Summary
Objective
Ovarian cancer is known as the seventh most common cancer among women, accounting for about 4% of all cancers associated with the females.
Method
This is a descriptive cross-sectional study based on cancer incidence data and cancer mortality rates from the Global Cancer Data in 2018. The incidence and mortality rates were estimated and ovarian cancer distribution maps for world countries were drawn. To analyze data, correlation and regression tests were used to evaluate association between its incidence and mortality with human development index (HDI)
Results
Results revealed a direct and significant correlation between ovarian cancer incidence (R = 0.409, P < 0.0001) and mortality (R = 0.193, P < 0.05) with HDI. It also projected a direct and significant correlation between incidence with Gross National Income per 1,000 capita (GNI), mean years of schooling (MYS), life expectancy at birth (LEB) and expected years of schooling (EYS) (P < 0.0001). The findings also demonstrated a direct and significant correlation between mortality and GNI, MYS, LEB as well as EYS (P < 0.05). The linear regression model showed that a higher MYS [B = 0.2, CI 95%: (-0.03, 0.5)] can significantly augment the incidence of ovarian cancer while an increased MYS [B = 0.2, CI 95% (0.03, 0.4)] can induce mortality.
Conclusions
Given the direct and significant correlation between ovarian cancer incidence and mortality with HDI, attention to risk factors in these countries can be effective in curbing its incidence and mortality.
Keywords: Incidence, Mortality, Ovarian cancer, Human development index
Introduction
Non-communicable diseases (NCDs) are among the leading causes of death around the globe. Due to major changes in fertility and life expectancy, the world’s population is growing rapidly and non-communicable diseases including cancers are accordingly increasing significantly [1]. According to WHO estimates in 2015, cancer is the first or second leading cause of mortality before the age of 70 in most countries. Population growth, aging, and economic development are among the reasons for the growing prevalence of cancer worldwide. Cancers are the leading cause of death in some developed countries and the second leading cause of death after cardiovascular disease in the developing countries [2]. Ovarian cancer is ranked seventh of the most common cancers among women thus accounting for about 4% of all cases associated with the females; it is the sixth malignant cause of cancer deaths in women [3]. As for its high prevalence, high mortality rate and its impact on patients’ quality of life and economic costs, ovarian cancer is known to be one of the important abnormalities that require special attention [4].
Among the most significant factors triggering the incidence of ovarian cancer is family history, pregnancy, childbirth and age [5, 6]. Environmental and socioeconomic factors also affect ovarian cancer mortality rate. The overall growing trend of ovarian cancer incidence and mortality can be observed in all regions of the world across different social and economic levels. Variations in ovarian cancer incidence rate in the world is not restricted to the above factors. It can also be due to differences in the cancer registration system in different countries [7].
Socio-economic developments can bear profound impact on the scale and features of cancer. The epidemiological transmission of cancer distribution around the world has virtually changed. Compared to high-income nations, the prevalence of global demographic and epidemiological trends in middle- and low-income countries has shown a crucial cancer decline in recent decades. In some regions of Africa, the standardized prevalence of ovarian cancer is lower than 5 per 100,000 whereas in eastern and central Europe it proves to be higher than 11 per 100,000 among women [4, 8].
Annually, 225,000 new cases of ovarian cancer are diagnosed 140,000 of whom die out [9]. The risk of complications resulting from this cancer is 1 in 71 and the chance of death amounts to 1 in 95 [9, 10]. Moreover, estimates of the disability-adjusted life-years (DALYs) lost in ovarian cancer are on the rise [11]. Ovarian cancer is more common in industrialized than in the developing countries. However, the highest proportion of age-specific mortality in ovarian cancer is seen in the developing countries [12]. According to the United Nations record on HDI, the disease burden associated with ovarian cancer in areas with a high HDI proves higher. HDI is a summary index for human development measurements; it measures the mean achievement of a country in three main dimensions of human development: long and healthy life, access to knowledge and living standards [13].
In regard with the global trend of ovarian cancer and its progress in recent years, awareness to its incidence and mortality and its relation to the HDI can be effective in planning and managing financial and human resources for its prevention. Despite the increasing burden of cancers in the developing countries, only 5% of global resources are appropriated for these countries [14]. It thus seems a requisite for all nations to conduct further research so as to uncover how socioeconomic conditions can affect the cancer risk factors.
The purpose of this study is to assess the impact of socioeconomic development (based on HDI) on the global trend of incidence and mortality resulting from ovarian cancer in the world based on Global Cancer Data for 2018. We intend to measure the key characteristics of ovarian cancer transmission in the world by examining the relationship between the incidence rate and the HDI, which consists of life expectancy, education, and gross national income.
Material and methods
Methods used for measuring incidence rate in terms of age and gender for each country are touched upon broadly as follows: 1) national incidence rate observed by 2018 (45 countries); 2) the most recent (national or regional) incidence rates on the basis of the population in 2018 (50 countries); 3) rates calculated using national mortality data through modeling and the ratio of mortality to incidence rate obtained from cancer records of countries (14 countries); 4) rates calculated using national mortality data through modeling and the ratio of mortality to incidence rate obtained from cancer records of neighboring countries (37 countries); and 5) national incidence rates in terms of age and gender for all cancers obtained by averaging the overall incidence rates from neighboring countries. These values were then analyzed to obtain the national incidence for each specific site using relative cancer frequency data (7 countries). Further, the rates were calculated as the median of selected neighboring countries.
MORTALITY
The methods deployed for measuring incidence rate for each country as to age and gender are ranked as follows: 1) the national mortality rate observed and published by 2018 (81 countries); 2) recent national mortality rates according to population in 2018 (20 countries); 3) rates calculated using national mortality data through modeling and the ratio of mortality to incidence rate obtained from cancer records of neighboring countries (81 countries); 4) the rates calculated as the median of selected neighboring countries (three countries).
HUMAN DEVELOPMENT INDEX (HDI)
Since 1990, a report called Human Development Report is published annually by the United Nations Development Program in which countries are compared on various indicators including education, health, as well as economic, social, environmental, political factors and the like. In the Human Development Report, countries are divided into groups of very high, high, moderate and low human development based on the Human Development Index [15].
The HDI numerical value is known to be between 0 and 1. This value reveals how much a country has attempted to achieve the highest possible value of 1 and also allows comparisons among the countries. The Human Development Index (HDI) is a summary measure of the average achievement in three dimensions of human development: having a long and healthy life, being knowledgeable and bearing a decent standard of living. The HDI is the geometric mean of normalized indices for each of the three dimensions, which measures the success of each dimension. Life expectancy is measured by life expectancy at birth, the education dimension is estimated by mean of years of schooling (elementary, secondary, and higher education), and living standard is measured by gross national income (GNI) per capita [16, 17].
STATISTICAL ANALYSIS
In this study, the correlation bivariate method was used to assess the correlation between the incidence and mortality rates of ovarian cancer and the HDI. Linear regression models were also used to assess the HDI effect on the incidence rate of ovarian cancer. Significance level was considered lower than 0.05. Data analysis was conducted by Stata software version 14.
Results
Based on cancer record results in 2018, 8,622,539 new cancer cases and 4,169,387 cancer deaths were recorded in women; 1.72 were new cases (4.4%) while 184,799 (2.08%) deaths were due to ovarian cancer. The highest number of new cancer cases and the highest death cases from ovarian cancer reported for Asia amounted to 153,075 (51.8%) and 92,527 (50.01%), respectively (Fig. 1).
