Abstract
The voltage-dependent anion channel (VDAC) is the most abundant protein of the mitochondrial outer membrane (MOM) where it regulates transport of ions and metabolites in and out of the organelle. VDAC function is extensively studied in a lipid bilayer system that allows conductance monitoring of reconstituted channels under applied voltage. The process of switching from a high-conductance state, open to metabolites, to a variety of low-conducting states, which excludes metabolite transport, is termed voltage gating and the mechanism remains poorly understood. Recent studies have implicated the involvement of the membrane-solvated residue E73 in the gating process through β-barrel destabilization. However, there has been no direct experimental evidence of E73 involvement in VDAC1 voltage gating. Here, using electrophysiology measurements, we exclude the involvement of E73 in murine VDAC1 (mVDAC1) voltage gating process. With an established protocol of assessing voltage gating of VDACs reconstituted into planar lipid membranes, we definitively show that mVDAC1 gating properties do not change when E73 is replaced by either a glutamine or an alanine. We further demonstrate that cholesterol has no effect on mVDAC1 gating characteristics, where E73 is coordinating residue in the cholesterol binding site. In contrast, we found a pronounced gating effect based on the charge of the phospholipid headgroup, where the positive charge stimulates and negative charge suppresses gating. These findings call for critical evaluation of the existing models of VDAC gating and contribute to our understanding of VDAC role in control of MOM permeability and regulation of mitochondrial respiration and metabolism.
Keywords: Voltage-Dependent Anion Channel, beta-barrel channel gating, mitochondrial outer membrane, point mutations, cholesterol, phospholipids
1. Introduction
The voltage–dependent anion channel (VDAC) is the most abundant protein in the mitochondrial outer membrane (MOM). VDAC’s main role is facilitating the exchange of ions and respiratory substrates such as ATP and ADP across the MOM, thus influencing mitochondrial respiration [1]. Despite being one of the most well characterized β-barrel channels, this large metabolite transport channel continues to stir an active debate regarding its gating mechanism [2–5] and its roles in mitochondria metabolism [6], calcium signaling [7, 8] as well as apoptosis [9–12]. Given its diverse and vital physiological roles, it is not surprising that there are number of diseases associating VDAC in their pathologies [13–16]. The 3D structure of VDAC1 was resolved in 2008 [17–19] revealing the location of E73, buried in the middle of the lipid bilayer hydrocarbon core (Figure 1A), thus underscoring this charged residue unique environment and motivating the continuing focus on its investigation.
Figure 1. The residue E73 is buried in the membrane and conserved among VDAC from different species.
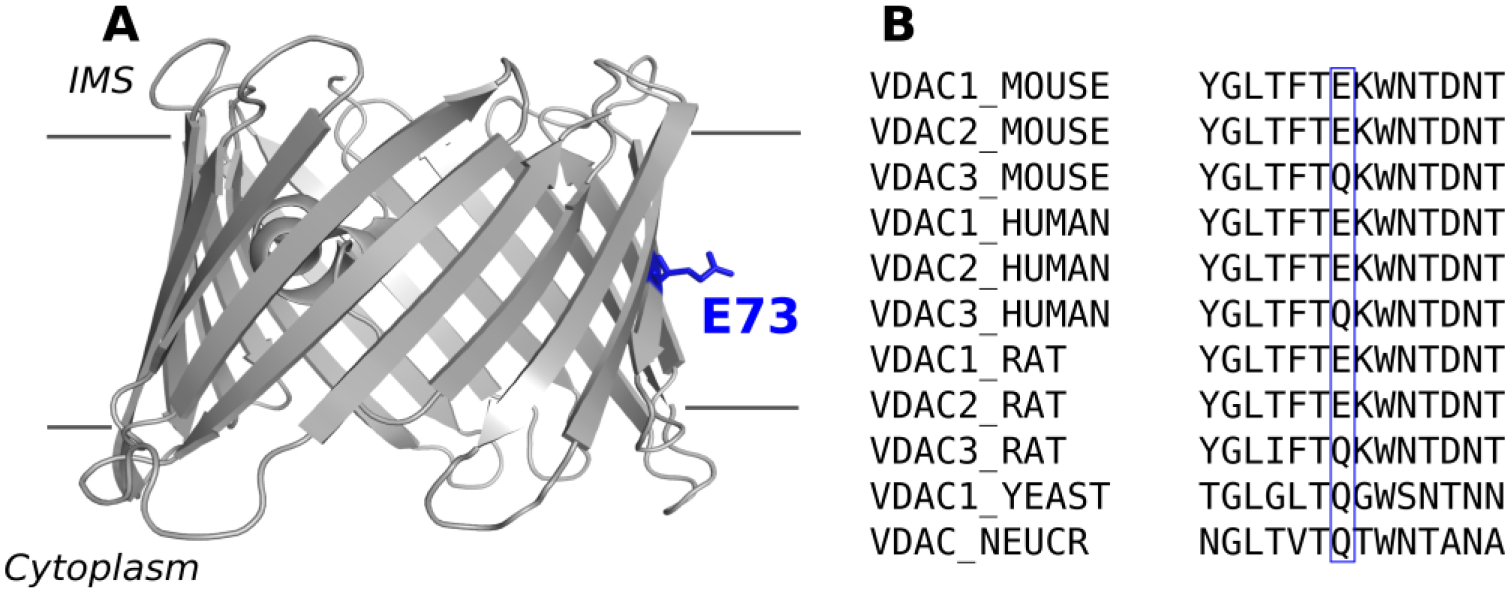
A) Cartoon representation of mVDAC1 (PDB ID 3EMN), view parallel to the membrane plane. The gray lines represent the limit of the membrane. B) Sequence alignment of different VDAC isoforms from mammals, yeast, and N. crassa around residue 73 (blue box).
There are 3 VDAC isoforms in mammals, which are known to have unique functional properties and tissue distributions [20]. One notable difference between mammalian VDAC isoforms is at residue 73, where isoforms 1 and 2 have a glutamate while isoform 3 has a non-charged glutamine residue—a feature conserved in both human and mouse VDAC as well as in yeast [20] and fungi [21] (Figure 1B). Using a combination of solution NMR spectroscopy with x-ray crystallography, Bayrhuber and colleagues showed that mutation of E73 in VDAC1 to a hydrophobic valine exhibited a reduction of β-barrel flexibility, presumably through stabilization of β-strands 1–4 [18]. Villinger and coauthors, using NMR spectroscopy with molecular dynamic (MD) simulations, showed that β-barrel dynamics are largely determined by the charge of E73 [22]. These and other studies concluded that β-barrel flexibility affects VDAC1 gating and, therefore, E73 influences the channel gating mechanism [18, 22, 23]. Supporting this hypothesis, a recent submicrosecond MD simulations of VDAC1 implicated E73 in voltage gating through its interaction with membrane lipids [24]. The importance of residue E73 goes beyond its involvement in structural stability of VDAC1. An NMR study identified multiple cholesterol binding sites [17] on VDAC1, which were later confirmed through computational studies [25] and more recently mapped to a binding pocket in murine VDAC1 (mVDAC1)—localized at residues E73, T83, and Y62—using photoaffinity labeling with mass spectrometry [26]. Importantly, a mutation of E73 to a glutamine abolished photolabeling thus confirming E73 as a specific cholesterol binding site residue. Similarly, this same group identified E73 as a binding site for the endogenous neurosteroid allopregnanolone [27], thus further emphasizing E73 functional significance as a specific binding site residue for natural steroids. In addition to E73 hypothesized role in channel gating and in binding of hydrophobic compounds, it has also been implicated in coordinating Ca2+ [28, 29] and binding of hexokinase-1 [10]. It is striking that one residue (E73) is implicated in such diverse structural and functional processes.
