Summary
In this retrospective study, we investigated the influence of chemotherapy and immunotherapy on thromboembolic risk among United States Veterans with lung cancer during their first 6 months (180 days) following initiation of systemic therapy. Included patients received treatment with common front-line agents that were divided into four groups: chemotherapy alone, immunotherapy alone, combination of chemo- and immunotherapies, and molecularly targeted therapies (control group). The cohort experienced a 74% overall incidence of thrombosis, but the analysis demonstrated significantly different rates among the different groups. We explored models incorporating multiple confounding variables as well as the competing risk of death, and these results indicated that both chemo- and immunotherapies were associated with an increased incidence of thrombosis, either alone or combined, compared with the control group (756%, P = 2.2 × 10−16; 10·2%, P = 2.2 × 10−16; and 7·87%, P = 2.4 × 10−14 respectively vs. 41·0%). The Khorana score was found to be associated with increased risk, as were vascular disease and metastases. We found an association between risk of thrombosis and the use of anticoagulation, accounting for several confounders, including history of thrombosis. Further study is warranted to better determine the drivers of thromboembolic risk and to identify ways to mitigate this risk for patients.
Keywords: anticoagulation, chemotherapy, immunotherapy, lung cancer, thromboembolism
Introduction
Venous thromboembolism (VTE) is a common complication of cancer.1 Incidence varies widely between patients with cancer and within the same patient at different time points during the cancer course, with an overall post-diagnosis incidence rate of 1–20%2 While randomised trials have shown prophylactic anticoagulation roughly halves the relative risk of cancer-associated VTE, the absolute risk reduction among ambulatory patients with cancer is relatively modest, given that the baseline VTE risk in this population is ∼1·4% in the first year after diagnosis.3 Bleeding complications associated with anticoagulation are more common in patients with cancer compared with those without cancer, and in patients at low risk of thromboembolism, this means that the risks of anticoagulation may exceed the benefits. Thus, risk stratification is an important consideration when deciding whether to recommend prophylactic anticoagulation in the cancer setting.
Arterial and venous thromboembolic events (TEEs) in patients with cancer are associated with several known risk factors such as age, type and stage of cancer, hospitalisation and indwelling catheters. Active chemotherapy treatment has been shown to increase the incidence of TEEs in patients with breast cancer4,5 and lung cancer.6,7 While cancer itself was associated with a 4·1-fold increase in the risk of thrombosis, the addition of chemotherapy increased the risk to 6·5-times the baseline level in a large population-based case control study over 15 years.8 Lung cancer is among the most common cancer types; an estimated 2·1 million new cases were diagnosed in 2018, resulting in an estimated 18 million deaths.9 Over half of newly diagnosed patients are considered incurable due to the presence of metastatic disease,10 but are eligible for systemic therapy.
Current standard of care for first-line treatment of advanced stage non-small cell lung cancer (NSCLC) varies depending on histology, the presence or absence of driver mutations, and programmed death ligand-1 (PD-L1) expression levels.11–13 Of NSCLCs 25–40% have a PD-L1 tumour proportional score (TPS) of ≥50%.14 Currently recommended first-line therapies for patients with higher PD-L1 TPS levels and without a driver mutation are either a single-agent checkpoint inhibitor (e.g. pembrolizumab, nivolumab) or checkpoint inhibitor plus pemetrexed and platinum regimen. For patients with a confirmed and actionable driver mutation, first-line therapy may employ molecularly targeted agents (e.g. crizotinib, erlotinib).
In the present large retrospective population-based cohort study, we determined the TEE incidence and identified associated VTE risk factors among 93 222 patients with lung cancer receiving systemic therapy.
Methods
Study population
We performed a retrospective cohort study of USA national health records available within the Veterans Health Administration’s (VA) Corporate Data Warehouse (CDW). Our VA CDW-derived database contains individually identifiable clinical and demographic information from the 1990s through to 2020 for ∼19 million individual Veterans who received care provided or paid for by the VA. All work was performed under local Institutional Review Board (IRB) approval.
The inclusion criteria for this study were a diagnosis of a primary malignant neoplasm of the lung or bronchus and subsequent treatment with any of 14 systemic therapies. The specific chemotherapy agents studied were cisplatin, carboplatin, paclitaxel, docetaxel, pemetrexed, etoposide and gemcitabine, and the immunotherapy agents studied were pembrolizumab and nivolumab. The molecularly targeted therapies included were crizotinib, alectinib, gefitinib, erlotinib and afatinib. We excluded patients with a diagnosis of thrombophilia. Diagnoses were identified in the database using the International Statistical Classification of Diseases and Related Health Problems (ICD) ICD-9 or ICD-10-CM codes (Tables SI and SII), and therapeutic agents were identified by Current Procedural Terminology (CPT) codes and name using RxNorm.15 Using custom Structured Query Language (SQL) and R scripts (Appendix S1), 93 222 Veterans meeting these criteria were identified (Figure S1).
We divided the overall cohort into four groups based on type of systemic therapy administered: i) conventional chemotherapy, ii) immunotherapy, iii) combination chemo- and immunotherapies and iv) molecularly targeted therapy (non-chemo- non-immunotherapy control group). Note that patients receiving sequential chemo- and immunotherapy were considered in the combined therapy cohort so long as both treatments preceded the event of interest or end of follow-up interval. Characteristics of the overall patient cohort are shown in Table I and Figures S2–S8.
Table I.
Characteristics of the 93 222 Veteran cohort.
| Characteristic | Chemotherapy alone N = 81 495 | Immunotherapy alone N = 2678 | Combined chemo- and immunotherapies N = 3449 | Control (molecularly targeted) N = 5600 |
|---|---|---|---|---|
| Platinum-based chemotherapy, n (%) | 75 542 (92–7) | N/A | 3321 (96–3) | N/A |
| Male, n (%) | 79 580 (97–7) | 2607 (97–3) | 3360 (97–4) | 5465 (97–6) |
| Age, years, median (MAD; range) | 66 (8–90; 21–97) | 71 (7–41; 30–96) | 69 (5–93; 32–92) | 75 (10–4; 28–99) |
| Year of diagnosis, median (MAD; range) | 2009 (7–4; 1992–2020) | 2017 (1–5; 1997–2020) | 2018 (1–5; 1999–2020) | 2007 (4–4; 1996–2020) |
| Months from diagnosis to treatment, mean (SD; range) | 6–34 (178–5; 0–260) | 16–5 (31–3; 0–266) | 8–42 (23–0; 0–246) | 11–7 (20–0; 0–198) |
| Khorana score, mean (SD; range) | 1–64 (0–80; 1–5) | 1–64 (0–82; 1–4) | 1–64 (0–79; 1–4) | 1–56 (0–76; 1–5) |
| CCI, mean (SD; range) | 9–77 (4–1; 2–30) | 12–4 (4–3; 2–25) | 11–6 (3–9; 2–29) | 9–11 (4–0; 1–24) |
| BMI, kg/m2, mean (SD; range) N (%) | 25–9 (5–4; 14–50) | 26–3 (5–7; 14–50) | 26–2 (5–4; 14–49) | 25–7 (5–0; 14–50) |
| Current smoker | 46 522 (57–1) | 1533 (57–2) | 2124 (61–6) | 2232 (39–9) |
| Concurrent anticoagulant use | 8971 (11–0) | 471 (17–5) | 490 (14–2) | 630 (11–3) |
| Concurrent aspirin use | 18 128 (22–2) | 519 (19–4) | 726 (21–0) | 748 (13–4) |
| Prior pulmonary embolism | 2203 (2–70) | 202 (7–54) | 146 (4–23) | 195 (3–48) |
| Prior deep vein thrombosis | 2021 (2–48) | 152 (5–68) | 138 (3–99) | 144 (2–57) |
| Prior cerebral infarction | 2479 (3–04) | 219 (8–16) | 204 (5–91) | 117 (2–08) |
| Prior myocardial infarction | 1721 (2–11) | 82 (3–05) | 82 (2–37) | 81 (1–44) |
| Metastases | 62 450 (76–6) | 2149 (80–2) | 3082 (89–4) | 2818 (50–3) |
| Pulmonary disease | 62 734 (77–0) | 2024 (75–6) | 2691 (78–0) | 3244 (57–9) |
| Congestive heart failure | 20 249 (21–7) | 867 (32–4) | 894 (25–9) | 1173 (20–9) |
| Liver disease | 15 673 (19–2) | 592 (22–1) | 744 (21–5) | 535 (9–55) |
| Diabetes | 28 232 (34–6) | 1116 (41–7) | 1338 (38–8) | 1766 (31–5) |
| Vascular disease | 38 820 (47–6) | 1513 (56–5) | 1821 (52–8) | 2210 (39–5) |
CCI, Charlson Comorbidity Index; MAD, median absolute deviation; SD, standard deviation.
