Abstract
Objectives.
The goal of the study was to determine whether reactive oxygen species (ROS) mediates cytomegalovirus (CMV)–induced labyrinthitis.
Study Design.
Murine model of CMV infection.
Setting.
University of Utah laboratory.
Subjects and Methods.
Nrf2 knockout mice were inoculated with murine CMV. Auditory brainstem response (ABR) and distortion product otoacoustic emissions (DPOAEs) were then performed on these and uninfected controls. BALB/c mice were inoculated with murine CMV to determine whether a marker for ROS production, dihydroethidium (DHE), is expressed 7 days after inoculation. Finally, 2 antioxidants—D-methionine and ACE-Mg (vitamins A, C, and E with magnesium)—were administered 1 hour before and after infection in inoculated mice for 14 days. Temporal bones were harvested at postnatal day 10 for DHE detection. ABR and DPOAE testing was done at postnatal day 30. Scanning electron microscopy was also performed at postnatal day 30 to evaluate outer hair cell integrity.
Results.
Nrf2-infected mice had worse hearing than uninfected mice (P < .001). A statistically significant increase in DHE fluorescence was detected in BALB/c-infected mice as compared with uninfected mice 7 days after inoculation. D-methionine- and ACE-Mg-treated mice demonstrated an attenuation of the DHE fluorescence and a significant improvement in ABR and DPOAE thresholds when compared with untreated infected controls (P <.0001). Scanning electron microscopy demonstrated less outer hair cell loss in the treated versus untreated infected controls.
Conclusion.
These results demonstrate for the first time that excessive ROS mediates CMV-induced hearing loss in a mouse model.
Keywords: cytomegalovirus, reactive oxygen species, auditory brainstem response, distortion product otoacoustic emissions, immunohistochemistry, D-methionine, ACE-Mg, outer hair Cells
Cytomegalovirus (CMV) infection is the most common congenital infection and nongenetic cause of sensorineural hearing loss (SNHL) in the United States.1–3 The mechanisms of injury leading to CMV-induced hearing loss have yet to be elucidated, and a better understanding of this mechanism may provide targets for novel therapy. A potential intervention target—excessive production of reactive oxygen species (ROS)—has been implicated in several types of hearing loss, including noise-induced hearing loss,4–7 oto-toxicity,4,8 and age-related hearing loss,9 but the role of ROS has not been extensively explored in CMV-induced SNHL.
Here we demonstrate that excessive ROS production contributes to CMV-induced hearing loss and that antioxidant treatment ameliorates hearing loss and outer ear hair cell damage in mice after CMV infection. These data suggest that antioxidant treatment may be an appropriate intervention for SNHL in patients with congenital CMV.
Materials and Methods
Viruses
Recombinant murine CMV (mCMV; strain K181 MC.55 [ie2– GFP+]) expressing green fluorescent protein (GFP) was used. Virus purification was carried out by Virapur (San Diego, California) as previously described.10
Animals
Inbred BALB/c and hybrid background Nrf2−/− mice were used for these experiments. Mice were housed and bred under specific-pathogen-free conditions under controlled temperature and humidity at the Central Animal Facility at the University of Utah. Nrf2-deficient (Nrf2−/−) mice were originally backcrossed with the C57/BL6 mice to the fifth generation as previously described.11 Genotype was verified by polymerase chain reaction amplification of genomic DNA with the following primers: Nrf2 forward, 5′-GCCTGAGAGCTGTAGGCCC-3′; Nrf2 reverse, 5′-GGAATGGAAAATAGCTCCTGCC-3′; and Nrf2 mutant, 5′-GGGTTTTCCCAGTCACGAC-3′. The University of Utah Institutional Animal Care and Use Committee approved all procedures in accordance with the standards established by the US Animal Welfare Act.
Viral Inoculation
Mice were injected via an intracerebral route with CMV at postnatal day 3 as previously described.10 Control animals (“uninfected”) received the same volume of normal saline. The injections were completed with a 10-μL Hamilton syringe with a 30-G needle.
Administration of D-methionine
D-methionine (D-met; Sigma-Aldrich, Inc, St Louis, Missouri) was dissolved in normal saline and delivered by intraperitoneal injection. Since doses of 400 mg/kg of D-met are effective in protecting against noise-induced hearing loss and cisplatin-induced auditory brainstem (ABR) threshold elevations, the same dose of D-met was administered 1 hour before and 1 hour after CMV inoculation.4 D-met administration was continued twice a day for 14 days.