While it is intuitively clear how a charged residue, facing the lipid environment, could destabilize the β-barrel, as of yet there is no functional data demonstrating that this leads to a change in channel gating properties. Here, we focus on the role E73 plays in voltage gating for mVDAC1. Under applied voltage, VDAC switches from a high-conductance open state to a variety of lower conductance states that are referred to as the “closed” states [30, 31]. The closed states are impermeable to ATP and other metabolites [32–34] due to steric and electrostatic barriers that limit their exchange across the MOM leading to impairment of mitochondrial respiration and cell metabolism [1, 6, 31]. In addition, E73 has been proposed to have a regulatory role, however there has been no quantitative functional analysis verifying this hypothesis.
In this study, we reconstitute mVDAC1 into planar lipid bilayers and compare single-channel conductance and voltage-gating properties of wild-type (WT) mVDAC1 and mutants E73Q and E73A. We show—contrary to previously proposed mechanism—that E73 is not involved in VDAC gating. Voltage-dependent channel properties are consistent for the WT protein compared to neutral (glutamine) or a hydrophobic (alanine) substitution at position 73. In addition, although cholesterol has been shown to bind at position E73, it too does not affect gating properties of WT and E73Q mutant. Therefore, we conclude that E73 does not influence channel gating properties of mVDAC1. At the same time, we demonstrate that VDAC gating is markedly sensitive, among other physical properties explored earlier, to the lipid headgroup charge.
2. Materials and methods
2.1. Cloning, recombinant protein production and purification
The mutants were prepared using the QuickChange site-directed Mutagenesis Kit from Stratagene (Agilent Technologies), using the pQE9-mVDAC1 plasmid. Each mutant was verified by DNA sequencing. Expression and purification of recombinant proteins was described previously [19]. In short, mVDAC1 native and mutants were expressed in inclusion bodies using M15 Escherichia coli cells and purified using a two-step protocol where solubilized IB were purified using Talon Metal affinity column, and eluted proteins were refolded by a 3-step dialysis in presence of LDAO. Refolded proteins were then centrifuged (200,000 × g for 45 min) and injected on a size exclusion chromatography column (superdex 200, GE healthcare) in 150 mM NaCl, 20 mM Tris-HCl pH 8.0, 0.1 % LDAO to remove misfolded proteins. The refolded peak was used for functional studies.
2.2. VDAC1 reconstitution and conductance measurements
Planar lipid membranes were formed from soybean Polar Lipid Extract (PLE) with or without 25% cholesterol (by weight), or from lipid mixtures of dioleoyl-trimethylammonium-propane (DOTAP), dioleoyl-phosphatidylcholine (DOPC), dioleoyl-phosphatidylethanolamine (DOPE), and dioleoyl-phosphatidylglycerol (DOPG), in pentane, and channel currents were analyzed as described [35]. All lipids were purchased from Avanti Polar Lipids (Alabaster, AL). Soybean PLE lipid headgroup composition is shown in Table 1 in comparison with lipid composition of the outer membrane from rat liver mitochondria: Channel insertion was achieved by adding 0.1–2 μL of mVDAC1 diluted 100x in buffer containing 10 mM Tris, 50 mM KCl, 1mM EDTA, 15% (v/v) DMSO, 2.5% (v/v) Triton X-100, pH 7.0 (VDAC1 stock solution was 5 mg/ml in 0.1% LDAO) to the ~1.8 ml aqueous phase of 1 M or 150 mM KCl buffered with 5 mM HEPES at pH 7.4 in the cis compartment while stirring. Potential is defined as positive when it is greater at the side of VDAC addition (cis-side). Current recordings were performed as described previously [35] using an Axopatch 200B amplifier (Axon Instruments, Inc.) in the voltage-clamp mode. Single-channel data were filtered by a low pass 8-pole Butterworth filter (Model 900 Frequency Active Filter, Frequency Devices, Inc.) at 15 kHz and saved with a sampling frequency of 50 kHz and analyzed using pClamp 10.7 software (Axon Instruments, Inc.).
Table 1.
Phospholipid composition of Avanti Polar Lipids soybean (PLE) and MOM from rat liver mitochondria, expressed as percentage of total lipid
| Soybean PLE1 | Mitochondrial Outer Membrane2 | |
|---|---|---|
| Phosphatidylcholine | 45.7 | 54.7 |
| Phosphatidylethanolamine | 22.1 | 27.5 |
| Phosphatidylinositol | 18.4 | 13.4 |
| Phosphatidic acid | 6.9 | 0.4 |
| Unknown / other | 6.9 | 4.0 |
From Avanti Polar Lipids website.
From ref. 37.
VDAC voltage-dependent properties were assessed following the protocol previously devised [30, 35] in which gating is inferred from the channels response to a slow symmetrical 5 mHz triangular voltage wave of ± 60 mV amplitude from an Arbitrary Waveform Generator 33220A (Agilent). Data were acquired at a sampling frequency of 2 Hz and analyzed as described previously [35, 36] using pClamp 10.7 software. Analysis of VDAC voltage-gating was performed following published protocols [35, 36] using an algorithm developed in-house. In each experiment, current records were collected from membranes containing 20–800 channels in response to 5–10 periods of voltage waves, to assure a minimum of 100 channels per experiment. Only the part of the wave during which the channels were reopening was used for the subsequent analysis [5]. Given the variable number of channels during experiment, the average conductance (G) was normalized to the maximum conductance (Gmax). The probability of the channel to be open, Popen, was defined as:
| (1) |
where Gmax and Gmin are the maximum and minimum conductances, corresponding to the channels mostly open at small voltages (<10 mV) and the channels mostly closed at high voltages (>30 mV), respectively. Popen plots were fit according to the Boltzmann equation:
| (2) |
where V0 is the voltage at which half of the channels are open, n is the effective gating charge, and R, T, and F are the gas constant, absolute temperature, and Faraday constant, respectively.
3. Results
Native recombinant mVDAC1 and the E73A and E73Q mutants were purified by a two-step purification process. Figure 2A shows a representative size exclusion chromatography elution profile displaying a clear peak of refolded VDAC protein as an indication of the quality of mVDAC1 samples. The expected molecular weights for mVDAC1 WT and the two mutants (E73Q and E73A) are shown in SDS-PAGE in Figure 2B.
Figure 2. Purification of mVDAC1 WT and E73 mutants.