Outcomes and covariates
Initial cancer diagnosis was defined as the first occurrence in the patient’s record of one of the specified lung cancer diagnoses, and elapsed time until treatment was calculated using the initial diagnosis date and the beginning of systemic therapy with any of this study’s specified agents.
Incidence of and number of days to any TEE was assessed for a 180-day period following a patient’s first treatment with one of the specified systemic therapies. The 1267 patients lost to follow-up during the study period were censored from the analyses at the time of last follow-up. Of the patients who did not die during the study, the average follow-up time was 178 days.
The TEEs were defined as deep vein thrombosis (DVT), pulmonary embolism (PE), stroke, or myocardial infarction (MI) and identified in the database using ICD-9 or ICD-10-CM codes (Table SIII). In the overall analysis of TEE incidence, patients who experienced two or more TEEs simultaneously were counted as a single event, and for those who experienced multiple events at different times, only the first event was considered. Further analyses considered each type of TEE separately, recording the time to first occurrence of each event. History of prior TEE was defined as the occurrence in the patient’s record of any of the specified TEE diagnoses at any time before the first day of systemic therapy with any of this study’s specified agents. Patients who experienced a TEE on the first day of systemic therapy were excluded.
We calculated the Khorana score (as per Table SIV) for prediction of VTE risk for our cohort.16 Pre-treatment platelet counts, haemoglobin levels, and leucocyte counts were extracted from the database, using the most recent value up to 90 days prior to start of systemic therapy. Body mass index (BMI) at the approximate time of treatment initiation was calculated using custom R and SQL scripts (Appendix S1). Due to incomplete records for Veterans treated at non-VA facilities, 25 501 patients lacked sufficient laboratory data for Khorana score calculation, resulting in 67 721 patients with Khorana scores. We performed a sensitivity analysis that indicated no impact from these missing data (Table SV).
The Charlson Comorbidity Index (CCI) score at the beginning of systemic cancer treatment was calculated against each patient’s full prior medical record using custom R and SQL scripts (Appendix S1), adapted from published methods.17–19 Diagnoses pertaining to the index categories and occurring before the start of cancer therapy were extracted from the database and scored according to the index algorithm (Table SVI).
We assessed patients’ smoking status at the time of initiation of systemic therapy using custom R and SQL scripts adapted from published methods (Appendix S1).20,21
We also examined the presence and effects of concurrent use of anticoagulation and aspirin during treatment. Anticoagulant drugs studied were warfarin, low-molecular-weight heparin, enoxaparin, dalteparin, apixaban, rivaroxaban, dabigatran and edoxaban (Figure S9). A patient was considered to be on anticoagulation or aspirin if they had a prescription filled within a window of 15 days prior to 180 days after the start of systemic therapy, so long as the prescription preceded (by at least 1 day) any diagnosed TEE while on treatment. All drugs were identified in the database with CPT codes or by name using RxNorm.15
Statistical analyses