Administration of Vitamins A, C, and E with Magnesium
A protocol for administration of vitamins A, C, and E with magnesium (ACE-Mg) was determined according to previous studies for noise-induced hearing loss.12 The compounds were administered separately according to their hydrophobic and hydrophilic properties. The hydrophilic vitamin C (200 mg/kg, L-ascorbic acid; Sigma-Aldrich) was prepared with magnesium sulfate (60 mg/kg; Sigma-Aldrich) dissolved in 0.9% sterile saline, filtered, and delivered by intraperitoneal injection with the 50-μL Hamilton syringe. The hydrophobic vitamins came in an oil suspension and were administered concurrently. Vitamin E (tocopherol; Sigma-Aldrich) was administered at 65 mg/kg. Vitamin A (retinol palmitate; Supelco/Sigma-Aldrich) was administered at a dose of 20 mg/kg. ACE-Mg was administered 1 hour before and 1 hour after CMV inoculation. To examine whether the administration of antioxidant is dependent on the duration and frequency of administration, infected BALB/c mice (2000 plaque-forming units [pfu]) were treated with ACE-Mg for 7 days daily and twice daily for 7 and 14 days. We found no difference with respect to hearing outcomes (Supplemental Figure S1, available online). However, for the data presented, we administered the agent twice a day for 14 days.
Hearing Assessment
To avoid potential confounding results due to immaturity of the auditory system in CMV-infected mice, we chose initial hearing tests beginning at 4 weeks of age. The methodology for our ABR and distortion product otoacoustic emissions (DPOAEs) was described previously.10
Dihydroethidium Staining Analysis of CMV-Induced Oxidative Damage in the Cochlea
Dihydroethidium (DHE) staining was used to assess the oxidative damage induced by CMV inoculation. Animals at 7 days postinoculation were deeply anesthetized and transcardially perfused with cold 100-U/L heparin in 1 × phosphate-buffered saline (PBS). The cochleae were rapidly removed, and the samples were then decalcified in EDTA (10%), incubated overnight in 5% sucrose, followed by 15% sucrose, and stored at 4°C until cryosectioned. The samples were embedded in porcine gelatin and cryosectioned at a thickness of 10 to 12 μm. The cochlear specimens were incubated with 5 μM DHE (Invitrogen) in PBS for 30 minutes at 37°C, washed twice with PBS, and then coverslipped. Fluorescent images of midmodiolar cochlear cryosections were acquired with a laser confocal microscope (model FV1000; Olympus, Tokyo, Japan). For immuno-fluorescence detection, a laser wavelength of 594 nm was used for excitation of Alexa Fluor 594, resulting in an emission of 618 nm. Cochlear cross sections from controls and CMV-infected mice were stained on the same day under the same condition to reduce variations. Fluorescent signals were subsequently taken with the same confocal imaging settings. Relative ROS production was expressed as the change in fluorescence of experimental groups as compared with the appropriate controls. Pixel intensity analysis was performed on the image from the center of the stack with ImageJ software (version 1.43u; National Institutes of Health, Bethesda, Maryland). The intensity of labeling of superoxide production with DHE was measured with an analysis of pixel intensity in 4 regions of interest: the organ of Corti, spiral ganglion, areas adjacent to the scala tympani, and the stria vascularis. Background pixel intensity was subtracted from these regions of interest. The mean pixel intensities were averaged for CMV-infected and D-met- and ACE-Mg-treated mice and presented as fold increase relative to the control group.
Scanning Electron Microscopy of Outer Hair Cell Damage
We previously described this procedure.10 Images were taken on a Hitachi S-4800 scanning electron microscope. Outer hair cells (OHCs) were counted from apical, middle, and basal cochlear turns from the same animals used for audiometric studies.
Statistical Analyses
A sample size of 8 pups per group would demonstrate a statistically significant difference with respect to ABR thresholds, assuming a power of 0.80, a P value <.05, a 15-dB difference in mean thresholds between the groups, and a standard deviation of 10 dB.13 A sample size of 5 pups per group would demonstrate a statistically significant difference with respect to number of GFP-infected cells per high-power field (40×), assuming a power of 0.80, a P value <.05, and 10-count difference in mean cells per high-power field. SPSS for Windows (version 19.0; IBM, Chicago, Illinois) and GraphPad Prism for Windows (version 6.00; GraphPad Software, La Jolla, California) were used for statistical analyses. Differences between groups were determined with nonparametric tests (Kruskal-Wallis or Wilcoxon rank sum tests) unless otherwise specified. Results were considered significant at P < .05.
