Abstract

It is a generally accepted perspective that type-II nanocrystal quantum dots (QDs) have low quantum yield due to the separation of the electron and hole wavefunctions. Recently, high quantum yield levels were reported for cadmium-based type-II QDs. Hence, the quest for finding non-toxic and efficient type-II QDs is continuing. Herein, we demonstrate environmentally benign type-II InP/ZnO/ZnS core/shell/shell QDs that reach a high quantum yield of ∼91%. For this, ZnO layer was grown on core InP QDs by thermal decomposition, which was followed by a ZnS layer via successive ionic layer adsorption. The small-angle X-ray scattering shows that spherical InP core and InP/ZnO core/shell QDs turn into elliptical particles with the growth of the ZnS shell. To conserve the quantum efficiency of QDs in device architectures, InP/ZnO/ZnS QDs were integrated in the liquid state on blue light-emitting diodes (LEDs) as down-converters that led to an external quantum efficiency of 9.4% and a power conversion efficiency of 6.8%, respectively, which is the most efficient QD-LED using type-II QDs. This study pointed out that cadmium-free type-II QDs can reach high efficiency levels, which can stimulate novel forms of devices and nanomaterials for bioimaging, display, and lighting.
Keywords: indium phosphide, quantum dots, type-II band alignment, liquid LED, color conversion
Introduction
Semiconductor nanocrystal (NC) quantum dots (QDs) have attracted significant attention for light-generating devices such as luminescent solar concentrators,1−3 light-emitting diodes (LEDs),4−7 and lasers.8,9 They show advantageous properties such as tunable emission color due to the quantum confinement effect, high photochemical stability, and solution processability.10−13 Especially, high photoluminescence quantum yield (PLQY), defined as the ratio of the number of photons emitted to the total number of generated excitons, is a key figure of merit. To reach high PLQYs, type-I QDs with straddling alignment that localize the electron and hole inside the core are the most widely investigated heterostructures.
Alternatively, type-II QDs have conduction and valence band extrema in different materials, and it is a generally accepted perspective that type-II QDs result in low PLQYs. So far, a wide variety of type-II heterojunctions composed of CdSe, CdS, CdTe, and ZnTe (e.g., CdTe/CdSe and ZnTe/CdSe) have been examined.14−17 However, their PLQY remained low, which is attributed to the spatial separation of the electrons and holes that leads to the increase in the nonradiative recombination rates and decrease in PLQY. Recently, high PLQY values of 61,18 68,19 and 88%20 were achieved with ZnSe/CdS/ZnS, CdSe/CdTe, and CdxZn1–xS/ZnSe/ZnS type-II QDs, respectively. However, the aforementioned type-II QDs have highly toxic heavy metal of cadmium.21−23 Alternatively, there were also a few cadmium-free QDs (e.g., ZnTe/ZnSe) investigated, but their PLQY reached up to 36%.24,25 Therefore, the quest for finding efficient and Cd-free type-II QDs, which can find widespread use to form environmentally benign light-generating devices and biocompatible luminescent markers, is continuing.
In this work, we demonstrate InP/ZnO/ZnS core/shell/shell QDs, which have type-II staggered line-up heterojunctions. We synthesized the ZnO shell with thermal decomposition and grow subsequent multiple ZnS shells with the Successive Ionic Layer Adsorption (SILAR) method. We optimized the ZnO and ZnS shell thickness that led to a high efficiency PLQY of 90.8%. Moreover, QDs were integrated in the liquid state on blue LEDs and QD-LEDs, demonstrating an external quantum efficiency (EQE) and a power conversion efficiency (PCE) of 9.4 and 6.6%, respectively.
Results and Discussion
Synthesis of InP/ZnO/ZnS Core/Shell/Shell QDs
InP core QDs were synthesized via a hot-injection method.1,26,27 For that, zinc undecylenate was used to passivate InP cationic dangling bonds and decrease trap states,26,28 and oleylamine (OAM) and oleic acid (OA) were used as stabilizing agents. In brief, indium chloride (InCl3), OAM, OA, and zinc undecylenate were dissolved in the 1-octadecene (ODE) solvent in a glovebox at room temperature. After degassing and heating to 200 °C, tris(trimethylsilyl)phosphine [P(TMS)3] was injected into the reaction mixture to initiate the nucleation and growth. To generate a type-II band alignment, the ZnO shell was selected; by considering the successful growth of CdTe/ZnSe core/shell QDs with a lattice mismatch of 14%,29 ZnO growth on InP with a lattice mismatch of 11% is also feasible.1,24,30 In this regard, ZnO was grown on the InP core by thermal decomposition of zinc acetylacetonate [Zn(acac)2].31,32 During the synthesis, OAM was used as the stabilizing agent in order to hinder ZnO aggregation.33 Furthermore, different amounts of ZnO precursor solution was added for reaching the highest PLQY (see Table S1). To further increase the PLQY, we performed ZnS growth on the InP/ZnO core/shell structure using a SILAR technique. For that, the reaction solution of InP/ZnO was cooled to 170 °C, zinc stearate–ODE [Zn(St)2–ODE] and sulfur–trioctylphosphine (S–TOP) stock solutions were added to reaction solution, and temperature was increased to 250 °C for 30 min. Similarly, to grow additional ZnS shells, the temperature followed the same thermal cycling.