Analyses were performed for overall TEE incidence, as well as for incidence of each type of TEE. Kaplan–Meier survival analyses were performed using the ‘survminer’ package (version 0·4.8)22 for R (version 4·0.2)23 and custom R and SQL scripts (Appendix S1). Risk was modelled using Cox proportional hazard regression (without interaction terms) including the following features: method of treatment (chemotherapy, immunotherapy, combined chemo- and immunotherapies, or non-chemo non-immunotherapy control), Khorana score, CCI score, prior TEE, anticoagulation or aspirin use, the time between initial cancer diagnosis and treatment, patient’s age at treatment, BMI, smoking status, and presence of comorbid conditions (metastases, pulmonary disease, congestive heart failure, liver disease, diabetes, and vascular disease). Further analyses were performed using the ‘cmprsk’ (version 2·2.10)24 and ‘aod’ (version 1·3.1)25 packages, and the ‘ggplot2’ (version 3·3.2)26 package was used for generating graphs. Cumulative probabilities of TEE and death during the first 180 days of systemic therapy were calculated for each treatment group using a competing risks framework. Fine–Gray subdistribution hazard ratios were computed for the covariates.27,28 We censored data according to date of last recorded clinical follow-up or date of death, whichever occurred first. We used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Checklist for cohort studies to ensure the comprehensiveness of this report (Appendix S1).29
Results
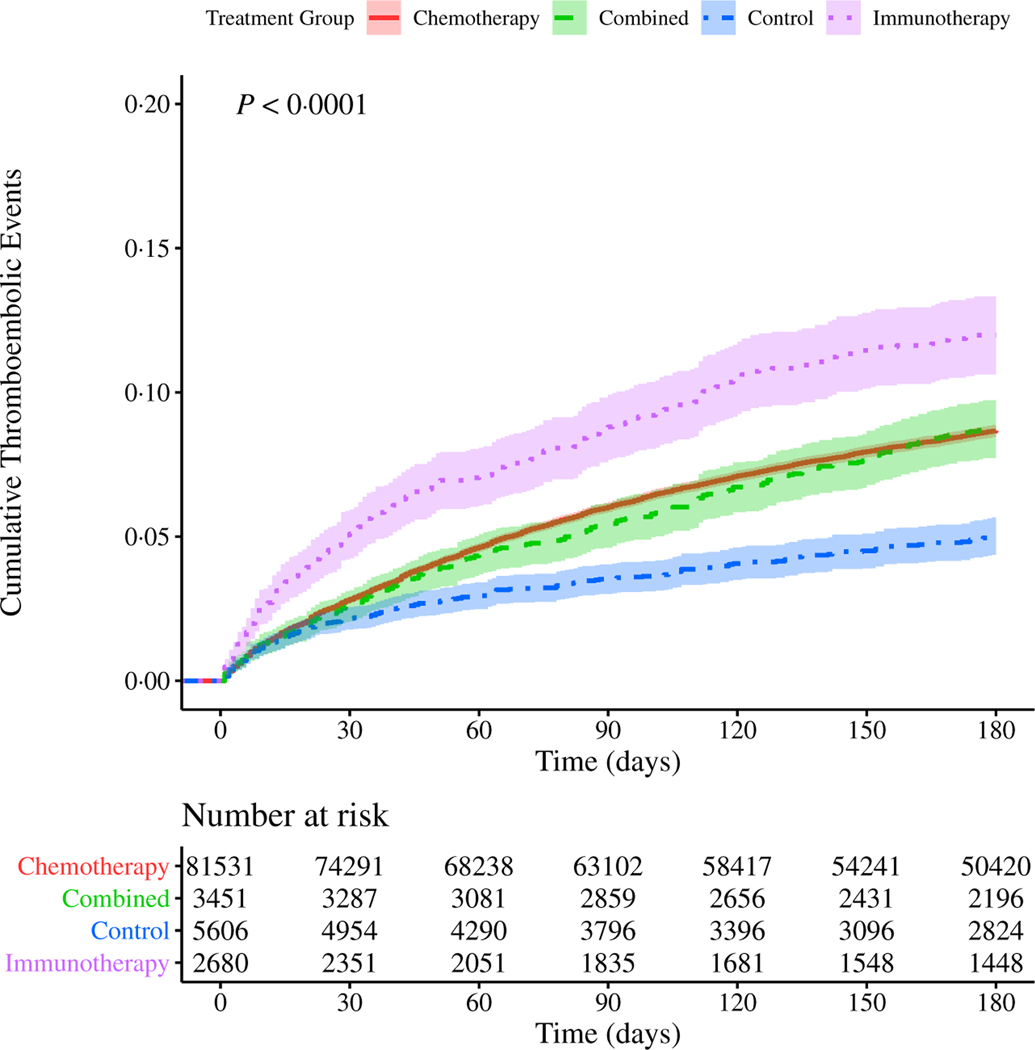
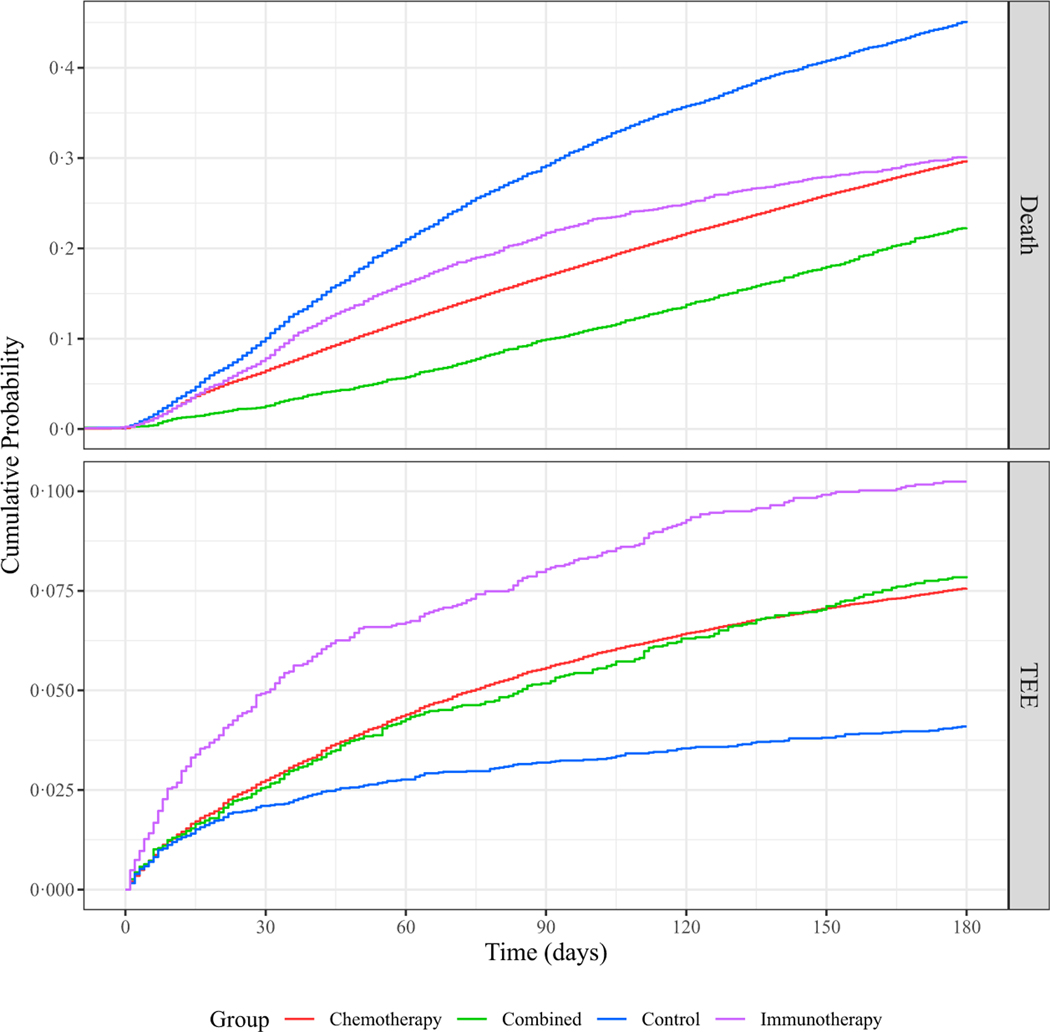
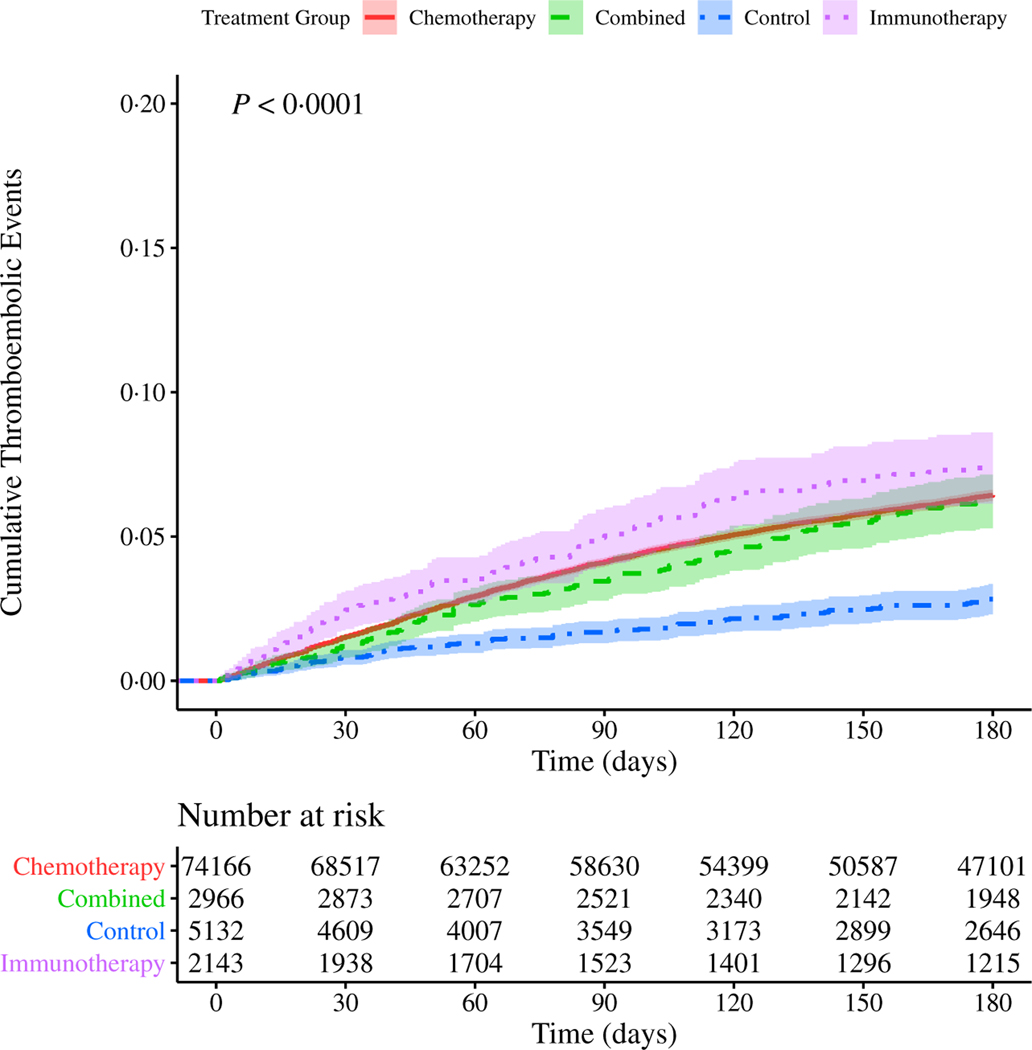
We first studied the incidence of TEE in our cohort, stratified by type of systemic therapy (chemotherapy, immunotherapy, combined chemo- and immunotherapies, or non-chemo non-immunotherapy control). Note that the majority of events observed were PE (49·2%), followed by DVT (27·5%), cerebral infarction (17·3%) and acute MI (5·97%); the remaining events were of multiple types. We identified an overall TEE rate of 7·4% (n = 6949) within the first 180 days of treatment initiation. Because the majority (87·4%) of our cohort received only chemotherapy, the TEE rate was similar in the chemotherapy alone group compared to the cohort overall. A significantly higher proportion of patients receiving immunotherapy alone (10·2%, n = 275; P = 2.8 × 10−7) experienced a TEE, and a significantly lower proportion of patients receiving the non-chemo non-immunotherapy control agents (4·10%, n = 230; P = 2.2 × 10−16) experienced a TEE. Results for all groups are summarised in Table II. Using both Kaplan–Meier cumulative risk analysis and a competing risk analysis accounting for an observed death rate of 30·4% by 180 days, we noted that patients who received immunotherapy alone were not only most likely to experience a TEE, but that TEE in this group occurred sooner after the start of treatment when compared to patients receiving chemotherapy alone, combined chemo- and immunotherapies, or neither of these therapies (Figs 1 and 2). These results were unchanged when accounting for immortal time bias, measuring time from date of first diagnosis (Figure S10). In order to account for changes in treatment strategies over time, as our cohort comprises nearly three decades’ worth of data, we also repeated the analysis for subsets of the cohort, stratified by time of treatment, without any appreciable changes in observed risk (Figure S11).
Table II.
Incidence of thromboembolic events (TEEs) stratified by treatment type.
| TEE, n (%) | Chemotherapy alone N = 81 602 | Immunotherapy alone N = 2685 | Combined Chemo- and Immunotherapies N = 3458 | Control (Molecularly Targeted) N = 5615 |
|---|---|---|---|---|
| - | 6172 (7–56) | 275 (10–2) | 272 (7–87) | 230 (4–10) |
| Pulmonary embolism | 3021 (48–9) | 148 (53–8) | 133 (48–9) | 118 (51–3) |
| Deep vein thrombosis | 1741 (28–2) | 47 (17–1) | 60 (22–1) | 64 (27–8) |
| Cerebral infarction | 1016 (16–5) | 75 (27–3) | 74 (27–2) | 37 (16–1) |
| Myocardial infarction | 394 (6–38) | 5 (1–82) | 5 (1–84) | 11 (4–78) |
The numbers of individuals with different types of thromboembolic events (TEEs) are shown, stratified by treatment group.
