Key Points
Question
Is there a benefit for patients who receive celecoxib as an addition to conventional therapy for women with ERBB2 (formerly HER2)–negative primary breast cancer?
Findings
In this randomized clinical trial, 2639 patients were randomized 2:1 to receive treatment with celecoxib or placebo for 2 years. Disease-free survival events were reported for 487 patients (19%): 18% for those receiving celecoxib vs 19% for those receiving placebo, a nonsignificant difference.
Meaning
Overall, no benefit was observed for patients who received celecoxib compared with placebo as adjuvant therapy for unselected ERBB2-negative primary breast cancer.
Abstract
Importance
Patients with breast cancer remain at risk of relapse after adjuvant therapy. Celecoxib has shown antitumor effects in preclinical models of human breast cancer, but clinical evidence is lacking.
Objective
To evaluate the role of celecoxib as an addition to conventional therapy for women with ERBB2 (formerly HER2)–negative primary breast cancer.
Design, Setting, and Participants
The Randomized European Celecoxib Trial (REACT) was a phase 3, randomized, double-blind study conducted in 160 centers across the UK and Germany testing 2 years of adjuvant celecoxib vs placebo among 2639 patients recruited between January 19, 2007, and November 1, 2012, with follow-up 10 years after treatment completion. Eligible patients had completely resected breast cancer with local and systemic therapy according to local practice. Patients with ERBB2-positive or node-negative and T1, grade 1 tumors were not eligible. Randomization was in a 2:1 ratio between celecoxib or placebo. Statistical analysis was performed from May 5, 2019, to March 5, 2020.
Interventions
Patients received celecoxib, 400 mg, or placebo once daily for 2 years.
Main Outcomes and Measures
The primary end point was disease-free survival (DFS), analyzed in the intention-to-treat population using Cox proportional hazards regression and log-rank analysis. Follow-up is complete.
Results
A total of 2639 patients (median age, 55.2 years [range, 26.8-86.0 years]) were recruited; 1763 received celecoxib, and 876 received placebo. Most patients’ tumors (1930 [73%]) were estrogen receptor positive or progesterone receptor positive and ERBB2 negative. A total of 1265 patients (48%) had node-positive disease, and 1111 (42%) had grade 3 tumors. At a median follow-up of 74.3 months (interquartile range, 61.4-93.6 years), DFS events had been reported for 487 patients (19%): 18% for those who received celecoxib (n = 323; 5-year DFS rate = 84%) vs 19% for those who received placebo (n = 164; 5-year DFS rate = 83%); the unadjusted hazard ratio was 0.97 (95% CI, 0.80-1.17; log-rank P = .75). Rates of toxic effects were low across both treatment groups, with no evidence of a difference.
Conclusions and Relevance
In this randomized clinical trial, patients showed no evidence of a DFS benefit for 2 years’ treatment with celecoxib compared with placebo as adjuvant treatment of ERBB2-negative breast cancer. Longer-term treatment or use of a higher dose of celecoxib may lead to a DFS benefit, but further studies would be required to test this possibility.
Trial Registration
ClinicalTrials.gov Identifier: NCT02429427 and isrctn.org Identifier: ISRCTN48254013
This randomized clinical trial evaluates the role of celecoxib as an addition to conventional therapy for women with ERBB2 (formerly HER2)–negative primary breast cancer.
Introduction
Chronic immune activation and associated inflammation have long been implicated in cancer progression, and there is a long history of anti-inflammatory drugs being used for patients with different cancers, albeit in the absence of direct clinical evidence of effectiveness. The principal mechanism explaining these effects of nonsteroidal anti-inflammatory drugs (NSAIDs) was their ability to inhibit prostaglandin (PG) synthesis. Prostaglandins are key mediators of inflammation; they are synthesized from phospholipids by the action of phospholipase A2 and cyclooxygenases (COXs). They have important functions in every organ system and regulate a variety of physiological functions, especially immunity.
At the inception of this trial, breast cancer had been a particular focus of interest for potential evaluation of the use of adjuvant NSAIDs because several studies had suggested an association between their use and decreased risk of breast cancer.1,2,3 At the time that these observational studies were reported, other potential mechanisms were uncovered to explain these findings, including the inhibition of procarcinogen activation and formation, tumor cell invasion and metastasis, angiogenesis, endothelial tube formation, and induction of apoptosis.4,5
The key regulatory step in PG synthesis is the enzymatic conversion of fatty acids to PGG2 and PGH2 by COX-1 and COX-2. Prostaglandin H2 is subsequently converted to 1 of several structurally related PGs, including PGE2, PGD2, PGF2, and thromboxane A2, by the activity of specific PG synthases. Prostaglandins have important functions in every organ system and regulate several physiological functions, such as immunity, maintenance of vascular integrity, and bone metabolism. COX-2 is not normally expressed in most tissues but is induced by a wide spectrum of growth factors and proinflammatory cytokines in specific pathophysiological conditions.6,7 The expression of COX-2 was found to be highly induced in v-src–transformed cells8 or after phorbol ester treatment.9
At the time that this trial was conceived, there was also considerable evidence that anti-inflammatory compounds could slow the growth of preclinical animal models of breast cancer. COX-2 is expressed in breast cancers,10,11 and the overexpression of COX-2 in mice has resulted in mammary tumor development,12 with other studies suggesting a prognostic effect of COX-2 expression in breast cancer.13,14 Furthermore, COX-2 expression was associated with aromatase content.15 These observations suggest that COX-2 inhibition plays a role in enhancing aromatase inhibitor treatment.
COX-1 inhibition predisposes to gastrointestinal ulceration, and for this reason, several specific COX-2 antagonists were developed, including celecoxib.16 Selective COX-2 antagonists (such as celecoxib) are associated with a lower incidence of adverse effects, especially gastrointestinal bleeding and dyspepsia, compared with other NSAIDs.17 In 2005, just before the start of the Randomized European Celecoxib Trial (REACT), reports suggested that NSAIDs may cause cardiac adverse events.18,19
In view of these considerations, the International Collaborative Cancer Group (ICCG) and the German Breast Group (GBG) initiated a trial to determine whether adjuvant celecoxib could improve disease outcomes among women with early breast cancer. In view of the controversy regarding adverse effects, we emphasized the importance of collecting data on gastrointestinal and cardiac adverse events and focused on women without cardiovascular risk factors.