Fig. 1.
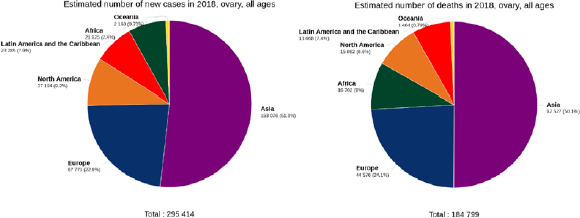
Pie charts presenting the distribution of new cases and deaths due to ovarian cancer in 2018 for all age groups in different continents [source: GLOBOCAN 2018].
Table I sets out the incidence and mortality rate of ovarian cancer in each continent. The results indicated the highest ovarian cancer incidence in the world being in Serbia (16.6 per 100,000), Brunei (16 per 100,000) and Belarus (15.4 per 100,000), respectively. The highest mortality rates for ovarian cancer were identified in Samoa (12 per 100,000), Solomon Islands (8.4 per 100,000) and Poland (8.7 per 100,000) (Tab. I, Fig. 2).
Tab. I.
Estimated age-standardized incidence and mortality rates due to ovarian cancer in the world in 2018 [source: GLOBOCAN 2018].
| Country | Incidence | Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Age group | Age group | |||||||
| 0-49 | 50-85 | 0-49 | 50-85 | |||||
| CR | ASR | CR | ASR | CR | ASR | CR | ASR | |
| Afghanistan | 1.5 | 2.2 | 13.1 | 13.1 | 0.93 | 1.5 | 12.4 | 12.5 |
| Albania | 2.1 | 1.6 | 12.6 | 12.7 | 0.53 | 0.42 | 7.1 | 6.6 |
| Algeria | 2.6 | 2.4 | 14.6 | 15 | 0.87 | 0.82 | 11.5 | 11.9 |
| Angola | 0.83 | 1.2 | 10.4 | 10.6 | 0.47 | 0.8 | 10.2 | 10.5 |
| Argentina | 3.8 | 3.3 | 27.2 | 25.9 | 1.1 | 0.94 | 18.3 | 16.1 |
| Armenia | 2.5 | 1.9 | 20.9 | 20.4 | 1.2 | 0.95 | 18.8 | 18.2 |
| Australia | 2.9 | 2.2 | 29.2 | 25.8 | 0.7 | 0.53 | 21.9 | 17.3 |
| Austria | 3.9 | 2.6 | 34.4 | 30.8 | 0.87 | 0.53 | 25.8 | 19.7 |
| Azerbaijan | 2.8 | 2.3 | 17.3 | 17.7 | 1.2 | 1 | 15.5 | 16.3 |
| Bahamas | 2.7 | 2 | 15.6 | 14.6 | 0.69 | 0.5 | 19 | 18.1 |
| Bahrain | 2.3 | 2.2 | 28 | 33 | 1 | 0.96 | 23.3 | 29.1 |
| Bangladesh | 2.4 | 2.2 | 10.5 | 10.8 | 1.5 | 1.4 | 9.1 | 9.5 |
| Barbados | 6.4 | 4.3 | 26.8 | 21.8 | 3.2 | 2.2 | 25 | 20 |
| Belarus | 11.4 | 7.9 | 44.3 | 45.6 | 1.9 | 1.3 | 18.4 | 17.7 |
| Belgium | 2.4 | 1.8 | 25.8 | 23.4 | 0.61 | 0.43 | 24.7 | 17.9 |
| Belize | 1.2 | 1.5 | 0 | 0 | 0.61 | 0.74 | 0 | 0 |
| Benin | 1.3 | 1.8 | 6.9 | 6.9 | 0.76 | 1.1 | 6.8 | 6.8 |
| Bhutan | 2.1 | 2.3 | 21.5 | 21.3 | 0.91 | 1.1 | 19.7 | 19.7 |
| Bolivia, Plurinational State of | 1.6 | 1.7 | 10.5 | 11.3 | 0.58 | 0.7 | 7 | 7.4 |
| Bosnia and Herzegovina | 6.6 | 4.4 | 34.3 | 34.5 | 1.4 | 0.93 | 23.6 | 22.2 |
| Botswana | 0.99 | 1.1 | 8.6 | 8.9 | 0.3 | 0.37 | 8.6 | 8.9 |
| Brazil | 2.6 | 2.1 | 16.6 | 15.9 | 0.94 | 0.76 | 12.4 | 11.7 |
| Brunei | 9.5 | 7.8 | 52.7 | 48.6 | 1.8 | 1.3 | 26.4 | 25.7 |
| Bulgaria | 7.9 | 5 | 34.6 | 34.8 | 3 | 1.9 | 25.1 | 22.9 |
| Burkina Faso | 1.3 | 1.8 | 9.9 | 9.9 | 0.85 | 1.3 | 9.8 | 9.8 |
| Burundi | 1 | 1.6 | 13.3 | 13.5 | 0.74 | 1.3 | 13.1 | 13.3 |
| Cabo Verde | 0 | 0 | 6.5 | 6.1 | 0 | 0 | 6.5 | 6.1 |
| Cambodia | 2.4 | 2.5 | 14.9 | 14.9 | 1 | 1.1 | 11.9 | 12.1 |
| Cameroon | 2.2 | 3.1 | 11.4 | 11.4 | 1.4 | 2.1 | 10.8 | 10.8 |
| Canada | 3.9 | 2.9 | 30.9 | 28.1 | 0.91 | 0.65 | 23.7 | 19.3 |
| Central African Republic | 1.7 | 2.5 | 14.8 | 14.9 | 1.3 | 2.1 | 14.8 | 14.9 |
| Chad | 2 | 3.2 | 17 | 17 | 1.4 | 2.5 | 17 | 17 |
| Chile | 3.5 | 2.8 | 22 | 21.5 | 0.96 | 0.75 | 14.5 | 13.4 |
| China | 4 | 2.8 | 15.4 | 15.5 | 1.1 | 0.72 | 11.5 | 11.4 |
| Colombia | 4.1 | 3.5 | 26.3 | 26.2 | 1.3 | 1.1 | 16.3 | 16.1 |
| Comoros | 0 | 0 | 6.5 | 6.1 | 0 | 0 | 6.5 | 6.1 |
| Congo, Democratic Republic of | 0.81 | 1.3 | 11.5 | 11.6 | 0.52 | 0.88 | 10.2 | 10.3 |
| Congo, Republic of | 1 | 1.4 | 13.1 | 13.2 | 0.46 | 0.64 | 12.7 | 12.9 |
| Costa Rica | 2.2 | 1.8 | 16.1 | 15.5 | 1 | 0.87 | 12 | 11 |