Quantum Mechanical Calculations and Optical Analysis
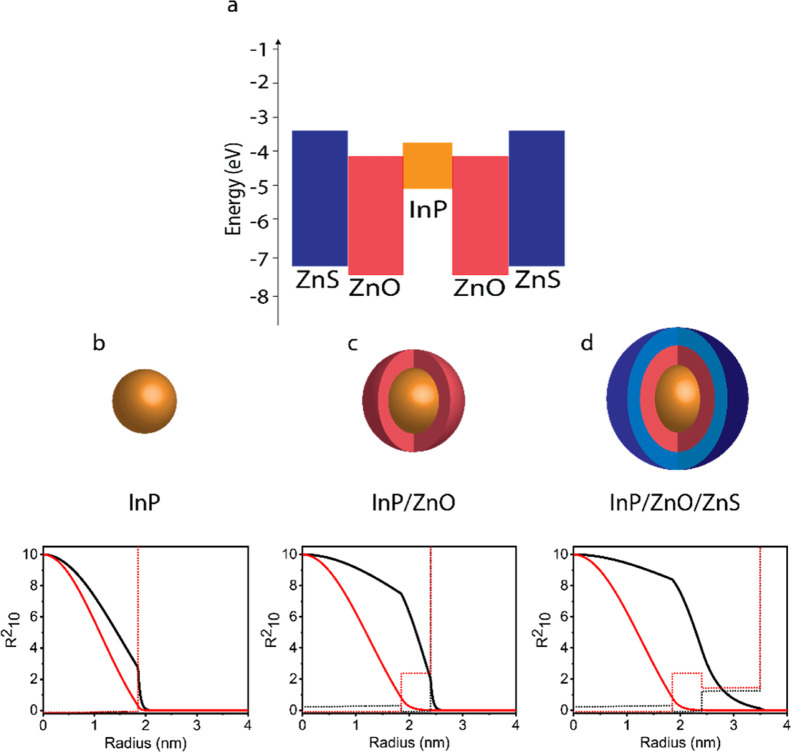
To understand the optical properties of the synthesized QDs, we calculated their quantum mechanical properties by self-consistently solving Poisson–Schrödinger equations in the effective mass approximation (Figure 1b–d) and correlated them with the optical measurements (Figure 2).24 InP core QD has type-I band alignment that led the confinement of electrons and holes in the same spatial core region with a high overlap of the wavefunctions of ∼90% (Figure 1b). After ZnO shell growth, the band alignment of the nanostructure transit from type-I to type-II, and while hole wavefunction is confined completely in the core region like in InP core QD, electron wavefunction expands toward the ZnO shell that reduces the wavefunction overlap to 60% (Figure 1c).34 Since the delocalization of the electron decreases the confinement energy level within the conduction band and the exciton binding energy, the photoluminescence peak red-shifted from 585 to 613 nm, while the full width at half-maximum (fwhm) was maintained at ∼74–75 nm for core and core/shell nanostructures. We investigated the PLQY of ZnO shell growth under different ZnO molar ratios, PLQY reached 25.8% (Figure 2a and see Supporting Information, Table S1).
Figure 1.
(a) Energy band diagram of InP/ZnO/ZnS core/shell/shell QDs. Quantum mechanical simulations of (b) InP core, (c) InP/ZnO core/shell, and (d) InP/ZnO/ZnS core/shell/shell QDs. Black lines correspond to the radial probability distribution of electrons, while red lines show the radial probability distribution of holes. Black and red dashed lines correspond to confinement potential profile for electrons and holes, respectively.
Figure 2.
(a) Absorbance and PL spectra of InP, InP/ZnO, and InP/ZnO/ZnS QDs with multiple shells. Here, QD refers to the InP/ZnO core/shell QDs and #ZnS refers to the number of SILAR growth cycles. (b) Normalized quenching factor of the QD-4ZnS core/shell NCs calculated by multiplication of reabsorption R and (1-PLQY). (c) PLQY (%) of QDs in hexane. Errors bars represent the standard deviation from three measurements.
Afterward to reach higher PLQYs, a variety of ZnS shell thickness was investigated. From 1 to 4 cycles of ZnS shell growth, the PLQY increased due to effective confinement of electron–hole pairs in the InP–ZnO nanostructure and surface passivation (Figure 2c).35 After the fourth growth cycle of ZnS, PLQY of InP/ZnO/4ZnS NCs (QD-4ZnS NCs) started to decrease, possibly due to higher interfacial strain caused by dislocations.29,36 By the fourth ZnS shell, PLQY reached the maximum level of 90.8%. QD-4ZnS showed a PL peak at 618 nm, which is slightly red-shifted (5 nm) with respect to InP/ZnO QDs due to the lower wavefunction overlap of 49%. We also investigated the effect of ZnO and ZnS shell growth on the normalized quenching factor [reabsorption × (1-PLQY)]37 for core and core/shell structures to select the appropriate nanostructure (Figure 2b). After ZnO shelling, due to red shifting of the PL peak, quenching factor decreased, and the minimum value is observed at QD-4ZnS NCs.
Structural Analysis
To understand the size and shape of the QDs, we performed small-angle X-ray scattering (SAXS) measurements in ethanol38 by probing up to 106 individual particles. The scattering data after subtracting the ethanol background are shown in Figure S1, which are plotted versus the reciprocal scattering vector q. As shown in Figure 3a, we plotted instead of the reciprocal form factor P(q) directly its Fourier transform, the pair distance distribution function (PDDF) in real space.38,39 The PDDF is the distribution of all distances within all single particles of the probed sample volume, which also contains the mean particle dimensions of the whole ensemble. From the maximum dimension found within the PDDF, we derive the maximum dimension found within the particle ensemble. The bell-like PDDFs of the InP-core and the InP/ZnO core/shell QDs indicate spherical QD shapes, whereas the asymmetric shape of the QD-4ZnS-PDDF is a hint for an elongated mean shape. The change from a spherical shape for the InP and InP/ZnO QDs to a more elliptical one for the QD-4ZnS NCs can also be seen in the fact that only the first two SAXS curves could be fitted with monomodal distributions of spheres, as shown in Figure 3b. In these distributions, we derive from the peak positions directly the mean radii and hence the mean diameters of the QDs, from the widths of the size distributions. For the InP-core, we get a sphere diameter of 3.6 ± 0.8 nm and for the InP/ZnO core/shell QDs 4.4 ± 0.9 nm with size distributions between 9 and 10%, which is in excellent accordance with the transmission electron microscopy (TEM) data shown in the inset of Figure 3c,d. The asymmetric PDDF of QD-4ZnS NCs, however, indicates a prolate like mean particle shape.40 The short diameter is only a bit larger than the spherical diameter of 4.4 nm found for the InP/ZnO NCs as can be deduced from the slightly shifted peak of the QD-4ZnS-PDDF with respect to InP/ZnO-PDDF (see Figure 3a). The long axis is derived from the long tail within the PDDF, from which we deduce maximum values between 9 and 10 nm. Most of the QDs within the ensemble depict long-axis values between 6 and 7 nm, which is within the broader mean size distribution found by TEM with a maximum at 7.02 ± 0.9 nm (Figure 3e). The elongated shape of the QD-4ZnS NCs together with their chain-like fractal aggregation (see Figure S1) can be explained by a slightly inhomogeneous growth of the ZnS shell, resulting in an overall prolate like NC shape. This kind of elliptical NC shape can be found also for other core/shell NC systems with thick shells, where the shell growth along specific crystallographic directions is energetically favored.41 The ellipsoidal shape of nanoparticles can potentially lead to radiation polarization42 and additionally allowed transitions forbidden in spherical QDs.43
Figure 3.

(a) PDDFs of the InP core (black), InP/ZnO core/shell QD (blue), and QD-4ZnS core/shell/shell NCs (red), as resulting from the P(q)-curves shown in Figure S1b. (b) Distributions of spheres that can describe the measured SAXS curves of the InP core and InP/ZnO-core/shell QDs. Size distribution histograms of the (c) InP core (inset: TEM image, scale bar is 2 nm), (d) InP/ZnO core/shell (inset: TEM image, scale bar is 2 nm), and (e) QD-4ZnS core/shell/shell NCs (inset: TEM image, scale bar is 2 nm) by high-resolution TEM measurements. Total number of 200 QDs are counted for size distribution. (f) XRD of InP, InP/ZnO, and InP/ZnO/4ZnS core/shell/shell NCs. XPS analysis of InP core QDs, (g) In 3d spectrum and (h) P 2p spectrum.
