Abstract
The field of microRNA research has evolved from studies aiming to gauge the importance of microRNAs to those focusing on understanding a subset of specific microRNAs that have emerged as potent regulators of molecular systems and pathophysiological conditions. In this article, we review the molecular features and regulation of miR-204 and the growing body of evidence for an important role of miR-204 in the regulation of cardiovascular and renal physiology and pathophysiological processes. miR-204 exhibits a highly tissue-specific expression pattern, and miR-204 abundance is regulated by several transcriptional and post-transcriptional mechanisms. Strong evidence supports a role for miR-204 in attenuating pulmonary arterial hypertension and hypertensive and diabetic renal injury while promoting hypertension and endothelial dysfunction in a wide range of model systems. miR-204 may influence these disease processes by targeting several biological pathways in a tissue-specific manner. miR-204 is dysregulated in patients with cardiovascular and renal diseases. The unequivocal functional roles and clear clinical relevance indicate that miR-204 is a high-value microRNA in cardiovascular and renal diseases.
Keywords: microRNA, hypertension, kidney, heart, blood vessel, gene expression
Introduction
MicroRNAs (miRNAs) are a class of small non-coding RNAs that regulate gene expression. Over 2000 distinct miRNAs have been identified in human (1), and more than half of mammalian messenger RNA (mRNA) are predicted to be subject to miRNA regulation (2,3). miRNAs often interact with 3’ untranslated region (UTR) of target mRNAs, which involves the seed region composed of nucleotides 2–7 of the miRNA and an RNA-induced silencing complex. The interaction may lead to mRNA degradation or translational repression (4,5). In some cases, miRNAs may bind to 5’ UTR, coding sequences or gene promoters (6–8)
miRNAs are involved in a variety of biological processes, such as development, differentiation, proliferation, cell survival and metabolism (9). Similar to transcriptional factors, an individual miRNA can regulate multiple target genes. A miRNA may modulate networks of target genes to influence several physiological or pathological processes. By orchestrating diverse aspects of cellular activities, miRNA-mediated gene expression regulation provides potent and dynamic mechanisms that allow cells to maintain homeostasis and adapt to challenges or contribute to pathophysiological development (10).
Cardiovascular and renal diseases contribute to large disease burden and rank among the leading causes of morbidity and mortality worldwide. Over the last two decades, the importance of miRNAs in the development of cardiovascular and renal diseases have been extensively studied. Dozens of miRNAs have been shown to be involved in these diseases, which has been reviewed elsewhere (11–15)
The field of miRNA research has evolved from initial efforts of gauging the general importance of miRNAs to focused and targeted efforts of understanding the role of specific miRNAs in defined pathophysiological conditions. In this context, several miRNAs have emerged as high-value miRNAs for cardiovascular or renal diseases because of their unequivocal functional roles and clinical relevance. miR-204 is one such miRNA.
In this article, we review the molecular features and regulation of miR-204 and the growing body of evidence for the role of miR-204 in the regulation of cardiovascular and renal physiology and pathophysiological processes. Studies of human specimens and a variety of model systems support an important role of miR-204 in these processes and have identified several molecular pathways that may mediate the effect of miR-204. miR-204 also has significant functional roles in other diseases and organ systems such as cancer, eye, and bone (16–21), which will not be reviewed in this article.
1. miR-204 Genomic organization, Expression, and Regulation
1.1. Genomic Organization of miR-204
The human gene MIR204, located in intron 9 of TRPM3, encodes a 110 bp pre-miR-204 stem loop (Figure 1A). . TRPM3 is one of the largest genes located on the long-arm of human chromosome 9 (9q21.12-q21.13) and has extensive alternative splicing and genetic variations (Figure 1B). Similarly, the gene encoding miR-204 in mouse, Mir204, which encodes a 68 bp pre-miR-204 stem loop, is located in an intron of Trpm3. The miR-204 gene is transcribed in the same direction as its host gene. Pre-miR-204 is processed by the cell to produce mature miR-204. miR-204-5p is the major strand of mature miR-204, and miR-204-3p is the minor strand and present at much lower abundance than miR-204-5p. The sequence of mature miR-204 is highly conserved across human, mouse and rat with an identical seed region sequence (Figure 1C). It is noteworthy that miR-204-5p has the same seed region sequence as miR-211-5p. The two miRNAs could, therefore, target the same mRNAs (22). The miR-211 gene is located in an intron of TRPM1 gene on human chromosome 15.