| Croatia | 7.3 | 4.9 | 42.3 | 40.6 | 2.1 | 1.4 | 30.9 | 26 |
| Cuba | 4.3 | 3.1 | 16.5 | 16.4 | 1.4 | 0.89 | 12.8 | 12.2 |
| Cyprus | 2.2 | 1.6 | 30.2 | 27.9 | 1.5 | 1.1 | 33.8 | 28.2 |
| Czech Republic | 4.9 | 3.1 | 38.7 | 35.3 | 1.8 | 1.1 | 34.9 | 29.1 |
| Côte d’Ivoire | 1 | 1.5 | 15.6 | 16.1 | 0.66 | 1 | 15.3 | 15.9 |
| Denmark | 2.5 | 1.8 | 33.1 | 29.5 | 0.99 | 0.69 | 30.7 | 24 |
| Djibouti | 3.1 | 3.2 | 21.8 | 21.9 | 1.7 | 1.8 | 21.8 | 21.9 |
| Dominican Republic | 1.3 | 1.3 | 7.9 | 7.9 | 0.34 | 0.34 | 6.7 | 6.6 |
| Ecuador | 2.9 | 2.7 | 22 | 21.3 | 0.98 | 0.95 | 14.8 | 13.9 |
| Egypt | 2.4 | 2.5 | 20.8 | 20.7 | 0.94 | 1 | 18.9 | 18.9 |
| El Salvador | 4.8 | 4.5 | 22 | 20.2 | 1.5 | 1.5 | 16.1 | 13.9 |
| Equatorial Guinea | 1.9 | 2.8 | 15.9 | 15.5 | 0.76 | 1.3 | 15.9 | 15.5 |
| Eritrea | 2.7 | 3.5 | 23.4 | 23.2 | 1.8 | 2.7 | 23.4 | 23.2 |
| Estonia | 5.4 | 3.6 | 41.6 | 36.9 | 1.6 | 1 | 36.8 | 28.5 |
| Ethiopia | 3.1 | 4.1 | 23.8 | 23.8 | 1.9 | 2.8 | 23.5 | 23.6 |
| Fiji | 8.8 | 8.3 | 27.6 | 27.9 | 2.8 | 2.8 | 21.5 | 21.6 |
| Finland | 2.9 | 2.3 | 32.2 | 27.9 | 0.76 | 0.58 | 25.6 | 19.2 |
| France | 2.8 | 2.1 | 32.5 | 27.4 | 0.74 | 0.54 | 27.7 | 19.5 |
| France, Guadeloupe | 3.5 | 2.7 | 25.3 | 25.8 | 1.4 | 0.78 | 16.2 | 13.6 |
| France, La Réunion | 2.9 | 2.3 | 25.6 | 24.8 | 0.64 | 0.49 | 12.4 | 11.3 |
| France, Martinique | 2.5 | 1.4 | 21 | 19.8 | 0.84 | 0.44 | 13.2 | 11.9 |
| France, New Caledonia | 5 | 3.9 | 36.7 | 37.2 | 0.99 | 0.73 | 15.7 | 16.9 |
| French Guyana | 0.84 | 0.85 | 38.4 | 39.1 | 0 | 0 | 23 | 22.9 |
| French Polynesia | 3.8 | 3.4 | 14.5 | 14.5 | 0 | 0 | 14.5 | 14.7 |
| Gabon | 2.1 | 2.5 | 13.1 | 12.2 | 1 | 1.3 | 13.9 | 12.8 |
| The Gambia | 0.1 | 0.16 | 2.1 | 2.1 | 0 | 0 | 2.1 | 2.1 |
| Gaza Strip and West Bank | 1.4 | 1.8 | 17.2 | 17.7 | 0.67 | 0.91 | 15.3 | 15.9 |
| Georgia | 2.9 | 2 | 21.9 | 21.4 | 1.3 | 0.93 | 19.6 | 18.5 |
| Germany | 3.2 | 2.2 | 31.1 | 27 | 1 | 0.66 | 26.5 | 20.1 |
| Ghana | 2.6 | 3.1 | 29.3 | 30 | 1.3 | 1.7 | 28.2 | 29.1 |
| Greece | 5.6 | 3.6 | 29.3 | 26.5 | 1.4 | 0.83 | 22.2 | 17.4 |
| Guam | 1.7 | 1.5 | 31.6 | 32.2 | 1.7 | 1.5 | 31.6 | 32.2 |
| Guatemala | 1.9 | 2.1 | 9.8 | 9.6 | 0.57 | 0.66 | 7.3 | 7 |
| Guinea | 0.88 | 1.2 | 7.9 | 7.9 | 0.59 | 0.89 | 8.1 | 8 |
| Guinea-Bissau | 1.3 | 1.8 | 11.8 | 11.6 | 0.81 | 1.2 | 11.8 | 11.6 |
| Guyana | 3.2 | 3.2 | 26.8 | 27.2 | 1.3 | 1.3 | 18.8 | 19.3 |
| Haiti | 2.8 | 2.8 | 7.7 | 7.8 | 1.8 | 1.8 | 7.4 | 7.6 |
| Honduras | 2.8 | 2.8 | 7.6 | 7.4 | 1.3 | 1.3 | 5.9 | 5.9 |
| Hungary | 8 | 5.1 | 49.7 | 45.6 | 2.3 | 1.4 | 33 | 27.6 |
| Iceland | 1.8 | 1.4 | 33.1 | 30.7 | 0 | 0 | 26.1 | 19.3 |
| India | 2.9 | 2.7 | 16.5 | 16.5 | 1.2 | 1.1 | 14 | 14.1 |
| Indonesia | 5 | 4.4 | 30.6 | 31.1 | 1.8 | 1.6 | 22.8 | 23.7 |
| Iran, Islamic Republic of | 2.2 | 1.9 | 13.8 | 14 | 0.59 | 0.54 | 8.9 | 9.3 |
| Iraq | 1.6 | 2 | 11.6 | 11.6 | 0.81 | 1 | 10.3 | 10.4 |
| Ireland | 4.3 | 3 | 49.1 | 45.1 | 1.2 | 0.88 | 35.5 | 28.5 |
| Israel | 1.9 | 1.7 | 22.6 | 20.9 | 0.87 | 0.81 | 25.5 | 21.2 |
| Italy | 5.5 | 3.5 | 28.4 | 26.1 | 1.3 | 0.74 | 21.1 | 16.7 |
| Jamaica | 4.2 | 3.7 | 27.1 | 25 | 1.5 | 1.3 | 23 | 20.3 |
| Japan | 8.7 | 5.7 | 24.5 | 25 | 1.8 | 1.1 | 14.6 | 12.2 |
| Jordan | 1.9 | 2.1 | 18.5 | 19.2 | 0.7 | 0.8 | 16.2 | 17 |
| Kazakhstan | 4.5 | 4 | 30.7 | 31 | 1.8 | 1.6 | 21.5 | 21.8 |
| Kenya | 1.6 | 2.1 | 23.8 | 24.7 | 0.79 | 1.1 | 23.4 | 24.3 |
| Korea, Democratic Republic of | 3.4 | 2.5 | 17.1 | 17.4 | 1.1 | 0.78 | 10.8 | 10.3 |
| Korea, Republic of | 5.4 | 3.7 | 18 | 17.8 | 1 | 0.62 | 10.6 | 9.5 |