A schematic of the experiments is shown in Figure 1.
Figure 1.
Schematic of experiments performed. ABR, auditory brainstem response; ACE-Mg, vitamins A, C, E with magnesium; DHE, dihydroethidium; D-met, D-methionine; DPOAE, distortion product otoacoustic emission; mCMV, murine cytomegalovirus.
Results
Nrf2-Deficient Mice Have Increased Susceptibility to CMV-Induced Hearing Loss
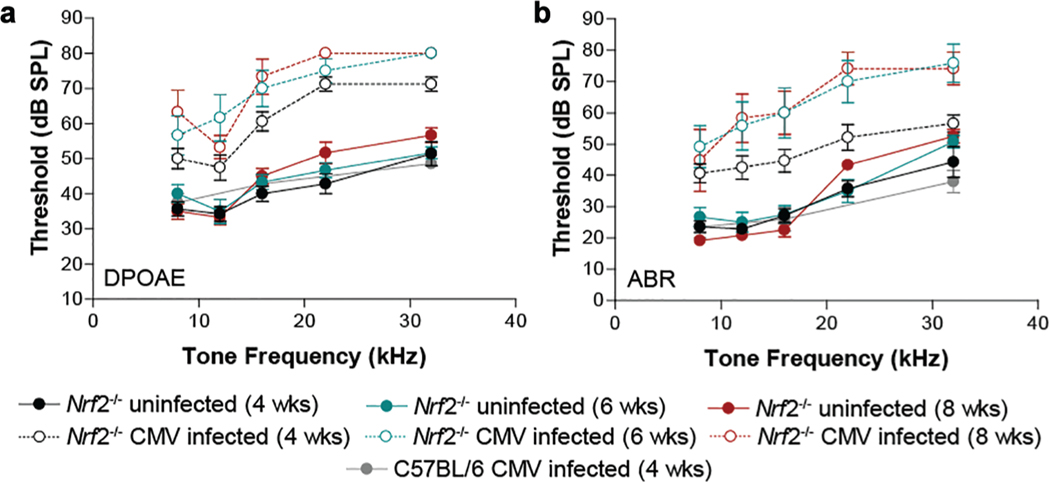
The Nrf2/Keap1 (Kelch-like ECH-associated protein 1) complex is a redox-sensing master regulator of the response to oxidative or electrophilic stresses,14 and stress-induced release of the Nrf2 transcription factor activates expression of several detoxifying and antioxidant genes.15 To address whether this antioxidant response plays a role in CMV-induced hearing loss, we tested hearing outcomes in Nrf2-deficient mice. Specifically, Nrf2−/− mice in C57BL/6 background were infected with 200 pfu of mCMV engineered to express GFP by intracerebral injection on postnatal day 3. Auditory function was assessed at 4, 6, and 8 weeks of age. Two standard electrophysiologic tests were used to monitor hearing performance: DPOAE and ABR. CMV-infected Nrf2−/− mice showed elevated thresholds as compared with uninfected Nrf2−/− mice at the 3 time points (P < .001) over the measured tone frequencies for DPOAE and ABR (Figure 2; Supplemental Table S1, online only). Notably, ABR and DPOAE thresholds for infected Nrf2−/− mice were also significantly elevated relative to infected wild-type C57BL/6 mice (Nrf2+/+). These data demonstrate that, in the absence of the Nrf2 transcription factor, an otherwise resistant C57BL/6 mouse strain10 was susceptible to CMV-induced hearing loss. This hearing loss was mild at 4 weeks of age and progressed to moderate to profound hearing loss by 6 and 8 weeks after CMV inoculation.
Figure 2.
Hearing assessment in Nrf2-deficient mice. (a) Distortion product otoacoustic emission (DPOAE) and (b) auditory brainstem response (ABR) at 4, 6, and 8 weeks of age (n = 8). Values are presented as mean ± SD. CMV, cytomegalovirus.
