Key Points
Question
Do current guidelines that direct the use of transvaginal ultrasonography as a gateway to endometrial biopsy among women with postmenopausal bleeding perform differently by patient race?
Findings
In this study of a simulated cohort of 367 073 Black and White women with postmenopausal bleeding, the use of 4-mm transvaginal ultrasonography endometrial thickness measurements to prompt biopsy resulted in a sensitivity of 47.5% among Black women compared with 87.9% among White women, with a negative predictive value of 92% among Black women vs 98% among White women.
Meaning
The findings of this study suggest that adherence to current clinical guidelines results in systematic underdiagnosis in Black women with endometrial cancer owing to measurement thresholds that fail to account for uterine fibroids and nonendometrioid histologic type.
Abstract
Importance
Black women in the US with endometrial cancer (EC) are more likely to be diagnosed with advanced-stage disease independent of insured status and histologic type. The most common way of diagnosing EC at early stages is through screening of people with postmenopausal bleeding to detect endometrial thickness (ET). This approach may disproportionately underperform in Black women secondary to a higher prevalence of fibroids and nonendometrioid EC in this population, both of which affect the quality of ET measurement.
Objective
To compare the performance of recommended transvaginal ultrasonography (TVUS) ET thresholds as a screening method to prompt endometrial biopsy by race in a simulated cohort of symptomatic women.
Design, Setting, and Participants
In a simulated retrospective cohort study, based on data from Surveillance, Epidemiology, and End Results (SEER) national cancer registry 2012-2016; the US census; and published estimates of ET distribution and fibroid prevalence, diagnostic test characteristics of the 3-mm or more, 4-mm or more, and 5-mm or more ET thresholds for biopsy to capture EC diagnoses were calculated. The simulated cohort was constructed from February 2, 2020 (date of access to SEER data), to August 31, 2020. Analysis occurred from September 30, 2020, to March 30, 2021, including the primary analysis and the sensitivity calculations.
Main Outcomes and Measures
The main outcome measured was accuracy of the TVUS ET threshold to accurately identify cases of EC, measured by sensitivity, negative predictive value, and area under the curve (AUC).
Results
A total of 367 073 simulated Black and White women with postmenopausal bleeding were evaluated, including 36 708 with EC. Among Black women, the currently recommended 4-mm or greater ET threshold prompted biopsy for fewer than half of EC cases (sensitivity, 47.5%; 95% CI, 46.0%-49.0%); of women referred for biopsy, 13.1% were EC cases (positive predictive value, 13.1%; 95% CI, 12.5%-13.6%). The AUC for the 4-mm or more threshold was 0.57 (95% CI, 0.56-0.57). In contrast, among the White women, the 4-mm or more threshold led to biopsy for most with EC (sensitivity, 87.9%; 95% CI, 87.6%-88.3%). Of those referred for biopsy, 14.6% had EC (positive predictive value, 14.6%; 95% CI, 14.4%-14.7%); AUC was 0.73 (95% CI, 0.73-0.74). The same variations held for the 3-mm or more and 5-mm or more ET thresholds: sensitivity, positive predictive value, and AUC were consistently lower for Black women than White women.
Conclusions and Relevance
The findings of this simulated cohort study suggest that use of ET as measured by TVUS to determine the need for EC diagnostic testing in symptomatic women may exacerbate racial disparities in EC stage at diagnosis. In simulated data, TVUS ET screening missed almost 5 times more cases of EC among Black women vs White women owing to the greater prevalence of fibroids and nonendometrioid histologic type in Black women.
This cohort study of a simulated population of Black and White women with postmenopausal bleeding examines the level and accuracy of transvaginal ultrasonography used to identify endometrial thickness indicating the need for biopsy.