| Kuwait | 1.7 | 1.3 | 14.3 | 17.7 | 0.65 | 0.49 | 11.9 | 16 |
| Kyrgyzstan | 3.7 | 3.8 | 24 | 24.3 | 1.4 | 1.6 | 19.3 | 19.9 |
| Lao People’s Democratic Republic | 2.6 | 2.8 | 17.5 | 17.6 | 1 | 1.2 | 13.6 | 13.9 |
| Latvia | 9.6 | 6.1 | 48.4 | 47 | 3 | 1.9 | 37 | 31.3 |
| Lebanon | 3.6 | 3.5 | 31.9 | 30.9 | 1.3 | 1.3 | 27.7 | 26 |
| Lesotho | 1 | 1.4 | 10.5 | 10.2 | 0.5 | 0.8 | 10.5 | 10.2 |
| Liberia | 1 | 1.4 | 12.5 | 12.5 | 0.65 | 0.95 | 12.5 | 12.5 |
| Libya | 2.5 | 2.2 | 15.3 | 15.3 | 0.59 | 0.51 | 11.1 | 11.3 |
| Lithuania | 7.7 | 5.1 | 42.8 | 40.6 | 3 | 1.9 | 36.3 | 31.3 |
| Luxembourg | 3.2 | 2.1 | 42.5 | 32.5 | 1.6 | 1 | 30.9 | 21.2 |
| Madagascar | 0.47 | 0.59 | 6.7 | 6.8 | 0.28 | 0.42 | 6.6 | 6.7 |
| Malawi | 1.6 | 2.2 | 8.1 | 8.2 | 0.96 | 1.5 | 8 | 8.1 |
| Malaysia | 3.7 | 3.4 | 26.3 | 26.3 | 1.5 | 1.4 | 19.9 | 20 |
| Maldives | 4.3 | 4 | 29.7 | 30.9 | 1.2 | 1.4 | 23.1 | 23.6 |
| Mali | 1.6 | 2.4 | 12.2 | 12.2 | 1 | 1.7 | 12.1 | 12.1 |
| Malta | 4 | 2.7 | 33.8 | 28.3 | 0 | 0 | 34.9 | 25.1 |
| Mauritania | 1.5 | 1.8 | 11.5 | 11.5 | 0.7 | 0.95 | 11.5 | 11.5 |
| Mauritius | 3.7 | 2.8 | 23.4 | 22.9 | 1.4 | 1 | 16.7 | 16.5 |
| Mexico | 4 | 3.5 | 20.2 | 20.2 | 1.4 | 1.2 | 15.5 | 15.2 |
| Mongolia | 2.5 | 2.3 | 14.6 | 14.7 | 1.1 | 1 | 10.3 | 11.2 |
| Montenegro | 1.5 | 1.1 | 24.7 | 24.6 | 0.5 | 0.37 | 15.3 | 13.4 |
| Morocco | 2.5 | 2.2 | 18.8 | 19.1 | 0.84 | 0.79 | 16.5 | 16.9 |
| Mozambique | 0.38 | 0.44 | 5.5 | 5.6 | 0.24 | 0.37 | 5.4 | 5.5 |
| Myanmar | 3.5 | 3.1 | 15.5 | 15.2 | 1.6 | 1.4 | 11.7 | 11.7 |
| Namibia | 0.86 | 1.1 | 11.8 | 12.2 | 0.35 | 0.47 | 11.2 | 11.7 |
| Nepal | 3.5 | 3.5 | 15.7 | 16.1 | 1.7 | 1.8 | 14.1 | 14.3 |
| New Zealand | 2.5 | 1.9 | 28.1 | 25.2 | 0.84 | 0.63 | 19.2 | 15.9 |
| Nicaragua | 2.9 | 2.7 | 13.1 | 13.1 | 1.1 | 1.1 | 11.7 | 11.6 |
| Niger | 2.5 | 4.1 | 21.9 | 22.4 | 1.9 | 3.4 | 22.1 | 22.5 |
| Nigeria | 1.8 | 2.5 | 12.5 | 12.2 | 1 | 1.5 | 12.1 | 11.8 |
| Norway | 2.7 | 2 | 29.1 | 26.8 | 0.89 | 0.64 | 29.5 | 24.3 |
| Oman | 1.6 | 1.7 | 9 | 9.7 | 0.62 | 0.69 | 9 | 9.7 |
| Pakistan | 2.4 | 2.8 | 18.1 | 18.2 | 1.3 | 1.6 | 16.3 | 16.5 |
| Panama | 2.4 | 2.1 | 16.4 | 15.5 | 0.8 | 0.73 | 12.6 | 11.4 |
| Papua New Guinea | 4.2 | 4.6 | 16.7 | 16.6 | 2.3 | 2.7 | 16.2 | 16.1 |
| Paraguay | 2.3 | 2.4 | 21.2 | 20.6 | 0.82 | 0.94 | 15.9 | 15.1 |
| Peru | 3.3 | 3 | 26.4 | 26 | 1.2 | 1.1 | 14.9 | 14.2 |
| Philippines | 4.5 | 4.7 | 34.1 | 34.3 | 1.6 | 1.7 | 25.9 | 26.4 |
| Poland | 9.5 | 6.4 | 49.8 | 47.9 | 2.6 | 1.7 | 36.4 | 32.4 |
| Portugal | 3.2 | 2.1 | 19.7 | 17.4 | 0.9 | 0.53 | 15.8 | 12.5 |
| Puerto Rico | 3.7 | 2.8 | 24.8 | 21.7 | 0.74 | 0.53 | 15.9 | 13.3 |
| Qatar | 1.8 | 1.8 | 19 | 23.4 | 0.82 | 0.82 | 17.4 | 22.4 |
| Republic of Moldova | 8.6 | 6.2 | 30.6 | 30.9 | 2.2 | 1.6 | 19.5 | 19.3 |
| Romania | 7.1 | 4.6 | 34.2 | 33.7 | 2 | 1.2 | 23.5 | 21.2 |
| Russian Federation | 7.4 | 5.2 | 34.3 | 34.8 | 2.1 | 1.5 | 23.1 | 22 |
| Rwanda | 0.67 | 0.92 | 9.9 | 10.1 | 0.39 | 0.61 | 9.6 | 9.8 |
| Saint Lucia | 0 | 0 | 15.9 | 16.1 | 0 | 0 | 11.9 | 12.1 |
| Samoa | 1.3 | 1.7 | 23.1 | 25.3 | 1.3 | 1.7 | 69.4 | 53.2 |
| Sao Tome and Principe | 5.3 | 6.4 | 9.3 | 7.5 | 2.1 | 2.2 | 9.3 | 7.5 |
| Saudi Arabia | 1.5 | 1.3 | 12.2 | 13 | 0.54 | 0.48 | 9.6 | 10.5 |
| Senegal | 1.6 | 2.1 | 16.5 | 16.7 | 0.93 | 1.4 | 16.2 | 16.5 |
| Serbia | 10.5 | 7.4 | 51.4 | 53.1 | 2.4 | 1.6 | 29.9 | 27.3 |
| Sierra Leone | 1.3 | 1.8 | 11.5 | 11.7 | 0.82 | 1.2 | 11.5 | 11.7 |
| Singapore | 7 | 4.6 | 38.9 | 34 | 1.8 | 1.1 | 24.1 | 22.6 |
| Slovakia | 6.5 | 4.3 | 43.7 | 40.6 | 1.3 | 0.87 | 32.7 | 28 |