CMV Infection Increases Production of ROS in the Cochlea, Which Is Attenuated by Antioxidant Treatment
We examined whether CMV infection causes an increased oxidative stress in BALB/c mice, a strain that is susceptible to CMV-induced hearing loss.10 We have demonstrated that mCMV susceptibility is mediated, at least in part, by natural killer (NK) cells in that blockade of the Ly49H receptor or infection with an m157-deficient viral strain rendered C57BL/6 mice susceptible to mCMV hearing loss and cochlear damage. Additionally, NK cell colocalization with GFP-expressing mCMV-infected cells dramatically increased in the cochlea of the normally resistant C57BL/6 mouse strain after blockade of the Ly49H receptor, consistent with the requirement for physical interaction between NK cells and mCMV-infected cells for effective Ly49H engagement of m157.10 The presence of ROS was measured at 7 days postinfection with a superoxide-sensitive fluorescent probe (DHE) on unfixed cryosectioned cochlear specimens from control and CMV-infected mice (Figure 3). DHE is selectively oxidized by superoxide anion. Cytosolic DHE exhibits blue fluorescence; however, when this probe is oxidized to ethidium, it intercalates with DNA, staining the nucleus a bright red fluorescence. In the presence of superoxide (O2−.), the cell-permeable nonfluorescent DHE is oxidized to fluorescent 2-hydroxyethidium, which is then detected by using the excitation emission filters appropriate for rhodamine. Animals for this experiment were inoculated with 2000 pfu of mCMV on postnatal day 3. In contrast to uninfected control cochleae, CMV infection significantly increased the production of intracellular ROS in the spiral ganglion cells, osseous spiral lamina, stria vascularis, regions adjacent to the scala tympanic, with very limited DHE staining in the hair cells. Next, we assessed whether the administration of the antioxidant D-met or ACE-Mg would reduce the production of ROS as a result of CMV infection. The CMV-induced increase in DHE staining was attenuated by treatment with the antioxidants ACE-Mg and D-met. The intensity of ROS production with DHE was quantified via an analysis of pixel intensity in 4 regions of interest and presented as the fold induction of the uninfected control. The increase in DHE staining was significantly elevated (P < .01) in CMV-infected mice relative to the uninfected and antioxidant-treated groups. We also verified that CMV caused an active infection of cells within the mouse cochlea, leading to activation of the apoptotic cascade. Immunohistochemical analyses with GFP expressed in CMV-infected cells showed that these cochlear regions, where ROS production was increased, are the major sites for active CMV infection when tested at 7 days postinfection as compared with uninfected controls. Furthermore, these cochlear midmodiolar sections were also stained with anticleaved caspase-3 antibodies (red fluorescence) as a marker for apoptosis. Cleaved caspase signal appears to colocalize with cells routinely expressing GFP signal in the spiral ganglion and stria vascularis and in regions adjacent to the scala tympani (Supplemental Figure S2, online only). There was no evidence of apoptosis amongst the hair cells. Taken together, these data indicate that CMV infection induced increased production of ROS in various regions and tissues in the cochlea and that administration of D-met or ACE-Mg antioxidants reduced CMV-induced oxidative stress 7 days following inoculation.
Figure 3.
Dihydroethidium fluorescence in mouse cochlea. (a) Uninfected. (b) CMV infected. (c) ACE-Mg only. (d) CMV1D-met. (e) CMV1ACE-Mg. (f) Quantification of fluorescence intensity. Scale bars indicate 50 mm. ACE-Mg, vitamins A, C, E with magnesium; CMV, cytomegalovirus; D-met, D-methionine.
Antioxidant Treatment Mitigates Hearing Loss in CMV-Infected Mice
To determine if treatment with antioxidants protects against CMV-induced hearing loss, BALB/c mice were infected with 2000 pfu of CMV at postnatal day 3 and treated with D-met or ACE-Mg 1 hour before and 1 hour after CMV inoculation, and antioxidant supplementation was continued for 14 days. At postnatal day 30, these mice were subjected to hearing assessment by DPOAE (Figure 4a) and ABR (Figure 4b). CMV infection resulted in marked DPOAE and ABR threshold elevations in response to all tone frequencies, particularly at higher tone frequencies, relative to uninfected controls. Statistically significant differences were seen between uninfected and infected mice over the measured tone frequencies for DPOAE and ABR thresholds (P < .0001), with substantially higher threshold elevations at the highest tone frequencies. Administration of ACE-Mg or D-met significantly reduced DPOAE and ABR thresholds at the measured tone frequencies (P < .0001; Supplemental Table S2, online only) as compared with untreated CMV-infected mice. DPOAE and ABR thresholds of mice in both antioxidant-treated groups (CMV + D-met and CMV + ACE-Mg) were significantly higher (P < .0001) as compared with the uninfected and ACE-Mg-only control groups. These results suggest that the antioxidant treatments provided partial protection against DPOAE and ABR threshold shifts. DPOAE and ABR thresholds were not significantly different for the uninfected and ACE-Mg-only injected control groups. Furthermore, mouse mass was measured before the hearing tests (postnatal day 30), and antioxidant treatment also protected mice from CMV-induced weight loss (Supplemental Figure S3, online only).