The crystal structure of the resulting QDs was investigated by analyzing the XRD patterns (Figures 3f and S2). The black vertical dashed lines and black crosses mark the positions of InP bulk peaks (ICDD 00-032-0452), the blue crosses of hexagonal ZnO (ICDD 00-036-1451), and the red crosses mark the positions of the cubic sphalerite phase of ZnS (a = 5.4093 Å ICDD 00-65-0309), respectively. From the only core QDs, we observe that the crystal structure corresponds to zinc blende (a = 5.869 Å, ICDD 00-32-0452). For InP/ZnO NCs, we still observed the InP zinc blende crystal structure, but due to the thin ZnO shell (∼0.2–0.3 nm), its diffraction peak (ICDD 00-036-1451) cannot be detected. This is not surprising as only one monolayer (ML) shell material on a crystalline core can grow epitaxially by maintaining the lattice constant of the core.44 For the InP/ZnO/ZnS NCs, a shift of the InP peaks occurred in the direction of the position of ZnS in the cubic sphalerite phase (ICDD 00-65-0309). This shift can be explained by the situation that the first MLs of the ZnS shell initially maintains epitaxial registry with the lattice parameter of InP and with subsequent ML of ZnS, tends to reach its bulk lattice constant.44 Because of the cubic lattice constant of ZnS is smaller than that of InP, the InP core is compressively strained, reflected by peak shifts to larger Q-values or diffraction angles (see Figure 3f). Additionally, we observe different peak-shift values as well as peak width values for the InP core along different crystallographic directions, indicating that the influence of the ZnS shell on the InP core is not isotropic. This result is suggestive of asymmetric ZnS shell growth as already derived from SAXS analysis.
X-ray photoelectron spectroscopy (XPS) analysis was performed to confirm the elemental surface chemistry of the InP core, InP/ZnO core/shell, and QD-4ZnS core/shell/shell NCs, respectively. For the analysis, all of the peaks have been spectrally corrected according to the carbon-1s standard peak. For the InP core QDs, the In 3d spectrum exhibits two peaks located at 444.5 eV (3d5/2) and 452.1 eV (3d3/2) (Figure 3g,h), which can be assigned to InP.45−47 The P 2p spectrum shows two doublets, which are related to the two different chemical environments of the phosphorus atoms. The first pre-dominant doublet that occurs at 127.8–128.9 eV (2p3/2) is the characteristic peak for InP.48,49 The doublet in the 132.4–133.4 eV range is associated with the P atoms in an oxidized medium, most probably InPOX.45,50 In the XPS spectra of InP/ZnO core/shell QDs, the peaks corresponding to Zn 2p are observed which indicates the Zn2+ bound to oxygen in the ZnO.51−53 The Zn 2p3/2 and 2p1/2 peaks are at 1022.09 and 1045.8 eV, respectively (Figure S3c). For the InP/ZnO core/shell QDs, the In 3d peak shifts to higher binding energies located at 444.7 eV (3d5/2) and 452.5 eV (3d3/2), compared to the InP core QD (Figure S3a). Furthermore, shifting to higher binding energies located at 127.9 and 132.4 eV also occurs in P 2p spectrum (Figure S3b). Similarly, the O 1s peak shifted to higher binding energy from 530.9 to 531.3 eV after formation of the ZnO shell coating (Figure S3d). The possible reason for this type of shifting is due to the additional oxidation process during the ZnO shelling procedure.45 For the QD-4ZnS core/shell/shell NCs, the S 2p spectrum exhibited two peaks located at 161.2–162.6 eV (Figure S4d). It was previously reported that these binding energies are associated with S2– anions present in the ZnS.45,54,55 In addition, after the ZnS shell formation, In 3d peaks shifted to lower binding energies located at 444.4 eV (3d5/2) and 452.0 eV (3d3/2) (Figure S4a). Similarly, binding energies of Zn 2p and O 1s (Figure S4b,c) had also displaced to lower values after ZnS shelling due to decrease in the InP–O bonds formed by oxidation.56
Ultrafast Decay Dynamics
The effect of shell growth on the ultrafast decay dynamics and the nonlinear absorption of QDs was further investigated using femtosecond pump–probe spectroscopy.37,57 In the experiments, the QD solutions (InP core, InP/ZnO core/shell, and QD-4ZnS core/shell/shell NCs) were excited with 320 nm, femtosecond pump pulses (incident fluence is 480 μJ·cm–2), and the difference between the pumped and unpumped absorbance (ΔA) of the QDs was measured over the 450–800 nm spectral range using a femtosecond visible continuum probe. Figure 4 summarizes the results of the femtosecond transient absorption (TA) measurements. Measured ΔA spectrum, temporal evolution of ΔA at the wavelength of maximum bleaching, and two-dimensional variation of ΔA as a function of probe delay and wavelength are shown in Figure 4a–c,d–f,g–i, for InP, InP/ZnO, and QD-4ZnS NCs, respectively. The peak wavelength of the measured ΔA spectra of the QDs exhibited a red-shift as a function of the probe delay due to the intra-band decay of the excited carriers to the conduction band edge58 (Figure 4a,d,g). The TA measurements showed that for the InP core, the steady-state absorption peak wavelength was at 490 nm, and the peak wavelength of bleach was at 520 nm (Figure 4a). However, after the growth of ZnO or ZnS shell over InP core, the peak wavelengths of steady-state absorption and bleach became nearly equal (Figure 4a,d,g), which can be explained based on the reduction of the biexciton and carrier-trap induced stark effect.59 The ultrafast temporal evolution of the carriers was further investigated for probe wavelengths at which the measured ΔA showed the maximum amount of bleach (520 and 585 nm) for the InP, InP/ZnO, and QD-4ZnS NCs. As can be seen in Figure 4b,e,h, following an initial rise, a biexponential decay occurred, which could be characterized by the decay times τ1 and τ2.60,61 Here, the decay times τ1 and τ2 are due to shallow and deeper traps, which originate due to the P and In dangling bonds.62 The decay times of τ1 and τ2 for the InP, InP/ZnO, and QD-4ZnS NCs were determined by performing a biexponential fit to the measured ultrafast decay data. The fit results showed that τ1 and τ2 both increased from 1 to 1.5 ps and from 65.7 to 76.5 ps, respectively, after the shell growth of ZnO on the InP core. Furthermore, for the case of QD-4ZnS NCs, τ1 and τ2 became 3.4 and 84.3 ps, respectively, indicating further passivation of the shallow and deeper traps as a result of shell growth.59,63−65 Note that the measured ΔA did not vanish completely for the longest probe delay of 80 ps since the carrier recombination for the QDs occur over nanosecond time scales (Figure S5).