| Slovenia | 4.2 | 2.8 | 29.9 | 26.8 | 1.5 | 0.93 | 28.2 | 23 |
| Solomon Islands | 4.8 | 5.3 | 19.9 | 20.6 | 4.8 | 5.3 | 19.9 | 20.6 |
| Somalia | 2.3 | 3.5 | 23.4 | 23.9 | 1.8 | 3 | 23.7 | 24.2 |
| South Africa | 1.9 | 1.8 | 16.7 | 16.5 | 0.71 | 0.7 | 14.6 | 14.4 |
| South Sudan | 1.9 | 2.6 | 20.3 | 20.5 | 1.3 | 1.9 | 20.2 | 20.4 |
| Spain | 5.2 | 3.2 | 26.9 | 24.9 | 1.3 | 0.75 | 19.1 | 15.6 |
| Sri Lanka | 3.7 | 3.1 | 18.4 | 18.6 | 1.4 | 1.2 | 13 | 13 |
| Sudan | 2.1 | 2.7 | 23.4 | 23.8 | 1 | 1.5 | 23.1 | 23.6 |
| Suriname | 1.8 | 1.6 | 22.9 | 22.7 | 0 | 0 | 19.8 | 19.1 |
| Swaziland | 1.3 | 1.8 | 8.6 | 8.7 | 0.47 | 0.71 | 8.6 | 8.7 |
| Sweden | 2.5 | 1.9 | 25.7 | 24.4 | 0.3 | 0.21 | 26.2 | 18.2 |
| Switzerland | 3.1 | 2.1 | 27.2 | 21.2 | 0.83 | 0.53 | 23.1 | 15.2 |
| Syrian Arab Republic | 2.2 | 2.6 | 20.4 | 20.6 | 1 | 1.3 | 18.8 | 19.1 |
| Tajikistan | 1.2 | 1.3 | 8 | 8 | 0.41 | 0.49 | 7 | 7.3 |
| Tanzania, United Republic of | 0.55 | 0.77 | 7.9 | 8.1 | 0.31 | 0.52 | 7.8 | 8 |
| Thailand | 4.8 | 3.3 | 17.6 | 17.5 | 2.4 | 1.6 | 11.3 | 11.3 |
| The Netherlands | 2.1 | 1.6 | 31.2 | 25.6 | 0.87 | 0.61 | 28.5 | 22.6 |
| The former Yugoslav Republic of Macedonia | 6.6 | 4.8 | 26.3 | 26.2 | 1.5 | 1 | 15.4 | 14.2 |
| Timor-Leste | 1.9 | 2.8 | 17.6 | 17.7 | 0.86 | 1.5 | 14.9 | 15.2 |
| Togo | 1.5 | 2 | 13.3 | 13.3 | 0.86 | 1.2 | 13.1 | 13.1 |
| Trinidad and Tobago | 5.7 | 4.5 | 25 | 24.6 | 2 | 1.6 | 33.6 | 32.2 |
| Tunisia | 1.8 | 1.5 | 12.8 | 12.6 | 0.61 | 0.51 | 10.8 | 10.7 |
| Turkey | 3.7 | 3.1 | 25.5 | 25.1 | 1.1 | 0.94 | 18.3 | 17.5 |
| Turkmenistan | 3.4 | 3.3 | 10.1 | 9.8 | 1.6 | 1.6 | 9.8 | 9.6 |
| Uganda | 1.3 | 2.3 | 19.2 | 19.2 | 0.9 | 1.7 | 18.4 | 18.6 |
| Ukraine | 9.3 | 6.3 | 34.6 | 36.4 | 2.7 | 1.8 | 23.2 | 23.1 |
| United Arab Emirates | 1.5 | 1.3 | 23.3 | 27.7 | 0.58 | 0.5 | 17.8 | 22.2 |
| United Kingdom | 3.7 | 2.7 | 42.9 | 37.5 | 0.78 | 0.56 | 30.3 | 22.4 |
| United States of America | 3.6 | 2.8 | 34.3 | 31.1 | 0.78 | 0.61 | 21.8 | 17.9 |
| Uruguay | 3.2 | 2.6 | 30.3 | 27.2 | 1.5 | 1.2 | 24.9 | 21.4 |
| Uzbekistan | 1.6 | 1.5 | 11.2 | 11.4 | 0.64 | 0.63 | 7.8 | 8 |
| Vanuatu | 1.7 | 1.7 | 9.9 | 10.4 | 1.7 | 1.7 | 9.9 | 10.4 |
| Venezuela, Bolivarian Republic of | 3.2 | 3 | 21 | 21 | 1.1 | 1.1 | 14.7 | 14.6 |
| Viet Nam | 1.8 | 1.5 | 7 | 7.4 | 0.64 | 0.55 | 5.1 | 5.4 |
| Yemen | 1.2 | 1.5 | 7 | 6.9 | 0.68 | 0.97 | 6.7 | 6.6 |
| Zambia | 0.82 | 1.1 | 6.5 | 6.5 | 0.41 | 0.65 | 6.2 | 6.3 |
| Zimbabwe | 1.2 | 1.5 | 16.9 | 17 | 0.55 | 0.83 | 16.6 | 16.8 |
Fig. 2.
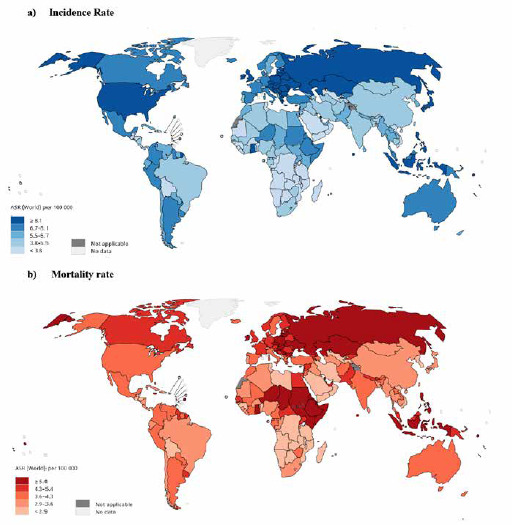
Map presenting (A) incidence and (B) mortality rates of ovarian cancer among women in the world in 2018 [source: GLOBOCAN 2018].
Based on the reported results for cancer in 2018, the highest incidence (8.6 per 1,000,000) and mortality (4.3 per 100,000) of the ovarian cancer are related to the very high HDI areas (Fig. 3).
Fig 3.
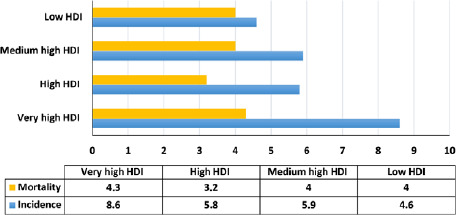
Distribution of incidence and mortality rates of ovarian cancer in terms of HDI [source: GLOBOCAN 2018].
The variance analysis detected the highest mean incidence (8.4 out of 100,000) being related to very high HDI areas and the lowest incidence (3.4 out of 100,000) related to low HDI areas, which was statistically significant (P < 0.0001). Moreover, the highest mortality rate (4.8 per 100,000) was pertinent to very high HDI areas while the lowest (3.6 per 100,000) was found for medium HDI areas; this difference was also statistically significant (P < 0.0001) (Tab. II).
Tab. II.