Fig 1.
Cumulative risk of thrombosis in patients with lung cancer stratified by treatment type. Risk of experiencing a thromboembolic event (TEE) during the first 180 days after the start of treatment for patients with lung cancer. The cohort is stratified by treatment group: immunotherapy alone (purple, dotted line), chemotherapy alone (red, solid line), or combination of both chemo- and immunotherapies (green, dashed line), as well as a non-chemo non-immunotherapy control group (blue, dot-dash line). Confidence intervals are represented by the shaded region around each line. Time (in days from start of treatment) is shown along the x-axis, with cumulative risk of TEE along the y-axis. The lower table depicts number of individuals at risk for each 30-day interval. P value reflects a significant difference in cumulative risk of TEE based on type of treatment. Pairwise P values computed by log-rank (Table SVII). [Colour figure can be viewed at wileyonlinelibrary.com]
Fig 2.
Cumulative risk of thrombosis in patients with lung cancer stratified by treatment type, accounting for competing risk of death. Risk of experiencing death or a thromboembolic event (TEE) during the first 180 days after the start of treatment for patients with lung cancer. The cohort is stratified by treatment group: immunotherapy alone (purple), chemotherapy alone (red), or combination of both chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue). Time (in days from start of treatment) is shown along the x-axis, with cumulative risk of death or TEE along the y-axes. The upper and lower plots depict the incidence of death or TEE respectively. [Colour figure can be viewed at wileyonlinelibrary.com]
We repeated these analyses for each type of TEE studied herein and note that immunotherapy and/or chemotherapy are associated with elevated risks of PE, DVT and cerebral infarction during the first 180 days of therapy (Figures S12 and S13).
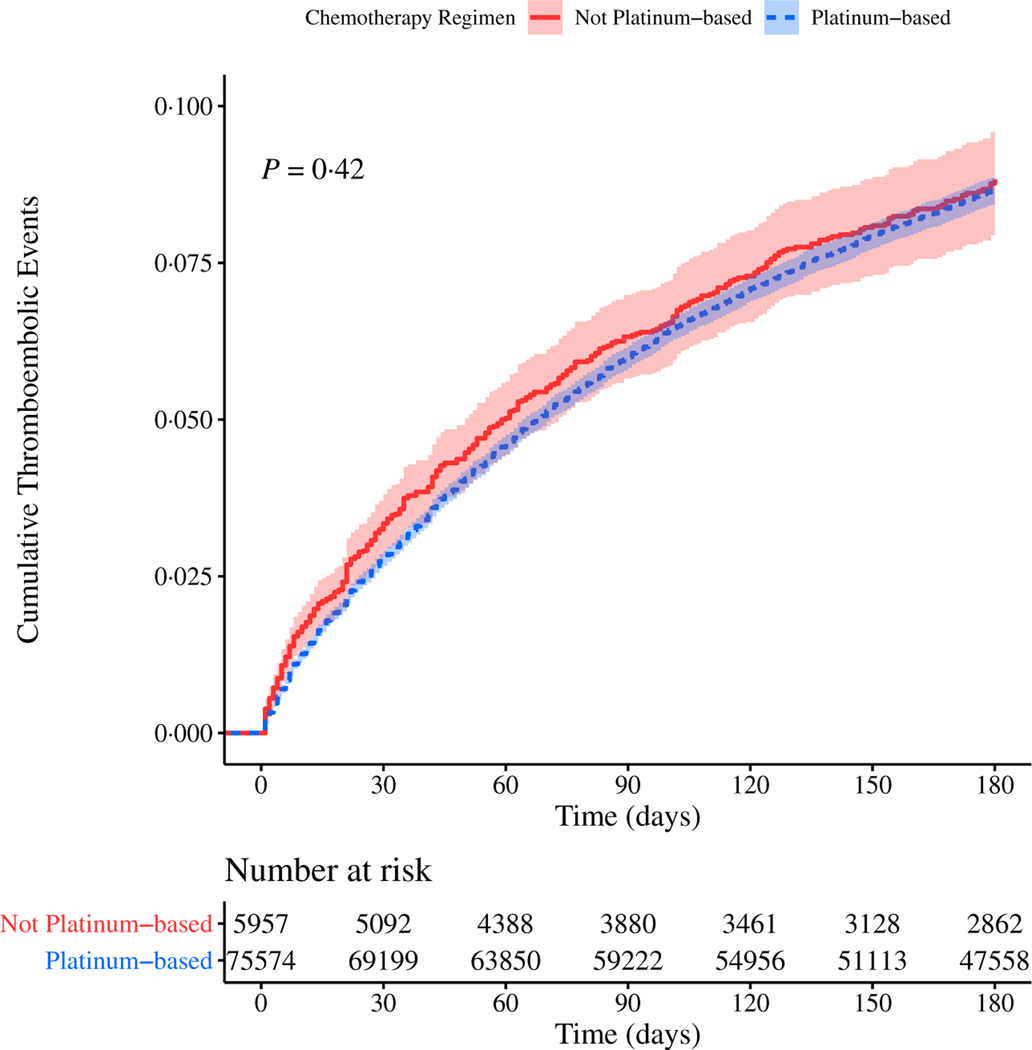
While platinum-based chemotherapy agents have been shown to be more thrombogenic than other chemotherapy agents,30–32 we found no difference in incidence of TEE between chemotherapy patients who received at least one platinum-based agent compared to those that received no platinum-based agents during their cancer treatment (Fig 3).
Fig 3.
Cumulative risk of thrombosis in patients with lung cancer stratified by chemotherapy regimen. Risk of experiencing a thromboembolic event (TEE) during the first 180 days after the start of treatment for patients with lung cancer. The cohort is stratified by chemotherapy regimen: not platinum-based agents (red, solid line) or platinum-based agents (blue, dashed line). Confidence intervals are represented by the shaded region around each line. P value computed by log-rank. [Colour figure can be viewed at wileyonlinelibrary.com]
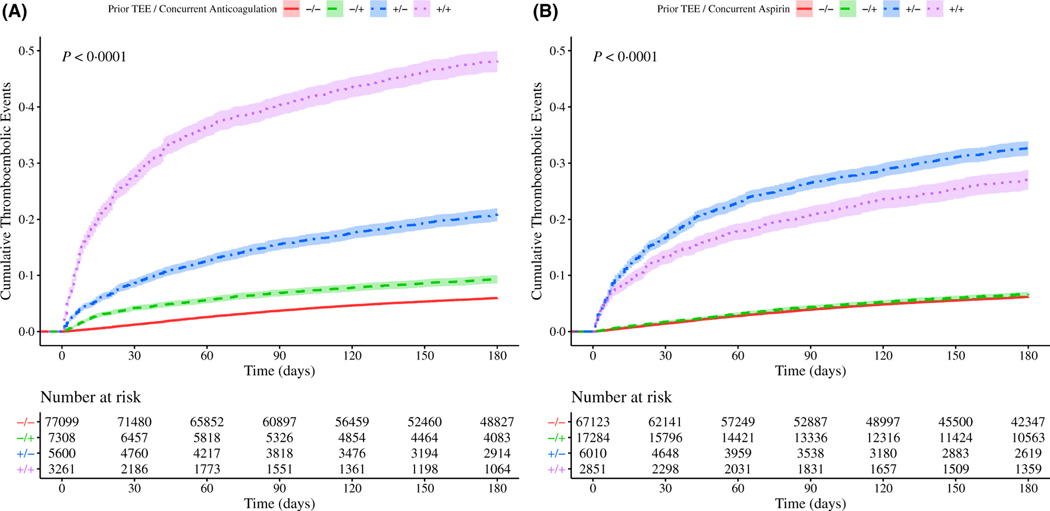
A prior history of TEE elevates a patient’s risk of experiencing a TEE during systemic therapy33,34 and may play a role in the decision to prescribe anticoagulant or aspirin. We hypothesised that either or both of these factors could significantly affect observed TEE rates in our cohort and could be influencing the differences we observed among systemic treatment groups. We therefore analysed TEE in our study cohort according to prior history of TEE and anticoagulation or aspirin use. Surprisingly, we found that patients receiving anticoagulation or aspirin experienced TEE at both a higher rate and a shorter interval than patients who did not (Figure S14). In particular, the cohort receiving anticoagulation had a much higher incidence of TEE compared to those receiving aspirin. While additional undetermined clinical risk factors could be significantly influencing patient TEE outcomes, we assessed the incidence of a prior history of TEE and its influence on subsequent TEE risk in this context and note that it accounts for the majority of increased TEE risk. However, the use of anticoagulation or aspirin does remain associated with a higher incidence of TEE (Fig 4).