Figure 1.

Sequences and genomic organization of miR-204. A. Stem-loop structure of human pre-miR-204 with the mature hsa-miR-204-5p in pink and mature hsa-miR-204-3p in blue. B. Primary transcripts of miR-204 in human, mouse and rat within their host gene TRPM3 or Trpm3. Exons and introns are shown as vertical light blue boxes and horizontal lines, respectively. Exons with dash line are expressed in some transcript variants only. The hairpins indicate the locations of the sequences encoding pre-miR-204. Number indicates the nucleotide position or length of an intron. Arrow indicates the direction of gene transcription. C. Mature sequences of miR-204-5p and −3p are highly conserved in human (hsa-), mouse (mmu-), and rat (rno-) with identical seed regions.
1.2. miR-204 Expression
miR-204 exhibits a highly tissue-specific expression pattern. In an analysis of human tissues (23), miR-204-5p expression was found to be highly abundant in several neural tissues including arachnoid mater, dura mater, brain and spinal cord. High expression levels of miR-204-5p were also detected in kidney, epididymis, and testis. Measurable levels of miR-204-5p were found in artery (Figure 2A, left panel). miR-204-3p exhibits a similar tissue pattern but at much lower abundance (Figure 2A, right panel). In a deep-sequencing analysis of a large collection of human primary cells (24), miR-204-5p was found to be one of the top 3 most abundant miRNAs in human retinal pigment epithelial cells but was not detected in hematopoietic or mesodermal cells.
Figure 2.
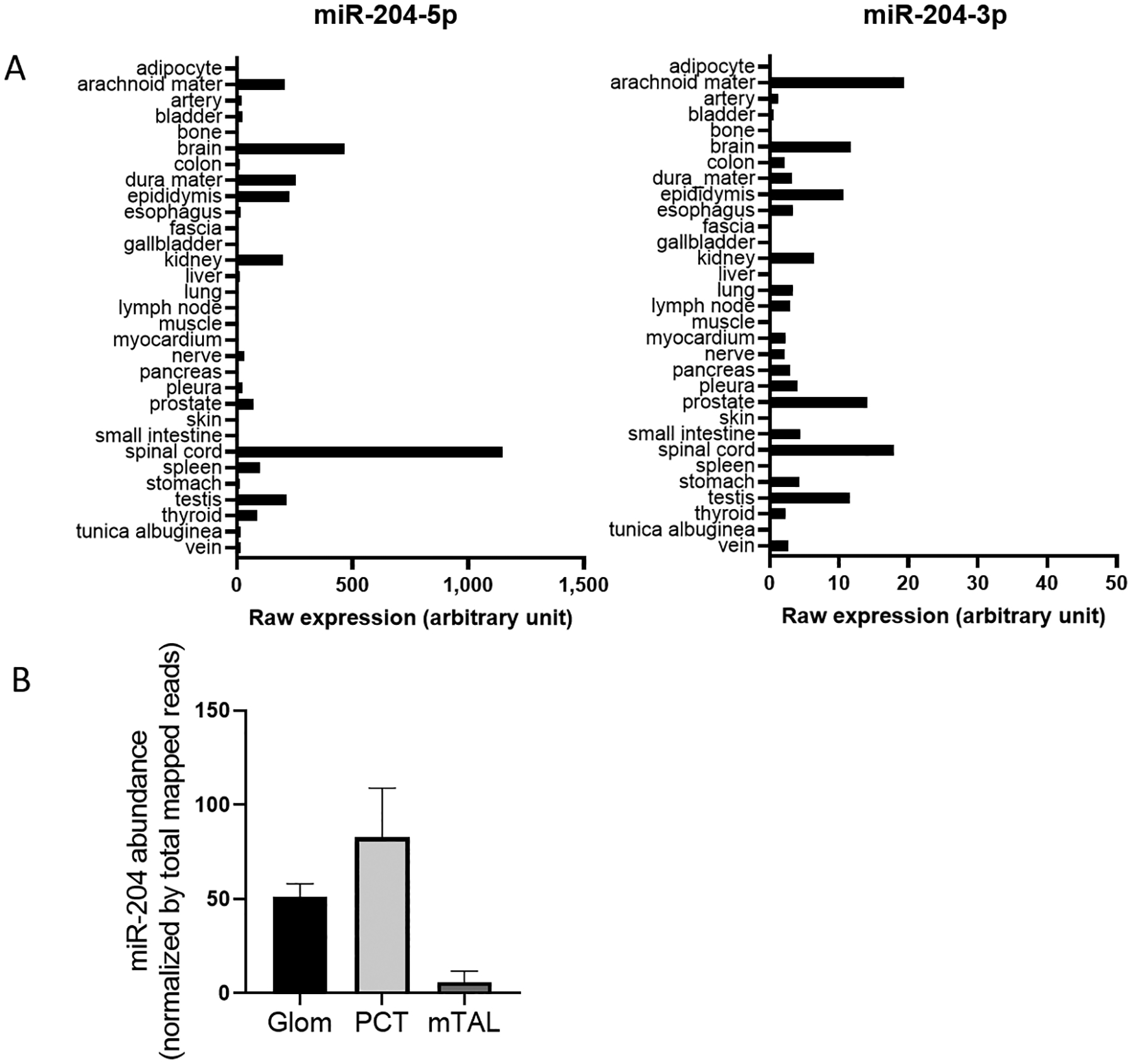
miR-204 expression is tissue-specific. A. Expression of miR-204-5p (left panel) and −3p (right panel) in human tissues from 2 males based on a microarray analysis. Note the different scales for miR-204-5p and −3p. Plotted based on data from reference 17. B. miR-204 abundance in the glomerulus (Glom), proximal convoluted tube (PCT) and medullary thick ascending limb (mTAL) in kidneys of male Sprague-Dawley rats. miR-204 expression was analyzed by small RNA deep-sequencing and normalized by total mapped reads in each sample. Plotted based on data from reference 20.