Methods
Study Design
REACT was a phase 3, multicenter, double-blind, placebo-controlled randomized clinical trial evaluating celecoxib for patients with primary breast cancer (trial protocol in Supplement 1). Patients were recruited between January 19, 2007, and November 1, 2012, with follow-up 10 years after treatment completion. The primary aim was to assess the disease-free survival (DFS) benefit of 2 years’ adjuvant therapy with the COX-2 inhibitor celecoxib. Secondary objectives included an assessment of the effect on overall survival (OS); to describe the safety profile of celecoxib in this population, particularly in combination with endocrine therapy; and to compare the incidence of second cancers. Patients were randomized in a 2:1 ratio in favor of celecoxib. The study was approved by the South West Multi-center Research Ethics Committee. The study was conducted in compliance with guidelines for Good Clinical Practice and in accordance with the recommendations adopted by the Declaration of Helsinki.20 Patients provided written informed consent.
The trial opened to recruitment in December 2004; however, in February 2005 after 3 patients were entered, randomization was suspended under advice from the ICCG Steering Committee after the Adenoma Prevention With Celecoxib Trial identified an increased risk of cardiovascular events with the use of COX-2 inhibitors.21 In December 2005, the trial reopened with changes to the eligibility criteria and a reduced dose of 400 mg of celecoxib (previous dose, 800 mg per day), with the first new patient recruited in 2007; the Independent Data Monitoring Committee (IDMC) agreed that this dose reduction was appropriate.
This trial was sponsored by Imperial College and run under the auspices of the ICCG, a member of the Breast International Group. The Clinical Trials Section of the Cancer Research UK Imperial Centre was responsible for central trial management and coordination of trial management activities for the UK sites. The GBG Forschungs GmbH was responsible for trial management activities in Germany and oversaw coordination at the German sites. The Institute of Cancer Research Clinical Trials and Statistics Unit provided statistical resources and expertise to the collaboration, hosted the UK trial database, combined the UK and German data sets, and conducted all interim and final statistical analyses.
Participants
Eligible patients were women aged older than 18 years who had had primary invasive breast cancer completely resected with no previous or current evidence for metastatic disease. Women with hormone receptor–negative breast cancer must have received prior chemotherapy. Exclusion criteria included ERBB2 (formerly HER2)–positive disease; node-negative and T1, grade 1 breast cancer; history of certain gastrointestinal and cardiovascular conditions; and current or planned chronic NSAID therapy (except low-dose aspirin). Prior nonbreast cancer was not considered an exclusion criterion. Eligibility was also restricted by duration since receipt of last active anticancer therapy (see eAppendix 1 in Supplement 2 for timelines and full eligibility criteria).
Randomization and Masking
Patients were allocated to celecoxib or matching placebo. Randomization was performed using computer-generated random permuted blocks with stratification by treating center and hormone receptor status. Randomization was performed centrally by each coordinating data center (ICCG or GBG). Treatment allocation was blinded, and sites were provided with code break cards for emergency unblinding.
Procedures
Celecoxib and matching placebo were given as 400 mg (2 × 200-mg capsules taken orally) once daily for 2 years. Both celecoxib and placebo were manufactured and provided by Pfizer Inc.
Patients with hormone receptor–positive breast cancer also received endocrine therapy according to local practice. Follow-up was every 3 months in year 1, every 6 months in years 2 and 3, and annually after year 3 for an additional 7 years. Starting in March 2012, 70 of 91 sites (77%) in Germany moved to self-reported follow-up for patients who had completed treatment. For consenting patients, follow-up questionnaires were sent out every 6 months to collect information regarding relapses, toxic effects, and deaths; 516 of the 813 patients recruited in Germany consented to follow-up via this method. Patients who did not consent were followed up through standard hospital-based methods. Patients consenting to self-reported follow-up also consented to family members completing information on their behalf in case of incapacity. Follow-up at UK sites was performed using standard hospital-based methods throughout the duration of the trial. Treatment adherence was assessed at each visit during the treatment phase, and cardiovascular assessments and electrocardiography were performed at 1 year after randomization and at the end of treatment with celecoxib or placebo. COX-2 immunostaining was analyzed on tissue samples at the Charité University Hospital in Berlin, Germany (Charité Directorate laboratory) using a prediluted rabbit monoclonal antibody (clone SP21; Cell Marque) (eAppendix 2 in Supplement 2).
Outcomes
The primary end point was DFS, defined as the time from randomization to the date of diagnosis of first local or distant metastasis at any site; second primary breast cancer; or death from any cause. Secondary end points included OS, defined as the time from randomization to death from any cause; toxic effects associated with long-term use of celecoxib in patients with primary breast cancer; cardiovascular mortality; and the incidence of second primary cancers.
Data on all adverse events assessed by the principal investigator (R.C.C.) as meeting National Cancer Institute Common Terminology Criteria for Adverse Events (version 3)22 at grade 2 or above were collected from the time of randomization until 30 days after discontinuation of trial treatment. Data on serious adverse events and serious adverse reactions were collected in accordance with regulatory guidelines.
Statistical Analysis
Statistical analysis was performed from May 5, 2019, to March 5, 2020. The trial was originally powered to detect an increase in 5-year DFS rates from 70% to 75.2% (hazard ratio, 0.8). For a 2:1 randomization (celecoxib vs placebo) with 80% power and 2-sided α = .05, 2590 patients (709 events) were required. It was anticipated that this would coincide with a median follow-up of 5 years. All P values were from 2-sided tests, and results were deemed statistically significant at P < .05 for the efficacy end points and P < .01 for adverse events.