Figure 4.
Measured ΔA spectrum, temporal evolution of ΔA at the wavelength of maximum bleaching, and two-dimensional variation of ΔA as a function of probe delay and wavelength for: InP core (a–c), InP/ZnO (d–f), and QD-4ZnS (g–i) NCs for InP, InP/ZnO, and QD-4ZnS NCs, respectively. Measured TA spectra of (a) InP core; (d) InP/ZnO and (g) QD-4ZnS at different probe delays. The excitation wavelength was 320 nm. The measured peak wavelength of the nonlinear absorption changed and matched the bleach peak after the shell growth which can be explained by the reduction of the carrier-trap-induced stark effect. (b,e,h) Measured and fitted temporal evolution of the carrier decay times at the probe wavelengths of 520 and 585 nm. For the InP core, the first excitonic peak was at 520 nm, and the τ1 and τ2 values were determined to be 1 and 65.7 ps. The ZnO shell growth reduced the trap states and hence, τ1 and τ2 increased to 1.5 and 76.5 ps, respectively. The multiple ZnS shell growth caused further trap passivation, resulting in short (τ1) and long (τ2) lifetimes of 3.4 and 84.3 ps, respectively. (c,f,i) Surface plots of the measured ΔA with respect to the probe delay and wavelength.
LED Applications
To prevent any efficiency drop due to the host material effect, we designed an architecture66 that can maintain QD solution in the liquid state on top of the blue LED (Figure 5a). For that we used a transparent polymeric lens made of polydimethylsiloxane (PDMS) due to its scalable and low cost fabrication.67,68 We placed the lens on top of the blue LED chip and sealed the edges with the UV-curable polymer. After the curing process was finished, we injected red-emitting QDs using a microsyringe into the blue LED capped with a PDMS polymeric lens. When the blue LED is turned on, the blue electroluminescence generated from the blue LED die optically pumps the QDs in the solution state, which generates photoluminescence in the red spectral region (Figure 5a,b). To analyze the optical performance of the fabricated QD-LED, we injected the QD solution with different optical densities. While the optical density increases, red emission becomes stronger in comparison with the blue electroluminescence [Figure 5c]. Finally, the (x, y) tristimulus coordinates reached the red region on the CIE chromaticity diagram by integrating QDs with an optical density of 0.4 (Figure 5d). The intensity spectra remained almost constant with a slight change in the PL peak position of 3 nm, while the current injection level increases from 5 to 150 mA (Figure 5e). The EQE was measured as 28.6, 25.3, 19.2, 13.4, and 9.43% for the optical densities of 0.04, 0.07, 0.13, 0.20, and 0.4, respectively. Moreover, PCE is calculated by the relation of PCE = ηext.λ1·(λ2)−1,69 where λ1 and λ2 are the emission peak wavelength of the blue EL and emission peak wavelength of the QDs, respectively. PCE of LEDs corresponded to 21.62, 18.85, 13.99, 9.85, and 6.86% for the respective OD values, respectively.
Figure 5.

(a) Schematic of the QD-based LED fabrication. (b) Photograph of the LED when it is turned on. (c) PCE (%) and EQE (%) at different OD values. Inset: Intensity spectra of red-emitting QD-based LEDs at different optical densities of 0.07 and 0.2. (d) (x, y) color coordinates of the QD-LEDs at different optical densities with an injection current of 10 mA. (e) Intensity spectra of the red-emitting QD-based LED with an optical density of 0.4 at different current injection levels ranging from 5 to 150 mA.
Till date, in terms of type-II QD-based LED studies, Jin et al. synthesized green-emitting alloyed CdxZn1–xS/ZnSe type-II QDs, and these LED results demonstrated EQE of 8.78%.20 Moreover, Lin et al. reported deep-red LEDs based on CdTe/CdSe core/shell QDs with a maximum EQE of 6.19%.19 Instead of cadmium-based QDs, Karatum et al. integrated InP/ZnO core/shell QDs as an emissive layer into LED applications, resulting in an EQE of 0.53%.30 Comparatively, the LED using InP-based type-II QDs in this study reached the EQE level, approaching to 10% due to the high efficiency in synthesis batch and conserving their efficiency levels by direct integration in the liquid state.
Conclusions
In summary, we demonstrated cadmium-free and efficient type-II InP/ZnO/ZnS core/shell/shell QDs. For that, initially InP core QDs with emission in the red spectral region were synthesized using a hot injection technique. Then, the sequential growth of ZnO and ZnS shells led to a high PLQY of 90.8%. ZnO and ZnS shell formation were confirmed by optical analysis that is supported by quantum mechanical simulations. Moreover, SAXS measurements showed that the ZnS shell induced the transition of the NC ensemble from spherical to elliptical shapes. Finally, we integrated the core/shell/shell QDs into LED die in the liquid form to reduce the host-material effect. The fabricated liquid-LED device demonstrated 9.4% of EQE and 6.8% of PCE, respectively, which is the most efficient type-II QD-based LED reported till date. Type-II QDs show high promise for future bioimaging, display, and laser applications.
Experimental Section
Chemicals
Zinc undecylenate (99%), OA (99%), OAM (99%), ODE (90%), indium(III) chloride (InCl3) (99%), P(TMS)3 (95%), Zn(St)2, TOP, sulfur (S) (reagent grade, powder, purified by refining, −100 mesh particle size), and zinc acetylacetonate hydrate [Zn(acac)2] (99.995%) were purchased from Sigma-Aldrich. ODE was purified at 120 °C by evacuating and refilling repeatedly with nitrogen for 1 h. All the procedures were performed in a glovebox under a nitrogen atmosphere.
Synthesis of InP Core
First, 43 mg of zinc undecylenate, 32 μL of OA, 66 μL of OAM, and 22 mg of InCl3 were mixed in 3 mL of ODE and heated to 120 °C. The flask was degassed 20 min at 120 °C and refilled with nitrogen. Afterward, it was heated to 200 °C, and 500 μL of phosphine stock solution [P(TMS)3-ODE 0.2 mmol mL–1] was rapidly injected and mixed 30 min at 200 °C. Then, the solution was cooled to 80 °C, and an aliquot was taken from the sample.
Synthesis of InP/ZnO and QD-4ZnS Core/Shell/Shell NCs
In order to prepare the ZnO stock solution, 245 μL of OAM, 8 μL of OA, and 6.5 mg of Zn(acac)2 were mixed in 1.6 mL of ODE at 80 °C. 500 μL of ZnO stock solution was added to InP core solution at 80 °C and heated to 250 °C. It was mixed 30 min at this temperature and cooled to 170 °C. For the zinc (Zn) and sulfur (S), precursor solutions were prepared separately. 510 mg of Zn(St)2 and 27 mg of S powder were dissolved in 8 mL of ODE and 8 mL of TOP, respectively. It should be noted that for fully dissolving sulfur in TOP, we applied heat which was around 100 °C. After preparation of stock solutions, they were added to InP/ZnO solution in a row and heated to 250 °C. The solution was mixed at 250 °C for 30 min. For each shelling procedure, the same method was employed. The injection amounts of precursors are shown in Table S2.