A deep-sequencing analysis by Smith et al (25) provides data on 1927 miRNAs in 23 different tissues from 14 organs extracted from male and female Sprague Dawley rats. Among tissues examined, miR-204-5p and miR-204-3p are enriched in brain regions including cerebellum, brainstem, hippocampus, and cerebrum. In addition, miR-204-5p is highly abundant in dorsal root ganglion (DGR). Kidney, especially the renal cortex, has one of the highest expressions of miR-204-5p outside of the nervous system. A study utilizing laser-capture microdissection to obtain specific tissue regions has shown that miR-204 is enriched in the glomerulus and proximal convoluted tube and detectable in the medullary thick ascending limb in the rat kidney (Figure 2B) (26).
1.3. Regulation of miR-204 Expression
Genomic deletion and epigenetic silencing.
A common feature of miRNAs observed in malignancies is defect of tumor repressive miRNAs. miR-204 is a well-known tumor-suppressor miRNA that is downregulated in a variety of cancers due to genetic deletion. Imam et al. (27) reported that miR-204-containing region on 9q21.12 chromosome was lost in 45% of ovarian cancers, 28% of breast cancers and 40% of pediatric renal tumors. Reduction of miR-204 level is strongly correlated with its genomic DNA content. DNA methylation may regulate gene expression (28). In cancer, tumoral cells may exhibit global hypomethylation of DNA, whereas CpG islands of promoter regions of tumor suppressive genes undergo DNA hypermethylation which may lead to gene silencing and promotion of cancer development (29–31). Ying et al. (32) showed that downregulation of miR-204 in most glioma specimens was associated with hypermethylation in the promoter CpG island in the TRPM3/miR-204 gene. Demethylation by inhibiting DNA methyltransferase in several glioma cells with low levels of miR-204 significantly promoted the expression of miR-204. A similar promoter methylation mechanism has been found by Zhang and colleague (33) as possibly mediating the downregulation of miR-204 in non-small-cell lung carcinoma (NSCLC).