After review of the emerging interim data by the IDMC, it became apparent that the incidence of DFS events in the control group was lower than anticipated. The IDMC therefore recommended in January 2016 that analysis should be performed at a median follow-up of 5 years owing to the time it would take to reach the prespecified number of events. Based on the updated estimates, it was estimated that there would be approximately 80% power to detect an absolute difference in DFS of 4% at this time.
Disease-free survival and OS are presented as Kaplan-Meier survival curves with hazard ratios and log-rank tests to assess the effect of celecoxib; Cox proportional hazards regression enabled adjustment for relevant clinical factors. Follow-up time is estimated via a reverse Kaplan-Meier method. Efficacy analyses were performed according to the intention-to-treat principle, with stratification by country and estrogen receptor (ER) status.
Toxic effects were coded according to the Medical Dictionary for Regulatory Activities, version 14.023 and compared between treatment groups using the Fisher exact test and a significance level of 1% to account for multiple comparisons. Prespecified cardiovascular events were reported as well as any other adverse events meeting 1 of the following criteria: statistically significant difference between groups, difference between groups greater than 1%, or greater than 5% prevalence in either treatment group.
Analyses were based on a database snapshot taken on May 30, 2019, and performed using Stata, version 15 (StataCorp LLC). The IDMC reviewed the emerging safety and efficacy data. A Trial Steering Committee brought together the respective research groups and investigators from selected sites and included independent members (chair, clinicians, and statistician). The trial was endorsed by Cancer Research UK and registered on trials databases (ISRCTN48254013; EUDRACT: 2004-00004939; and ClinicalTrials.gov: NCT02429427).
Results
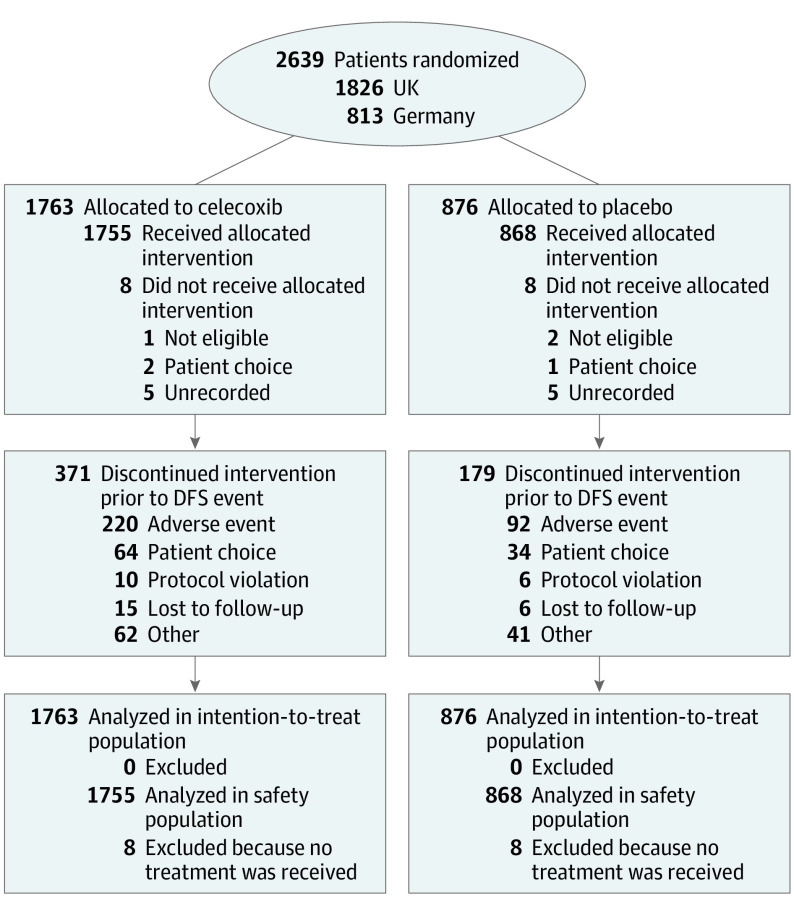
Between January 19, 2007, and November 1, 2012, 2639 patients were recruited (1763 received celecoxib, and 876 received placebo) from 160 centers across the UK and Germany (1826 patients from 69 UK centers and 813 patients from 91 German centers) (Figure 1). At the time of the database lock, the overall median follow-up was 74.3 months (interquartile range, 61.4-93.6 months).
Figure 1. CONSORT Diagram.
DFS indicates disease-free survival.
The median age of patients at trial entry was 55.2 years (range, 26.8-86.0 years), and most patients (1810 [69%]) were postmenopausal (Table). Most patients’ tumors were ER or progesterone (PgR) positive and ERBB2 negative (celecoxib group, 1279 [73%]; placebo group, 651 [74%]; 16 of these tumors were ER negative but PgR positive), while the remaining patients’ tumors were ER, PgR, and ERBB2 negative. A total of 838 patients (48%) in the celecoxib group and 427 patients (49%) in the placebo group had node-positive disease; 741 patients (42%) in the celecoxib group and 370 patients (42%) in the placebo group had grade 3 tumors.