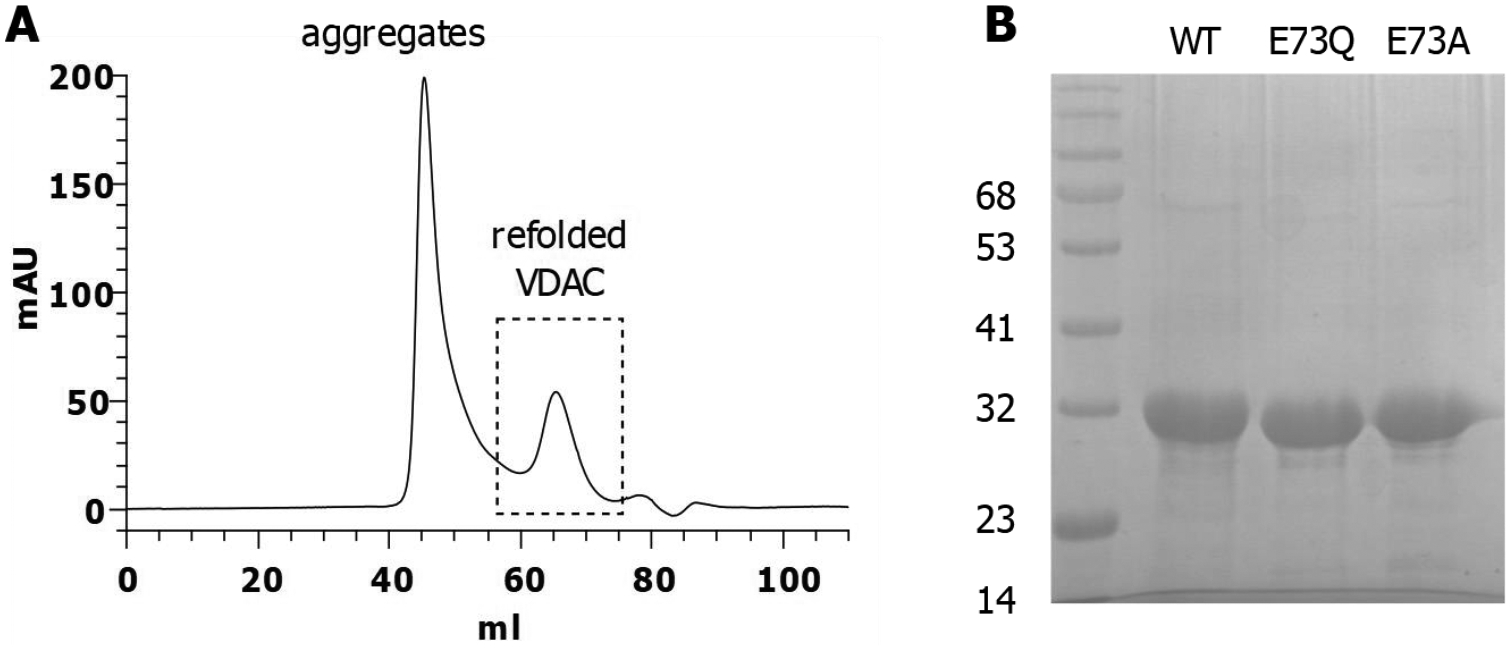
A) Size Exclusion Chromatography elution profile. The dashed box represents the homogeneous population of refolded proteins (~20 %) used for functional studies. B) 12% SDS-PAGE stained with Coomassie blue showing the three purified proteins.
Single- and multi-channel experiments were conducted on WT and the two mutants (E73Q and E73A) of mVDAC1, under identical experimental conditions to compare channel properties. For the membrane-forming lipid, a soybean PLE was used, which closely mimics the phospholipid polar headgroup composition of MOM of rat liver mitochondria [37] (Table 1) and provides a natural mixture of phospholipid acyl chains. It contains phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol and phosphatidic acid as its main components. Figure 3A shows single channel current traces obtained with the WT and the two mutants (E73Q and E73A) of mVDAC1 reconstituted into the planar lipid membrane. At low applied voltage (±10 mV), WT and both mutants exhibit the typical open state conductance for VDAC1 (4.0 ± 0.2 nS in 1 M KCl) [30]. As applied voltage is increased (±50 mV), WT and both mutants transition to multiple closed states again exhibiting typical VDAC gating behavior routinely observed on single channels of VDAC1 [3, 9, 38, 39]. Notably, all traces recorded at ±50 mV show more gating events at negative polarities, thus establishing a slight gating asymmetry typical for VDACs isolated from different species such as rat liver, fungus, and plant as well as for recombinant human VDAC1 [35, 36, 38, 40].
Figure 3. Mutations of E73 residue do not affect mVDAC1 voltage gating.
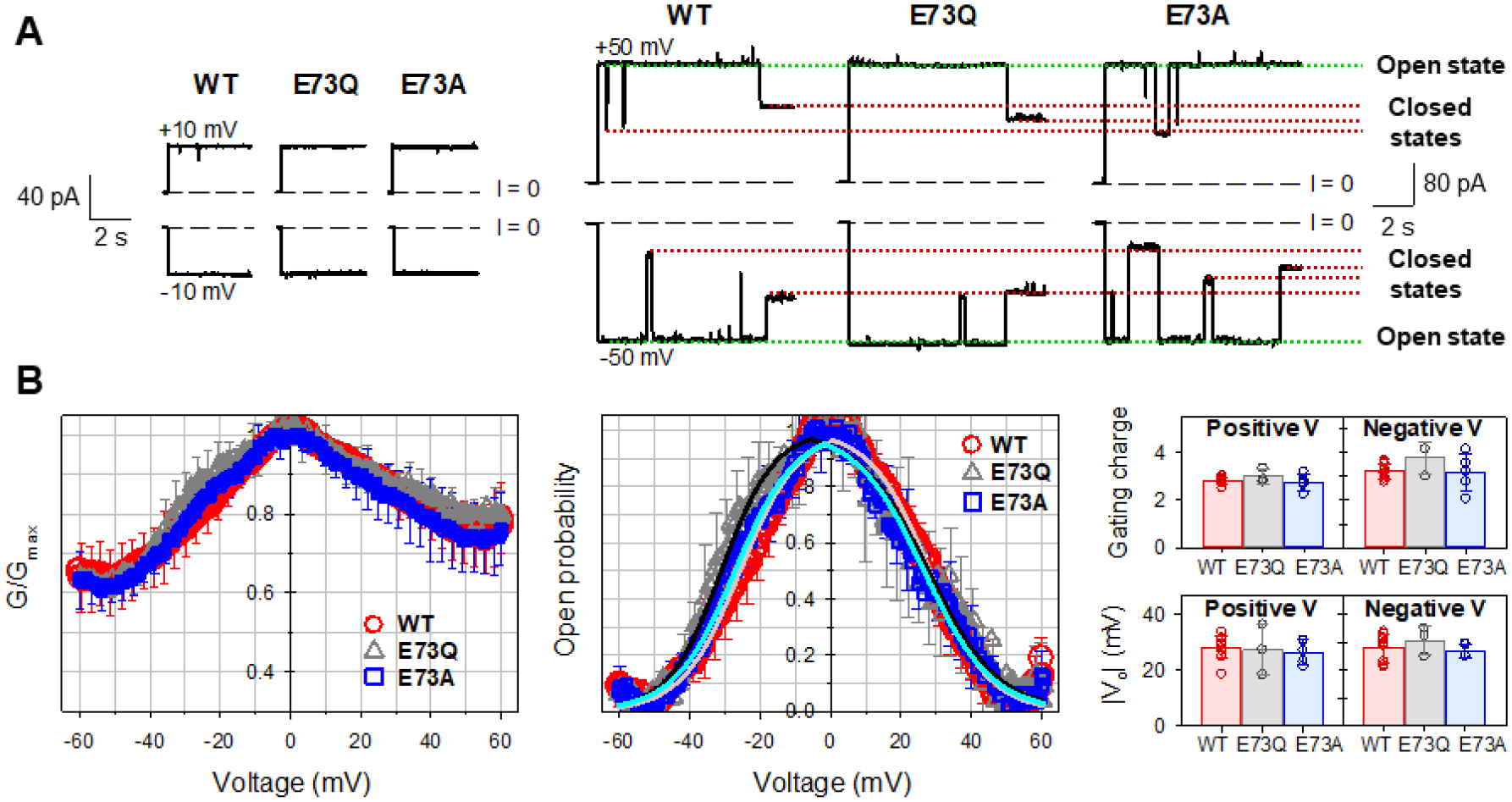
A, Representative single-channel current traces obtained with reconstituted mVDAC1 wild type (WT) and E73Q and E73A mutants at low (±10 mV) and high (±50 mV) applied voltages. Dashed line indicates zero current level. Current records were digitally filtered at 100 Hz using a low pass Bessel (8-pole) filter. B, Characteristic bell-shaped plots of normalized average conductance (left panel) and probability to be open (middle panel) as functions of the applied voltage for mVDAC1 WT, E73Q, and E73A mutants. Solid lines in the middle panel (light red for WT; black for E73Q; cyan for E73A) are the fits of Popen plots with the Boltzmann equation (eq. 2) using the effective gating charge, n, and the voltage at which half of the channels are open, V0, as fitting parameters, shown in the right panel. There is no significant difference between gating parameters n and V0 (p>0.05, t-test with unequal variance and two-tailed distribution). In all panels, the membrane-bathing solutions consisted of 1M KCl buffered with 5 mM HEPES at pH 7.4. Data are means of 3–9 experiments ± SD (here and elsewhere error bars shown every 5 points for clarity).
