthermore, the effect of infliximab was attenuated; thus, repetitive infusion of infliximab was required in shorter intervals. The dosage was also increased to 7.5 mg/kg, and mycophenolate mofetil (MMF, 20 mg/kg) was added to prevent the production of neutralizing antibodies against infliximab. Later, MMF was switched with 5 mg of prednisolone, which helped reduce the dosage and frequency. Treatment with 5 mg/kg infliximab every 8 wk and 5 mg of oral prednisolone allowed the maintenance of remission for the following 2 years. Since the ulcerative lesion persisted despite symptom relief, another partial resection of the intestinal part with longitudinal ulcerative lesions was performed at the same time as ileostomy reversal 11 years after ITx (when the patient was 22 years old). The serum level of TNF-α and changes in body weight are shown in Figure 2. The trough serum TNF-α levels ranged from 14.8 to 246 pg/mL, which rapidly declined to 0.55-4.17 pg/mL (immediately after each infusion). The resected specimen of the graft revealed no clinical features of ACR or CR (such as submucosal vasculopathy, intimal thickening, or fibrosis) (Figure 3). Endoscopy performed for surveillance 3 mo after the resection showed the recurrence of several ulcerative lesions in the graft, which prompted the resumption of treatment with infliximab (5 mg/kg) followed by symptom-based administration. Currently, the patient is successfully off parenteral nutrition. Infliximab is being administered as needed (average 8-10 wk), and the patient’s condition has been stable without any obvious adverse events for 5 years since the first administration infliximab. The test for neutralizing antibodies against infliximab has been negative to date.
Figure 2.

Clinical course during infliximab administration. A: Height and body weight indicating a steady growth, in line with the initiation of infliximab (5 mg/kg). Hemoglobin (g/dL) level rapidly recovered. Following attenuation in the clinical response, the dose was increased to 7.5 mg/kg and immunomodulatory agents were added (mycophenolate mofetil was later switched with oral prednisolone); B: Dots (●) indicate the trough serum tissue necrosis factor alpha level, which declined immediately after each infliximab infusion (indicated by squares). In contrast, almost normal white blood cell counts and C-reactive protein level were observed throughout the course of treatment. Partial resection and stoma reversal were performed (indicated by dotted lines). IFN: Infliximab; MMF: Mycophenolate mofetil; PSL: Prednisolone; TAC: Tacrolimus; Hb: Hemoglobin.
Figure 3.
Pathological findings of the ulcerative lesion. A: The resected specimen of the graft showing ulcerative inflammation with fibrotic tissue; B: No signs of chronic rejection are observed.
DISCUSSION
The four major indications of infliximab for ITx can be considered as follows: (1) Induction therapy; (2) Salvage treatment for rejection refractory to treatment with lymphocyte-depleting antibodies [anti-thymocyte globulin (ATG) or OKT3]; (3) Preemptive treatment for steroid-refractory rejection to avoid the use of treatment with lymphocyte-depleting antibodies; and (4) Treatment for ulcerative inflammation not meeting the criteria for rejection. Regarding indication I, some treatment centers use infliximab as part of induction therapy based on results of animal studies[13-15]. however, thus far, clear benefits in human studies remain to be elucidated[16]. The data of 22 ITx patients treated with infliximab due to indications 2, 3, and 4 are summarized in Table 1[17-23]. Ten patients received infliximab after being treated with lymphocyte-depleting antibodies (indication 2), six received infliximab before treatment with lymphocyte-depleting antibodies or did not receive lymphocyte-depleting antibodies (indication 3), and two received infliximab for chronic ulcerative conditions, which did not meet the criteria for ACR (indication 4). No clinical details were available for four patients[24].
Table 1.
Summary of the literature related to treatment with infliximab for ulcerative lesion after intestinal transplantation
|
Ref.