Table. Baseline Patient and Tumor Characteristics.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Placebo (n = 876) | Celecoxib (n = 1763) | Total (N = 2639) | |
| Age, median (IQR) [range], y | 55.3 (48.6-63.0) [27.9-86.0] | 55.2 (48.7-62.6) [26.8-84.0] | 55.2 (48.6-62.7) [26.8-86.0] |
| Age, categorical, y | |||
| 18-64 | 718 (82) | 1463 (83) | 2181 (83) |
| 65-84 | 156 (18) | 300 (17) | 456 (17) |
| ≥85 | 2 (0.2) | 0 | 2 (0.1) |
| Weight, median (IQR), kga | 72 (63-82) | 72 (64-83) | 72 (64-82) |
| Height, median (IQR), cmb | 164 (160-168) | 164 (159-168) | 164 (159-168) |
| Menopausal status (at diagnosis) | |||
| Premenopausal | 213 (24) | 433 (25) | 646 (25) |
| Perimenopausalc | 15 (2) | 37 (2) | 52 (2) |
| Permenopausalc | 47 (5) | 84 (5) | 131 (5) |
| Postmenopausal | 601 (69) | 1209 (69) | 1810 (69) |
| WHO performance status | |||
| 0 = Normal activity | 816 (93) | 1667 (95) | 2483 (94) |
| 1 = Restricted activity | 53 (6) | 81 (5) | 134 (5) |
| 2 = In bed <50% of time | 1 (0.1) | 3 (0.2) | 4 (0.2) |
| Not done | 6 (0.7) | 12 (0.7) | 18 (0.7) |
| Tumor size, median (IQR) [range], mmd | 21 (14-30) [0-160] | 21 (14-30) [0-150] | 21 (14-30) [0-160] |
| Tumor size category, cm | |||
| <2 | 406 (46) | 852 (48) | 1258 (48) |
| 2-5 | 381 (44) | 770 (44) | 1151 (44) |
| >5 | 63 (7) | 108 (6) | 171 (7) |
| Missing or unknown | 26 (3) | 33 (2) | 59 (2) |
| Type of surgery | |||
| Breast conserving | 586 (67) | 1221 (69) | 1807 (69) |
| Has received or will receive RT | 525 (60) | 1062 (60) | 1587 (60) |
| No RT | 59 (7) | 151 (9) | 210 (8) |
| RT missing | 2 (0.2) | 8 (0.5) | 10 (0.4) |
| Mastectomy | 290 (33) | 540 (31) | 830 (32) |
| Has received or will receive RT | 200 (23) | 363 (21) | 563 (21) |
| No RT | 90 (10) | 176 (10) | 266 (10) |
| RT missing | 0 | 1 (0.1) | 1 (0.1) |
| Unknown | 0 | 2 (0.1) | 2 (0.1) |
| Has received or will receive RT | 0 | 2 (0.2) | 2 (0.1) |
| Histologic findings | |||
| Ductal | 671 (77) | 1356 (77) | 2027 (77) |
| Lobular | 107 (12) | 237 (13) | 344 (13) |
| Mixed ductal | 35 (4) | 65 (4) | 100 (4) |
| Other | 61 (7) | 102 (6) | 163 (6) |
| Missing | 2 (0.2) | 3 (0.2) | 5 (0.2) |
| Grading | |||
| G1 | 29 (3) | 93 (5) | 122 (5) |
| G2 | 471 (54) | 917 (52) | 1388 (53) |
| G3 or 4 | 370 (42) | 741 (42) | 1111 (42) |
| Unknown | 6 (0.7) | 12 (0.7) | 18 (0.7) |
| No. of nodes involved | |||
| 0 | 444 (51) | 911 (52) | 1355 (51) |
| 1-3 | 285 (33) | 589 (33) | 874 (33) |
| ≥4 | 142 (16) | 249 (14) | 391 (15) |
| Unknown | 5 (0.6) | 14 (0.8) | 19 (0.7) |
| ER status | |||
| Positive | 646 (74) | 1270 (72) | 1916 (73) |
| Negative | 230 (26) | 493 (28) | 723 (27) |
| PgR status | |||
| Positive | 398 (45) | 777 (44) | 1175 (45) |
| Negative | 281 (32) | 575 (33) | 856 (32) |
| Unknown | 197 (23) | 411 (23) | 608 (23) |
| ERBB2 status | |||
| Negative | 875 (99.9) | 1760 (99.8) | 2635 (99.8) |
| Unknowne | 1 (0.1) | 3 (0.2) | 4 (0.2) |
| Biological subtypef | |||
| ER positive, PgR positive, and ERBB2 negative | 651 (74) | 1279 (73) | 1930 (73) |
| Triple negativeg | 224 (26) | 481 (27) | 705 (27) |
Abbreviation: ER, estrogen receptor; IQR, interquartile range; PgR, progesterone receptor; RT, radiotherapy; WHO, World Health Organization.
Data missing for 83 patients.
Data missing for 107 patients.
Perimenopausal: when the menopausal process begins at 45 years of age or younger; permenopausal: when the menopausal process begins at older than 45 years.
Data missing for 59 patients.
Identified as such after trial entry.
Excludes the 4 patients with unknown or missing ERBB2 status (1 is ER negative and PgR negative, and 1 is ER positive and PgR positive).
Includes 43 patients with PgR status unknown.
With regard to systemic therapy, 829 of 1270 women (65%) in the celecoxib group with ER-positive disease and 438 of 646 women (68%) in the placebo group with ER-positive disease received cytotoxic chemotherapy (Table). A total of 490 of 493 women (99%) in the celecoxib group with ER-negative disease and 227 of 230 women (99%) in the placebo group with ER-negative disease received cytotoxic chemotherapy.