Purification and Storage
After synthesis, QD solution was directly centrifuged at 9000 rpm for 10 min two times. The precipitated part was removed from the QD solution, and ethanol was added to the solution until it became turbid and centrifuged again with same parameters. The precipitated QDs were dispersed in hexane and kept at 4 °C and covered with the aluminum foil.
Lens Making Procedure
In order to make hemispherical lens with outer diameter of 9 nm and inner diameter of 7 nm, the PDMS-SYLGARD 184 elastomer was mixed with the SYLGARD 184 curing agent with a ratio of 10:1, and then, the mixture was stirred vigorously until the bubbles appear. Afterward, to dispel the bubbles completely, the mixture was degassed 20 min. The mixture was poured into the aluminum mold and cured at 70 °C 1 h. After curing, lens was taken out from the mold. The thickness of the as-prepared lens is 1 mm.
Fabrication of LED Devices and Close-Packed LEDs
Before soldering of the electrical wires for connection to the voltage supply, the blue chip was mounted on board. Afterward, PDMS lens was attached on LEDs by using an NOA 68 UV-curable polymer. To cure LEDs with PDMS, lens were kept under UV radiation at 365 nm for 20 min. To prevent any leakage from the PDMS lens, dripping of the curable polymer and keeping under radiation were performed two times.
LED Measurements
For LED measurements, an EP-B4040F-A3 InGaN GaN blue LED chip from Secol Company with an illumination wavelength at 465 nm was used. LED measurements were performed with a multi-port Ocean Optics integrating sphere. The detector was an Ocean Optics Torus with an optical resolution of 1.6 nm fwhm. The signal to noise ratio was 250:1. The EQE was calculated based on the literature, as mentioned above.
Structural and Optical Characterizations of QDs
Powder X-ray diffraction (XRD) measurements of the InP, InP/ZnO, and QD-4ZnS NCs were performed by a Bruker D8 eco X-ray diffractometer (λCu Kα = 1.54 Å radiation) with a scan speed of 0.14° min–1. Before XRD measurements, they were purified by three times to remove excess impurities. Then, the colloidal NC dispersion was drop-cast on a Si-miscut wafer, a zero-background holder. XRD measurements were carried out at room temperature. Steady-state absorbance and PL measurements were performed using an Edinburgh Instruments FL-50 spectrofluorometer with an excitation wavelength of 375 nm. QE measurements were carried out using an integrating sphere with an inner diameter of 150 mm.
For TEM measurements, samples were deposited as 10 μL of 1 mM of QD in hexane solution on a copper support grid. TEM analysis was performed using a JEOL JEM-ARM200CFEG UHR microscope with a spherical aberration-corrected probe and equipped with a Gatan UltraScan camera model 994 US1000X.
Bright-field images were collected using an accelerating voltage of 200 keV. For the SAXS measurements about 100 μL of NC-dispersions have been filled in quartz glass capillaries with 1.5 mm diameter and have been sealed with epoxy resin. The capillaries have been inserted into our laboratory SAXS system (Nanostar from Bruker AXS) and have been measured in vacuum to suppress air scattering. Two-dimensional SAXS images have been recorded using Cu Kα X-ray radiation with a wavelength of 1.5 Å and a 2D Vantec detector. The 2D scattering patterns have been azimuthal integrated to obtain 1D scattering intensity I(q) data as a function of the reciprocal scattering vector q. All 1D data have been corrected for the different X-ray transmission values.
Transient absorption (TA) studies were performed with an ultrafast pump–probe spectrometer to measure the change ΔA in the nonlinear absorbance spectrum (ΔA = Apumped – Aunpumped; Apumped = absorbance spectrum of the pumped sample and Aunpumped = small-signal absorbance spectrum). Further details of the pump–probe setup and measurement procedure are described in ref (37). Here, free charge carriers were excited with a 320 nm femtosecond pump pulse (fluence of 480 μJ·cm–2), and the resulting nonlinear absorbance change was measured with a femtosecond white-light probe in the 450–800 nm spectral window.
Acknowledgments
S.N. acknowledges support by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (grant agreement no. 639846). S.N. also acknowledges the support by the Turkish Academy of Sciences (TÜBA-GEBIP; The Young Scientist Award Program); the Science Academy of Turkey (BAGEP; The Young Scientist Award Program); and Bilim Kahramanları Derneği (The Young Scientist Award Program).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.1c08118.