Introduction
With an estimated 61 880 newly diagnosed cases and 12 160 cancer deaths in 2019, endometrial cancer (EC) is the fourth most common cancer in the US and is increasing in incidence each year.1 In the US, Black women with EC have a 90% higher 5-year mortality after diagnosis, compared with White women in the US.2 The magnitude of the racial inequity in survival is larger than that within cervical, breast, or colon cancers2 and is increasing.3,4 Two independent factors in excess death among Black women are the higher likelihood of advanced stage at diagnosis and the greater prevalence of high-risk EC (nonendometrioid histologic, or type 2) among Black women compared with White women.5,6 Black women in the US are diagnosed with more-advanced stages of disease independently of insurance coverage and health care access.5,6,7
Postmenopausal bleeding (PMB) (ie, vaginal bleeding after the onset of menopause) occurs in approximately 90% of women diagnosed with EC, making this symptom a sensitive indicator to prompt diagnostic testing.7,8,9,10,11,12 Currently, the American College of Obstetrics and Gynecology (ACOG) recommends 1 of 3 strategies in the event an individual presents with postmenopausal bleeding: endometrial biopsy, uterine dilation and curettage, or use of transvaginal ultrasonography (TVUS); TVUS is the only noninvasive choice in the current algorithm. Transvaginal ultrasonography is used to measure endometrial thickness that must meet a certain threshold (4 mm) to prompt one of the invasive tests (biopsy or dilation and curettage). In published guidelines and earlier studies, arguments are made for using TVUS to avoid unnecessary invasive procedures among women with PMB, with reported 99% to 100% negative predictive values of this strategy.8,12
The TVUS strategy using the ET threshold to prompt biopsy is based on large population-based studies from Scandinavia,13,14 Italy,15 and Hong Kong.16 The findings of these studies were recently supported in a systematic review and meta-analysis including more than 24 countries.12 These data, however, may be suboptimal sources from which to base guidelines for US Black women. The presence of uterine fibroids can distort the endometrial cavity, resulting in poor visualization of the endometrium—a key component of the TVUS strategy.17 Nonendometrioid cancers can be focal lesions that are less likely to cause the global hypertrophy of the endometrium that is captured in the ET measurement.18,19 Given the high prevalence of both fibroids20 and nonendometrioid histologic type3,4 among US Black women20 in comparison with White women, we hypothesized that current TVUS screening guidelines for PMB may have worse performance for Black women contributing to ongoing racial inequity in EC outcomes. In light of the consistent pattern of Black women with EC diagnosed at an advanced stage, despite insurance and health care access,6 we sought to compare the performance of ACOG–recommended TVUS ET thresholds for biopsy by race in a simulated cohort of symptomatic women.
Methods
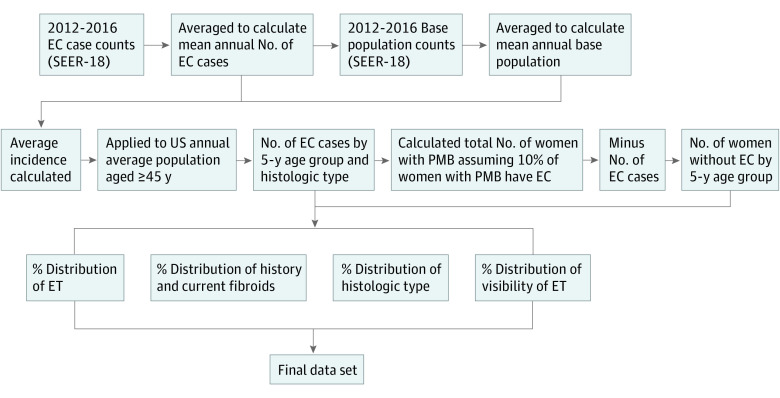
We constructed a simulated cohort of postmenopausal White and Black women aged 45 years and older who were experiencing PMB. We used the definitions of Black and White race as social constructs21 with substantial health consequences secondary to differential exposure to interpersonal, institutional, structural, and internalized racism across generations,22 with self-reported race as the most accurate measurement of group belonging. We defined the parameters of the simulation using national cancer registry data, population-based cohorts of women with PMB, and published TVUS measurements of ET among women with and without EC from several retrospective cohort studies.8,13,14,15,17,20,23,24 Our steps for cohort creation mirror those from a prior publication25 and are reproduced here. Using EC case counts from 2012 to 2016 in the Surveillance, Epidemiology, and End Results (SEER) National Cancer Registry (SEER-18 in SEER*Stat), we estimated the mean number of EC cases in a given year.23 Using the SEER base population, we then calculated average age–based incidence rates among Black and White women over the same time.22 The total number of incident cases in the US was then calculated by multiplying by this incidence rate × corresponding US Black and White female population × age from the US Census.26 We used the stable estimate that 10% of women with PMB have EC7,8,9,10,27 to calculate the total number of symptomatic Black and White women in a given year and categorized them into 5-year age groups from ages 45 to 85 years and older (Table 1). This stepwise process was conducted for overall EC counts and by histologic type as reported in SEER (Figure 1). Histologic type was classified as endometroid (type 1) and nonendometrioid (type 2: serous, carcinosarcoma, and clear cell) types using International Classification of Diseases for Oncology, 3rd Edition codes (eTable 1 in the Supplement).26 According to the self-determination policies of the University of Washington Human Subjects Division, this study did not meet the criteria for human research and was exempt from institutional review board approval. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Table 1. Parameters for Cohort Simulation of White and Black Women in the US With Postmenopausal Bleeding.