Ovarian cancer incidence and mortality rate in different HDI regions in 2018.
| HDI levels | Incidence rate | Mortality rate | ||
|---|---|---|---|---|
| CR | ASR | CR | ASR | |
| Very high human development | 14.7 | 8.4 | 10.1 | 4.8 |
| High human development | 8.7 | 6.7 | 5.6 | 4.1 |
| Medium human development | 4.5 | 5.1 | 2.9 | 3.6 |
| Low human development | 2.9 | 4.4 | 2.3 | 3.8 |
| P-value (F-test) | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 |
CR: Crude Rate; ASR: Age-Standardized Rates per 100,000.
Displayed by the results, there is a positive and significant correlation between incidence (r = 0.409, P < 0.05) and ovarian cancer mortality (r = 0.193, P < 0.05) with HDI (Fig. 4).
Fig 4.
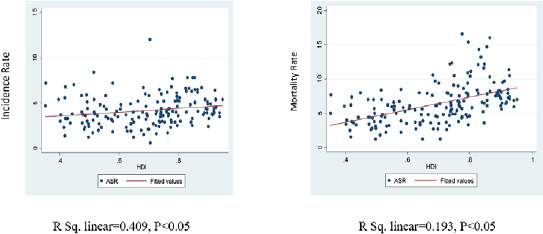
Human Development Index, incidence and mortality rates of ovarian cancer in the world in 2018.
The results demonstrated a positive and significant correlation between incidence with GNI (r = 0.326, P < 0.0001), MYS (r = 0.503, P < 0.0001), LEB (r = 0.42, P < 0.0001) and EYS (r = 0.439, P < 0.0001). Also a positive and significant correlation was identified between the ovarian cancer mortality with GNI (r = 0.169, P < 0.05), MYS (r = 0.249, P < 0.05), LEB (r = 0.163, P < 0.05) and EYS (r = 0.118, P < 0.05) (Tab. III).
Tab. III.
Pearson correlation between HDI components and dependent variable.
| HDI components | ASIR* | ASMR* | ||
|---|---|---|---|---|
| r | P-value | r | P-value | |
| Gross national income per 1,000 capita | 0.326 | P < 0.0001 | 0.169 | P < 0.05 |
| Mean years of schooling | 0.503 | P < 0.0001 | 0.249 | P < 0.05 |
| Life expectancy at birth | 0.420 | P < 0.0001 | 0.163 | P < 0.05 |
| Expected years of schooling | 0.439 | P < 0.0001 | 0.118 | P > 0.05 |
* Dependent variables: ASIR and ASMR.
The results of linear regression analysis illustrated that boosting one standard unit in mean years of schooling would escalate the incidence and mortality of ovarian cancer by 0.2 (P < 0.05). However, no statistically significant relationship was identified between gross national income per 1,000 capita, life expectancy at birth and expected years of schooling with the incidence and mortality of ovarian cancer (Tab. IV).
Tab. IV.
Effect of HDI components on ovarian cancer incidence and mortality in the world in 2018.
| HDI components | Incidence | Mortality | ||||
|---|---|---|---|---|---|---|
| B | CI 95% | P-value | B | CI 95% | P-value | |
| HDI | 7.6 | (-5.6, 21) | 0.2 | 1.1 | (-7.1, 9.5) | 0.7 |
| Gross national income per 1,000 capita | -0.004 | (-0.04, 0.002) | 0.7 | -0.004 | (-0.0001, 0.002) | 0.6 |
| Mean years of schooling | 0.2 | (-0.03, 0.5) | 0.03 | 0.2 | (0.03, 0.4) | 0.02 |
| Life expectancy at birth | -0.01 | (0.1, 0.09) | 0.7 | 0.006 | (0.06, 0.08) | 0.8 |
| Expected years of schooling | -0.1 | (0.4, 0.2) | 0.4 | -0.2 | (-0.4, -0.01) | 0.04 |
The results demonstrated the mean incidence and mortality rates in women over 50 being significantly higher than in those under 50 (P < 0.05) (Tab. V).
Tab. V.
Incidence and mortality rates of ovarian cancer for different age groups in the world in 2018.
| Incidence | Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | CR | ASR | CR | ASR | |||||
| Mean ± SD | P-value | Mean ± SD | P-value | Mean ± SD | P-value | Mean ± SD | P-value | ||
| Age group | 0-49 | 3.1 ± 2.1 | P < 0.0001 | 2.7 ± 1.4 | P<0.0001 | 1.1 ± 0.6 | P < 0.0001 | 1 ± 0.04 | P < 0.0001 |
| 50-85 | 21.6 ± 10.8 | 21.05 ± 10 | 17.7 ± 8.8 | 16.4 ± 8.1 | |||||
CR: Crude Rate; ASR: Age-Standardized Rates.
Discussion
The results uncovered the highest ovarian cancer incidence in the world pertaining to Serbia (16.6 per 100,000), Brunei (16 per 100,000) and Belarus (15.4 per 100,000), respectively. In addition, the highest death rate for ovarian cancer was related to Samoa (12 per 100,000), Solomon Islands (8.4 per 100,000) and Poland (7.8 per 100,000), respectively. The highest ovarian cancer incidence reported for Asia amounts to (51.8%), followed by Europe (22.9%), North America (9.2%), Latin America (7.9%), Africa (7.4%) and Oceania (0.73%). The lowest incidence is pertinent to Oceania.
Differences in the incidence of cancer in these areas can be attributed to variation in the economic situation of the individuals. An indicator for measuring a country’s condition is the human development index (HDI). This index examines the conditions of a country in three fundamental aspects of development including life expectancy, education and living standard. Life expectancy is measured with life expectancy at birth, and years of potential education, and standards of living with per capita income or GDP. In countries with high levels of HDI, ovarian cancer incidence and mortality proves to be higher. Causes of high incidence include environmental and genetic factors, early screening, proper health care, early-stage disease diagnosis, and accurate registration systems [18].
The incidence of ovarian cancer in the United States in 2015 was 21,290 whereas the death rate levelled at 14,180. The cancer is reported as 11.4 percent for Eastern Europe and 6% for Central Europe [18]. Eighty-four percent of cancerous cases are reported for Asian countries. The highest standardized rate of ovarian cancer incidence is related to Asian countries such as Singapore and Kazakhstan. Standardized ovarian cancer mortality comprises five countries including India, China, Indonesia, Japan and Pakistan bearing the highest rate of ovarian cancer mortality [16].
The results of our study identified a positive correlation between human development index and the incidence and mortality of ovarian cancer.
The results of Rahmani’s et al. study in 2018 revealed a significant positive relationship between the prevalence of ovarian cancer and HDI (p < 0.001) [12]. A study by Momenimovahed et al. (2019) demonstrated that although the prevalence of ovarian cancer is higher in areas with higher HDI, it is inversely proportional to the mortality rate [19]. Moreover, a 2016 study by Razi et al. identified a significant positive relationship between HDI levels and ovarian cancer. Ovarian rates appear to be greater in high-income individuals. The findings of the above study are in line with those of ours [1]. As these investigations project, cancer is still a global issue thereby requiring more extensive and comprehensive probes [4].