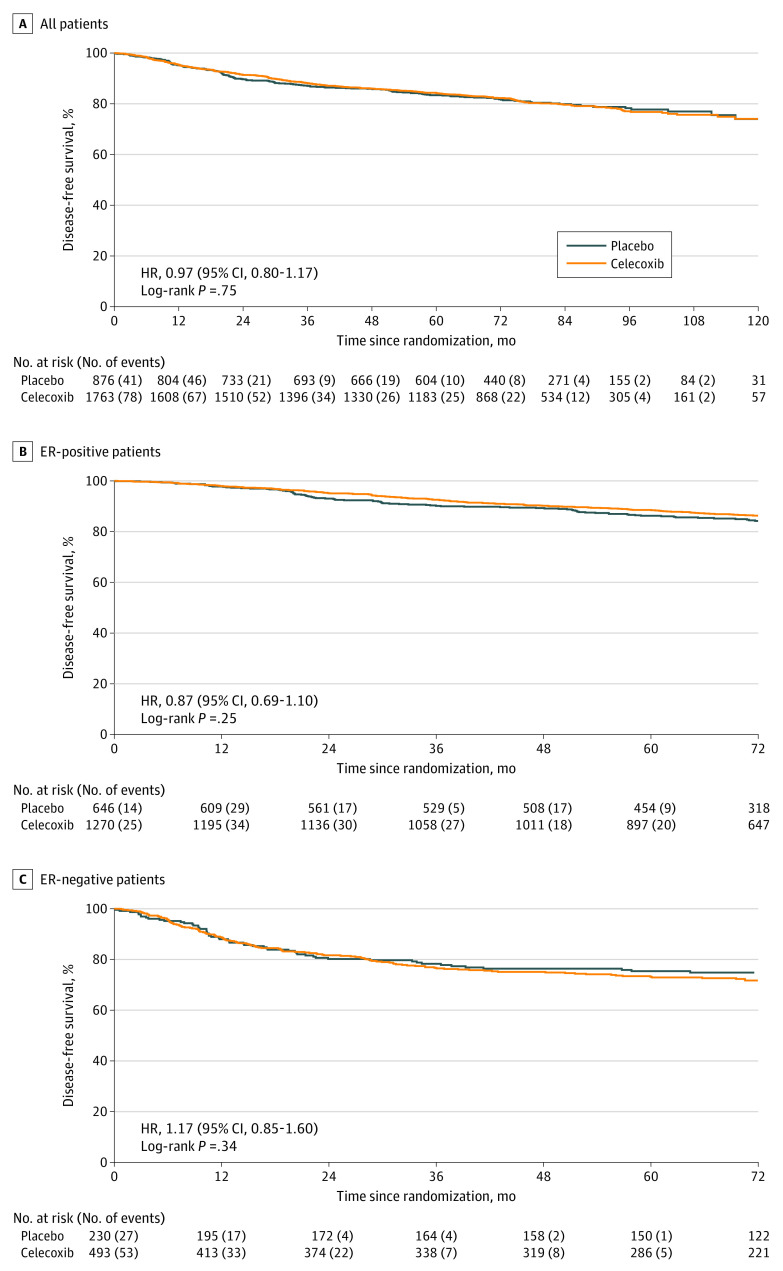
A total of 487 patients (19%) had a DFS event reported (celecoxib group, 323 of 1763 [18%]; 5-year DFS rate = 84%; placebo group, 164 of 876 [19%]; 5-year DFS rate = 83%). There was no evidence of a significant difference in DFS between celecoxib and placebo (unadjusted hazard ratio, 0.97; 95% CI, 0.80-1.17; log-rank P = .75) (Figure 2A). No evidence of a differential effect according to ER status was observed (patients with ER-positive disease: hazard ratio, 0.87; 95% CI, 0.69-1.10; patients with ER-negative disease: hazard ratio, 1.17; 95% CI, 0.85-1.60; P = .12 for interaction; Figure 2B and C), although the study was not powered to test this outcome.
Figure 2. Kaplan-Meier Curves of Disease-Free Survival.
A-C, Hazard ratio (HR) stratified by estrogen receptor (ER) status and country for all patients; stratified by country only for ER-positive and ER-negative patients.
The treatment effect was also examined within prespecified subgroups defined by menopausal status (premenopausal or perimenopausal vs postmenopausal), nodal involvement (0 vs 1-3 vs ≥4), and chemotherapy use (neoadjuvant vs adjuvant vs none). No evidence of differential treatment effects in any subgroup was observed for DFS (eFigure in Supplement 2).
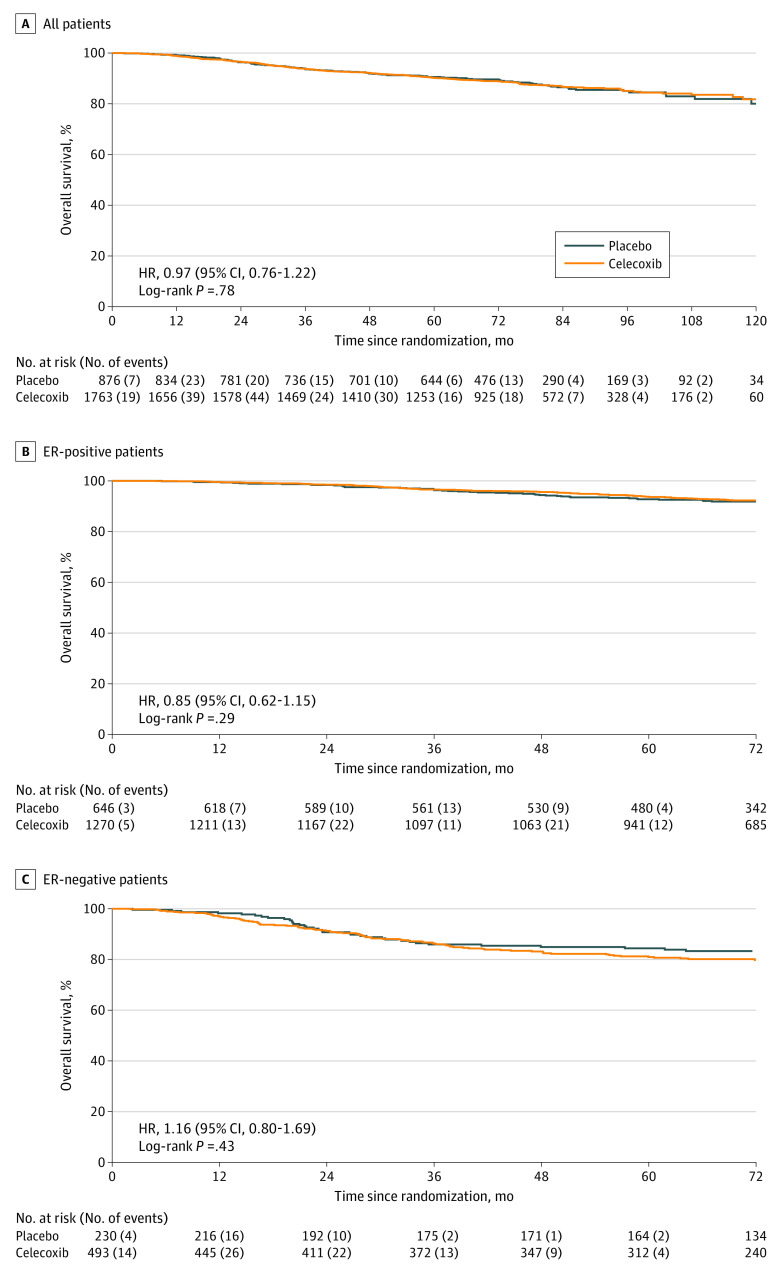
There were 307 deaths reported overall (celecoxib group, 203 of 1763 [12%]; 5-year OS rate = 90%; placebo group, 104 of 876 [12%]; 5-year OS rate = 91%). Consistent with the DFS result, no evidence of a difference in OS was found between treatment groups (unadjusted hazard ratio, 0.97; 95% CI, 0.76-1.22; log-rank P = .78; Figure 3A). This finding remained when analyzed separately by ER status, with no evidence of an interaction (patients with ER-positive disease: hazard ratio, 0.85; 95% CI, 0.62-1.15; patients with ER-negative disease: hazard ratio, 1.16; 95% CI, 0.80-1.69; P = .17) (Figure 3B and C).