| Parameter | Value | Source | |
|---|---|---|---|
| Black women | White women | ||
| Average US annual population (2012-2016), No. | 8 213 830 | 49 299 503 | US Census Bureau, 201726 |
| Average annual No. of women with EC overall (2012-2016), No. | 4462 | 32 246 | National Cancer Institute, 201923 |
| Endometrioid (type 1) | 2848 | 27 926 | |
| Nonendometrioid (type 2) | 1614 | 4320 | |
| Women with EC with PMB, % | 90 | 90 | ACOG, 20188; Goldstein et al, 200110; Clarke et al, 201811; Musonda et al, 201127 |
| ET distribution for EC type 1, average % | |||
| <3 mm | 0.6 | 0.7 | ACOG, 20188; Gull et al, 200313; Karlsson et al, 199514; Ferrazzi et al, 199615; Wong et al, 201616; Phillip et al, 200424 |
| 3 mm | 5.4 | 0.5 | |
| 4 mm | 5.8 | 1.0 | |
| 5 mm | 5.6 | 2.4 | |
| >5 mm | 82.6 | 95.4 | |
| ET distribution for EC type 2, average % | |||
| <3 mm | 9.2 | 9.2 | Wang et al, 200618; Billingsley et al, 201519 |
| 3 mm | 9.2 | 9.2 | |
| 4 mm | 9.2 | 9.2 | |
| 5 mm | 19.8 | 19.8 | |
| >5 mm | 52.6 | 52.6 | |
| ET distribution for women without EC, average % | |||
| <3 mm | 15.5 | 16.8 | ACOG, 20188; Gull et al, 200313; Karlsson et al, 199514; Ferrazzi et al, 199615; Wong et al, 201616; Phillip et al, 200424 |
| 3 mm | 18.5 | 19.0 | |
| 4 mm | 17.4 | 17.7 | |
| 5 mm | 10.5 | 8.5 | |
| >5 mm | 38.1 | 38.1 | |
| % Women | |||
| With history of fibroids | 74.0 | 42.0 | Baird et al, 200320 |
| With current fibroids with fibroid history | 87.0 | 78.0 | Baird et al, 200320 |
| With current fibroids without fibroid history | 60.0 | 43.0 | Baird et al, 200320 |
| % Of endometria visible | |||
| Without fibroids present | 63.7 | 97.0 | ACOG, 20188; Gull et al, 200313; Karlsson et al, 199514; Ferrazzi et al, 199615; Wong et al, 201616; Rotenberg et al, 201717 |
| With fibroids present | 50.6 | 83.9 | ACOG, 20188; Gull et al, 200313; Karlsson et al, 199514; Ferrazzi et al, 199615; Wong et al, 201616; Rotenberg et al, 201717 |
Abbreviations: ACOG, American College of Obstetricians and Gynecologists; EC, endometrial cancer; ET, endometrial thickness; PMB, postmenopausal bleeding.
Figure 1. Schema of Stepwise Creation of Simulated Population Cohort of Symptomatic US Black and White Women.
This figure represents the order in which the parameters from Table 1 were applied to create the final population, using Surveillance, Epidemiology, and End Results (SEER-18) data, US Census data, and data from cited published studies. EC indicates endometrial cancer; ET, endometrial thickness; PMB, postmenopausal bleeding.
We used ET measurements from the 4 population-based studies on which the ACOG guidelines that recommend the use of TVUS were developed. Owing to the lack of representation or report on Black women in these studies (based in Scandinavia,13,14 Italy,15 and Hong Kong16), we added an additional article from our comprehensive literature search set in Jamaica.24 These data determined the value parameters and proportional distribution for TVUS ET measurements in categories of less than 3, 3, 4, 5, and greater than 5 mm among women overall with PMB with and without EC. Data on ET distribution by race were available for endometrioid (type 1) EC,8,13,14,15,16,24 with a greater proportion of Black women with thinner endometrial measurements on TVUS compared with White women. In contrast, the ET measurements for nonendometrioid (type 2) EC were the same for both groups given the lack of such data available specifically for Black women.18,19
The fibroid prevalence in the cohort was calculated in the following manner. We first estimated the proportion of women with a history of fibroids (Black women, 74%; White women, 42%) from the National Institute of Environmental Health Sciences uterine fibroid study.20 Based on the presence or absence of a history of fibroids, we could then calculate the proportion of women with current fibroids, using estimates from this same cohort. Next, we assigned EC or no EC based on population-level risk of EC diagnosis with and without a history of fibroids and, within the EC cases, the known distribution of type 1 vs type 2 EC by race.28 In addition, the visibility of the endometrium (required to have an accurate measurement) on TVUS was assigned based on fibroid history given the potential of fibroids to distort the endometrium, thereby causing an inability to visualize and measure the thickness. Endometrial visibility was assigned as visible or not visible based on published estimations of the proportion of visible endometrium during TVUS measurement of women with and without fibroids.13,14,15,17 For Black women, we used ET visibility estimates that were reported directly by the presence or absence of fibroids in a study of 440 women of whom 85% were Black race or Hispanic ethnicity.17 For White women, the proportion of nonvisible ET in women without fibroids was averaged from 3 studies13,14,15 of the 4 population-based investigations that reported this parameter, and we then used the same decrement of visibility (13.1%) in the presence of fibroids to estimate visibility in the setting of fibroids. An inclusive list of parameters used to generate the simulated data set is detailed in Table 1 and a subset of the calculated population inputs is reported in Table 2. The simulated cohort was constructed from February 2, 2020 (date of access to SEER data), to August 31, 2020. Analysis occurred from September 30, 2020, to March 30, 2021, including the primary analysis and the sensitivity calculations.