Fig 4.
Cumulative risk of thrombosis in patients with lung cancer stratified by prior thromboembolic events (TEEs) and anticoagulation or aspirin use. Risk of experiencing a TEE during the first 180 days after the start of treatment patients with for lung cancer. The cohort is stratified by occurrence of prior TEEs and either anticoagulation (panel A) or aspirin (panel B) use concurrently with systemic therapy: the use of anticoagulation or aspirin without a history of TEEs (‘−/+’green, dashed line), neither (‘−/−’ red, solid line), the presence of a prior TEE and anticoagulant or aspirin use (‘+/+’ purple, dotted line), or the presence of a prior TEE without anticoagulant or aspirin use (‘+/−’ blue, dot-dash line). Confidence intervals are represented by the shaded region around each line. Time (in days from start of treatment) is shown along the x-axes, with cumulative risk of TEE along the y-axes. The lower tables depict number of individuals at risk for each 30-day interval. Pairwise P values computed by log-rank (Tables SXVI-SXVII). [Colour figure can be viewed at wileyonlinelibrary.com]
Because anticoagulation therapy carries a known risk of haemorrhage, we analysed the incidence of haemorrhagic events during the first 180 days of systemic cancer therapy, stratified by treatment group and by presence of anticoagulants or aspirin. We found that immunotherapy and chemotherapy were both associated with a significant increase in haemorrhagic events (Figure S15), as were the use of anticoagulants or aspirin (Figure S16).
To assess the relative importance of additional clinical covariates, we used a multivariate Cox proportional hazard model to quantify the effects of immunotherapy on patients’ risk of TEE (Table III). When stratifying for chemotherapy, Khorana Score, anticoagulation (warfarin, enoxaparin, dalteparin, apixaban, dabigatran or rivaroxaban), BMI, prior TEE (PE, cerebral infarction or DVT), and delay to treatment, and accounting for additional covariates (e.g. age, year of diagnosis, aspirin use), the overall influence of immunotherapy no longer appeared significant. Older age demonstrated a slight protective effect, likely due to a more robust underlying health of patients who could either safely delay systemic treatment or be considered good candidates for systemic treatment even at an advanced age.
Table III.
Cox proportional hazard model of effect on thromboembolic events (TEEs) of treatment type and covariates.
| Variable | HR ( 95% CI) |
|---|---|
| Immunotherapy alone | 0·918 (0·788–1·07) |
| Age | 0·994 (0·990–0·999) |
| CCI score | 1·02 (1·01–1·03) |
| Smoking status | 0993 (0·927–1·06) |
| Edoxaban | 6·84 (0·424–110) |
| LMW heparin | 177 (16·0–1967) |
| Aspirin | 0·992 ( 0·915–1·07) |
| Prior myocardial infarction | 1·29 (1·06–1·56) |
| Metastases | 1·21 (1·10–1·34) |
| Pulmonary disease | 0·978 (0·899–1·06) |
| Congestive heart failure | 1·08 (0·999–1·17) |
| Liver disease | 1·03 (0·945–1·12) |
| Diabetes | 0·906 (0·839–0·978) |
| Vascular disease | 1·41 (1·31–1·52) |
CCI, Charlson Comorbidity Index; CI, confidence interval; HR, hazard ratio; LMW, low molecular weight.
We also used a competing risks model to determine Fine–Gray subdistribution hazards of TEE for the systemic treatments and covariates in consideration of the competing risk of death (Table IV). Treatment with chemotherapy demonstrated 1·3-times greater risk of TEE under this model, but the effect of treatment with immunotherapy no longer showed a significant effect; the hazard ratios of the covariates were similar to those developed in the Cox model.
Table IV.
Fine–Gray subdistribution hazard ratios (HRs) for thromboembolic events (TEEs) considering competing risk of death.
| Variable | HR (95% CI) |
|---|---|
| Chemotherapy alone | 1·31 (1·11–1·55) |
| Immunotherapy alone | 0·986 (0·793–1·23) |
| Combined therapies | 0·750 (0·605–0·930) |
| Age | 0·992 (0·988–0·995) |
| Year of diagnosis | 1·06 (1·06–1·07) |
| Time since diagnosis | 1·00 (1 · 00–1 · 00) |
| Khorana score | 1·09 (1·06–1·13) |
| CCI score | 0·981 (0·973–0·990) |
| BMI | 1·01 (1·01–1·02) |
| Smoking status | 0·934 (0·882–0·988) |
| Anticoagulation | 1·91 (1·79–2·05) |
| Aspirin | 1·02 (0·959–1·09) |
| Prior pulmonary embolism | 4·45 (4·05–4·90) |
| Prior deep vein thrombosis | 2·15 (1·93–2·39) |
| Prior cerebral infarction | 2·14 (1·93–2·38) |
| Prior myocardial infarction | 1·17 (1·00–1·37) |
| Metastases | 1·39 (1·28–1·50) |
| Pulmonary disease | 0·977 (0·911–1·05) |
| Congestive heart failure | 1·08 (1·01–1·15) |
| Liver disease | 1·01 (0·942–1·08) |
| Diabetes | 0·932 (0·875–0·993) |
| Vascular disease | 1·36 (1·28–1·45) |
CCI, Charlson Comorbidity Index; CI, confidence interval; HR, hazard ratio.
Finally, in recognition of the strong effect in our study cohort of prior history of TEE on the risk of TEE during systemic treatment of lung cancer, we eliminated the patients who had experienced a TEE before beginning lung cancer therapy, resulting in a reduced cohort of 84 496 Veterans. This change substantially reduced the cumulative risk of TEE experienced by the group who received immunotherapy alone; nonetheless, both chemotherapy and immunotherapy retained an increased risk of TEE (Fig 5).
Fig 5.
Cumulative risk of thrombosis in patients with lung cancer with no prior history of thromboembolic events (TEEs), stratified by treatment type. Risk of experiencing a TEE during the first 180 days after the start of treatment for patients with lung cancer. The cohort is stratified by treatment group: chemotherapy alone (red, solid line), immunotherapy alone (purple, dotted line), or combination of both chemotherapy and immunotherapies (green, dashed line), as well as a non-chemo non-immunotherapy control group (blue, dot-dash line). Confidence intervals are represented by the shaded region around each line. Time (in days from start of treatment) is shown along the x-axis, with cumulative risk of TEE along the y-axis. The lower table depicts number of individuals at risk for each 30-day interval. Pairwise P values computed by log-rank (Table SXIX). [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
To the best of our knowledge, this is the largest study to date exploring thromboembolic risk in the setting of lung cancer, and the first to report on these risks among a cohort of USA Veterans. While this is also the largest study to suggest a correlation in patients with cancer between checkpoint inhibitor immunotherapy and increased thromboembolic risk, our present findings are consistent with those among other cohorts.35,36 We surprisingly found that patients with lung cancer on prophylactic anticoagulation or aspirin had a higher TEE incidence compared with those not on prophylaxis, and we speculate this counterintuitive finding may be due to latent clinical variables associated with a higher baseline thrombosis risk, in particular, but not wholly, the existence of a prior TEE.
Thromboembolic events have not been reported in phase III trials evaluating checkpoint inhibitors in patients with cancer, and little is known about thromboembolic risk in this setting. In one secondary analysis within a prospective observational protocol of 217 NSCLC patients treated with checkpoint inhibitors, an elevated thrombosis incidence of 13·8% was noted.37 Other small, single institutional studies have also observed a correlation between checkpoint inhibitor therapy and thrombosis in patients with cancer.35,36,38 In our present study, we found a potential increased incidence of thromboembolic events among patients with lung cancer who received first-line checkpoint inhibitor monotherapy compared with chemotherapy either alone or combined with immunotherapy; however, these effects may be largely influenced by other clinical covariates. These data likely merit further clinical and translational studies to investigate the potential thrombogenicity of checkpoint inhibitors in the lung cancer setting (whether alone or in combination with chemotherapy) and argue for incorporating this therapeutic class into thrombosis risk models and guidelines for prophylactic anticoagulation.
In the present study, we also found evidence of a weak correlation between thrombosis risk and the Khorana score (a widely utilised VTE risk model in patients with cancer). However, we note that other studies have been unable to demonstrate a significant association between the Khorana score and VTE in patients with lung cancer.39–41 These data call into question the routine clinical utility of the Khorana score in the lung cancer setting.