|
Number of patients
|
Age of the patients at the time of events (yr)
|
Disease
|
Baseline immunosuppressive therapy at the time of the event
|
Indications
|
Serum TNF-α (pg/mL)
|
Dose
|
Number of infusions
|
Interval after ITx
|
Outcome
|
Adverse events and F/u
|
| Fishbein et al[17], 2006 | 4 | N/A | N/A | N/A | Late-onset resistant rejection (no details available) | N/A | N/A | N/A | N/A | Full recovery | N/A |
| Giovanelli et al[18], 2008 | 1 | 11 (m) | Chronic intestinal pseudo-obstruction | Tacrolimus, sirolimus | Steroid-resistant ACR | N/A | N/A | 1 | 18 mo | No response, remission after OKT3 treatment | EBV viremia, graft loss due to chronic rejection in 5 mo |
| Gerlach et al[19], 2011 | 9 | 27-44 (7: m 2: f) | Short bowel syndrome, chronic intestinal pseudo-obstruction | Steroid, tacrolimus, ATG, daclizumab, alemtuzumab, sirolimus, MMF | Late-onset OKT3-resistant ACR (2), early-onset OKT3-resistant ACR, humoral rejection (1), early-onset OKT3-resistant ACR (1), steroid-resistant ACR (3), chronic ulcerative ileitis/anastomositis (2) | Late-onset OKT3-resistant ACR (60-170), steroid-resistant ACR (80-140), chronic ulcerative ileitis (60-114), early-onset OKT3-resistant rejection (100-167) | 5 mg/kg | 12 ± 11.3 | 0-40 mo (18.2 + 14.1 mo) | 6/9 sustained remission, 2/9 repeated transient remission, 1/9 graft loss | EBV infection (3), cutaneous mycosis, pneumonia, 6 mo to 10 yr |
| De Greef et al[20], 2012 | 2 | 13 (m), 5 (f) | Microvillous inclusion disease, short bowel syndrome | Tacrolimus monotherapy, rapamycin + daclizumab + steroid | Steroid- and ATG-resistant ACR | N/A | 5 mg/kg, 4 mg/kg + 3 mg/kg 2-wk interval | 1, 3 | 5 yr and 6 mo | Complete remission | 27/22 mo |
| Avsar et al[21], 2014 | 1 | 52 (f) | Short bowel syndrome | Steroid, tacrolimus, everolimus, daclizumab, MMF | Graft rejection after CMV infection | N/A | 5 mg/kg | 1 | 6 mo | No response, graft explantation (no ATG) | Graft loss |
| Rao et al[22], 2016 | 1 | 38 (f) | Crohn’s disease | Steroid, tacrolimus | Late-onset steroid- and ATG-resistant ACR | N/A | Adalimumab | < 2 | 10 mo | Remission with maintenance adalimumab treatment | 6 mo |
| Narang et al[23], 2019 | 1 | 20 (f) | Total intestinal aganglionosis + solitary kidney | Steroid, tacrolimus | Late-onset steroid-resistant ACR (moderate to severe), ulcerative ileitis | N/A | 5 mg/kg, 10 mg/kg | More than 3 | 13 yr | No response with 5 mg/kg, remission after ATG, 10 mg/kg induced remission for another ACR episode later | PTLD (remission), 2 yr |
| Current case, 2020 | 1 | 20 (m) | Isolated hypoganglionosis | Tacrolimus | Chronic ulcerative ileitis, anastomositis | 14.8-246 pg/mL | 5-7.5 mg/kg | > 17 | 6 yr | Repeated transient remission | 5 yr |
TNF-α: Tissue necrosis factor alpha; ITx: Intestinal transplantation; ACR: Acute cellular rejection; OKT3: Anti-CD3 monoclonal antibody; EBV: Epstein-Barr virus; ATG: Anti-thymocyte globulin; MMF: Mycophenolate mofetil; CMV: Cytomegalovirus; PTLD: Posttransplant lymphoproliferative disorder; N/A: Not available.
Eight out of the 10 patients (dosage: 3 mg/kg to 5 mg/kg) treated for indication 2 achieved complete remission, one was in transient remission and being treated, and one lost the graft. The patient who lost the graft had developed severe exfoliative ACR accompanied by humoral rejection 15 d after transplantation. The graft did not recover even after the administration of seven doses of infliximab along with plasma pheresis and intravenous immunoglobulin (high dose). Although based on only a single case, it can be speculated that infliximab may have a limited effect on humoral rejection. Among the 10 rejection episodes, two occurred early after ITx (1 d and 15 d) and the other eight episodes occurred 6 mo to 5 years after ITx.
Among six patients who received infliximab for indication III, graft recovery was achieved in three patients (dosage: 5 mg/kg) without the need for treatment with lymphocyte-depleting antibodies. Two patients needed ATG/OKT-3 treatment and achieved remission, and one lost the graft without receiving ATG because of underlying CMV disease. Whether earlier treatment with infliximab might have prevented the need for treatment with lymphocyte-depleting antibodies remains unclear, because, unfortunately, data on the interval between the onset of ACR and infliximab administration were unavailable. For now, early application of infliximab in cases of steroid-resistant ACR with an aim to avoid treatment with lymphocyte-depleting antibodies remains controversial and should be prudent because a delay in treatment with ATG might have detrimental effects.