Table 2. Subset of Calculated Population Inputs for Simulated Cohort of US Black and White Women With Postmenopausal Bleeding, EC, and Fibroids.
| Variable | Women, No. (%) | ||
|---|---|---|---|
| Total | Black | White | |
| Total population with postmenopausal bleeding | 367 073 | 44 611 (12.2) | 322 462 (87.8) |
| Total population with current fibroids | 221 741 | 35 680 (15.9) | 186 061 (82.7) |
| EC population with current fibroids | 18 161 | 3643 (20.0) | 14 518 (79.9) |
| Type 1 (endometrioid) | 14 777 | 2272 (15.3) | 12 505 (84.6) |
| Type 2 (nonendometrioid) | 3384 | 1371 (40.5) | 2013 (59.4) |
| ET visible | |||
| Among non-EC with current fibroids | 160 151 | 16 210 (10.1) | 143 941 (89.9) |
| Among EC with current fibroids | 14 023 | 1841 (13.1) | 12 182 (86.8) |
Abbreviations: EC, endometrial cancer; ET, endometrial thickness.
Statistical Analysis
We calculated the sensitivity, specificity, positive predictive value, negative predictive value, receiver operating characteristic curves, and area under the curve (AUC) of the theoretical performance of endometrial thickness measurement thresholds of greater than or equal to 3, greater than or equal to 4, and greater than or equal to 5 mm in prompting diagnostic workup among Black and White women with EC, using Stata, version 15.1.29 In addition, we compared the racial difference in performance at each ET measurement threshold level. For sensitivity analyses, we first examined TVUS performance within age subgroups of those younger than 50 years (age 45-49 years) compared with those 50 years and older, as well as those younger than 60 years (age 45-59 years) compared with those 60 years and older. We then considered that not all fibroids would have the same potential to distort the endometrial cavity, with submucosal location (close to the endometrium) being most likely to do so. Using estimates of the proportion of fibroids that have submucosal location by race (Black women: 63.6%; White women: 43.6%),30 we did additional test calculations assuming only submucosal fibroids would have the potential to affect ET measurement and again compared TVUS performance by race. In addition, considering the influence of ET visibility parameters on the results and the potential overestimate of 97% ET visibility for US White women, we examined performance varying this parameter down to 83%. Findings are reported as 95% CIs for each parameter
Results
Using the average annual US population of White and Black women, our simulated cohort comprised 367 073 theoretical women with PMB, among whom 36 708 had EC (Table 1). The distribution of endometrioid (type 1) and nonendometrioid (type 2) histologic characteristics by race is reported in Table 1, with expected 86.6% (n = 27 926) of White women and 63.8% (n = 2848) of Black women with type 1 (endometrioid) histologic types.
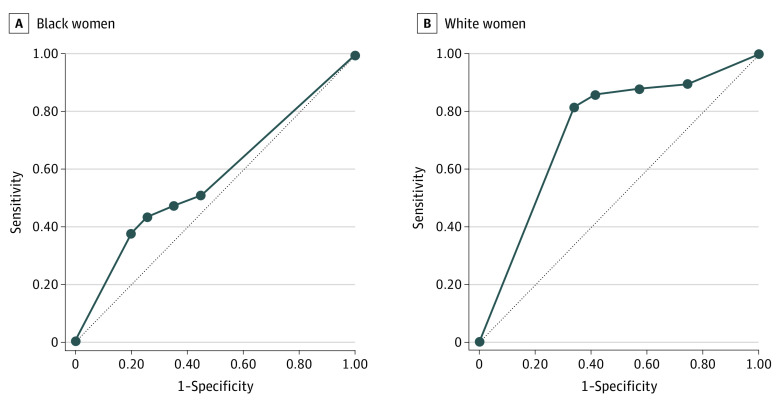
Among all Black women, the ACOG–recommended 4-mm or more threshold resulted in a sensitivity of 47.5% (95% CI, 46.0%-49.0%); of women referred for biopsy, 13.1% were EC cases: positive predictive value, 13.1% (95% CI, 12.5%-13.6%). The AUC for the 4 mm or more threshold among the simulated Black women was 0.57 (95% CI, 0.56-0.57) (Table 3). In contrast, among White women, the 4-mm or more threshold led to biopsy for most with EC (sensitivity, 87.9%; 95% CI, 87.6%-88.3%). Of those referred for biopsy, 14.6% had EC (positive predictive value, 14.6%; 95% CI, 14.4%-14.7%); AUC was 0.73 (95% CI, 0.73-0.74) (Table 3). The receiver operating characteristic curves for Black and White women are displayed in Figure 2.