Figure 3. Kaplan-Meier Curves of Overall Survival.
A, All patients. In the celecoxib group, 203 patients died, and in the placebo group, 104 patients died. B, Estrogen receptor (ER)–positive patients. In the celecoxib group, 110 patients died, and in the placebo group, 65 patients died. C, ER-negative patients. In the celecoxib group, 93 patients died, and in the placebo group, 39 patients died. Hazard ratio (HR) stratified by ER status and country for all patients; stratified by country only for ER-positive and ER-negative patients.
Analysis of prespecified cardiovascular events did not find any evidence that celecoxib treatment was associated with an increase in cardiac events compared with placebo (258 of 1755 [15%] vs 114 of 868 [13%]). There was also no evidence of an increase in individual events (including myocardial infarction, heart failure, coronary heart disease, hypertension, cardiac rhythm abnormality, peripheral vascular disease, stroke, or carotid disease) in this patient population (eTable 1 in Supplement 2). There was no evidence that celecoxib use was associated with an excess of gastrointestinal adverse effects. Rates of other toxic effects were low across both treatment groups, with no statistical evidence of a difference in incidence between treatment groups for any toxic effect (eTable 2 in Supplement 2).
Of the 307 deaths, cardiac involvement was cited as contributing to the cause of death for 11 patients (4%) (celecoxib group, 6 of 203 [3%]; placebo group, 5 of 104 [5%]). The incidence of new, primary nonbreast cancers was low, with no evidence of a difference between treatment groups (celecoxib group, 61 of 1763 [4%]; placebo group, 31 of 876 [4%]).
The allocated 2 years of treatment was completed by 1277 of 1763 patients (72%) in the celecoxib group and 620 of 876 patients (71%) in the placebo group, with 107 of 1763 patients (6%) in the celecoxib group and 69 of 876 patients (8%) in the placebo group discontinuing prior to 2 years owing to a DFS event. In the celecoxib group, 220 of 1755 patients (13%) who started treatment discontinued owing to adverse events; in the placebo group, 92 of 868 patients (11%) discontinued owing to adverse events (eTable 3 in Supplement 2).
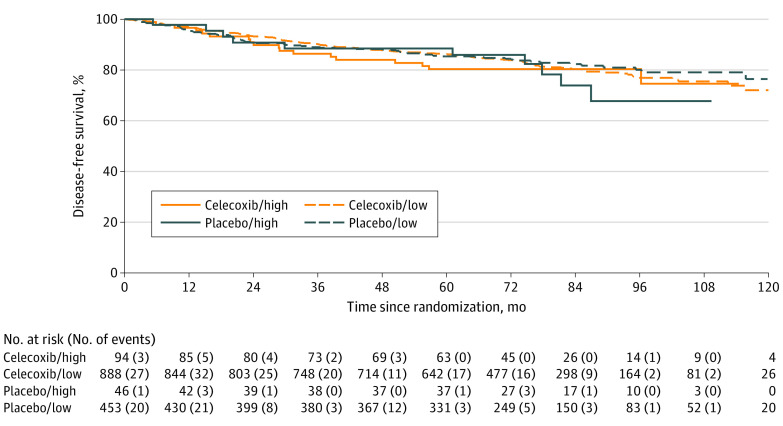
The results of COX-2 expression were available for 1471 patients (low COX-2 expression, 1341 patients; high COX-2 expresson, 140 patients). Subgroup analysis by COX-2 status showed no significant difference in DFS outcomes between treatment groups among patients with low or high COX-2 expression, with a hazard ratio of 1.04 (95% CI, 0.80-1.36) for the subgroup with low expression and 0.83 (95% CI, 0.37-1.86) for the subgroup with high expression (Figure 4). There was no observed interaction between COX-2 status and treatment (P = .69).
Figure 4. Kaplan-Meier Curve of Disease-Free Survival.
Disease-free survival curves presented by allocated treatment group and dichotomized COX-2 status (high vs low).
Discussion
Overall, this study observed no benefit from adding celecoxib to conventional adjuvant treatment for this cohort of patients with ERBB2-negative primary invasive breast cancer after total resection. Contrary to expectations, no significant increase in adverse effects was observed; in particular, there was no evidence of an increase in gastrointestinal or cardiac adverse effects with celecoxib.
The MA.27 study also failed to show any benefit with celecoxib as adjuvant therapy for breast cancer.24 In that trial, 1622 patients were randomly assigned in a 2 × 2 factorial design to receive 400 mg of celecoxib or placebo and exemestane or anastrozole. Randomization between celecoxib and placebo was, however, discontinued prematurely after approximately 18 months in view of concern about potential adverse cardiac events. The MA.27 study also included more than 25% of patients who were taking low-dose aspirin (2% [56 of 2639] reported in the REACT trial) and excluded patients with ER-negative disease and premenopausal patients.