Transcriptional factors.
miRNA expression could also be regulated by transcription factors (TFs). Pax6 is one of the key transcription factors in regulating genetic networks for the normal development of central nervous system, pancreas, and eye (34,35). It is reported that expression of TRPM3/miR-204 requires Pax6, and systemic deletion or lens-specific mutants of PAX6 led to the loss of TRPM3 and miR-204 expression during lens development (36). STAT3 is a critical transcription factor that mediates a variety of biological processes. Activation of STAT3 by phosphorylation of cytoplasmic STAT3 leads to STAT3 dimerization and nuclear translocation and regulates gene expression including miR-204. Courboulin et al. (37) showed that activated STAT3 bound directly to the regulatory regions of TRPM3 and diminished the expression of miR-204 in pulmonary artery smooth muscle cells (PASMCs). The phenotype is sustained by a positive feedback loop attributed to the activation of Src-STAT3 caused by downregulation of miR-204. Inhibition of STAT3 by Dehydroepiandrosterone (DHEA) partially reversed pulmonary arterial hypertension (PAH) (38). Transcriptional repression of miR-204 by activated STAT3 has also been observed in cancers (39,40).
Feedback loop.
An interesting observation on miR-204 regulation is the regulatory circuit between miR-204 and its targets. Endoplasmic reticulum (ER) stress upregulated vascular miR-204 which impaired endothelial-dependent vasodilation by targeting Sirtuin 1 (Sirt1) (41). Sirt1 is an NAD-dependent deacetylase and plays an important role in vascular function. Interestingly, Sirt1 could downregulate miR-204, suppress ER stress and preserve vasorelaxation (42). In this miR-204/Sirt1 feed forward loop, reduction of vascular Sirt1 by miR-204 leads to amplification of miR-204 during ER stress, and endothelial dysfunction is attributed to the further downregulation of Sirt1. In addition, miR-204/Six1 loop contributes to the epithelial-mesenchymal transition (EMT) during migration and invasion of breast cancer (43). As a direct target of miR-204, Six1 expression is upregulated in breast cancer specimens and negatively correlated with miR-204 level. The overexpression of Six1 mediates downregulation miR-204.
Long non-coding RNAs.
Long non-coding RNAs (LncRNAs) are a class of transcripts (> 200 nt in length) with very limited or no protein coding potential but acting as potent regulators in diverse biological processes (28,44) Yu et al. showed LncRNA TUG1 (Taurine Upregulated Gene 1) sponged (i.e., bound and prevented the functioning of) miR-204, interfered with the interaction of miR-204 and its target Runt-related transcription factor 2 (RUNX2), promoted osteogenic differentiation in calcific aortic valve disease (45).
These reported mechanisms for the regulation of miR-204 expression are summarized in Figure 3.
Figure 3.