Table 3. Performance of TVUS ET Thresholds for Identifying Endometrial Cancer Among a Simulated Cohort of US Black and White Women With Postmenopausal Bleeding.
| Threshold, mm | % (95% CI) | AUC (95% CI) | |||
|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | ||
| Black women | |||||
| ≥3 | 51.1 (49.6-52.6) | 55.0 (54.5-55.5) | 11.2 (10.8-11.6) | 91.0 (90.6-91.4) | 0.57 (0.56-0.57) |
| ≥4 | 47.5 (46.0-49.0) | 64.9 (64.4-65.3) | 13.1 (12.5-13.6) | 91.7 (91.4-92.1) | |
| ≥5 | 43.7 (42.3-45.2) | 74.1 (73.7-74.5) | 16 (15.2-16.5) | 92.2 (91.9-92.5) | |
| White women | |||||
| ≥3 | 89.5 (89.1-89.8) | 25.7 (25.6-25.9) | 11.8 (11.7-11.9) | 95.6 (95.5-95.8) | 0.73 (0.73-0.74) |
| ≥4 | 87.9 (87.6-88.3) | 42.7 (42.5-42.9) | 14.6 (14.4-14.7) | 97.0 (96.9-97.0) | |
| ≥5 | 86.0 (85.6-86.4) | 58.5 (58.3-58.7) | 18.7 (18.5-18.9) | 97.4 (97.3-97.5) | |
Abbreviations: AUC, area under the curve; ET, endometrial thickness; NPV, negative predictive value; PPV, positive predictive value; TVUS, transvaginal ultrasound.
Figure 2. Receiver operating characteristic Curves for Performance of Transvaginal Ultrasonography (TVUS) Endometrial Thickness (ET) Thresholds for Identifying Endometrial Cancer Among a Simulated Cohort of US Black and White Women With Postmenopausal Bleeding.
Sensitivity and specificity in Black (area under the receiver operating characteristic [ROC] curve, 0.57) (A) and White (ROC, 0.73) (B) women. The thickened line represents test performance by area under the curve (AUC) in which the further distance from the thinner reference line (AUC, 0.5, no discriminatory power) demonstrates greater discriminatory power of a test. An AUC greater than 0.7 is considered the threshold for fair performance. With regard to the use of TVUS to measure ET among women with postmenopausal bleeding, the AUC is greater for White women compared with Black women, and the AUC for Black women does not meet the threshold of fair performance.
Overall, for both Black and White women, when decreasing the endometrial thickness threshold (ie, moving from the ≥5 mm to the ≥3 mm cutoff), sensitivity increased to detect EC cases and negative predictive value decreased. Although the direction of this association in performance was the same among Black and White women, the magnitude of change differed. The 3-mm or more threshold resulted in a sensitivity of 51.1% (95% CI, 49.6%-52.6%) at best among Black women, compared with a sensitivity of 89.5% (95% CI, 89.1%-89.8%) at best among White women. Similarly, the negative predictive value was 92.2% (95% CI, 91.9%-92.5%) at best (≥5 mm) among Black women compared with 97.4% (95% CI, 97.3%-97.5%) at best among White women. Based on the scenarios examined herein, there was none in which the TVUS ET strategy performed equally as well among Black women as it did among White women.
When examining age subgroups of women aged 45 to 49, 45 to 59, and 60 years and older, there was minimal change in diagnostic performance, with an AUC range of 0.73 to 0.75 for White women and 0.57 to 0.58 for Black women. With incorporation of fibroid location to assume only submucosal fibroids had the potential to distort the ET measurement, the performance for Black women improved to a sensitivity of 60.9%, negative predictive value of 92.7%, and AUC of 0.60 at the 4 mm or more threshold, compared with a sensitivity of 91.9%, negative predictive value of 97.8%, and AUC of 0.75 among White women (eTable 2 in the Supplement). Although test parameters improved for both groups, the racial disparity remained. In addition, when we used a lower ET visibility estimate (83%) for White women, the AUC declined expectedly to 0.68. Compared with the same parameters used for Black women in the sensitivity analyses of a prior publication (AUC = 0.61),25 the racial disparity persisted, albeit narrowed.
Discussion
In this simulated cohort based on population-based data, we found inequity in the performance of TVUS as a screening tool for EC diagnosis among US women. Over 4 times more cases among Black women with EC were missed compared with those among White women based on the difference in performance at the guideline-recommended 4 mm or more threshold. The racial difference in AUC for this diagnostic strategy reflects this inequity, with a fair performance (AUC 0.73) for White women compared with very poor discriminatory performance (AUC, 0.57) for Black women, implying that TVUS does not meaningfully predict risk of EC for Black women. In our simulation model, this discrepancy in performance is based on the difference in prevalence of both nonendometrioid (type 2) histology types and uterine fibroids among Black women,20 both of which are higher than among White women.20 These data suggest that the current clinical guidelines that allow for TVUS as a primary evaluation for endometrial cancer may disproportionately underperform for Black women in the US and represent an example of structural racial inequity in care.