Only three patients, including our current patient, received infliximab for indication 4, known as ulcerative ileititis/anastomositis, which is clearly distinct from ACR. Results of histological analyses were consistent in three individuals, and were characterized as an ulcer with granulation tissue and fibrinoid necrosis on the surface. The events occurred 17, 24, and 72 mo after ITx. Of note, although one patient achieved complete remission after two infusions of infliximab, the other two patients continue to be treated for recurrent ulcerative lesions at the time of submission. One patient received regular treatment every 6 mo[19], and our patient receives the treatment every 8-10 wk as of now. The macroscopic findings and the clinical response induced by infliximab led us to speculate about the potential commonality of immune responses in the transplanted intestine and consider IBD. The first description of such similarity was reported by Fishbein et al[25]. They reported that 10% of 30 ITx recipients showed gross features of graft ulceration and a reduction in the number of Paneth cells on histological examination, and required on average 6 wk of infliximab treatment until remission; the success rate was 75%. A subsequent study reported the potential underlying mechanism of NOD2 polymorphism in such inflammatory lesions[25].
The optimal management for recurrent ulcerative inflammation under ITx settings by using anti-TNF-α therapy needs further elucidation. Previous studies in patients with Crohn’s disease revealed that anti-TNF-α therapy provides better outcomes when used in combination with immunomodulatory agents and that therapeutic drug monitoring might help optimization of dosing[26]. Our patient received MMF (500 mg) for 6 mo without additional improvement. MMF has since been switched with 5 mg of prednisolone. The dose was once escalated to 7.5 mg/kg based on clinical symptoms. Five years have passed since the patient was first administered infliximab, and treatment has been effective thus far. Options for future treatment include dose escalation or short-interval based on therapeutic drug monitoring, combination treatment with other immunomodulatory agents (such as azathioprine), and switching to other anti-TNF antibodies (such as adalimumab)[23]. With regard to the safety of infliximab addition in highly immunocompromised recipients, EBV viremia and cutaneous mycosis have been reported as adverse events. Studies on the addition of anti-TNF-α treatment for immunosuppression maintenance among other solid-organ transplant recipients, the indications for which mostly included coexistent IBD and rheumatoid arthritis, showed essentially no additional risk of infectious complications and malignancy in liver transplant[27,28] and kidney transplant patients[29]. Nevertheless, as stated in the guideline for Crohn’s disease management, a thorough assessment for opportunistic infections and malignancy is necessary among ITx recipients before the initiation of anti-TNF-α therapy.
CONCLUSION
Treatment with infliximab and immunomodulatory agents in addition to conventional immunosuppressive therapy is safe and effective for chronic ulcerative conditions after ITx. Anti-TNF-α therapy should be considered as a powerful option in immunosuppressive treatment strategy against various conditions associated with immunologically challenging ITx.
Footnotes
Informed consent statement: Consent was obtained from the patient for the publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: February 3, 2021
First decision: March 6, 2021
Article in press: May 17, 2021
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Quaglio AEV S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
Contributor Information
Takumi Fujimura, Department of Pediatric Surgery, National Saitama Hospital, Wako Shi, Saitama 351-0102, Japan; Department of Pediatric Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Yohei Yamada, Department of Pediatric Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan. yohei.z7@keio.jp.
Tomoshige Umeyama, Department of Pediatric Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Yumi Kudo, Department of Pediatric Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Hiroki Kanamori, Department of Pediatric Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Teizaburo Mori, Department of Pediatric Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Takahiro Shimizu, Department of Pediatric Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Mototoshi Kato, Department of Pediatric Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Miho Kawaida, Department of Pathology, Keio University School of Medicine, Tokyo 160-8582, Japan.
Naoki Hosoe, Center for Diagnostic and Therapeutic Endoscopy, Keio University School of Medicine, Tokyo 160-8582, Japan.
Yasushi Hasegawa, Department of Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Kentaro Matsubara, Department of Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Naoki Shimojima, Department of Pediatric Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Masahiro Shinoda, Department of Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan; Digestive Diseases Center, International University of Health and Welfare, Mita Hospital, Tokyo 108-8329, Japan.
Hideaki Obara, Department of Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Makoto Naganuma, Department of Gastroenterology and Hepatology, Keio University School of Medicine, Tokyo 160-8582, Japan.