Since the start of this study, celecoxib therapy has been used in several breast cancer studies, albeit in smaller numbers. For example, in the REMAGUS 2 trial, 340 patients were randomly assigned to receive celecoxib or placebo, but no benefit was observed.25 Aristarco et al26 compared the effects of neoadjuvant celecoxib, exemestane, or placebo on Ki67 and other proliferative indices but saw no beneficial effects of celecoxib. De Cremoux et al27 showed that pathologic complete response due to celecoxib was more common in tumors that expressed COX-2. A phase 2 study has also been reported in which preoperative adjuvant therapy with celecoxib resulted in the downregulation of matrix metalloproteinase-2 and matrix metalloproteinase-9.28 Other smaller studies have also reported on the use of celecoxib in breast cancer studies29,30; however, these studies suggest either a small effect or no effect of celecoxib treatment on breast cancer disease outcomes despite the fact that, in several of the studies, the short duration of therapy allowed the use of a daily 800-mg dose of celecoxib.
In an era of increased reliance on real-world data, the result of our study is a further illustration of how plausible associations identified through observational studies do not always translate to clinically meaningful effects in randomized clinical trials. This disparity may be assessed through the use of observational studies with sophisticated study designs31; however, these studies cannot fully address confounders, and randomized clinical trials remain the criterion standard.
Analysis by expression of COX-2 and by ER status did not identify any differential effects in our study. However, increasing evidence suggests that the PGs that maintain the inflammatory phenotype also undermine the body’s immune reaction to the breast cancer. Therefore, it is likely, for example, that the patients who may benefit from anti-inflammatory compounds are those whose breast cancers have a pronounced inflammatory infiltrate. COX-2 can mediate immunosuppressive effects. Zelenay et al32 discovered that a COX-2 signature exists in tumors in that COX-2 levels correlated positively with messenger RNA expression levels for known tumor-promoting inflammatory factors and correlated negatively with factors associated with cytotoxic T-lymphocyte infiltration and type 1 interferon signaling. The results suggest that PGs could exert an immune-suppressive effect on the tumor microenvironment.
Despite the lack of evidence for a benefit of COX-2 inhibitors, to date, this outcome should have no implications for the ongoing aspirin studies, which should continue to run to completion given the different mechanisms of action. Updated results with more mature follow-up are highly anticipated.
Limitations
This study has some limitations. The relapse rate observed in the control group was lower than anticipated based on historical data. For this reason, the IDMC recommended that the primary analysis be conducted when a median follow-up of at least 5 years was reached. Updated power calculations, however, were reviewed and deemed appropriate by the IDMC. Self-reported follow-up was used by some centers in Germany, but previous validation work within REACT identified that, although fewer deaths are reported by self-reporting methods, no effect of follow-up method on DFS rates was seen.33
Finally, we were obliged to reduce the dose of celecoxib in our study because of potential cardiac adverse effects, and we have not excluded the possibility that a higher dose of celecoxib may result in improved results. The dose of 400 mg daily is, however, effective in many disease indications34 and is the recommended dose for analgesia.
Conclusions
Despite much preclinical evidence suggesting that COX-2 inhibitors would improve the outlook of patients with early breast cancer, this randomized clinial trial found no evidence of benefit when given at a dose of 400 mg per day for 2 years to patients with ERBB2-negative breast cancer.
Trial Protocol
eAppendix 1. Eligibility Criteria
eAppendix 2. COX2 Analysis
eReference
eTable 1. Cardiovascular Toxicities
eTable 2. Other Toxicities Meeting Reporting Criteria
eTable 3. Reasons for Discontinuations if 2 Years Not Completed
eFigure. Forest Plot of Other Subgroup Analyses Stratified by ER Status and Country
Nonauthor Collaborators. REACT Trial Management Group and Investigators
Data Sharing Statement
References
- 1.Harris RE, Kasbari S, Farrar WB. Prospective study of nonsteroidal anti-inflammatory drugs and breast cancer. Oncol Rep. 1999;6(1):71-73. doi: 10.3892/or.6.1.71 [DOI] [PubMed] [Google Scholar]
- 2.Harris RE, Namboodiri KK, Farrar WB. Nonsteroidal antiinflammatory drugs and breast cancer. Epidemiology. 1996;7(2):203-205. doi: 10.1097/00001648-199603000-00017 [DOI] [PubMed] [Google Scholar]
- 3.Sharpe CR, Collet JP, McNutt M, Belzile E, Boivin JF, Hanley JA. Nested case-control study of the effects of non-steroidal anti-inflammatory drugs on breast cancer risk and stage. Br J Cancer. 2000;83(1):112-120. doi: 10.1054/bjoc.2000.1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder DJ, Halton DE, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)–selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clin Cancer Res. 1997;3(10):1679-1683. [PubMed] [Google Scholar]
- 5.Sheng H, Shao J, Kirkland SC, et al. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99(9):2254-2259. doi: 10.1172/JCI119400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145-182. doi: 10.1146/annurev.biochem.69.1.145 [DOI] [PubMed] [Google Scholar]
- 7.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271(52):33157-33160. doi: 10.1074/jbc.271.52.33157 [DOI] [PubMed] [Google Scholar]
- 8.Xie WL, Chipman JG, Robertson DL, Erikson RL, Simmons DL. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991;88(7):2692-2696. doi: 10.1073/pnas.88.7.2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991;266(20):12866-12872. doi: 10.1016/S0021-9258(18)98774-0 [DOI] [PubMed] [Google Scholar]