Figure 4.
Effect of antioxidant treatment on hearing thresholds in CMV-infected mice: (a) DPOAE and (b) ABR tests. Values are presented as mean ± SD. ABR, auditory brainstem response; ACE-Mg, vitamins A, C, E with magnesium; CMV, cytomegalovirus; D-met, D-methionine; DPOAE, distortion product otoacoustic emission.
Antioxidant Treatment Attenuates CMV-Induced OHC Loss
DPOAEs reflect OHC function, and the elevated DPOAE thresholds indicate a pathologic change to the organ of Corti, particularly the OHCs. To assess the magnitude of OHC damage caused by CMV infection (2000 pfu) and whether treatment with antioxidants protects cochlear morphology, scanning electron microscopy was performed on cochlear whole mounts at postnatal day 30. Representative scanning electron microscopy micrographs corresponding to apical, middle, and basal cochlear turns from control, uninfected mice, CMV-infected, CMV + D-met–treated mice, and CMV + ACE-Mg–treated BALB/c mice are shown in Figure 5a, and the quantitative OHC survival in percentage is presented in Figure 5b. Cochlea from uninfected mice exhibited 3 well-defined rows of OHCs, whereas CMV-infected mice exhibited substantial OHC loss in all cochlear turns (P < .0001; Supplemental Table S3, online only). Treatment with antioxidants resulted in significantly less OHC loss relative to CMV-infected mice (P = .0003) but more loss for the D-met- and ACE-Mg-treated groups than uninfected controls (CMV + D-met, P = .0026; CMV + ACE-Mg, P = .0004). Similar results were obtained in mice infected with CMV at 200 pfu (Supplemental Figure S4, online only). These data indicate that antioxidant treatment resulted in significant but not complete protection from OHC loss after CMV infection.
Figure 5.
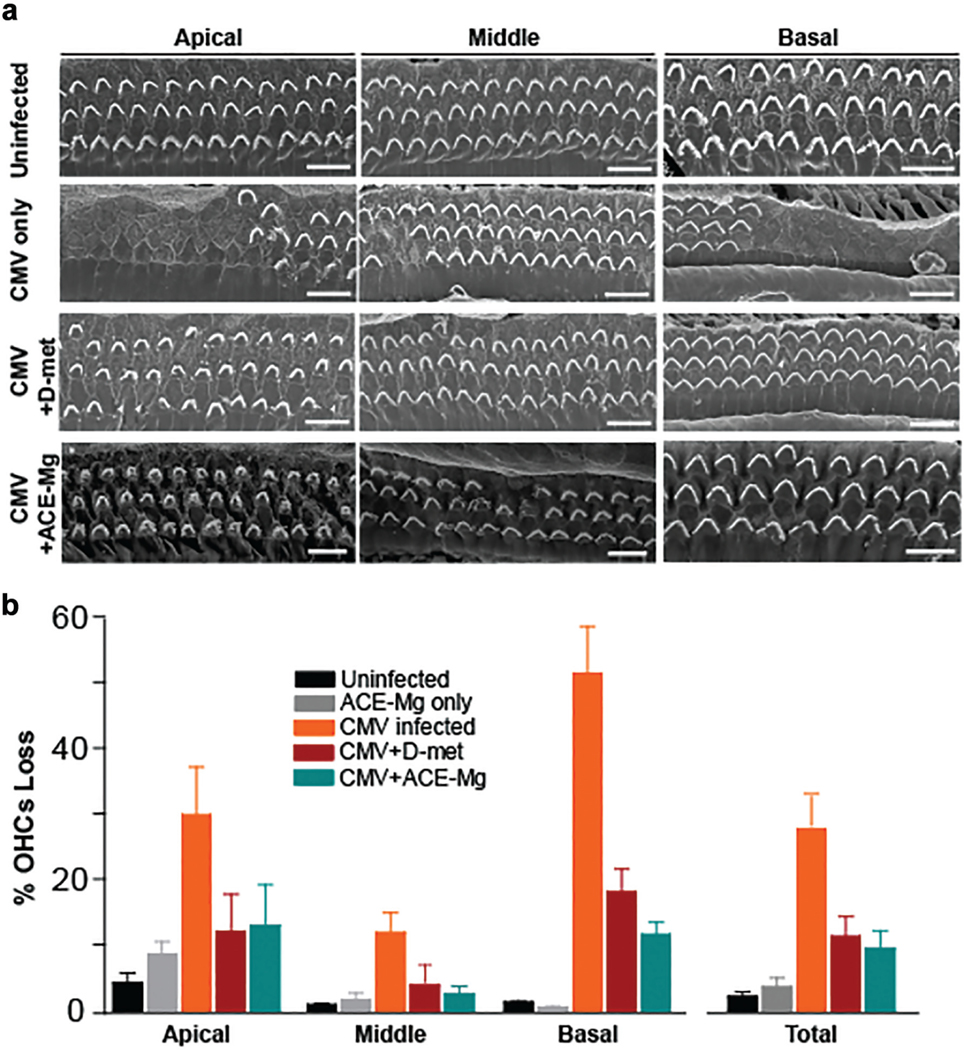
Outer hair cell counts (OHCs) in infected, uninfected, and antioxidant-treated mice: (a) scanning electron microscopy micrographs and (b) quantitative OHC survival in percentages. Scale bars indicate 10 μm. Values are presented as mean ± SD. ACE-Mg, vitamins A, C, E with magnesium; CMV, cytomegalovirus; D-met, D-methionine.