Socio-economic changes have had profound effects on cancer incidence and mortality. In countries with low or average income, the risk of cancer exhibits a rising trend. Cancer-induced infections account for about 26% of cancers in low-income countries. Low physical activity levels, obesity, malnutrition, infant lactation and the affluent social class are known as some of the risk factors for ovarian cancer [19]. The disease is associated with the industrialization of societies, and statistics have constantly shown that most of the deaths from cancer occur in the developing countries. Our results also disclosed a positive correlation between HDI and ovarian cancer incidence (r = 0.409, P < 0.0001) as well as mortality (r = 0.0771, P < 0.05) in 2018.
Global cancer rates and the distribution of different types of cancer are clearly linked to HDI, and planners need to be vigilant about the continually changing features and priorities of cancer as countries move toward higher levels of human development.
The incidence of cancer in different geographical areas can partly be attributed to differences in lifestyle-related risk factors. Related risk factors in the developed countries include smoking, pattern of nutritional and reproductive behaviors, and infectious agents in the developing countries; however, the pattern of the disease is being under change [20].
The greater ovarian cancer incidence in countries with higher HDIs could be ascribed to appropriate infrastructure, optimal health care, use of modern medical equipment, high standards of living and screening for early detection of the disease. The reason for higher mortality in the developing countries pertains to lack of access to optimal health care, changing lifestyle and high diagnostic and therapeutic costs. HDI is the mean for normal indexes, which measures optimal lifestyle, knowledge, and life expectancy.
The results of our study manifested a significant positive correlation between the incidence and mortality of ovarian cancer and GDP (living standards) (P < 0.05). In a study by Leitzmann et al., the findings revealed that obesity with its hormonal mechanism elevates the risk of ovarian cancer, and hence escalates death [21].
Socioeconomic status is one of the predictors of ovarian cancer incidence and maintenance. Access to health care, patient awareness of ovarian cancer symptoms, timely response to symptoms, lifestyle and underlying diseases justify the link between socioeconomic status and ovarian cancer [22, 23].
Our study could also indicate a positive and significant correlation between life expectancy index and the incidence and mortality of ovarian cancer (P < 0.05).
Most cancers seem to be more common in the elderly. Cancer is also associated with population aging and socio-economic development. Further, the United Nations Development Program (UNDP) has now considered life expectancy as important [24, 25]. The Human Development Index (HDI) has also been taken into account to assess the impact of social development on health issues (including cardiovascular diseases and cancer) in different countries. Despite augmenting life expectancy and shrinking standardized mortality in men and women, the incidence of cancer has relatively remained stable [26]. The relationship between cancer and life expectancy in the general population has also been extensively studied [17, 27]
In addition to what was explained, the greater incidence of ovarian cancer in areas with higher life expectancy than those with lower and moderate levels could possibly indicate an improvement in diagnosis. Nevertheless, over-diagnosis of the ovarian cancer can also play a role [26]. Our findings, additionally, demonstrated a significant positive correlation between education level and the incidence and mortality of the ovarian cancer (p < 0.05). Furthermore, in some investigations by Keng et al. [28] and Fallowfield et al. [29] a positive relationship was observed between ovarian cancer awareness and education level.
Because there is to date no effective way to prevent or screen ovarian cancer, early detection of the symptoms is of crucial importance. In countries where women enjoy greater levels of knowledge and education, there may be higher report of ovarian cancer incidence and mortality resulting from diagnosis of the disease, hence justifying this correlation.
As for some recent advancements and the fact that the true purpose of any development plan is achieving a healthy creative and happy life, HDI is needed to be considered. Genetic factors are known as the major causes of ovarian cancer. Therefore, early prevention strategies for the disease can be effective in reducing its incidence. Despite the relationship between these factors, caution must be taken regarding the interpretation and generalizability of the findings. In addition to the epidemiological risk factors of ovarian cancer, the inherent constraints of ecological studies should also be considered. On balance, it can be said that ovarian cancer incidence and mortality rate has seen a growing trend in many countries. Through adopting preventive measures, conducting epidemiological studies, performing timely treatment, and monitoring patients affected with ovarian cancer particularly with individuals residing in less-developed countries, the burden of the disease could very likely be reduced and effective steps could also be taken to improve the health systems of these countries.
Conclusions
Given the positive and significant correlation between ovarian cancer incidence and mortality with HDI, exercising attention to risk factors in these countries can be effective in containing its incidence and mortality. Higher incidence in these countries may be due to better and earlier detection of the disease. It can, therefore, be said that life expectancy, education, and GDP are the factors virtually associated with the disease accordingly contributing to depress its incidence and mortality.
Global cancer rates and the distribution of different types of cancer are clearly linked to HDI thus planners need to be vigilant about proceeding to change cancer features and priorities as countries move toward higher levels of human development.
LIMITATIONS OF THE STUDY
Limitations associated with our study largely comprise the data sources deployed. This is an ecological study special limitation of which include ecological misleading and lack of linkage between group results and the subjects.
RECOMMENDATIONS
Further research into the association between cancer (incidence, mortality, and survival) and HDI is warranted, and research using specific HDI components may provide meaningful insights into the relative importance of each factor pertinent to cancer. In addition, examining the incidence and mortality of cancer with increasing human development index can lead to a better understanding of cancer patterns and the way they change in terms of the rate of human development. Research on cancer changes considering human development at the regional and national levels can shed light on the relative importance of social, economic, cultural, and environmental factors playing a role in the scale and characteristics of the disease.
Figures and tables
Acknowledgements
Funding sources: Yazd University of Medical Sciences, Yazd, Iran.
The authors gratefully acknowledge the score of cancer registries worldwide as well as their staff for their willingness to contribute their data to this probe.
Footnotes
List of abbreviations
HDI: Human development Index
NCDs: Non-communicable diseases
GNI: Gross national income per 1000 capita
MYS: Mean years of schooling
LEB: Life expectancy at birth
LYS: Expected years of schooling
ASIR: Age Standard Incidence Rate
ASMR: Age Standard Mortality Rate
CR: Crude Rate
ASR: Age-Standardized Rate
Ethical statement
Code of Ethics: IIR.SSU.SPH.REC.1398.141.
Conflicts of interest statement
The authors declare no conflict of interest.
Authors’ contributions
EG and ZKH data extraction, performed data analysis and prepared the original manuscript. EG, ZKH, SMN and RB extracted Data, summarized and interpreted data and wrote the first draft of the manuscript. HN, SMB, EG and ZKH contributed to consultations and data collection and supervised the project. They also monitored the implementation of study and were involved in the preparation of the manuscript. All author(s) read and approved the final manuscript.