VDAC voltage gating has a stochastic nature [41, 42], meaning that the timing of a gating event of the same or another VDAC channel (Figure 3A) cannot be unambiguously predicted. Therefore, a consistent quantification of VDAC gating must rely on strong statistics, which we achieve using a well-established protocol where gating is acquired by monitoring the response of multiple VDAC channels (more than 100 in total) to a slowly changing periodic voltage wave [30, 35, 43]. The slow progressing voltage wave (over seconds) assures that the kinetics of channel reopening are much quicker (in the milliseconds range) providing reliable results independent of the voltage-applying protocol [5].
The normalized conductance plots (G/Gmax) versus applied voltage for mVDAC1 WT, E73Q, and E73A obtained on membranes containing many channels are shown in Figure 3B (left panel). All mVDAC1 samples display the characteristic bell-shaped voltage dependence of normalized conductance with all data sets (WT, E73Q, and E73A) overlapping within the error bars. As could be seen in single-channel recordings, there is a slight asymmetry in the minimum conductance (the closed state conductance (Gmin), showing lower Gmin values at negative potentials than at positive). To quantitatively describe VDAC gating, G vs V plots are presented as voltage dependences of open probabilities, Popen (eq. 1) (Figure 3B, middle panel). The gating parameters – the effective gating charge, n, and the voltage at which half of the channels are open, V0 – were obtained from the fitting of Popen vs V plot with the Boltzmann equation (eq. 2) (solid lines in Figure 3B, middle panel) and are shown in the right panel in Figure 3B. There is no significant difference between gating parameters obtained for mVDAC1 WT and the two mutants. Altogether, the data presented in Figure 3A and B demonstrate that mVDAC1 gating is not affected by substitution of residue E73 with a neutral (E73Q) or hydrophobic (E73A) residue.
Recently, E73 was identified as an essential component for a cholesterol binding site in mVDAC1, that displayed greatly reduced affinities in the E73Q and E73A mutant backgrounds [26], thus assigning a specific role for E73 in VDAC-cholesterol interaction. To quantify the effects of cholesterol on mVDAC1 gating, and to further assess the role of residue E73, we evaluated the voltage-gating properties of mVDAC1 WT and E73Q mutant in the membranes formed from PLE and 25% (w/w) cholesterol. Similar molar fractions of cholesterol were used in the study by Mlayeh and coauthors where the effect of sterols on plant VDAC voltage gating was characterized [44]. The voltage dependences of normalized conductance obtained in membranes with cholesterol are indistinguishable for both mVDAC1 WT and E73Q mutant (left panels of Figure 4A and B, respectively). Correspondingly, there is no significant difference in the open probabilities and gating parameters calculated from the open probability plots obtained with or without cholesterol, for both mVDAC1 WT and E73Q mutant (middle and right panels of Figure 4). Thus, cholesterol at relative concentrations up to 25% has no noticeable effect on mVDAC1 gating.
Figure 4. Cholesterol does not affect mVDAC1 voltage gating.
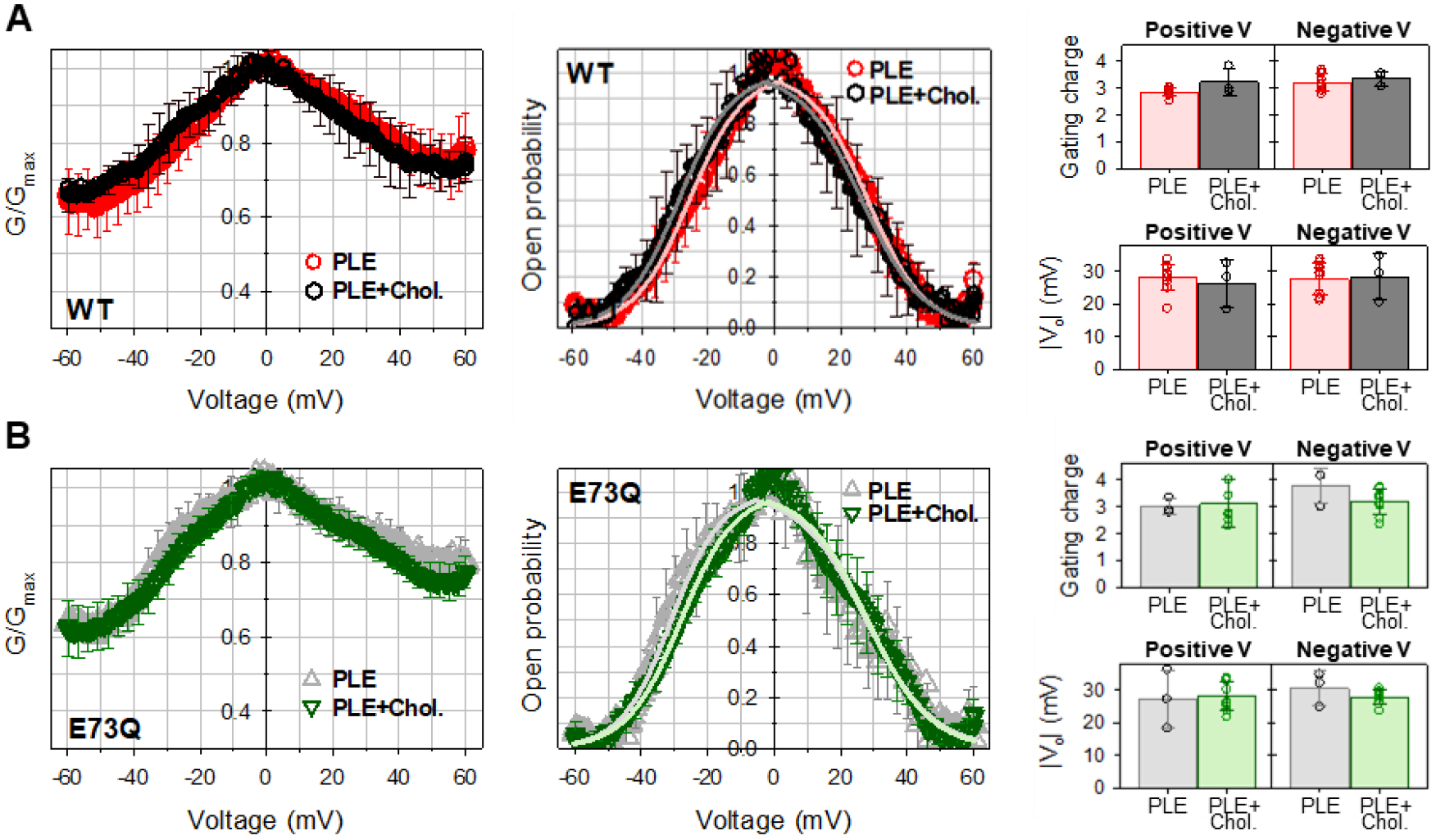
Normalized conductance (left panels), open probability (middle panels), and gating parameters (right panels) obtained for mVDAC1 WT (A) and E73Q mutant (B) on multichannel membranes formed of PLE in the absence and presence of 25% (w/w) of cholesterol in 1 M KCl. In all panels, the membrane-bathing solutions were buffered with 5 mM HEPES at pH 7.4. Data are means of 3–9 experiments ± SD. Solid lines in middle panels (in A: light red for PLE and grey for PLE with cholesterol; in B: light grey for PLE and light green for PLE with cholesterol) are the fits of Popen plots with the Boltzmann equation (eq. 2) and fitting parameters n and V0 are shown in right panels. Right panels: There is no significant difference between n and V0 gating parameters (p>0.05, t-test with unequal variance and two-tailed distribution).