PLQY values according to the injected ZnO precursor; zinc and sulfur precursor solution concentration for ZnS shells; SAXS data (symbols) and fits of InP core, InP/ZnO, and QD-4ZnS; XRD of InP core, InP/ZnO, and QD-4ZnS; XPS analysis of InP/ZnO QDs; XPS analysis of QD-4ZnS QDs; and surface plots of the measured TA spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sadeghi S.; Bahmani Jalali H.; Melikov R.; Ganesh Kumar B.; Mohammadi Aria M.; Ow-Yang C. W.; Nizamoglu S. Stokes-shift-engineered indium phosphide quantum dots for efficient luminescent solar concentrators. ACS Appl. Mater. Interfaces 2018, 10, 12975–12982. 10.1021/acsami.7b19144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.; Mazzaro R.; Wang Y.; Zhao H.; Vomiero A. High efficiency sandwich structure luminescent solar concentrators based on colloidal quantum dots. Nano Energy 2019, 60, 119–126. 10.1016/j.nanoen.2019.03.038. [DOI] [Google Scholar]
- Meinardi F.; McDaniel H.; Carulli F.; Colombo A.; Velizhanin K. A.; Makarov N. S.; Simonutti R.; Klimov V. I.; Brovelli S. Highly efficient large-area colourless luminescent solar concentrators using heavy-metal-free colloidal quantum dots. Nat. Nanotechnol. 2015, 10, 878–885. 10.1038/nnano.2015.178. [DOI] [PubMed] [Google Scholar]
- Rogach A. L.; Gaponik N.; Lupton J. M.; Bertoni C.; Gallardo D. E.; Dunn S.; Li Pira N.; Paderi M.; Repetto P.; Romanov S. G.; O’Dwyer C.; Sotomayor Torres C. M.; Eychmüller A. Light-emitting diodes with semiconductor nanocrystals. Angew. Chem., Int. Ed. 2008, 47, 6538–6549. 10.1002/anie.200705109. [DOI] [PubMed] [Google Scholar]
- Shirasaki Y.; Supran G. J.; Bawendi M. G.; Bulović V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 2013, 7, 13. 10.1038/nphoton.2012.328. [DOI] [Google Scholar]
- Won Y.-H.; Cho O.; Kim T.; Chung D.-Y.; Kim T.; Chung H.; Jang H.; Lee J.; Kim D.; Jang E. Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. Nature 2019, 575, 634–638. 10.1038/s41586-019-1771-5. [DOI] [PubMed] [Google Scholar]
- Sadeghi S.; Mutcu S. E.; Srivastava S. B.; Aydindogan G.; Caynak S.; Karslı K.; Melikov R.; Nizamoglu S. High quality quantum dots polymeric films as color converters for smart phone display technology. Mater. Res. Express 2018, 6, 035015. 10.1088/2053-1591/aaf3ef. [DOI] [Google Scholar]
- Sabatini R. P.; Bappi G.; Bicanic K. T.; Fan F.; Hoogland S.; Saidaminov M. I.; Sagar L. K.; Voznyy O.; Sargent E. H. Temperature-Induced Self-Compensating Defect Traps and Gain Thresholds in Colloidal Quantum Dots. ACS Nano 2019, 13, 8970–8976. 10.1021/acsnano.9b02834. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Ta V. D.; Leck K. S.; Tan B. H. I.; Wang Z.; He T.; Ohl C.-D.; Demir H. V.; Sun H. Robust whispering-gallery-mode microbubble lasers from colloidal quantum dots. Nano Lett. 2017, 17, 2640–2646. 10.1021/acs.nanolett.7b00447. [DOI] [PubMed] [Google Scholar]
- Murray C. B.; Norris D. J.; Bawendi M. G. Synthesis and characterization of nearly monodisperse CdE (E= sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. 10.1021/ja00072a025. [DOI] [Google Scholar]
- Yin Y.; Talapin D. The chemistry of functional nanomaterials. Chem. Soc. Rev. 2013, 42, 2484–2487. 10.1039/c3cs90011h. [DOI] [PubMed] [Google Scholar]
- Dai X.; Zhang Z.; Jin Y.; Niu Y.; Cao H.; Liang X.; Chen L.; Wang J.; Peng X. Solution-processed, high-performance light-emitting diodes based on quantum dots. Nature 2014, 515, 96–99. 10.1038/nature13829. [DOI] [PubMed] [Google Scholar]
- Han M.; Jalali H. B.; Yildiz E.; Qureshi M. H.; Şahin A.; Nizamoglu S. Photovoltaic neurointerface based on aluminum antimonide nanocrystals. Commun. Mater. 2021, 2, 19. 10.1038/s43246-021-00123-4. [DOI] [Google Scholar]
- Zhang Y.; Li Y.; Yan X.-P. Photoactivated CdTe/CdSe quantum dots as a near infrared fluorescent probe for detecting biothiols in biological fluids. Anal. Chem. 2009, 81, 5001–5007. 10.1021/ac900394e. [DOI] [PubMed] [Google Scholar]
- Gentle C. M.; Wang Y.; Haddock T. N.; Dykstra C. P.; van der Veen R. M. Internal Atomic-Scale Structure Determination and Band Alignment of II–VI Quantum Dot Heterostructures. J. Phys. Chem. C 2020, 124, 3895–3904. 10.1021/acs.jpcc.9b11443. [DOI] [Google Scholar]
- Long T.; Cao J.; Jiang Z.-J. Predictable spectroscopic properties of type-II ZnTe/CdSe nanocrystals and electron/hole quenching. Phys. Chem. Chem. Phys. 2019, 21, 5824–5833. 10.1039/c9cp00026g. [DOI] [PubMed] [Google Scholar]
- Ca N. X.; Hien N. T.; Luyen N. T.; Lien V. T. K.; Thanh L. D.; Do P. V.; Bau N. Q.; Pham T. T. Photoluminescence properties of CdTe/CdTeSe/CdSe core/alloyed/shell type-II quantum dots. J. Alloys Compd. 2019, 787, 823–830. 10.1016/j.jallcom.2019.02.139. [DOI] [Google Scholar]
- Tyrakowski C. M.; Shamirian A.; Rowland C. E.; Shen H.; Das A.; Schaller R. D.; Snee P. T. Bright Type II Quantum Dots. Chem. Mater. 2015, 27, 7276–7281. 10.1021/acs.chemmater.5b02040. [DOI] [Google Scholar]
- Lin Q.; Song B.; Wang H.; Zhang F.; Chen F.; Wang L.; Li L. S.; Guo F.; Shen H. High-efficiency deep-red quantum-dot light-emitting diodes with type-II CdSe/CdTe core/shell quantum dots as emissive layers. J. Phys. Chem. C 2016, 4, 7223–7229. 10.1039/c6tc01531j. [DOI] [Google Scholar]
- Jin X.; Li H.; Huang S.; Gu X.; Shen H.; Li D.; Zhang X.; Zhang Q.; Li F.; Li Q. Bright alloy type-II quantum dots and their application to light-emitting diodes. J. Colloid Interface Sci. 2018, 510, 376–383. 10.1016/j.jcis.2017.09.080. [DOI] [PubMed] [Google Scholar]
- Chen N.; He Y.; Su Y.; Li X.; Huang Q.; Wang H.; Zhang X.; Tai R.; Fan C. The cytotoxicity of cadmium-based quantum dots. Biomaterials 2012, 33, 1238–1244. 10.1016/j.biomaterials.2011.10.070. [DOI] [PubMed] [Google Scholar]