- 10.Parrett M, Harris R, Joarder F, Ross M, Clausen K, Robertson F. Cyclooxygenase-2 gene expression in human breast cancer. Int J Oncol. 1997;10(3):503-507. doi: 10.3892/ijo.10.3.503 [DOI] [PubMed] [Google Scholar]
- 11.Soslow RA, Dannenberg AJ, Rush D, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89(12):2637-2645. doi: [DOI] [PubMed] [Google Scholar]
- 12.Liu CH, Chang SH, Narko K, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276(21):18563-18569. doi: 10.1074/jbc.M010787200 [DOI] [PubMed] [Google Scholar]
- 13.Denkert C, Winzer KJ, Müller BM, et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer. 2003;97(12):2978-2987. doi: 10.1002/cncr.11437 [DOI] [PubMed] [Google Scholar]
- 14.van Nes JG, de Kruijf EM, Faratian D, et al. COX2 expression in prognosis and in prediction to endocrine therapy in early breast cancer patients. Breast Cancer Res Treat. 2011;125(3):671-685. doi: 10.1007/s10549-010-0854-7 [DOI] [PubMed] [Google Scholar]
- 15.Brueggemeier RW, Quinn AL, Parrett ML, Joarder FS, Harris RE, Robertson FM. Correlation of aromatase and cyclooxygenase gene expression in human breast cancer specimens. Cancer Lett. 1999;140(1-2):27-35. doi: 10.1016/S0304-3835(99)00050-6 [DOI] [PubMed] [Google Scholar]
- 16.Emery P, Zeidler H, Kvien TK, et al. Celecoxib versus diclofenac in long-term management of rheumatoid arthritis: randomised double-blind comparison. Lancet. 1999;354(9196):2106-2111. doi: 10.1016/S0140-6736(99)02332-6 [DOI] [PubMed] [Google Scholar]
- 17.Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. JAMA. 2000;284(10):1247-1255. doi: 10.1001/jama.284.10.1247 [DOI] [PubMed] [Google Scholar]
- 18.Lévesque LE, Brophy JM, Zhang B. The risk for myocardial infarction with cyclooxygenase-2 inhibitors: a population study of elderly adults. Ann Intern Med. 2005;142(7):481-489. doi: 10.7326/0003-4819-142-7-200504050-00113 [DOI] [PubMed] [Google Scholar]
- 19.Solomon DH. Selective cyclooxygenase 2 inhibitors and cardiovascular events. Arthritis Rheum. 2005;52(7):1968-1978. doi: 10.1002/art.21132 [DOI] [PubMed] [Google Scholar]
- 20.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21.Solomon SD, McMurray JJ, Pfeffer MA, et al. ; Adenoma Prevention with Celecoxib (APC) Study Investigators . Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071-1080. doi: 10.1056/NEJMoa050405 [DOI] [PubMed] [Google Scholar]
- 22.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Published August 9, 2006. Accessed June 7, 2021. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- 23.MedDRA. Medical Dictionary for Regulatory Activities. Accessed June 7, 2021. https://www.meddra.org/
- 24.Strasser-Weippl K, Higgins MJ, Chapman JW, et al. Effects of celecoxib and low-dose aspirin on outcomes in adjuvant aromatase inhibitor–treated patients: CCTG MA.27. J Natl Cancer Inst. 2018;110(9):1003-1008. doi: 10.1093/jnci/djy017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giacchetti S, Hamy AS, Delaloge S, et al. Long-term outcome of the REMAGUS 02 trial, a multicenter randomised phase II trial in locally advanced breast cancer patients treated with neoadjuvant chemotherapy with or without celecoxib or trastuzumab according to HER2 status. Eur J Cancer. 2017;75:323-332. doi: 10.1016/j.ejca.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 26.Aristarco V, Serrano D, Gandini S, et al. A randomized, placebo-controlled, phase II, presurgical biomarker trial of celecoxib versus exemestane in postmenopausal breast cancer patients. Cancer Prev Res (Phila). 2016;9(5):349-356. doi: 10.1158/1940-6207.CAPR-15-0311 [DOI] [PubMed] [Google Scholar]
- 27.De Cremoux P, Hamy AS, Lehmann-Che J, et al. COX2/PTGS2 expression is predictive of response to neoadjuvant celecoxib in HER2-negative breast cancer patients. Anticancer Res. 2018;38(3):1485-1490. [DOI] [PubMed] [Google Scholar]
- 28.Brandão RD, Veeck J, Van de Vijver KK, et al. A randomised controlled phase II trial of pre-operative celecoxib treatment reveals anti-tumour transcriptional response in primary breast cancer. Breast Cancer Res. 2013;15(2):R29. doi: 10.1186/bcr3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falandry C, Debled M, Bachelot T, et al. Celecoxib and exemestane versus placebo and exemestane in postmenopausal metastatic breast cancer patients: a double-blind phase III GINECO study. Breast Cancer Res Treat. 2009;116(3):501-508. doi: 10.1007/s10549-008-0229-5 [DOI] [PubMed] [Google Scholar]
- 30.Dirix LY, Ignacio J, Nag S, et al. Treatment of advanced hormone-sensitive breast cancer in postmenopausal women with exemestane alone or in combination with celecoxib. J Clin Oncol. 2008;26(8):1253-1259. doi: 10.1200/JCO.2007.13.3744 [DOI] [PubMed] [Google Scholar]
- 31.Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70-75. doi: 10.1016/j.jclinepi.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelenay S, van der Veen AG, Böttcher JP, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162(6):1257-1270. doi: 10.1016/j.cell.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tovey HL, Bliss J, Coombes C, von Minckwitz G, Steffen J, Kilburn L. Sensitivity analysis assessing the impact of patient self-reported follow up in the REACT trial. Presented at: 4th International Clinical Trials Methodology Conference (ICTMC); May 7-10, 2017; Liverpool, UK. [Google Scholar]
- 34.Shi S, Klotz U. Clinical use and pharmacological properties of selective COX-2 inhibitors. Eur J Clin Pharmacol. 2008;64(3):233-252. doi: 10.1007/s00228-007-0400-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Eligibility Criteria
eAppendix 2. COX2 Analysis
eReference
eTable 1. Cardiovascular Toxicities
eTable 2. Other Toxicities Meeting Reporting Criteria
eTable 3. Reasons for Discontinuations if 2 Years Not Completed
eFigure. Forest Plot of Other Subgroup Analyses Stratified by ER Status and Country
Nonauthor Collaborators. REACT Trial Management Group and Investigators
Data Sharing Statement