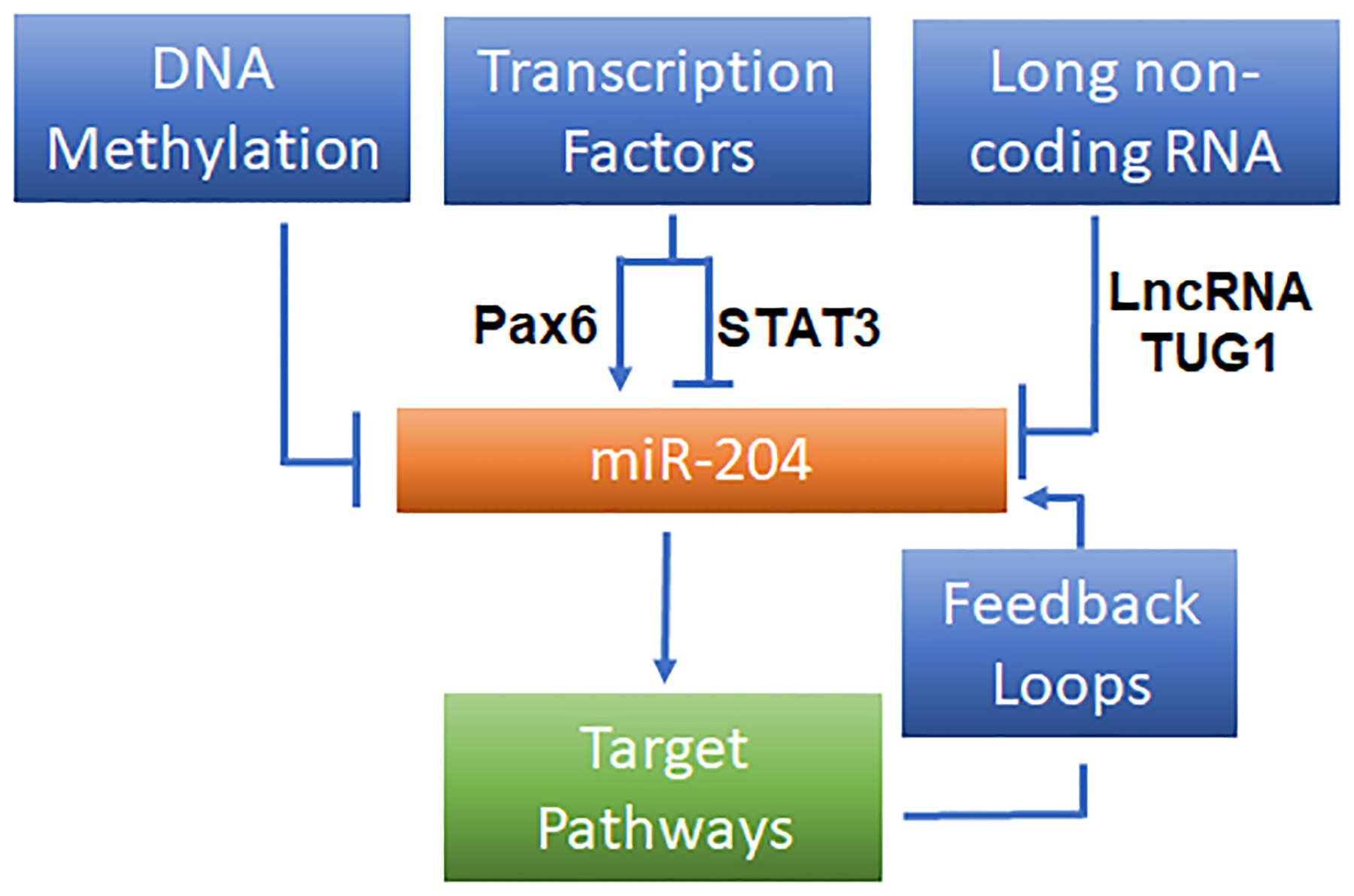
Reported mechanisms that regulate miR-204 expression.
2. miR-204 in Cardiovascular Diseases
2.1. Pulmonary Arterial Hypertension
PAH is a severe health condition exhibiting a complex and heterogeneous vasculopathy in pulmonary vasculature that affects a large number of patients worldwide (46,47). PAH is characterized by intimal thickening and fibrotic lesions, medial smooth muscle cell hypertrophy, adventitial collagen deposition and variable venous obstruction (48). These pathological processes will ultimately lead to pulmonary arterial remodeling and pressure increase, right ventricle dysfunction and in some cases patient death. These complex pathological changes observed in PAH is initiated by seemingly disparate signaling pathways (49). Several studies support an important role of miR-204 in the development of PAH (Figure 4).