References
- [1].Fidler MM, Soerjomataram I, Bray F. A global view on cancer incidence and national levels of the human development index. Int J Cancer 2016;139:2336-46. https://doi.org/10.1002/ijc.30382 10.1002/ijc.30382 [DOI] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. https://doi.org/10.3322/caac.21492 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- [3].Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:359-86. https://doi.org/0.1002/ijc.29210 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- [4].Razi S, Ghoncheh M, Mohammadian-Hafshejani A, Aziznejhad H, Mohammadian M, Salehiniya H. The incidence and mortality of ovarian cancer and their relationship with the Human Development Index in Asia. Ecancermedicalscience 2016;10:155. https://doi.org/10.3332/ecancer.2016.628 10.3332/ecancer.2016.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Poorolajal J, Jenabi E, Masoumi SZ. Body mass index effects on risk of ovarian cancer: a meta-analysis. Asian Pac J Cancer Prev 2014;15:65-71. [DOI] [PubMed] [Google Scholar]
- [6].Riman T, Nilsson S, Persson IR. Review of epidemiological evidence for reproductive and hormonal factors in relation to the risk of epithelial ovarian malignancies. Acta Obstet Gynecol Scand 2004;83:783-95. https://doi.org/10.1111/j.0001-6349.2005.0838b.x 10.1111/j.0001-6349.2005.0838b.x [DOI] [PubMed] [Google Scholar]
- [7].Tsilidis K, Allen N, Key T, Dossus L, Lukanova A, Bakken K, Lund E, Fournier A, Overvad K, Hansen L, Tjonneland A, Fedirko V, Rinaldi S, Romieu I, Clavel-Chapelon F, Engel P, Kaaks R, Schütze M, Steffen A, Bamia C, Trichopoulou A, Zylis D, Masala G, Pala V, Galasso R, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, van Duijnhoven FJB, Braem MGM, Onland-Moret NC, Gram IT, Rodríguez L, Travier N, Sánchez M-J, Huerta JM, Ardanaz E, Larrañaga N, Jirström K, Manjer J, Idahl A, Ohlson N, Khaw K-T, Wareham N, Mouw T, Norat T, Riboli E. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer 2011;105:1436. https://doi.org/10.1038/bjc.2011.371 10.1038/bjc.2011.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Garcia M, Jemal A, Ward E, Center M, Hao Y, Siegel R, Thun MJ. Global Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society; 2007, p. 52. [Google Scholar]
- [9].Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. https://doi.org/10.1002/ijc.25516 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- [10].Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. https://doi.org/10.3322/caac.20107 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- [11].Murray CJ, Lopez AD, World Health Organization; World Bank & Harvard School of Public Health . The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020: summary. World Health Organization 1996. https://apps.who.int/iris/handle/10665/41864
- [12].Rahmani K, Moradi-Lakeh M, Mansori K, Bidokhti F, Asadi-Lari M. Global inequalities in incidence and mortality of ovarian cancer and associated factors: an ecological study. Alzheimers Parkinsonism Res Ther 2018;1:1001. [Google Scholar]
- [13].Mao Y, Desmeules M, Semenciw R, Hill G, Gaudette L, Wigle D. Increasing brain cancer rates in Canada. CMAJ 1991;145: 1583. [PMC free article] [PubMed] [Google Scholar]
- [14].Greiman AK, Rosoff JS, Prasad SM. Association of Human Development Index with global bladder, kidney, prostate and testis cancer incidence and mortality. BJU international 2017;120:799-807. https://doi.org/10.1111/bju.13875 10.1111/bju.13875 [DOI] [PubMed] [Google Scholar]
- [15].Sagar AD, Najam A. The human development index: a critical review. Ecol Econ 1998;25:249-64. [Google Scholar]
- [16].Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol 2012;13:790-801. https://doi.org/10.1016/S1470-2045(12)70211-5 10.1016/S1470-2045(12)70211-5 [DOI] [PubMed] [Google Scholar]
- [17].Goodarzi E, Moslem A, Feizhadad H, Jarrahi AM, Adineh HA, Sohrabivafa M, Khazaei Z. Epidemiology, incidence and mortality of thyroid cancer and their relationship with the human development index in the world: an ecology study in 2018. Adv Hum Biol 2019;9:162. https://doi.org/10.4103/AIHB.AIHB_2_19 10.4103/AIHB.AIHB_2_19 [DOI] [Google Scholar]
- [18].Kiadaliri AA. Social disparity in breast and ovarian cancer incidence in iran, 2003-2009: a time trend province-level study. J Breast Cancer 2013;16:372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health 2019;11:287. https://doi.org/10.2147/IJWH.S197604 10.2147/IJWH.S197604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hajmanoochehri F, Asefzadeh S, Kazemifar AM, Ebtehaj M. Clinicopathological features of colon adenocarcinoma in Qazvin, Iran: a 16 year study. Asian Pac J Cancer Prev 2014;15:951-5. https://doi.org/10.7314/apjcp.2014.15.2.951 10.7314/apjcp.2014.15.2.951 [DOI] [PubMed] [Google Scholar]
- [21].Leitzmann MF, Koebnick C, Danforth KN, Brinton LA, Moore SC, Hollenbeck R, Schatzkin A, Lacey JV, Jr. Body mass index and risk of ovarian cancer. Cancer 2009;115:812-22. https://doi.org/10.1002/cncr.24086 10.1002/cncr.24086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Booth CM, Li G, Zhang-Salomons J, Mackillop WJ. The impact of socioeconomic status on stage of cancer at diagnosis and survival: a population-based study in Ontario, Canada. Cancer 2010;116:4160-7. https://doi.org/10.1002/cncr.25427 10.1002/cncr.25427 [DOI] [PubMed] [Google Scholar]
- [23].Morris CR, Sands MT, Smith LH. Ovarian cancer: predictors of early-stage diagnosis. Cancer Causes Control 2010;21:1203-11. https://doi.org/10.1007/s10552-010-9547-0 10.1007/s10552-010-9547-0 [DOI] [PubMed] [Google Scholar]
- [24].WHO. World health statistics 2016: monitoring health for the SDGs sustainable development goals. World Health Organization; 2016. [Google Scholar]
- [25].Koh HK, Blakey CR, Roper AY. Healthy People 2020: a report card on the health of the nation. Jama 2014;311:2475-6. https://doi.org/10.1001/JAMA.2014.6446 10.1001/JAMA.2014.6446 [DOI] [PubMed] [Google Scholar]
- [26].Gu X, Zheng R, Xia C, Zeng H, Zhang S, Zou X, Yang Z, Li H, Chen W. Interactions between life expectancy and the incidence and mortality rates of cancer in China: a population-based cluster analysis. Cancer Commun 2018;38:44. https://doi.org/10.1186/s40880-018-0308-x 10.1186/s40880-018-0308-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Khazaei Z, Sohrabivafa M, Mansori K, Naemi H, Goodarzi E. Incidence and mortality of cervix cancer and their relationship with the human development index in 185 countries in the world: An ecology study in 2018. Adv Hum Biol 2019;9:222. https://doi.org/10.4103/AIHB.AIHB_15_19 10.4103/AIHB.AIHB_15_19 [DOI] [Google Scholar]
- [28].Keng SL, Wahab SBA, Chiu LB, Yusuf A. Awareness of ovarian cancer risk factors among women in Malaysia: a preliminary study. Asian Pac J Cancer Prev 2015;16:537-40. https://doi.org/10.7314/apjcp.2015.16.2.537 10.7314/apjcp.2015.16.2.537 [DOI] [PubMed] [Google Scholar]
- [29].Fallowfield L, Fleissig A, Barrett J, Menon U, Jacobs I, Kilkerr J, Farewell V, UKCTOCS Trialists. Awareness of ovarian cancer risk factors, beliefs and attitudes towards screening: baseline survey of 21 715 women participating in the UK Collaborative Trial of Ovarian Cancer Screening. Br J Cancer 2010;103:454-61. https://doi.org/10.1038/sj.bjc.6605809 10.1038/sj.bjc.6605809 [DOI] [PMC free article] [PubMed] [Google Scholar]