In the context of the larger problem of excess mortality among Black women with EC, these findings have important implications. The 2 largest known factors in the racial disparity in endometrial cancer are stage at diagnosis and histologic type.5,31,32 Stage at diagnosis has multiple influences, from the onset of symptoms to the point of confirmed diagnosis.33 Black women, within both types of EC, consistently are found to have a more advanced stage of EC at diagnosis on average than White women. In addition to other factors that may contribute to delay in diagnosis, this study suggests that even when Black women would present with symptoms and have appropriate diagnostic procedures ordered—in this case TVUS—the clinical algorithm is more likely to fail among Black women. If the ET is not visualized, the appropriate next step according to the guidelines is endometrial sampling via biopsy or dilation and curettage8; however, it is unknown with what fidelity this occurs, especially in the setting of a TVUS finding of fibroids, another common cause of abnormal vaginal bleeding.
Earlier work noted that TVUS as a strategy to screen and avoid unnecessary endometrial biopsy is based on a high likelihood of ET visibility and low-risk consequences if the test is wrong: delay in diagnosis of a relatively low-risk cancer.25 When considering the overall US population, low-risk type 1 EC predominates and is known to have an indolent course. For Black women, however, nearly a third have aggressive type 2 EC and more than 50% have high-risk EC (including high-grade type 1) at the time of diagnosis.2,6 In addition, among both Black and White women, the number with nonendometrioid (type 2) histologic types is increasing.4 Based on earlier work18,19,34 and this study, we question whether the use of an ET measurement threshold is a realistic goal for these histologic types. It is therefore important to consider whether a strategy that may be least effective for the most aggressive histologic type, which is increasing in prevalence, is still ideal.
In this simulated analysis, at most, there was a 5-fold difference in performance at the population level (sensitivity) and 2-fold difference at the individual level (negative predictive value) by race. Our findings should be considered with other recent work questioning the current TVUS strategy for EC risk screening. In a cross-sectional study of 1163 women older than 45 years presenting with PMB, Clarke et al34 found that ET measurement from TVUS did not provide meaningful risk stratification for endometrial intraepithelial neoplasia (a precursor lesion) or EC for any woman older than 60 years. In contrast we noted apparent utility in TVUS for White women older than 60 years with an AUC greater than 0.7; however, our analysis did not include precursor lesions, which may have altered the assessment of test performance. The study of Clarke et al34 included few Black women and did not report on the prevalence or outcome of fibroids or time since symptom onset. These factors and others are important to include in any efforts at developing a risk-based approach to diagnostic algorithms so as to not reify the racial inequity in clinical strategy that we report in this analysis.
Limitations
Our study is limited in its design as a simulated cohort, by necessity. Several parameters, including history of fibroids, concurrent presence of fibroids, and visibility of ET among Black women were based on few studies and might not be generalizable to a larger population. We strove to account for the uncertainty in these reference data by varying the most notable parameters in our sensitivity analyses. For US White women, we noted that our calculated sensitivity and negative predictive value differ from prior published meta-analyses, up to 10% for sensitivity and 5% for negative predictive value. We believe this variance is the result of our exclusion of nonspecific histologic types, which lowered the overall number of cases in our simulated cohort, including women with fibroids who were excluded from several of the population-based studies, and including a lack of ET visibility as a missed result to assess real-world performance. Likelihood ratios instead of predictive values are often useful in the clinical setting because they are not dependent on disease prevalence within a population.35 However, because we simulated the entire US population of Black and White women with PMB with the goal of assessing the diagnostic performance of TVUS in this population, we chose to report the more easily interpretable predictive values. If cases of EC are underreported in SEER-18 or are not reflective of other states or regions not captured in SEER-18, our predictive values may be biased.
The focus of this analysis was on Black women given their uniquely poor outcomes after EC diagnosis compared with all other racial and ethnic groups in the US.4 Given this design, we are unable to comment on how TVUS guidelines may affect other racial and ethnic minority groups, such as Asian, Hispanic, Native American, and Other Pacific Islander women.
Conclusions
Race-based health inequities are often the result of interlocking and overlapping influences of structural racism that combine to affect overall outcomes.36 In the case of EC, the long-standing mortality gap between Black and White individuals mandates that each step along the cancer care continuum be reevaluated with respect to specific performance among Black women, with the goal of racially equitable performance. In this study, the results suggest that the current option of TVUS to determine the appropriateness of endometrial biopsy is neither sufficiently accurate nor racially equitable. There is growing evidence from this simulated study and others that this approach does not meaningfully stratify by risk. However, we are cautious to recommend tissue sampling as the only strategy for Black women in all settings. First, community input is required to foster a collaborative and respectful approach to intervention to ensure feasibility and acceptability among historically mistreated groups. Second, additional studies designed to identify the potential for other important factors that may affect TVUS that may be racially patterned but are not exclusive to Black women should be undertaken and are underway. This work can then inform a risk-based approach in which all people presenting with PMB can be assessed for such factors and triaged appropriately for expedited biopsy.