Discussion
Excessive ROS and Antioxidant Effects
This study demonstrates the role of oxidative stress in mCMV-induced labyrinthitis and the protective effect of antioxidants. In the absence of the Nrf2 transcription factor, a key regulator of the oxidative stress, an otherwise resistant C57BL/6 mouse strain10 was shown to be susceptible to CMV-induced hearing loss. Both tested antioxidant regimens mitigated DHE staining and CMV-mediated hearing loss and attenuated OHC loss. D-met, the optical isomer of the essential amino acid L-methionine, has direct and indirect effects as a free radical scavenger. Combinations of antioxidants demonstrated better outcomes as compared with single agents in a guinea pig model for noise-induced hearing loss and a murine model for hereditary hearing loss.10,16 Vitamin A scavenges singlet oxygen and prevents its interaction with lipids to form lipid hydroperoxides, while vitamin E is a donor antioxidant that reacts with and reduces peroxyl radicals, inhibiting the propagation cycle of lipid peroxidation.17 Vitamin C reduces free radicals in the aqueous phase and supports the antioxidant activity of vitamin E.18 Magnesium itself has antivasoconstrictive properties, and its administration with vitamins A, C, and E for noise-induced hearing loss showed a significant reduction in hearing loss and cell death in a guinea pig model.19 The hydrogen peroxide scavenger N-acetylcysteine reduced CMV replication in mice,20 suggesting that antioxidants function, at least in part, by reducing viral load.
ROS Mediates CMV-Induced Hearing Loss
ROS describes a number of reactive molecules and free radicals derived from molecular oxygen. These molecules are by-products of normal metabolism and are important signal transduction mediators, resulting in altered gene expression.21 They are also an important component of the host cell defense mechanism, especially for phagocytic cells. Uncontrolled excess production of ROS may produce collateral damage through oxidation of lipids, proteins, and DNA, eventually inducing apoptotic cell death. This oxidative stress has been shown to be a major factor in noise-induced, age-related, aminoglycoside-induced, and cisplatin-induced SNHL.6,22–24 Antioxidants have also been shown to be safe and effective in a variety of species and against several ototoxic insults.4 Our results are consistent with previous reports citing otoprotection against noise-induced25 and cisplatin-induced hearing loss.4
A recent study demonstrated increased ROS production in response to CMV infection in cultured mouse spiral ganglion neurons.26 Our DHE staining experiments demonstrated ROS production in spiral ganglion cells in the osseous spiral lamina and stria vascularis of CMV-infected mice. These findings indicate high oxidative stress in regions that we and others have established as primary targets for CMV infection in the mouse inner ear.10,26–29 These sites are also important targets for immune cells, which will migrate to these regions ostensibly to maintain cochlear homeostasis and function.30 For instance, macrophages have been found to reside in the spiral ligament, stria vascularis, spiral limbus, and neural tissues uniformly along the apex to base of the cochlea.31,32 Morphologic analysis had revealed micropores on the surface of the spiral ligament facing the scala tympani.33 These fenestrae may facilitate an interface between the spiral ligament leukocytes and perilymph. Macrophages in the stria vascularis may serve as a perivascular barrier around endothelial cells and participate in maintaining the blood-labyrinth barrier.34,35
Identifying Treatment for Reduced CMV-Induced Hearing Loss
The probable source of ROS is the macrophages. Schachtele et al noted that macrophages displayed high levels of 2’,7’-DCFH-DA, a marker for ROS production, 7 and 14 days following inoculation.28 They concluded that the overwhelming number of macrophages caused sufficient inflammation to mediate cochlear hair cell death. Macrophages have been implicated as important innate immune cells involved in the pathogenesis of type 1 diabetes.36 Jun et al demonstrated resistance to type 1 diabetes in nonobese diabetic mice depleted of macrophages.37 Lehuen et al found that macrophages contribute to the destruction of insulin-secreting β cells by generating ROS and proinflammatory cytokines via the activation of the redox-sensitive signaling pathways.38
Characterization of this source with loss-of-function experiments and elucidation of affected regions, as in the spiral ganglion and other sites, will need to be performed to understand this mechanism. The preservation of cochlear OHCs and hearing outcomes in CMV-infected mice treated with D-met and ACE-Mg demonstrated here is consistent with antioxidant protection in other model systems. The data suggest potential therapeutic utility and promising possibilities for clinical trials of antioxidants or targeting of macrophages to protect hearing in humans with CMV infection.