- Derfus A. M.; Chan W. C. W.; Bhatia S. N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner C.; Liedl T.; Kudera S.; Pellegrino T.; Muñoz Javier A.; Gaub H. E.; Stölzle S.; Fertig N.; Parak W. J. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005, 5, 331–338. 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- Bahmani Jalali H.; Mohammadi Aria M.; Dikbas U. M.; Sadeghi S.; Ganesh Kumar B.; Sahin M.; Kavakli I. H.; Ow-Yang C. W.; Nizamoglu S. Effective neural photostimulation using indium-Based type-II quantum dots. ACS Nano 2018, 12, 8104–8114. 10.1021/acsnano.8b02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang J.; Park J.; Lee J. H.; Won N.; Nam J.; Lim J.; Chang B. Y.; Lee H. J.; Chon B.; Shin J.; Park J. B.; Choi J. H.; Cho K.; Park S. M.; Joo T.; Kim S. ZnTe/ZnSe (core/shell) type-II quantum dots: their optical and photovoltaic properties. Chem. Mater. 2010, 22, 233–240. 10.1021/cm9027995. [DOI] [Google Scholar]
- Xu S.; Ziegler J.; Nann T. Rapid synthesis of highly luminescent InP and InP/ZnS nanocrystals. J. Mater. Chem. 2008, 18, 2653–2656. 10.1039/b803263g. [DOI] [Google Scholar]
- Bahmani Jalali H.; Karatum O.; Melikov R.; Dikbas U. M.; Sadeghi S.; Yildiz E.; Dogru I. B.; Ozgun Eren G.; Ergun C.; Sahin A.; Kavakli I. H.; Nizamoglu S. Biocompatible Quantum Funnels for Neural Photostimulation. Nano Lett. 2019, 19, 5975–5981. 10.1021/acs.nanolett.9b01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B. G.; Sadeghi S.; Melikov R.; Aria M. M.; Jalali H. B.; Ow-Yang C. W.; Nizamoglu S. Structural control of InP/ZnS core/shell quantum dots enables high-quality white LEDs. Nanotechnology 2018, 29, 345605. 10.1088/1361-6528/aac8c9. [DOI] [PubMed] [Google Scholar]
- Smith A. M.; Mohs A. M.; Nie S. Tuning the optical and electronic properties of colloidal nanocrystals by lattice strain. Nat. Nanotechnol. 2009, 4, 56–63. 10.1038/nnano.2008.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatum O.; Jalali H. B.; Sadeghi S.; Melikov R.; Srivastava S. B.; Nizamoglu S. Light-Emitting Devices Based on Type-II InP/ZnO Quantum Dots. ACS Photonics 2019, 6, 939–946. 10.1021/acsphotonics.8b01618. [DOI] [Google Scholar]
- Shirasaki Y.; Supran G. J.; Bawendi M. G.; Bulović V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 2013, 7, 13–23. 10.1038/nphoton.2012.328. [DOI] [Google Scholar]
- Dai X.; Zhang Z.; Jin Y.; Niu Y.; Cao H.; Liang X.; Chen L.; Wang J.; Peng X. Solution-processed, high-performance light-emitting diodes based on quantum dots. Nature 2014, 515, 96–99. 10.1038/nature13829. [DOI] [PubMed] [Google Scholar]
- Liu J. F.; Bei Y. Y.; Wu H. P.; Shen D.; Gong J. Z.; Li X. G.; Wang Y. W.; Jiang N. P.; Jiang J. Z. Synthesis of relatively monodisperse ZnO nanocrystals from a precursor zinc 2, 4-pentanedionate. Mater. Lett. 2007, 61, 2837–2840. 10.1016/j.matlet.2007.03.028. [DOI] [Google Scholar]
- Barak Y.; Meir I.; Shapiro A.; Jang Y.; Lifshitz E. Fundamental properties in colloidal quantum dots. Adv. Mater. 2018, 30, 1801442. 10.1002/adma.201801442. [DOI] [PubMed] [Google Scholar]
- Li L. S.; Pradhan N.; Wang Y.; Peng X. High quality ZnSe and ZnS nanocrystals formed by activating zinc carboxylate precursors. Nano Lett. 2004, 4, 2261–2264. 10.1021/nl048650e. [DOI] [Google Scholar]
- Rawalekar S.; Kaniyankandy S.; Verma S.; Ghosh H. N. Effect of Surface States on Charge-Transfer Dynamics in Type II CdTe/ZnTe Core-Shell Quantum Dots: A Femtosecond Transient Absorption Study. J. Phys. Chem. C 2011, 115, 12335–12342. 10.1021/jp202916v. [DOI] [Google Scholar]
- Sadeghi S.; Bahmani Jalali H.; Srivastava S. B.; Melikov R.; Baylam I.; Sennaroglu A.; Nizamoglu S. High-Performance, Large-Area, and Ecofriendly Luminescent Solar Concentrators Using Copper-Doped InP Quantum Dots. Iscience 2020, 23, 101272. 10.1016/j.isci.2020.101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatter O.Scattering Methods and Their Application in Colloid and Interface Science; Elsevier, 2018. [Google Scholar]
- Fritz G.; Glatter O. Structure and interaction in dense colloidal systems: evaluation of scattering data by the generalized indirect Fourier transformation method. J. Phys.: Condens. Matter 2006, 18, S2403. 10.1088/0953-8984/18/36/s14. [DOI] [Google Scholar]
- Fritz G.; Bergmann A. Interpretation of small-angle scattering data of inhomogeneous ellipsoids. J. Appl. Crystallogr. 2004, 37, 815–822. 10.1107/s0021889804017959. [DOI] [Google Scholar]
- Ludescher L.; Dirin D. N.; Kovalenko M. V.; Sztucki M.; Boesecke P.; Lechner R. T. Impact of crystal structure and particles shape on the photoluminescence intensity of CdSe/CdS core/shell nanocrystals. Front. Chem. 2019, 6, 672. 10.3389/fchem.2018.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantele G.; Piacente G.; Ninno D.; Iadonisi G. Optical anisotropy of ellipsoidal quantum dots. Phys. Rev. B: Condens. Matter Mater. Phys. 2002, 66, 113308. 10.1103/physrevb.66.113308. [DOI] [Google Scholar]
- van den Broek M.; Peeters F. M. Confined states in two-dimensional flat elliptic quantum dots and elliptic quantum wires. Phys. E 2001, 11, 345–355. 10.1016/s1386-9477(01)00169-2. [DOI] [Google Scholar]
- Lechner R. T.; Fritz-Popovski G.; Yarema M.; Heiss W.; Hoell A.; Schülli T. U.; Primetzhofer D.; Eibelhuber M.; Paris O. Crystal phase transitions in the shell of PbS/CdS core/shell nanocrystals influences photoluminescence intensity. Chem. Mater. 2014, 26, 5914–5922. 10.1021/cm502521q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virieux H.; Le Troedec M.; Cros-Gagneux A.; Ojo W.-S.; Delpech F.; Nayral C.; Martinez H.; Chaudret B. InP/ZnS nanocrystals: coupling NMR and XPS for fine surface and interface description. J. Am. Chem. Soc. 2012, 134, 19701–19708. 10.1021/ja307124m. [DOI] [PubMed] [Google Scholar]