Figure 4.
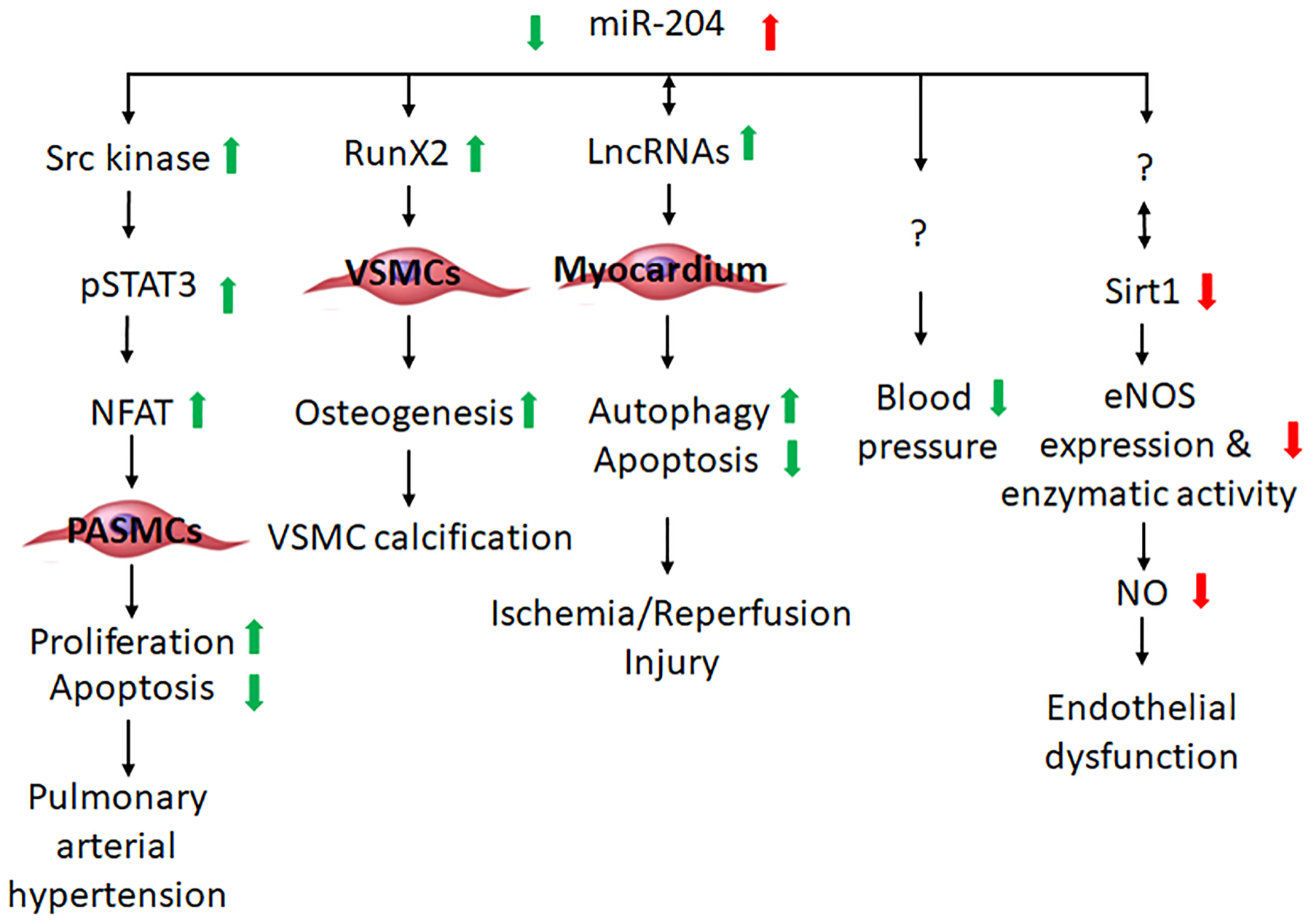
miR-204 may influence the development of cardiovascular diseases through several cellular processes and signaling pathways.