Yuko Kitagawa, Department of Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Ken Hoshino, Department of Pediatric Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
Tatsuo Kuroda, Department of Pediatric Surgery, Keio University School of Medicine, Tokyo 160-8582, Japan.
References
- 1.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Kuijpers TW, Hakkert BC, Hart MH, Roos D. Neutrophil migration across monolayers of cytokine-prestimulated endothelial cells: a role for platelet-activating factor and IL-8. J Cell Biol. 1992;117:565–572. doi: 10.1083/jcb.117.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paleolog EM, Delasalle SA, Buurman WA, Feldmann M. Functional activities of receptors for tumor necrosis factor-alpha on human vascular endothelial cells. Blood. 1994;84:2578–2590. [PubMed] [Google Scholar]
- 4.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 5.Van Deventer SJ. Tumour necrosis factor and Crohn's disease. Gut. 1997;40:443–448. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 7.Myatt N, Coghill G, Morrison K, Jones D, Cree IA. Detection of tumour necrosis factor alpha in sarcoidosis and tuberculosis granulomas using in situ hybridisation. J Clin Pathol. 1994;47:423–426. doi: 10.1136/jcp.47.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berns M, Hommes DW. Anti-TNF-α therapies for the treatment of Crohn's disease: the past, present and future. Expert Opin Investig Drugs. 2016;25:129–143. doi: 10.1517/13543784.2016.1126247. [DOI] [PubMed] [Google Scholar]
- 9.Mitoma H, Horiuchi T, Tsukamoto H, Ueda N. Molecular mechanisms of action of anti-TNF-α agents - Comparison among therapeutic TNF-α antagonists. Cytokine. 2018;101:56–63. doi: 10.1016/j.cyto.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 10.ten Hove T, van Montfrans C, Peppelenbosch MP, van Deventer SJ. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn's disease. Gut. 2002;50:206–211. doi: 10.1136/gut.50.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Brande JM, Koehler TC, Zelinkova Z, Bennink RJ, te Velde AA, ten Cate FJ, van Deventer SJ, Peppelenbosch MP, Hommes DW. Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn's disease. Gut. 2007;56:509–517. doi: 10.1136/gut.2006.105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascher A, Radke C, Dignass A, Schulz RJ, Veltzke-Schlieker W, Adler A, Sauer IM, Platz K, Klupp J, Volk HD, Neuhaus P, Mueller AR. Successful infliximab treatment of steroid and OKT3 refractory acute cellular rejection in two patients after intestinal transplantation. Transplantation. 2003;76:615–618. doi: 10.1097/01.TP.0000072804.41125.82. [DOI] [PubMed] [Google Scholar]
- 13.Pech T, Fujishiro J, Finger T, Ohsawa I, Praktiknjo M, Abu-Elmagd K, von Websky M, Overhaus M, Kalff JC, Schaefer N. Combination therapy of tacrolimus and infliximab reduces inflammatory response and dysmotility in experimental small bowel transplantation in rats. Transplantation. 2012;93:249–256. doi: 10.1097/TP.0b013e31823e7abb. [DOI] [PubMed] [Google Scholar]
- 14.Gerlach UA, Atanasov G, Wallenta L, Polenz D, Reutzel-Selke A, Kloepfel M, Jurisch A, Marksteiner M, Loddenkemper C, Neuhaus P, Sawitzki B, Pascher A. Short-term TNF-alpha inhibition reduces short-term and long-term inflammatory changes post-ischemia/reperfusion in rat intestinal transplantation. Transplantation. 2014;97:732–739. doi: 10.1097/TP.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 15.Pech T, Fujishiro J, Finger T, Ohsawa I, Praktiknjo M, von Websky M, Wehner S, Abu-Elmagd K, Kalff JC, Schaefer N. Perioperative infliximab application has marginal effects on ischemia-reperfusion injury in experimental small bowel transplantation in rats. Langenbecks Arch Surg. 2012;397:131–140. doi: 10.1007/s00423-011-0853-0. [DOI] [PubMed] [Google Scholar]
- 16.Gerlach UA, Lachmann N, Sawitzki B, Arsenic R, Neuhaus P, Schoenemann C, Pascher A. Clinical relevance of the de novo production of anti-HLA antibodies following intestinal and multivisceral transplantation. Transpl Int. 2014;27:280–289. doi: 10.1111/tri.12250. [DOI] [PubMed] [Google Scholar]