One limitation is that although antioxidant administration provided otoprotection against viral infection in the cochlea, hearing was not completely preserved, as DPOAE and ABR thresholds increased modestly but significantly in mCMV-infected, antioxidant-treated mice as compared with uninfected controls. The increased DPOAE thresholds in the treated mice were consistent with OHC loss, which eventually reached about 12%. These data indicate that antioxidant treatment is not sufficient to completely protect inner ear structures from damage after mCMV infection and that either clearance of infected cells was incomplete or sequelae of infection contributed to subsequent SNHL. Furthermore, direct evidence of mCMV infection in the hair or supporting cells within the organ of Corti was not seen, suggesting that secondary effects of mCMV infection are responsible for hair cell loss.
Supplementary Material
Acknowledgments
We thank Dr Li Wang (University of Utah) for generously providing Nrf2−/− breeding pairs.
Sponsorships: None of the sponsors or funding source were involved in the study design, conduct, collection, analysis, or interpretation of the data.
Funding source: Albert H. Park—Career Development Award from the Triological Society; Jun Yang—NIH grant EY020853; grants from the NIH (EY014800) and the Research to Prevent Blindness Fund for support of imaging facilities.
Footnotes
Disclosures
Competing interests: Pranav D. Mathur, Jun Yang, Namakkal S. Rajasekaran, and Matthew A. Firpo, National Institutes of Health (NIH); Albert H. Park, Merck consultant, NIH Small Business Technology Transfer consultant, NIH principal investigator, Roche consultant.
Supplemental Material
Additional supporting information is available in the online version of the article.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- 1.Engman ML, Malm G, Engstrom L, et al. Congenital CMV infection: prevalence in newborns and the impact on hearing deficit. Scand J Infect Dis. 2008;40:935–942. [DOI] [PubMed] [Google Scholar]
- 2.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253–276. [DOI] [PubMed] [Google Scholar]
- 3.Stehel EK, Shoup AG, Owen KE, et al. Newborn hearing screening and detection of congenital cytomegalovirus infection. Pediatrics. 2008;121:970–975. [DOI] [PubMed] [Google Scholar]
- 4.Campbell KC, Meech RP, Klemens JJ, Gerberi MT, Dyrstad SS. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226:92–103. [DOI] [PubMed] [Google Scholar]
- 5.Fetoni AR, De Bartolo P, Eramo SL, et al. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. J Neurosci. 2013;33:4011–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006; 27:1–19. [DOI] [PubMed] [Google Scholar]
- 7.Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4:229–236. [DOI] [PubMed] [Google Scholar]
- 8.Choung YH, Taura A, Pak K, Choi SJ, Masuda M, Ryan AF. Generation of highly-reactive oxygen species is closely related to hair cell damage in rat organ of Corti treated with gentamicin. Neuroscience. 2009;161:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol Aging. 2007;28:1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almishaal AA, Mathur PD, Hillas E, et al. Natural killer cells attenuate cytomegalovirus-induced hearing loss in mice. PLoS Pathog. 2017;13:e1006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf hetero-dimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. [DOI] [PubMed] [Google Scholar]
- 12.Tamir S, Adelman C, Weinberger JM, Sohmer H. Uniform comparison of several drugs which provide protection from noise induced hearing loss. J Occup Med Toxicol. 2010;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park AH, Gifford T, Schleiss MR, et al. Development of cytomegalovirus-mediated sensorineural hearing loss in a guinea pig model. Arch Otolaryngol Head Neck Surg. 2010; 136:48–53. [DOI] [PubMed] [Google Scholar]
- 14.Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715–748. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T, Yamamoto M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J Biol Chem. 2017;292:16817–16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green KL, Swiderski DL, Prieskorn DM, et al. ACEMg diet supplement modifies progression of hereditary deafness. Sci Rep. 2016;6:22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schafer FQ, Wang HP, Kelley EE, Cueno KL, Martin SM, Buettner GR. Comparing beta-carotene, vitamin E and nitric oxide as membrane antioxidants. Biol Chem. 2002;383:671–681. [DOI] [PubMed] [Google Scholar]