We further verified these findings under more physiological like conditions by lowering the salt concentration to 150 mM KCl (Figure 5). Under these conditions, there is less gating asymmetry and a slightly higher Gmin than observed in 1 M KCl (compare left panels in Figure 5 with left panels in Figures 3B and 4). In addition, the open probability and the calculated gating parameters do not change when lowering salt concentration (compare middle and right panels in Figure 5 with corresponding panels in Figure 3B and 4). Despite these minimal differences, there is no significant influence of residue E73Q mutation (Figure 5A) nor of cholesterol (Figure 5B) on mVDAC1 gating at physiological lower salt concentration.
Figure 5. Mutation of E73Q or presence of cholesterol in the membrane do not affect mVDAC1 gating in 150 mM KCl.
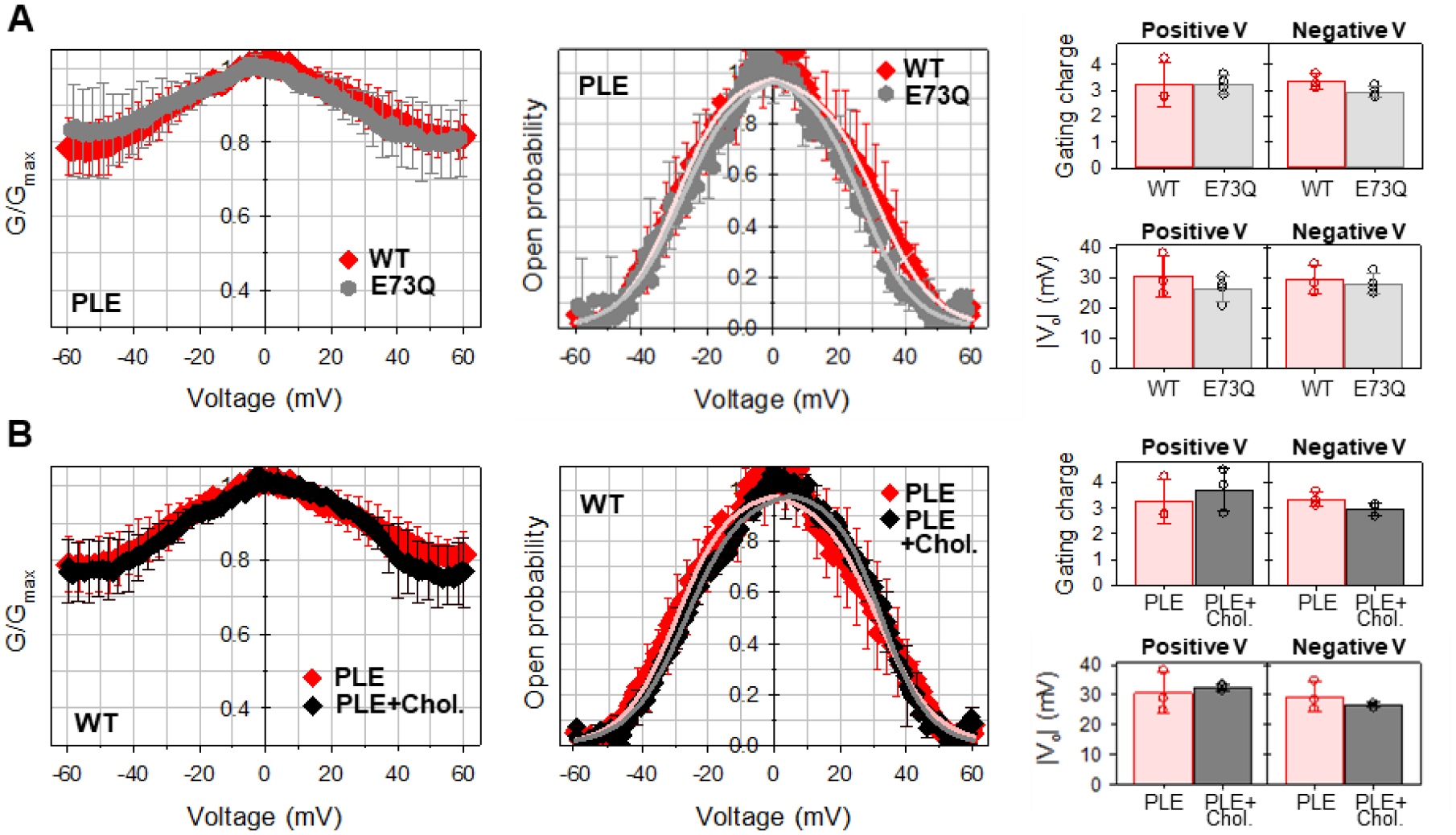
Normalized conductance (left panels), open probability (middle panels), and calculated gating parameters (right panels) obtained for mVDAC1 WT (A, B) and E73Q mutant (A) on multichannel membranes formed of PLE without (A, B) or with 25% (w/w) of cholesterol (B). In all panels, the membrane-bathing solution was 150 mM KCl buffered with 5 mM HEPES at pH 7.4. Data are means of 3–9 experiments ± SD. Solid lines in middle panels in A and B (in A: light red for WT and light grey for E73Q; in B: light red for PLE and grey for PLE with cholesterol) are the fits of Popen plots with the Boltzmann equation (eq. 2) and fitting parameters n and V0 are shown in right panels. Right panels: There is no significant difference between n and V0 gating parameters (p>0.05, t-test with unequal variance and two-tailed distribution).
Although cholesterol does not impact mVDAC1 gating, the phospholipid composition has a substantial effect as seen in Figure 6A. While maintaining a consistent acyl chain, the head group charge was varied using mixtures of dioleoyl-PC (DOPC) and dioleoyl-PE (DOPE) with a negatively charged dioleoyl-PG (DOPG) or with synthetic positively charged dioleoyl-trimethylammonium-propane (DOTAP). DOTAP was chosen as an antipode of DOPG to contrast the effect of lipid headgroup charge on VDAC gating. The purpose of these experiments was to test the effect of lipid headgroup charge on VDAC gating and to compare these results with our finding that cholesterol, at comparably high content (25% w/w) in the lipid membrane, does not affect gating (Figures 4 and 5B). Thus, while there is essentially no gating in the negatively charged membranes (green triangles in Figure 6A), in the positively charged membranes gating is strong (cyan squares) with Gmin decrease for 50–60% in comparison with the negatively charged membranes, and gating is intermediate in the net neutral membranes (yellow diamonds). Enhanced gating in cationic DOTAP/DOPC/DOPE membranes is also seen in single-channel experiments as shown in Figure 6B where transitions to the closed states occur at relatively low voltages of 20 and 30 mV (compare with single-channel traces at 50 mV in Figure 3A).
Figure 6. The charge of lipid headgroup affects mVDAC1 WT voltage gating.