We note differences in the overall observed TEE rates in our lung cancer cohort treated with systemic therapy compared to the published literature. For instance, a retrospective analysis of 204 patients with lung cancer treated with cisplatin-based chemotherapy, found an 11·8% incidence of TEE during treatment or within 4 weeks of the last treatment.30 While this incidence is higher than the cumulative incidence we observed (7·5%), we note this discrepancy could be explained by different length of observation between the two studies (our study only investigated events occurring within 180 days of treatment initiation) and the small cohort size in the prior study.
There are limitations associated with administrative databases, including numerous sources of possible bias and missing or inaccurate data. Additionally, while the cohort we analysed was national in scope, it is most reflective of the USA Veteran population, and therefore contains relatively few women. We also acknowledge that many Veterans receive a portion of their care outside of the VA system and that this could have been a source of missing data in the present study. Moreover, we did not explicitly study the influence of initial disease staging, prior cancer therapies including surgery or radiotherapy, potentially relevant laboratory results such as D-dimer, family history of VTE, or non-prescription medications. The use of molecularly targeted therapy as a control cohort also invites the potential confounder of differential tumour biology. We only analysed the impact of clinical predictors at the time of treatment initiation and did not assess risk factors that may have arisen later in the treatment course. Finally, we confined analysis to lung cancers, as they are known to be at increased risk for TEEs compared to most other malignancies16 and did not discriminate between lung cancer subtypes (e.g. adenocarcinoma vs. small cell).
In conclusion, given the continued high rate of TEEs in lung cancer and the increased risks associated with chemotherapy and immunotherapy, future thromboprophylaxis risk-adapted trials in this setting may be warranted, particularly accounting for immunotherapy-based treatment regimens. Until such additional data becomes available, the use of immune checkpoint inhibitors likely warrants particular consideration during shared clinical decision-making about thromboprophylaxis.
Supplementary Material
This appendix comprises our completed STROBE checklist and the custom SQL and R statements we developed in performing the study.
Cohort Development. This figure describes the inclusion and exclusion processes used when building the study cohort.
Distribution of Patient Age in Years at the Start of Treatment. The patient cohort is stratified by treatment group: chemotherapy alone (red), immunotherapy alone (purple), combined chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue).
Distribution of Year of Lung Cancer Diagnosis. The patient cohort is stratified by treatment group: chemotherapy alone (red), immunotherapy alone (purple), combined chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue).
Distribution of Elapsed Time from Diagnosis to Systemic Treatment. The cohort is stratified by treatment group: chemotherapy alone (red), immunotherapy alone (purple), combined chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue).
Distribution of Khorana Score at Initiation of Systemic Therapy. The cohort is stratified by treatment group: chemotherapy alone (red), immunotherapy alone (purple), combined chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue).
Distribution of Charlson Comorbidity Score at Initiation of Systemic Therapy. The cohort is stratified by treatment group: chemotherapy alone (red), immunotherapy alone (purple), combined chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue).
Distribution of Body Mass Index at Initiation of Systemic Therapy. The cohort is stratified by treatment group: chemotherapy alone (red), immunotherapy alone (purple), combined chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue).
Distribution and Overlap of Comorbid Conditions at Initiation of Systemic Therapy. This figure describes the numbers of patients with various comorbid conditions, alone and in combination.
Cumulative Risk of Thrombosis Following Initial Diagnosis of Lung Cancer Patients, Stratified by Treatment Type. Risk of experiencing a thromboembolic event during the first ten years after diagnosis of lung cancer. The cohort is stratified by ultimate treatment group: immunotherapy alone (purple, dotted line), chemotherapy alone (red, solid line), or combination of both chemo- and immunotherapies (green, dashed line), as well as a non-chemo non-immunotherapy control group (blue, dot-dash line). Confidence intervals are represented by the shaded region around each line. Time (in days from initial cancer diagnosis) is shown along the x-axis, with cumulative risk of TEE along the y-axis. The lower table depicts number of individuals at risk for each 1-year interval. Pairwise P-values were computed by log-rank test (Table SVIII).
Cumulative Risk of Thrombosis in Lung Cancer Patients of Different Eras. Risk of experiencing a thromboembolic event during the first 180 days after the start of treatment for lung cancer patients. Each panel in this figure represents the cumulative risk during an era of time; the cohort was subdivided into smaller groups of approximately 23,000 patients each. Panel A is patients treated 2001–2006, Panel B is patients treated 2006–2011, Panel C is patients treated 2011–2015, and Panel D is patients treated 2015–2020. The sub-cohorts are stratified by treatment group: immunotherapy alone (purple, dotted line), chemotherapy alone (red, solid line), or combination of both chemo- and immunotherapies (green, dashed line), as well as a non-chemo non-immunotherapy control group (blue, dot-dash line). Confidence intervals are represented by the shaded region around each line. Time (in days from initial cancer diagnosis) is shown along the x-axes, with cumulative risk of TEE along the y-axes. The lower tables depict number of individuals at risk for each 30-day interval. Pairwise p-value comparisons were by log-rank test (Supplementary Tables SIX–SXI).
Cumulative Risk of Different Types of Thrombosis. Risk of experiencing a thromboembolic event during the first 180 days after the start of treatment for lung cancer patients. Each panel in this figure represents the cumulative risk of a particular type of thrombosis; Panel A is pulmonary embolism, Panel B is deep vein thrombosis, Panel C is cerebral infarction, and Panel D is myocardial infarction. The cohort is stratified by treatment group: immunotherapy alone (purple, dotted line), chemotherapy alone (red, solid line), or combination of both chemo- and immunotherapies (green, dashed line), as well as a non-chemo non-immunotherapy control group (blue, dot-dash line). Confidence intervals are represented by the shaded region around each line. Time (in days from initial cancer diagnosis) is shown along the x-axes, with cumulative risk of TEE along the y-axes. The lower tables depict number of individuals at risk for each 30-day interval. Pairwise P-value comparisons were by log-rank test (Supplementary Tables SXII–SXV).
Cumulative Risk of Different Types of Thrombosis in Lung Cancer Patients Stratified by Treatment Type, Accounting for Competing Risk of Death. Risk of experiencing death or a TEE during the first 180 days after the start of treatment for lung cancer patients. Each panel in this figure represents the cumulative risk of a particular type of thromboembolic event; Panel A is pulmonary embolism, Panel B is deep vein thrombosis, Panel C is cerebral infarction, and Panel D is myocardial infarction. The cohort is stratified by treatment group: immunotherapy alone (blue), chemotherapy alone (red), or combination of both chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (purple). Time (in days from start of treatment) is shown along the x-axis, with cumulative risk of death or TEE along the y-axes. The upper and lower plots depict the incidence of death or TEE, respectively.
Cumulative Risk of Thrombosis in Lung Cancer Patients, Stratified by Use of Anticoagulants or Aspirin. Risk of experiencing a thromboembolic event during the first 180 days after the start of treatment for lung cancer patients. The cohort is stratified by anticoagulation (panel A) or aspirin (panel B) use concurrently with systemic therapy: absence (“−/−” red, solid line), presence (“+/−” blue, dotted line). Confidence intervals are represented by the shaded region around each line. Time (in days from start of treatment) is shown along the x-axes, with cumulative risk of TEE along the y-axes. The lower tables depict number of individuals at risk for each 30-day interval. P-values computed by log-rank.
Cumulative Risk of Hemorrhage in Lung Cancer Patients. Risk of experiencing a hemorrhage during the first 180 days after the start of treatment for lung cancer patients. The cohort is stratified by treatment group: immunotherapy alone (blue, dot-dash line), chemotherapy alone (red, solid line), or combination of both chemo- and immunotherapies (green, dashed line), as well as a non-chemo non-immunotherapy control group (purple, dotted line). Confidence intervals are represented by the shaded region around each line. Time (in days from start of treatment) is shown along the x-axis, with cumulative risk of hemorrhage along the y-axis. The lower table depicts number of individuals at risk for each 30-day interval. P-value reflects a significant difference in cumulative risk of TEE based on type of treatment. Pairwise p-values computed by log-rank (Supplementary Table SXVIII).
Cumulative Risk of Hemorrhage in Lung Cancer Patients, Stratified by Use of Anticoagulants or Aspirin. Risk of experiencing a hemorrhagic event during the first 180 days after the start of treatment for lung cancer patients. The cohort is stratified by anticoagulation (panel A) or aspirin (panel B) use concurrently with systemic therapy: absence (“−/−” red, solid line), presence (“+/−” blue, dotted line). Confidence intervals are represented by the shaded region around each line. Time (in days from start of treatment) is shown along the x-axes, with cumulative risk of TEE along the y-axes. The lower tables depict number of individuals at risk for each 30-day interval. P-values computed by log-rank.