- 18.Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins: vitamins E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci. 1992;669:7–20. [DOI] [PubMed] [Google Scholar]
- 19.Le Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic Biol Med. 2007;42:1454–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao J, Deng J, Lv L, et al. Hydrogen peroxide induce human cytomegalovirus replication through the activation of p38-MAPK signaling pathway. Viruses. 2015;7:2816–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirrier AL, Pincemail J, Van Den Ackerveken P, Lefebvre PP, Malgrange B. Oxidative stress in the cochlea: an update. Curr Med Chem. 2010;17:3591–3604. [DOI] [PubMed] [Google Scholar]
- 22.Jensen-Smith HC, Hallworth R, Nichols MG. Gentamicin rapidly inhibits mitochondrial metabolism in high-frequency cochlear outer hair cells. PLoS One. 2012;7:e38471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Lee JH, Kim SJ, et al. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and oto-toxicity. J Neurosci. 2010;30:3933–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coling D, Chen S, Chi LH, Jamesdaniel S, Henderson D. Age-related changes in antioxidant enzymes related to hydrogen peroxide metabolism in rat inner ear. Neurosci Lett. 2009;464: 22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rewerska A, Pawelczyk M, Rajkowska E, Politanski P, Sliwinska-Kowalska M. Evaluating D-methionine dose to attenuate oxidative stress-mediated hearing loss following overexposure to noise. Eur Arch Otorhinolaryngol. 2013:1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhuang W, Wang C, Shi X, et al. MCMV triggers ROS/NLRP3-associated inflammasome activation in the inner ear of mice and cultured spiral ganglion neurons, contributing to sensorineural hearing loss. Int J Mol Med. 2018; 41:3448–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford RD, Bantug GB, Jonjic S, Britt WJ. Hearing loss and sensorimotor coordination abnormalities in a murine model of cytomegalovirus infection. Presented at: Congenital Cytomegalovirus Conference; September 2010; Paris, France. [Google Scholar]
- 28.Schachtele SJ, Mutnal MB, Schleiss MR, Lokensgard JR. Cytomegalovirus-induced sensorineural hearing loss with persistent cochlear inflammation in neonatal mice. J Neurovirol. 2011;17:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Patel R, Ren C, et al. A comparison of different murine models for cytomegalovirus-induced sensorineural hearing loss. Laryngoscope. 2013;123:2801–2806. [DOI] [PubMed] [Google Scholar]
- 30.Hu BH, Zhang C, Frye MD. Immune cells and non-immune cells with immune function in mammalian cochleae. Hear Res. 2018;362:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okano T, Nakagawa T, Kita T, et al. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J Neurosci Res. 2008;86:1758–1767. [DOI] [PubMed] [Google Scholar]
- 32.Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol. 2005;489:180–194. [DOI] [PubMed] [Google Scholar]
- 33.Lim DJ. Surface ultrastructure of the cochlear perilymphatic space. J Laryngol Otol. 1970;84:413–428. [DOI] [PubMed] [Google Scholar]
- 34.Shi X. Resident macrophages in the cochlear blood-labyrinth barrier and their renewal via migration of bone-marrow-derived cells. Cell Tissue Res. 2010;342:21–30. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Dai M, Fridberger A, et al. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc Natl Acad Sci U S A. 2012;109:10388–10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seleme MC, Lei W, Burg AR, et al. Dysregulated TLR3-dependent signaling and innate immune activation in superoxide-deficient macrophages from nonobese diabetic mice. Free Radic Biol Med. 2012;52:2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jun HS, Yoon CS, Zbytnuik L, et al. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Exp. Med. 1999;189:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell cross-talk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.