eTable 1. List of ICD-O-3 Codes Included to Calculate Endometrial Cancer Case Counts
eTable 2. Performance of TVUS ET Thresholds for Identifying Endometrial Cancer Among a Simulated Cohort of US Black and White Women With Postmenopausal Bleeding: Sensitivity Analysis by Submucosal Fibroid Prevalence
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211-233. doi: 10.3322/caac.21555 [DOI] [PubMed] [Google Scholar]
- 3.Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R. The growing burden of endometrial cancer: a major racial disparity affecting Black women. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1407-1415. doi: 10.1158/1055-9965.EPI-15-0316 [DOI] [PubMed] [Google Scholar]
- 4.Clarke MA, Devesa SS, Harvey SV, Wentzensen N. Hysterectomy-corrected uterine corpus cancer incidence trends and differences in relative survival reveal racial disparities and rising rates of nonendometrioid cancers. J Clin Oncol. 2019;37(22):1895-1908. doi: 10.1200/JCO.19.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doll KM, Snyder CR, Ford CL. Endometrial cancer disparities: a race-conscious critique of the literature. Am J Obstet Gynecol. 2018;218(5):474-482.e2. doi: 10.1016/j.ajog.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 6.Rauh-Hain JA, Buskwofie A, Clemmer J, Boruta DM, Schorge JO, Del Carmen MG. Racial disparities in treatment of high-grade endometrial cancer in the Medicare population. Obstet Gynecol. 2015;125(4):843-851. doi: 10.1097/AOG.0000000000000605 [DOI] [PubMed] [Google Scholar]
- 7.Doll KM, Khor S, Odem-Davis K, et al. Role of bleeding recognition and evaluation in Black-White disparities in endometrial cancer. Am J Obstet Gynecol. 2018;219(6):593.e1-593.e14. doi: 10.1016/j.ajog.2018.09.040 [DOI] [PubMed] [Google Scholar]
- 8.The American College of Obstetricians and Gynecologists . ACOG Committee Opinion 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131(5):e124-e129. doi: 10.1097/AOG.0000000000002631 [DOI] [PubMed] [Google Scholar]
- 9.Coates RJ, Click LA, Harlan LC, et al. Differences between Black and White patients with cancer of the uterine corpus in interval from symptom recognition to initial medical consultation (United States). Cancer Causes Control. 1996;7(3):328-336. doi: 10.1007/BF00052938 [DOI] [PubMed] [Google Scholar]
- 10.Goldstein RB, Bree RL, Benson CB, et al. Evaluation of the woman with postmenopausal bleeding: Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. J Ultrasound Med. 2001;20(10):1025-1036. doi: 10.7863/jum.2001.20.10.1025 [DOI] [PubMed] [Google Scholar]
- 11.Clarke MA, Long BJ, Del Mar Morillo A, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(9):1210-1222. doi: 10.1001/jamainternmed.2018.2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long B, Clarke MA, Morillo ADM, Wentzensen N, Bakkum-Gamez JN. Ultrasound detection of endometrial cancer in women with postmenopausal bleeding: systematic review and meta-analysis. Gynecol Oncol. 2020;157(3):624-633. doi: 10.1016/j.ygyno.2020.01.032 [DOI] [PubMed] [Google Scholar]
- 13.Gull B, Karlsson B, Milsom I, Granberg S. Can ultrasound replace dilation and curettage? a longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol. 2003;188(2):401-408. doi: 10.1067/mob.2003.154 [DOI] [PubMed] [Google Scholar]
- 14.Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding—a Nordic multicenter study. Am J Obstet Gynecol. 1995;172(5):1488-1494. doi: 10.1016/0002-9378(95)90483-2 [DOI] [PubMed] [Google Scholar]
- 15.Ferrazzi E, Torri V, Trio D, Zannoni E, Filiberto S, Dordoni D. Sonographic endometrial thickness: a useful test to predict atrophy in patients with postmenopausal bleeding: an Italian multicenter study. Ultrasound Obstet Gynecol. 1996;7(5):315-321. doi: 10.1046/j.1469-0705.1996.07050315.x [DOI] [PubMed] [Google Scholar]
- 16.Wong AS, Lao TT, Cheung CW, et al. Reappraisal of endometrial thickness for the detection of endometrial cancer in postmenopausal bleeding: a retrospective cohort study. BJOG. 2016;123(3):439-446. doi: 10.1111/1471-0528.13342 [DOI] [PubMed] [Google Scholar]
- 17.Rotenberg O, Escobar P, Fridman D, Dar P. Factors associated with inconsistency in visibility of the endometrial echo on transvaginal ultrasound of postmenopausal women [ISUOG Education abstract P14.10]. Ultrasound Obstet Gynecol. 2017;50(suppl 1):199-200. doi: 10.1002/uog.18140 [DOI] [Google Scholar]
- 18.Wang J, Wieslander C, Hansen G, Cass I, Vasilev S, Holschneider CH. Thin endometrial echo complex on ultrasound does not reliably exclude type 2 endometrial cancers. Gynecol Oncol. 2006;101(1):120-125. doi: 10.1016/j.ygyno.2005.09.042 [DOI] [PubMed] [Google Scholar]
- 19.Billingsley CC, Kenne KA, Cansino CD, et al. The use of transvaginal ultrasound in type II endometrial cancer. Int J Gynecol Cancer. 2015;25(5):858-862. doi: 10.1097/IGC.0000000000000423 [DOI] [PubMed] [Google Scholar]
- 20.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in Black and White women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100-107. doi: 10.1067/mob.2003.99 [DOI] [PubMed] [Google Scholar]
- 21.Harawa NT, Ford CL. The foundation of modern racial categories and implications for research on Black/White disparities in health. Ethn Dis. 2009;19(2):209-217. [PubMed] [Google Scholar]
- 22.Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212-1215. doi: 10.2105/AJPH.90.8.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Institute Surveillance Epidemiology and End Results (SEER) Program . SEER*Stat Database: Incidence—SEER 18 Regs Research Data + Hurricane Katrina impacted Louisiana Cases, November 2018: 1975-2016 varying sub-linked to county attributes—total US, 1969-2017 counties. Released April 2019. Accessed x. http://www.seer.cancer.gov.