Diagnostic Inclusion Criteria: Lung Cancer. Patients included in the study experienced at least one of the lung cancer diagnoses listed in this table and specified by ICD-9 or ICD-10-CM codes.
Diagnostic Exclusion Criteria: Thrombophilia. Patients who experienced at least one of the thrombophilia diagnoses listed in this table and specified by ICD-9 or ICD-10-CM codes were excluded from the study.
Diagnostic Criteria: Thromboembolic Event. The thromboembolic diagnoses listed in this table and specified by ICD-9 or ICD-10-CM codes were considered events of interest during the 180-day period following initiation of systemic therapy.
Khorana Score Calculation.
Sensitivity Analysis on Khorana Score Missing Data.
Charlson Score Calculation.
Groupwise Comparisons of Incidence of TEE. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of TEE After Diagnosis. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of TEE in Sub-Cohort Treated 2006–2011. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of TEE in Sub-Cohort Treated 2011–2015. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of TEE in Sub-Cohort Treated 2015–2020. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Pulmonary Embolism. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Deep Vein Thrombosis. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Cerebral Infarction. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Myocardial Infarction. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Prior TEE and Anticoagulation. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Prior TEE and Aspirin. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Hemorrhage. P-values were computed by log rank test.
Groupwise Comparisons of Incidence of TEE in Sub-Cohort with No Prior History of TEE. P-values were computed by log rank test.
Acknowledgements
This work was supported by VA Career Development Award (1 IK2 CX002049-01) to Reid F. Thompson.
All authors made substantial contributions to the research and approved the final version of the manuscript. Cecelia J. Madison, Reid F. Thompson and David C. Calverley designed the research question. Cecelia J. Madison performed the research. Cecelia J. Madison, Michael J. Conlin, Kenneth R. Gundle and Reid F. Thompson created the SQL and R programming tools used to extract and analyse the data. Cecelia J. Madison, Kenneth R. Gundle and Reid F. Thompson analysed the data. Cecelia J. Madison, Ryan A. Melson, Reid F. Thompson and David C. Calverley wrote the paper.
This product uses publicly available data courtesy of the United States National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; the NLM is not responsible for the product and does not endorse or recommend this or any other product.
All authors declare that they have neither financial nor competing interests.
Footnotes
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Cronin-Fenton DP, Søndergaard F, Pedersen LA, Fryzek JP, Cetin K, Acquavella J, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997–2006. British Journal of Cancer. 2010;103:947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost. 2017;117:219–30. [DOI] [PubMed] [Google Scholar]
- 3.Di Nisio M, Porreca E, Candeloro M, De Tursi M, Russi I, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2016;12: CD008500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine MN, Gent M, Hirsh J, Arnold A, Goodyear MD, Hryniuk W, et al. The thrombogenic effect of anticancer drug therapy in women with stage II breast cancer. N Engl J Med. 1988;318:404–7. [DOI] [PubMed] [Google Scholar]
- 5.Saphner T, Tormey DC, Gray R. Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer. J Clin Oncol. 1991;9:286–94. [DOI] [PubMed] [Google Scholar]
- 6.Hicks LK, Cheung MC, Ding K, Hasan B, Seymour L, Le Maître A, et al. Venous thromboembolism and non-small cell lung cancer: a pooled analysis of National Cancer Institute of Canada Clinical Trials Group trials. Cancer. 2009;115:5516–25. [DOI] [PubMed] [Google Scholar]
- 7.Zer A, Moskovitz M, Hwang DM, Hershko-Klement A, Fridel L, Korpanty GJ, et al. ALK-rearranged non-small-cell lung cancer is associated with a high rate of venous thromboembolism. Clin Lung Cancer. 2017;18:156–61. [DOI] [PubMed] [Google Scholar]
- 8.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–15. [DOI] [PubMed] [Google Scholar]
- 9.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 10.Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. J Thorac Oncol. 2016;11:1653–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:504–35. [DOI] [PubMed] [Google Scholar]
- 12.Hanna N, Johnson D, Temin S, Baker S, Brahmer J, Ellis PM, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35:3484–515. [DOI] [PubMed] [Google Scholar]
- 13.Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v1–27. [DOI] [PubMed] [Google Scholar]
- 14.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- 15.Nelson SJ, Zeng K, Kilbourne J, Powell T, Moore R. Normalized names for clinical drugs: RxNorm at 6 years. J Am Med Inform Assoc. 2011;18:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 18.Søgaard M, Thomsen RW, Bossen KS, Sørensen HT, Nørgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5 (Suppl 1):3–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 20.McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golden SE, Hooker ER, Shull S, Howard M, Crothers K, Thompson RF, et al. Validity of Veterans Health Administration structured data to determine accurate smoking status. Health Informatics J. 2020;26:1507–15. [DOI] [PubMed] [Google Scholar]
- 22.Kassambara A, Kosinski M, Biecek P, Fabian S. suvminer: Drawing Survival Curves using ‘ggplot2’. 2020. Available at: https://CRAN.R-project.org/package=survminer
- 23.R Core Team. R. A language and environment for statistical computing. R-Foundation for Statistical Computing. 2020. https://www.R-project.org/ [Google Scholar]
- 24.Gray B cmprsk: Subdistribution Analysis of Competing Risks. 2020. https://CRAN.R-project.org/package=cmprsk
- 25.Lesnoff M, Lancelot R, aod: Analysis of Overdispersed Data. 2012. https://CRAN.R-project.org/package=aod
- 26.Wickham H ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag, 2016. [Google Scholar]
- 27.Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;45:1388–95. [DOI] [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 29.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observation Studies in Epidemiology (STROBE) statement: guidelines for reporting observation studies. J Clin Epidemiol. 2008;61:344–9. [DOI] [PubMed] [Google Scholar]
- 30.Moore RA, Adel N, Riedel E, Bhutani M, Feldman DR, Tabbara NE, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29:3466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jafri M, Protheroe A. Cisplatin-associated thrombosis. Anticancer Drugs. 2008;19:927–9. [DOI] [PubMed] [Google Scholar]
- 32.Starling N, Rao S, Cunningham D, Iveson T, Nicolson M, Coxon F, et al. Thromboembolism in patients with advanced gastroesophageal cancer treated with anthracycline, platinum, and fluoropyrimidine combination chemotherapy: a report from the UK National Cancer Research Institute Upper Gastrointestinal Clinical Studies Group. J Clin Oncol. 2009;27:3786–93. [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Proctor MC, Varma M, Greenfield LJ, Upchurch GR Jr, Henke PK. Factors associated with recurrent venous thromboembolism in patients with malignant disease. J Vasc Surg. 2003;37:976–83. [DOI] [PubMed] [Google Scholar]
- 34.Cella CA, Di Minno G, Carlomagno C, Arcopinto M, Cerbone AM, Matano E, et al. Preventing venous thromboembolism in ambulatory cancer patients: the ONKOTEV study. Oncologist. 2017; 22:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sussman TA, Li H, Hobbs B, Funchain P, McCrae KR, Khorana AA. Incidence of thromboembolism in patients with melanoma on immune checkpoint inhibitor therapy and its adverse association with survival. J Immunother Cancer. 2021;9:e001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moik F, Chan WE, Weidemann S, Hoeller C, Tuchmann F, Aretin MB, et al. Incidence, risk factors, and clinical outcome of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. 2021;137;1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nichetti F, Ligorio F, Zattarin E, Signorelli D, Prelaj A, Proto C, et al. Is there an interplay between immune checkpoint inhibitors, thromboprophylactic treatments, and thromboembolic events? Mechanisms and impact in non-small cell lung cancer patients. Cancers (Basel). 2020;12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ibrahini S, Machiorlatti M, Vesely SK, Malla M, Modhia F, Jones SA, et al. Incidence of vascular thromboembolic events in patients receiving immunotherapy: a single institution experience. Blood. 2017;130(Suppl 1):4864. [Google Scholar]
- 39.Kuderer NM, Poniewierski MS, Culakova E, Lyman GH, Khorana AA, Pabinger I, et al. Predictors of venous thromboembolism and early mortality in lung cancer: Results from a global prospective study (CANTARISK). Oncologist. 2018;23:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansfield AS, Tafur AJ, Wang CE, Kourelis TV, Wysokinska EM, Yang P, et al. Predictors of active cancer thromboembolic outcomes: Validation of the Khorana score among patients with lung cancer. J Thromb Haemost. 2016;14:1773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noble S, Alikhan R, Robbins A, Macbeth F, Hood K, et al. Predictors of active cancer thromboembolic outcomes: Validation of the Khorana score among patients with lung cancer: comment. J Thromb Haemost. 2017;15:590–1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This appendix comprises our completed STROBE checklist and the custom SQL and R statements we developed in performing the study.