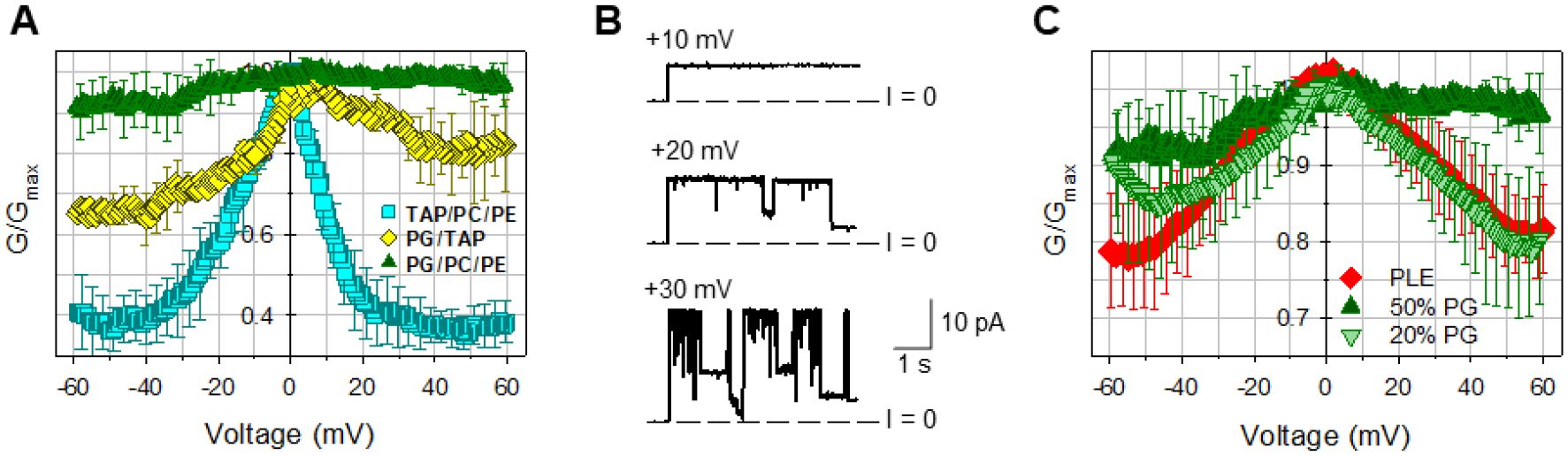
A, Normalized conductance obtained on multichannel membranes formed of anionic (DOPG/DOPC/DOPE 2:1:1), cationic (DOTAP/DOPC/DOPE 2:1:1), or neutral (DOPG/DOTAP 1:1) lipid mixtures. B, Representative single-channel traces in cationic DOTAP/DOPC/DOPE 2:1:1 membrane. C, mVDAC1 gating decreases with negative lipid content in the membranes. Normalized conductance obtained on multichannel membranes with 20 and 50% of the negatively charged DOPG in DOPC/DOPE mixtures in comparison with membranes formed of natural soybean PLE with ~25% of charged lipids. The membrane-bathing solutions consisted of 150 mM KCl buffered with 5 mM HEPES at pH 7.4. In A and C, data are means of 3–9 experiments ± SD.
Figure 6C compares the effect of different contents of negatively charged lipid on mVDAC1 gating. While mVDAC1 gating is very poor in membranes with a 50% DOPG, it is partially recovered when DOPG content is reduced to a more physiological 20%. Interestingly, in the membranes with 20% DOPG the gating resembles that obtained on the membranes made of soybean PLE (Figure 6C), which contains ~ 25% of negatively charged lipids, although fatty acid composition (a natural mixture of acyl chains) is different from pure DOPG.
4. Discussion
VDAC is a complex channel with many diverse properties. Along with measuring single-channel conductance and selectivity, monitoring VDAC’s voltage gating has become the gold standard to assess VDAC’s function. It has successfully identified individual amino acid residues involved in VDAC’s ion selectivity and voltage gating [45, 46]. Most recently, the membrane-solvated residue E73 has been proposed to be implicated in the gating mechanism through β-barrel destabilization [18, 22, 23], yet the data substantiating this claim is still lacking. Zaid and coauthors demonstrated that E73Q mVDAC1 could characteristically gate when reconstituted in planar lipid membranes [10]. However, there is no quantification of gating parameters nor direct comparison between the WT and E73Q mutant gating properties. Nevertheless, other groups in following publications [18, 22] interpreted this data as an established difference between WT and E73Q mutant gating, despite the lack of supporting data. Here, using electrophysiology measurements with dependable statistics, we exclude the involvement of E73 in the voltage gating process. Both single- and multi-channel data demonstrate qualitatively and quantitatively similar behavior for WT mVDAC1 and the E73A and E73Q mutants. These data are consistent with electrophysiological recordings on VDACs isolated from N. crassa and yeast which have a glutamine at position 73 and display a typical voltage-gating behavior in reconstituted experiments [35, 46, 47].
In addition to its putative role in gating, E73 coordinates a binding site for cholesterol and endogenous neurosteroids along the hydrophobic surface of the VDAC pore. The MOM contains a relatively high content of cholesterol (up to 10 % of total MOM lipid) in comparison to the inner membrane (~ 2%) [37, 48] and changes in lipidic environment have been previously shown to affect voltage gating of VDAC isolated from N. crassa and bean seeds [35, 40], thus an impact of cholesterol binding may alter the gating properties. Again, using electrophysiology measurements we have shown that the doping of cholesterol into lipid bilayers does not cause any measurable alterations in the gating properties of WT mVDAC1 or the E73Q mutant. This indicates that the reported cholesterol binding at E73 [26] does not affect channel gating characteristics, which, given our result with E73 VDAC mutation, was to be expected.
These results were also verified under more physiologically relevant conditions with the lower salt concentration (150 mM KCl), where a slight reduction of channel gating is observed. A decrease of voltage gating in low salt has been shown for plant VDAC [40, 44] and interpreted as a result of a different screening of VDAC charged residues (presumably located in the loops exposed to the lipid headgroups/water interface) and phospholipid headgroup charges, which changes the protein-lipid interactions and thus affects the channel voltage gating. Still, the reduced gating in physiological low salt concentration is independent of residue E73 as well as of the presence of cholesterol in the membrane. The absence of cholesterol effect agrees with a previously published study on plant VDACs which were unresponsive to mammalian and plant sterols (sitosterol) [44]. However, the lack of effect on mVDAC1 gating properties reported here does not exclude a different role of cholesterol bound to E73 in VDAC function, such as regulation of the interaction with partner proteins in the MOM or dimerization.
In contrast to cholesterol, mVDAC1 gating is sensitive to the charge of the phospholipid headgroup, where negatively charged lipids prevent gating and positively charged lipids promote it. mVDAC1 gating in the membranes made with 20% DOPG resembles those obtained with soybean PLE membranes, which contains ~25% of negatively charged lipids, but has very different acyl chain content in comparison with pure DOPG. These results suggest that phospholipid headgroup charge influences mVDAC1 gating profoundly while acyl chain composition has a residual effect. Interestingly, these results do not support a previously proposed model of VDAC gating where under applied voltage the positively-charged voltage sensor domain translocates out of the pore towards the membrane surface [21, 49, 50], where it should be exposed to the membrane surface charge. Consequently, according to this model, negatively charged lipids should attract a positively-charged domain and promote gating. An opposite situation is expected for positively-charged membranes. Such obvious inconsistency between the experimental data and predicted gating models underscores the urgency in obtaining structural and computational data on the VDAC gating mechanism and elusive closed states of this channel. Our results clearly demonstrate that phospholipid charge is another modulator of VDAC gating in addition to the previously shown effects of lipid packing stress [35] and pH alterations [36, 51]. Sensitivity to phospholipid headgroup charge could arise from specific protein-lipid interactions, as previously was proposed for plant VDAC gating [40]. Thus, membrane phospholipid composition has a pronounced effect on VDAC gating, while cholesterol has not. It is well-known that the mitochondrial lipid composition changes in response to a number of physiological conditions including apoptosis [52], neurodegeneration, and in various types of cancers [53–55], providing a key to the biological relevance of our in vitro results.