- 24.Phillip H, Dacosta V, Fletcher H, Kulkarni S, Reid M. Correlation between transvaginal ultrasound measured endometrial thickness and histopathological findings in Afro-Caribbean Jamaican women with postmenopausal bleeding. J Obstet Gynaecol. 2004;24(5):568-572. doi: 10.1080/01443610410001722671 [DOI] [PubMed] [Google Scholar]
- 25.Romano SS, Doll KM. The Impact Of Fibroids And Histologic Subtype On The Performance of US clinical guidelines for the diagnosis of endometrial cancer among Black women. Ethn Dis. 2020;30(4):543-552. doi: 10.18865/ed.30.4.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United States Census Bureau Population Division . Annual estimates of the resident population by sex, age, race, and Hispanic origin for the United States and states: April 1, 2010 to July 1, 2017. US Census Bureau. Accessed February 24, 2020. https://data.census.gov/cedsci/
- 27.Musonda P, Burbos N, Duncan TJ, Crocker SG, Morris EP, Nieto JJ. Comparing the performance of two clinical models in estimating the risk of endometrial cancer in symptomatic postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):433-438. doi: 10.1016/j.ejogrb.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 28.Wise LA, Sponholtz TR, Rosenberg L, et al. History of uterine leiomyoma and risk of endometrial cancer in Black women. Cancer Causes Control. 2016;27(4):545-552. doi: 10.1007/s10552-016-0728-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stata Statistical Software . Version 15.1. StataCorp; 2017. [Google Scholar]
- 30.Moorman PG, Leppert P, Myers ER, Wang F. Comparison of characteristics of fibroids in African American and white women undergoing premenopausal hysterectomy. Fertil Steril. 2013;99(3):768-776.e1. doi: 10.1016/j.fertnstert.2012.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smotkin D, Nevadunsky NS, Harris K, Einstein MH, Yu Y, Goldberg GL. Histopathologic differences account for racial disparity in uterine cancer survival. Gynecol Oncol. 2012;127(3):616-619. doi: 10.1016/j.ygyno.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong K, Randall TC, Polsky D, Moye E, Silber JH. Racial differences in surgeons and hospitals for endometrial cancer treatment. Med Care. 2011;49(2):207-214. doi: 10.1097/MLR.0b013e3182019123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen BL, Cacioppo JT. Delay in seeking a cancer diagnosis: delay stages and psychophysiological comparison processes. Br J Soc Psychol. 1995;34(Pt 1):33-52. doi: 10.1111/j.2044-8309.1995.tb01047.x [DOI] [PubMed] [Google Scholar]
- 34.Clarke MA, Long BJ, Sherman ME, et al. Risk assessment of endometrial cancer and endometrial intraepithelial neoplasia in women with abnormal bleeding and implications for clinical management algorithms. Am J Obstet Gynecol. 2020;223(4):549.e1-549.e13. doi: 10.1016/j.ajog.2020.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dujardin B, Van den Ende J, Van Gompel A, Unger JP, Van der Stuyft P. Likelihood ratios: a real improvement for clinical decision making? Eur J Epidemiol. 1994;10(1):29-36. doi: 10.1007/BF01717448 [DOI] [PubMed] [Google Scholar]
- 36.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453-1463. doi: 10.1016/S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. List of ICD-O-3 Codes Included to Calculate Endometrial Cancer Case Counts
eTable 2. Performance of TVUS ET Thresholds for Identifying Endometrial Cancer Among a Simulated Cohort of US Black and White Women With Postmenopausal Bleeding: Sensitivity Analysis by Submucosal Fibroid Prevalence