Cohort Development. This figure describes the inclusion and exclusion processes used when building the study cohort.
Distribution of Patient Age in Years at the Start of Treatment. The patient cohort is stratified by treatment group: chemotherapy alone (red), immunotherapy alone (purple), combined chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue).
Distribution of Year of Lung Cancer Diagnosis. The patient cohort is stratified by treatment group: chemotherapy alone (red), immunotherapy alone (purple), combined chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue).
Distribution of Elapsed Time from Diagnosis to Systemic Treatment. The cohort is stratified by treatment group: chemotherapy alone (red), immunotherapy alone (purple), combined chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue).
Distribution of Khorana Score at Initiation of Systemic Therapy. The cohort is stratified by treatment group: chemotherapy alone (red), immunotherapy alone (purple), combined chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue).
Distribution of Charlson Comorbidity Score at Initiation of Systemic Therapy. The cohort is stratified by treatment group: chemotherapy alone (red), immunotherapy alone (purple), combined chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue).
Distribution of Body Mass Index at Initiation of Systemic Therapy. The cohort is stratified by treatment group: chemotherapy alone (red), immunotherapy alone (purple), combined chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (blue).
Distribution and Overlap of Comorbid Conditions at Initiation of Systemic Therapy. This figure describes the numbers of patients with various comorbid conditions, alone and in combination.
Cumulative Risk of Thrombosis Following Initial Diagnosis of Lung Cancer Patients, Stratified by Treatment Type. Risk of experiencing a thromboembolic event during the first ten years after diagnosis of lung cancer. The cohort is stratified by ultimate treatment group: immunotherapy alone (purple, dotted line), chemotherapy alone (red, solid line), or combination of both chemo- and immunotherapies (green, dashed line), as well as a non-chemo non-immunotherapy control group (blue, dot-dash line). Confidence intervals are represented by the shaded region around each line. Time (in days from initial cancer diagnosis) is shown along the x-axis, with cumulative risk of TEE along the y-axis. The lower table depicts number of individuals at risk for each 1-year interval. Pairwise P-values were computed by log-rank test (Table SVIII).
Cumulative Risk of Thrombosis in Lung Cancer Patients of Different Eras. Risk of experiencing a thromboembolic event during the first 180 days after the start of treatment for lung cancer patients. Each panel in this figure represents the cumulative risk during an era of time; the cohort was subdivided into smaller groups of approximately 23,000 patients each. Panel A is patients treated 2001–2006, Panel B is patients treated 2006–2011, Panel C is patients treated 2011–2015, and Panel D is patients treated 2015–2020. The sub-cohorts are stratified by treatment group: immunotherapy alone (purple, dotted line), chemotherapy alone (red, solid line), or combination of both chemo- and immunotherapies (green, dashed line), as well as a non-chemo non-immunotherapy control group (blue, dot-dash line). Confidence intervals are represented by the shaded region around each line. Time (in days from initial cancer diagnosis) is shown along the x-axes, with cumulative risk of TEE along the y-axes. The lower tables depict number of individuals at risk for each 30-day interval. Pairwise p-value comparisons were by log-rank test (Supplementary Tables SIX–SXI).
Cumulative Risk of Different Types of Thrombosis. Risk of experiencing a thromboembolic event during the first 180 days after the start of treatment for lung cancer patients. Each panel in this figure represents the cumulative risk of a particular type of thrombosis; Panel A is pulmonary embolism, Panel B is deep vein thrombosis, Panel C is cerebral infarction, and Panel D is myocardial infarction. The cohort is stratified by treatment group: immunotherapy alone (purple, dotted line), chemotherapy alone (red, solid line), or combination of both chemo- and immunotherapies (green, dashed line), as well as a non-chemo non-immunotherapy control group (blue, dot-dash line). Confidence intervals are represented by the shaded region around each line. Time (in days from initial cancer diagnosis) is shown along the x-axes, with cumulative risk of TEE along the y-axes. The lower tables depict number of individuals at risk for each 30-day interval. Pairwise P-value comparisons were by log-rank test (Supplementary Tables SXII–SXV).
Cumulative Risk of Different Types of Thrombosis in Lung Cancer Patients Stratified by Treatment Type, Accounting for Competing Risk of Death. Risk of experiencing death or a TEE during the first 180 days after the start of treatment for lung cancer patients. Each panel in this figure represents the cumulative risk of a particular type of thromboembolic event; Panel A is pulmonary embolism, Panel B is deep vein thrombosis, Panel C is cerebral infarction, and Panel D is myocardial infarction. The cohort is stratified by treatment group: immunotherapy alone (blue), chemotherapy alone (red), or combination of both chemo- and immunotherapies (green), as well as a non-chemo non-immunotherapy control group (purple). Time (in days from start of treatment) is shown along the x-axis, with cumulative risk of death or TEE along the y-axes. The upper and lower plots depict the incidence of death or TEE, respectively.
Cumulative Risk of Thrombosis in Lung Cancer Patients, Stratified by Use of Anticoagulants or Aspirin. Risk of experiencing a thromboembolic event during the first 180 days after the start of treatment for lung cancer patients. The cohort is stratified by anticoagulation (panel A) or aspirin (panel B) use concurrently with systemic therapy: absence (“−/−” red, solid line), presence (“+/−” blue, dotted line). Confidence intervals are represented by the shaded region around each line. Time (in days from start of treatment) is shown along the x-axes, with cumulative risk of TEE along the y-axes. The lower tables depict number of individuals at risk for each 30-day interval. P-values computed by log-rank.
Cumulative Risk of Hemorrhage in Lung Cancer Patients. Risk of experiencing a hemorrhage during the first 180 days after the start of treatment for lung cancer patients. The cohort is stratified by treatment group: immunotherapy alone (blue, dot-dash line), chemotherapy alone (red, solid line), or combination of both chemo- and immunotherapies (green, dashed line), as well as a non-chemo non-immunotherapy control group (purple, dotted line). Confidence intervals are represented by the shaded region around each line. Time (in days from start of treatment) is shown along the x-axis, with cumulative risk of hemorrhage along the y-axis. The lower table depicts number of individuals at risk for each 30-day interval. P-value reflects a significant difference in cumulative risk of TEE based on type of treatment. Pairwise p-values computed by log-rank (Supplementary Table SXVIII).
Cumulative Risk of Hemorrhage in Lung Cancer Patients, Stratified by Use of Anticoagulants or Aspirin. Risk of experiencing a hemorrhagic event during the first 180 days after the start of treatment for lung cancer patients. The cohort is stratified by anticoagulation (panel A) or aspirin (panel B) use concurrently with systemic therapy: absence (“−/−” red, solid line), presence (“+/−” blue, dotted line). Confidence intervals are represented by the shaded region around each line. Time (in days from start of treatment) is shown along the x-axes, with cumulative risk of TEE along the y-axes. The lower tables depict number of individuals at risk for each 30-day interval. P-values computed by log-rank.
Diagnostic Inclusion Criteria: Lung Cancer. Patients included in the study experienced at least one of the lung cancer diagnoses listed in this table and specified by ICD-9 or ICD-10-CM codes.
Diagnostic Exclusion Criteria: Thrombophilia. Patients who experienced at least one of the thrombophilia diagnoses listed in this table and specified by ICD-9 or ICD-10-CM codes were excluded from the study.
Diagnostic Criteria: Thromboembolic Event. The thromboembolic diagnoses listed in this table and specified by ICD-9 or ICD-10-CM codes were considered events of interest during the 180-day period following initiation of systemic therapy.
Khorana Score Calculation.
Sensitivity Analysis on Khorana Score Missing Data.
Charlson Score Calculation.
Groupwise Comparisons of Incidence of TEE. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of TEE After Diagnosis. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of TEE in Sub-Cohort Treated 2006–2011. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of TEE in Sub-Cohort Treated 2011–2015. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of TEE in Sub-Cohort Treated 2015–2020. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Pulmonary Embolism. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Deep Vein Thrombosis. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Cerebral Infarction. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Myocardial Infarction. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Prior TEE and Anticoagulation. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Prior TEE and Aspirin. P-values were computed by log-rank.
Groupwise Comparisons of Incidence of Hemorrhage. P-values were computed by log rank test.
Groupwise Comparisons of Incidence of TEE in Sub-Cohort with No Prior History of TEE. P-values were computed by log rank test.