- 17.Fishbein T, Novitsky G. Innate immunity is altered after intestinal transplantation causing a new inflammatory bowel disease. Transplantation. 2006;82:372. [Google Scholar]
- 18.Giovanelli M, Gupte GL, Sharif K, Mayer DA, Mirza DF. Chronic rejection after combined liver and small bowel transplantation in a child with chronic intestinal pseudo-obstruction: a case report. Transplant Proc. 2008;40:1763–1767. doi: 10.1016/j.transproceed.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 19.Gerlach UA, Koch M, Müller HP, Veltzke-Schlieker W, Neuhaus P, Pascher A. Tumor necrosis factor alpha inhibitors as immunomodulatory antirejection agents after intestinal transplantation. Am J Transplant. 2011;11:1041–1050. doi: 10.1111/j.1600-6143.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 20.De Greef E, Avitzur Y, Grant D, De-Angelis M, Ng V, Jones N, Ngan B, Shapiro R, Steinberg R, Gana JC. Infliximab as salvage therapy in paediatric intestinal transplant with steroid- and thymoglobulin-resistant late acute rejection. J Pediatr Gastroenterol Nutr. 2012;54:565–567. doi: 10.1097/MPG.0b013e3182293d73. [DOI] [PubMed] [Google Scholar]
- 21.Avsar Y, Cicinnati VR, Kabar I, Wolters H, Anthoni C, Schmidt HH, Beckebaum S. Small bowel transplantation complicated by cytomegalovirus tissue invasive disease without viremia. J Clin Virol. 2014;60:177–180. doi: 10.1016/j.jcv.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Rao B, Jafri SM, Kazimi M, Mullins K, Raoufi M, Segovia MC. A Case Report of Acute Cellular Rejection Following Intestinal Transplantation Managed With Adalimumab. Transplant Proc. 2016;48:536–538. doi: 10.1016/j.transproceed.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 23.Narang A, Xi D, Mitsinikos T, Genyk Y, Thomas D, Kohli R, Lin CH, Soufi N, Warren M, Merritt R, Yanni G. Severe Late-Onset Acute Cellular Rejection in a Pediatric Patient With Isolated Small Intestinal Transplant Rescued With Aggressive Immunosuppressive Approach: A Case Report. Transplant Proc. 2019;51:3181–3185. doi: 10.1016/j.transproceed.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto CS, Zasloff MA, Fishbein TM. Chronic mucosal inflammation/inflammatory bowel disease-like inflammation after intestinal transplantation: where are we now? Curr Opin Organ Transplant. 2014;19:276–280. doi: 10.1097/MOT.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 25.Fishbein T, Novitskiy G, Mishra L, Matsumoto C, Kaufman S, Goyal S, Shetty K, Johnson L, Lu A, Wang A, Hu F, Kallakury B, Lough D, Zasloff M. NOD2-expressing bone marrow-derived cells appear to regulate epithelial innate immunity of the transplanted human small intestine. Gut. 2008;57:323–330. doi: 10.1136/gut.2007.133322. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481–517. doi: 10.1038/ajg.2018.27. [DOI] [PubMed] [Google Scholar]
- 27.Altwegg R, Combes R, Laharie D, De Ledinghen V, Radenne S, Conti F, Chazouilleres O, Duvoux C, Dumortier J, Leroy V, Treton X, Durand F, Dharancy S, Nachury M, Goutorbe F, Lamblin G, Boivineau L, Peyrin-Biroulet L, Pageaux GP. Effectiveness and safety of anti-TNF therapy for inflammatory bowel disease in liver transplant recipients for primary sclerosing cholangitis: A nationwide case series. Dig Liver Dis. 2018;50:668–674. doi: 10.1016/j.dld.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Westerouen van Meeteren MJ, Hayee B, Inderson A, van der Meulen AE, Altwegg R, van Hoek B, Pageaux GP, Stijnen T, Stein D, Maljaars PWJ. Safety of Anti-TNF Treatment in Liver Transplant Recipients: A Systematic Review and Meta-analysis. J Crohns Colitis. 2017;11:1146–1151. doi: 10.1093/ecco-jcc/jjx057. [DOI] [PubMed] [Google Scholar]
- 29.Quinn CS, Jorgenson MR, Descourouez JL, Muth BL, Astor BC, Mandelbrot DA. Management of Tumor Necrosis Factor α Inhibitor Therapy After Renal Transplantation: A Comparative Analysis and Associated Outcomes. Ann Pharmacother. 2019;53:268–275. doi: 10.1177/1060028018802814. [DOI] [PubMed] [Google Scholar]