The data presented here answer the question of the putative role of E73 residue in VDAC gating. It is important to note that although we have shown that E73 and cholesterol composition has no effect on VDAC gating, there may be other mitochondrial regulatory functions associated with them. The protonation state of E73 was recently shown to regulate VDAC1 dimerization where upon acidification—often occurring during cellular stressful events like apoptosis—E73 becomes protonated forming a specific pH-dependent dimer [56]. Similarly, E73 has been shown to influence apoptotic cell death by coordinating interaction with the cytosolic protein hexokinase-I influencing its recruitment to the MOM [10]. In mammalian VDACs, the established interaction of E73 with cholesterol, as well as with other ligands, may modulate mitochondria functions in vivo, although channel gating does not appear to be one of them. Emerging data implicate VDAC, in complex with the 18 kDa Translocator Protein (TSPO), the steroidogenic acute regulatory program (STAR), and 14-3-3ε signaling protein, in trafficking cholesterol and natural steroids across MOM and in hormone stimulation [57–59]. Further testing is required to determine if E73 is essential for TSPO-orchestrated cholesterol trafficking.
Acknowledgements
M.Q.M., D.J., S.M.B., and T.K.R. were supported by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development. L.B. and J.A were supported by the NIH R01GM078844, R01GM124783, and the UCLA Cardiovascular Theme Discovery Award.
Abbreviations:
- VDAC
Voltage Dependent Anion Channel
- MOM
Mitochondrial Outer Membrane
- PLE
Polar Lipid Extract
- DOPC
dioleoyl-phosphatidylcholine
- DOPE
dioleoyl-phosphatidylethanolamine
- DOPG
dioleoyl-phosphatidylglycerol
- DOTAP
dioleoyl-trimethylammonium-propane
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- [1].Lemasters JJ, Holmuhamedov E, Voltage-dependent anion channel (VDAC) as mitochondrial governator--thinking outside the box, Biochim Biophys Acta, 1762 (2006) 181–190. [DOI] [PubMed] [Google Scholar]
- [2].Zachariae U, Schneider R, Briones R, Gattin Z, Demers JP, Giller K, Maier E, Zweckstetter M, Griesinger C, Becker S, Benz R, de Groot BL, Lange A, β-Barrel mobility underlies closure of the voltage-dependent anion channel, Structure, 20 (2012) 1540–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Teijido O, Ujwal R, Hillerdal CO, Kullman L, Rostovtseva TK, Abramson J, Affixing N-terminal alpha-helix to the wall of the voltage-dependent anion channel does not prevent its voltage gating, J Biol Chem, 287 (2012) 11437–11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mertins B, Psakis G, Grosse W, Back KC, Salisowski A, Reiss P, Koert U, Essen LO, Flexibility of the N-terminal mVDAC1 segment controls the channel’s gating behavior, PLoS One, 7 (2012) e47938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rappaport SM, Teijido O, Hoogerheide DP, Rostovtseva TK, Berezhkovskii AM, Bezrukov SM, Conductance hysteresis in the voltage-dependent anion channel, Eur Biophys J, 44 (2015) 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lemasters JJ, Holmuhamedov EL, Czerny C, Zhong Z, Maldonado EN, Regulation of mitochondrial function by voltage dependent anion channels in ethanol metabolism and the Warburg effect, Biochim Biophys Acta, 1818 (2012) 1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tan W, Colombini M, VDAC closure increases calcium ion flux, Biochim Biophys Acta, 1768 (2007) 2510–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G, Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC), J Biol Chem, 281 (2006) 17347–17358. [DOI] [PubMed] [Google Scholar]
- [9].Rostovtseva TK, Tan W, Colombini M, On the role of VDAC in apoptosis: fact and fiction, J Bioenerg Biomembr, 37 (2005) 129–142. [DOI] [PubMed] [Google Scholar]
- [10].Zaid H, Abu-Hamad S, Israelson A, Nathan I, Shoshan-Barmatz V, The voltage-dependent anion channel-1 modulates apoptotic cell death, Cell Death Differ, 12 (2005) 751–760. [DOI] [PubMed] [Google Scholar]
- [11].McCommis KS, Baines CP, The role of VDAC in cell death: friend or foe?, Biochim Biophys Acta, 1818 (2012) 1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Galluzzi L, Kroemer G, Mitochondrial apoptosis without VDAC, Nat Cell Biol, 9 (2007) 487–489. [DOI] [PubMed] [Google Scholar]
- [13].Shoshan-Barmatz V, Ben-Hail D, VDAC, a multi-functional mitochondrial protein as a pharmacological target, Mitochondrion, 12 (2012) 24–34. [DOI] [PubMed] [Google Scholar]
- [14].Camara AKS, Zhou Y, Wen PC, Tajkhorshid E, Kwok WM, Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target, Front Physiol, 8 (2017) 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Das S, Steenbergen C, Murphy E, Does the voltage dependent anion channel modulate cardiac ischemia-reperfusion injury?, Biochim Biophys Acta, 1818 (2012) 1451–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reina S, De Pinto V, Anti-Cancer Compounds Targeted to VDAC: Potential and Perspectives, Curr Med Chem, 24 (2017) 4447–4469. [DOI] [PubMed] [Google Scholar]
- [17].Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G, Solution structure of the integral human membrane protein VDAC-1 in detergent micelles, Science, 321 (2008) 1206–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M, Zeth K, Structure of the human voltage-dependent anion channel, Proc Natl Acad Sci U S A, 105 (2008) 15370–15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, Ping P, Abramson J, The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating, Proc Natl Acad Sci U S A, 105 (2008) 17742–17747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Messina A, Reina S, Guarino F, De Pinto V, VDAC isoforms in mammals, Biochim Biophys Acta, 1818 (2012) 1466–1476. [DOI] [PubMed] [Google Scholar]
- [21].Colombini M, The published 3D structure of the VDAC channel: native or not?, Trends Biochem Sci, 34 (2009) 382–389. [DOI] [PubMed] [Google Scholar]
- [22].Villinger S, Briones R, Giller K, Zachariae U, Lange A, de Groot BL, Griesinger C, Becker S, Zweckstetter M, Functional dynamics in the voltage-dependent anion channel, Proc Natl Acad Sci U S A, 107 (2010) 22546–22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jaremko M, Jaremko L, Villinger S, Schmidt CD, Griesinger C, Becker S, Zweckstetter M, High-Resolution NMR Determination of the Dynamic Structure of Membrane Proteins, Angew Chem Int Ed Engl, 55 (2016) 10518–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Briones R, Weichbrodt C, Paltrinieri L, Mey I, Villinger S, Giller K, Lange A, Zweckstetter M, Griesinger C, Becker S, Steinem C, de Groot BL, Voltage Dependence of Conformational Dynamics and Subconducting States of VDAC-1, Biophys J, 111 (2016) 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Weiser BP, Salari R, Eckenhoff RG, Brannigan G, Computational investigation of cholesterol binding sites on mitochondrial VDAC, J Phys Chem B, 118 (2014) 9852–9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Budelier MM, Cheng WWL, Bergdoll L, Chen ZW, Janetka JW, Abramson J, Krishnan K, Mydock-McGrane L, Covey DF, Whitelegge JP, Evers AS, Photoaffinity labeling with cholesterol analogues precisely maps a cholesterol-binding site in voltage-dependent anion channel-1, J Biol Chem, 292 (2017) 9294–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Budelier MM, Cheng WW, Bergdoll L, Chen ZW, Abramson J, Krishnan K, Qian M, Covey DF, Janetka JW, Evers AS, Click Chemistry Reagent for Identification of Sites of Covalent Ligand Incorporation in Integral Membrane Proteins, Anal Chem, 89 (2017) 2636–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Israelson A, Abu-Hamad S, Zaid H, Nahon E, Shoshan-Barmatz V, Localization of the voltage-dependent anion channel-1 Ca2+-binding sites, Cell Calcium, 41 (2007) 235–244. [DOI] [PubMed] [Google Scholar]