- Kazmerski L. L.; Ireland P. J.; Sheldon P.; Chu T. L.; Chu S. S.; Lin C. L. Comparison of low-temperature oxides on polycrystalline InP by AES, SIMS, and XPS. J. Vac. Sci. Technol. 1980, 17, 1061–1066. 10.1116/1.570591. [DOI] [Google Scholar]
- Pietra F.; De Trizio L.; Hoekstra A. W.; Renaud N.; Prato M.; Grozema F. C.; Baesjou P. J.; Koole R.; Manna L.; Houtepen A. J. Tuning the lattice parameter of In X Zn Y P for highly luminescent lattice-matched core/shell quantum dots. ACS Nano 2016, 10, 4754–4762. 10.1021/acsnano.6b01266. [DOI] [PubMed] [Google Scholar]
- Xi L.; Cho D.-Y.; Besmehn A.; Duchamp M.; Grützmacher D.; Lam Y. M.; Kardynał B. E. Effect of zinc incorporation on the performance of red light emitting InP core nanocrystals. Inorg. Chem. 2016, 55, 8381–8386. 10.1021/acs.inorgchem.6b00747. [DOI] [PubMed] [Google Scholar]
- Granada-Ramirez D. A.; Arias-Cerón J. S.; Pérez-González M.; Luna-Arias J. P.; Cruz-Orea A.; Rodríguez-Fragoso P.; Herrera-Pérez J. L.; Gómez-Herrera M. L.; Tomás S. A.; Vázquez-Hernández F.; Durán-Ledezma A. A.; Mendoza-Alvarez J. G. Chemical synthesis and optical, structural, and surface characterization of InP-In2O3 quantum dots. Appl. Surf. Sci. 2020, 530, 147294. 10.1016/j.apsusc.2020.147294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra P.; Dung M. X.; Choi J.-K.; Jeong S.-H.; Jeong H.-D. Effects of curing temperature on the optical and charge trap properties of InP quantum dot thin films. Bull. Korean Chem. Soc. 2011, 32, 263–272. 10.5012/bkcs.2011.32.1.263. [DOI] [Google Scholar]
- Karatum O.; Eren G. O.; Melikov R.; Onal A.; Ow-Yang C. W.; Sahin M.; Nizamoglu S. Quantum dot and electron acceptor nano-heterojunction for photo-induced capacitive charge-transfer. Sci. Rep. 2021, 11, 2460. 10.1038/s41598-021-82081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J.; Stickle W.; Sobol P.; Bomben K.. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; PerkinElmer Corporation, 1995. [Google Scholar]
- Gao D.; Zhang Z.; Fu J.; Xu Y.; Qi J.; Xue D. Room temperature ferromagnetism of pure ZnO nanoparticles. J. Appl. Phys. 2009, 105, 113928. 10.1063/1.3143103. [DOI] [Google Scholar]
- Liang Y.-C.; Wang C.-C. Surface crystal feature-dependent photoactivity of ZnO–ZnS composite rods via hydrothermal sulfidation. RSC Adv. 2018, 8, 5063–5070. 10.1039/c7ra13061a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. I.; Lee S.; Kim S. K.; Kim Y.-I.; Cho D. W.; Khan M. M.; Sohn Y. Fabrication of ZnO, ZnS, Ag-ZnS, and Au-ZnS microspheres for photocatalytic activities, CO oxidation and 2-hydroxyterephthalic acid synthesis. J. Alloys Compd. 2016, 675, 46–56. 10.1016/j.jallcom.2016.03.070. [DOI] [Google Scholar]
- Kim T.; Kim S. W.; Kang M.; Kim S.-W. Large-scale synthesis of InPZnS alloy quantum dots with dodecanethiol as a composition controller. J. Phys. Chem. Lett. 2012, 3, 214–218. 10.1021/jz201605d. [DOI] [Google Scholar]
- Baylam I.; Cizmeciyan M. N.; Kakenov N.; Kocabas C.; Sennaroglu A. Ultrafast spectroscopy of voltage reconfigurable graphene saturable absorbers in the visible and near infrared. 2D Materials 2019, 6, 035013. 10.1088/2053-1583/ab1532. [DOI] [Google Scholar]
- Zhu H.; Song N.; Rodríguez-Córdoba W.; Lian T. Wave function engineering for efficient extraction of up to nineteen electrons from one CdSe/CdS quasi-type II quantum dot. J. Am. Chem. Soc. 2012, 134, 4250–4257. 10.1021/ja210312s. [DOI] [PubMed] [Google Scholar]
- Rawalekar S.; Kaniyankandy S.; Verma S.; Ghosh H. N. Ultrafast Charge Carrier Relaxation and Charge Transfer Dynamics of CdTe/CdS Core– Shell Quantum Dots as Studied by Femtosecond Transient Absorption Spectroscopy. J. Phys. Chem. C 2010, 114, 1460–1466. 10.1021/jp909118c. [DOI] [Google Scholar]
- Dutta A.; Bera R.; Ghosh A.; Patra A. Ultrafast Carrier Dynamics of Photo-Induced Cu-Doped CdSe Nanocrystals. J. Phys. Chem. C 2018, 122, 16992–17000. 10.1021/acs.jpcc.8b05422. [DOI] [Google Scholar]
- Peng P.; Sadtler B.; Alivisatos A. P.; Saykally R. J. Exciton Dynamics in CdS– Ag2S Nanorods with Tunable Composition Probed by Ultrafast Transient Absorption Spectroscopy. J. Phys. Chem. C 2010, 114, 5879–5885. 10.1021/jp9116722. [DOI] [Google Scholar]
- Jang E.; Kim Y.; Won Y.-H.; Jang H.; Choi S.-M. Environmentally Friendly InP-Based Quantum Dots for Efficient Wide Color Gamut Displays. ACS Energy Lett. 2020, 5, 1316–1327. 10.1021/acsenergylett.9b02851. [DOI] [Google Scholar]
- Klimov V.; Hunsche S.; Kurz H. Biexciton effects in femtosecond nonlinear transmission of semiconductor quantum dots. Phys. Rev. B: Condens. Matter Mater. Phys. 1994, 50, 8110. 10.1103/physrevb.50.8110. [DOI] [PubMed] [Google Scholar]
- Klimov V. I.; McBranch D. W.; Leatherdale C. A.; Bawendi M. G. Electron and hole relaxation pathways in semiconductor quantum dots. Phys. Rev. B: Condens. Matter Mater. Phys. 1999, 60, 13740. 10.1103/physrevb.60.13740. [DOI] [Google Scholar]
- Burda C.; Link S.; Green T. C.; El-Sayed M. A. New transient absorption observed in the spectrum of colloidal CdSe nanoparticles pumped with high-power femtosecond pulses. J. Phys. Chem. B 1999, 103, 10775–10780. 10.1021/jp991503y. [DOI] [Google Scholar]
- Sadeghi S.; Ganesh Kumar B.; Melikov R.; Mohammadi Aria M.; Bahmani Jalali H.; Nizamoglu S. Quantum dot white LEDs with high luminous efficiency. Optica 2018, 5, 793–802. 10.1364/optica.5.000793. [DOI] [Google Scholar]
- McDonald J. C.; Whitesides G. M. Poly (dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- Cai D. K.; Neyer A.; Kuckuk R.; Heise H. M. Optical absorption in transparent PDMS materials applied for multimode waveguides fabrication. Opt. Mater. 2008, 30, 1157–1161. 10.1016/j.optmat.2007.05.041. [DOI] [Google Scholar]
- Schubert E. F.; Gessmann T.; Kim J. K.. Light emitting diodes. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley, 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.