- [29].De Pinto V, al Jamal JA, Palmieri F, Location of the dicyclohexylcarbodiimide-reactive glutamate residue in the bovine heart mitochondrial porin, J Biol Chem, 268 (1993) 12977–12982. [PubMed] [Google Scholar]
- [30].Colombini M, Voltage gating in the mitochondrial channel, VDAC, J Membr Biol, 111 (1989) 103–111. [DOI] [PubMed] [Google Scholar]
- [31].Colombini M, Mannella CA, VDAC, the early days, Biochim Biophys Acta, 1818 (2012) 1438–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rostovtseva T, Colombini M, ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane, J Biol Chem, 271 (1996) 28006–28008. [DOI] [PubMed] [Google Scholar]
- [33].Rostovtseva T, Colombini M, VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function, Biophys J, 72 (1997) 1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hodge T, Colombini M, Regulation of metabolite flux through voltage-gating of VDAC channels, J Membr Biol, 157 (1997) 271–279. [DOI] [PubMed] [Google Scholar]
- [35].Rostovtseva TK, Kazemi N, Weinrich M, Bezrukov SM, Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes, J Biol Chem, 281 (2006) 37496–37506. [DOI] [PubMed] [Google Scholar]
- [36].Teijido O, Rappaport SM, Chamberlin A, Noskov SY, Aguilella VM, Rostovtseva TK, Bezrukov SM, Acidification asymmetrically affects voltage-dependent anion channel implicating the involvement of salt bridges, J Biol Chem, 289 (2014) 23670–23682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].de Kroon AI, Dolis D, Mayer A, Lill R, de Kruijff B, Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane?, Biochim Biophys Acta, 1325 (1997) 108–116. [DOI] [PubMed] [Google Scholar]
- [38].Eddy MT, Ong TC, Clark L, Teijido O, van der Wel PC, Garces R, Wagner G, Rostovtseva TK, Griffin RG, Lipid dynamics and protein-lipid interactions in 2D crystals formed with the beta-barrel integral membrane protein VDAC1, J Am Chem Soc, 134 (2012) 6375–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rostovtseva TK, Bezrukov SM, VDAC inhibition by tubulin and its physiological implications, Biochim Biophys Acta, 1818 (2012) 1526–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mlayeh L, Krammer EM, Leonetti M, Prevost M, Homble F, The mitochondrial VDAC of bean seeds recruits phosphatidylethanolamine lipids for its proper functioning, Biochim Biophys Acta, 1858 (2017) 786–794. [DOI] [PubMed] [Google Scholar]
- [41].Pustovoit MA, Berezhkovskii AM, Bezrukov SM, Analytical theory of hysteresis in ion channels: two-state model, J Chem Phys, 125 (2006) 194907. [DOI] [PubMed] [Google Scholar]
- [42].Banerjee K, Dynamic memory of a single voltage-gated potassium ion channel: A stochastic nonequilibrium thermodynamic analysis, J Chem Phys, 142 (2015) 185101. [DOI] [PubMed] [Google Scholar]
- [43].Schein SJ, Colombini M, Finkelstein A, Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria, J Membr Biol, 30 (1976) 99–120. [DOI] [PubMed] [Google Scholar]
- [44].Mlayeh L, Chatkaew S, Leonetti M, Homble F, Modulation of plant mitochondrial VDAC by phytosterols, Biophys J, 99 (2010) 2097–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Blachly-Dyson E, Peng S, Colombini M, Forte M, Selectivity changes in site-directed mutants of the VDAC ion channel: structural implications, Science, 247 (1990) 1233–1236. [DOI] [PubMed] [Google Scholar]
- [46].Thomas L, Blachly-Dyson E, Colombini M, Forte M, Mapping of residues forming the voltage sensor of the voltage-dependent anion-selective channel, Proc Natl Acad Sci U S A, 90 (1993) 5446–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zizi M, Byrd C, Boxus R, Colombini M, The voltage-gating process of the voltage-dependent anion channel is sensitive to ion flow, Biophys J, 75 (1998) 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ardail D, Lerme F, Louisot P, Further characterization of mitochondrial contact sites: effect of short-chain alcohols on membrane fluidity and activity, Biochem Biophys Res Commun, 173 (1990) 878–885. [DOI] [PubMed] [Google Scholar]
- [49].Song J, Midson C, Blachly-Dyson E, Forte M, Colombini M, The sensor regions of VDAC are translocated from within the membrane to the surface during the gating processes, Biophys J, 74 (1998) 2926–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Colombini M, Blachly-Dyson E, Forte M, VDAC, a channel in the outer mitochondrial membrane, Ion Channels, 4 (1996) 169–202. [DOI] [PubMed] [Google Scholar]
- [51].Bowen KA, Tam K, Colombini M, Evidence for titratable gating charges controlling the voltage dependence of the outer mitochondrial membrane channel, VDAC, J Membr Biol, 86 (1985) 51–59. [DOI] [PubMed] [Google Scholar]
- [52].Cosentino K, Garcia-Saez AJ, Mitochondrial alterations in apoptosis, Chem Phys Lipids, 181 (2014) 62–75. [DOI] [PubMed] [Google Scholar]
- [53].Barrera G, Gentile F, Pizzimenti S, Canuto RA, Daga M, Arcaro A, Cetrangolo GP, Lepore A, Ferretti C, Dianzani C, Muzio G, Mitochondrial Dysfunction in Cancer and Neurodegenerative Diseases: Spotlight on Fatty Acid Oxidation and Lipoperoxidation Products, Antioxidants (Basel), 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Aufschnaiter A, Kohler V, Diessl J, Peselj C, Carmona-Gutierrez D, Keller W, Buttner S, Mitochondrial lipids in neurodegeneration, Cell Tissue Res, 367 (2017) 125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Eckmann J, Eckert SH, Leuner K, Muller WE, Eckert GP, Mitochondria: mitochondrial membranes in brain ageing and neurodegeneration, Int J Biochem Cell Biol, 45 (2013) 76–80. [DOI] [PubMed] [Google Scholar]
- [56].Bergdoll LA, Lerch MT, Patrick JW, Belardo K, Altenbach C, Bisignano P, Laganowsky A, Grabe M, Hubbell WL, Abramson J, Protonation state of glutamate 73 regulates the formation of a specific dimeric association of mVDAC1, Proc Natl Acad Sci U S A, 115 (2018) E172–E179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gatliff J, East D, Crosby J, Abeti R, Harvey R, Craigen W, Parker P, Campanella M, TSPO interacts with VDAC1 and triggers a ROS-mediated inhibition of mitochondrial quality control, Autophagy, 10 (2014) 2279–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Aghazadeh Y, Martinez-Arguelles DB, Fan J, Culty M, Papadopoulos V, Induction of androgen formation in the male by a TAT-VDAC1 fusion peptide blocking 14-3-3varepsilon protein adaptor and mitochondrial VDAC1 interactions, Mol Ther, 22 (2014) 1779–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Campanella M, Peptide targeting of mitochondria elicits testosterone formation, Mol Ther, 22 (2014) 1727–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]