Caruso and colleagues screened lung miRNA profiles using rat PAH model induced by hypoxia and monocrotaline (MCT) (50). Interestingly, chronic hypoxia exposure led to a marked and sustained decrease in Dicer which is critical to miRNA biogenesis. This finding suggests an overall downregulation of miRNA in the development of PAH. Subsequently, the altered miRNA expression was reported in human diseased pulmonary artery smooth muscle cells (PASMCs) and animal models (37). Among 377 miRNAs being examined, 6 miRNAs (miR-450a, −145a, −302b, −27b, 367, and −138) were found to be upregulated in PAH-PASMCs compared with control PASMCs, while miR-204 was the only miRNA that was downregulated in PAH-PASMCs. Notably, in both human subjects and rodents the decreased miR-204 expression level is not only a biomarker for PAH, but also closely correlated with the severity of PAH (37,51). Recently, Estephan et al. (51) showed a stepwise elevation of circulating miR-204 across pulmonary vasculature and a decrease of intracellular miR-204 in PASMCs in World Health organization (WHO) Group I PAH patients. Changes in circulating miR-204 levels also occur in other disease conditions including type 1 diabetes (52). It remains to be determined whether miR-204 in extracellular fluids may contribute to intercellular signaling. These finding suggest pathological excretion and depletion of miR-204 in PASMCs may attribute to the downregulation of miR-204 observed in human and animal models of PAH.
The imbalance of proliferation and apoptosis observed in PAH-PASMCs has been implicated in the pathogenesis of PAH, and the activation of Src-STAT3-BMPR2 and NFAT pathways may be involved (53–55). In line with depressed miR-204 expression, increased activity of Src, STAT3 and NFATc2 has been observed in PAH-PASMCs which could be reversed by miR-204 mimic. Courboulin and colleagues reported that as a direct target of miR-204 in PAH, Src activator SHP2 was upregulated following miR-204 suppression and led to activation of the Src-STAT3-NFAT pathway. Furthermore, the restoration of miR-204 in MCT-induced PAH rat model attenuates the SHP2, STAT3, NFATc2 activation, PASMC proliferation and resistance to apoptosis as well as the development of PAH (37). In addition to the aberrant STAT3 activity in the pathogenesis of PAH, the abnormal activation of HIF-1α has also been reported (56) and may be mediated through miR-204 downregulation. RUNX2, a well-known direct target of miR-204 (57,58), is involved in the activation of HIF-1α (59–61). Potus and colleagues (62) showed that synthetic miR-204 treatment inhibited RUNX2-HIF-1α in a rat model of PAH.
These studies highlight miR-204 as potentially a diagnostic biomarker, severity indicator, mechanistic mediator, and therapeutic agent for PAH. However, the cell type-specific mechanistic pathways mediating the effect of miR-204 on PAH awaits experimental validation by using knockout or transgenic animal models, and the clinical value of miR-204 in PAH requires further investigation.
2.2. Vascular Calcification
Vascular calcification in peripheral artery and coronary artery is highly prevalent in aging populations and is associated with increased risk of cardiovascular diseases, such as myocardial infarction and arterial wall dissection after balloon angioplasty procedure (63–65). Vascular calcification is an active and regulated biomineralization process that shares similar pathways as bone formation (66). Vascular smooth muscle cells (VSMCs) play a pivotal and integral role in mediating vascular calcification by undergoing trans-differentiation into osteoblast-like cells, secretion of osteogenic proteins, depositing calcified matrix material and inhibiting mineral reabsorption activity (67). Growing evidence suggests miRNAs are important regulators in the pathogenesis of vascular calcification by mediating osteoblastic differentiation.
miR-204 overexpression decreases, while miR-204 depletion increases, alkaline phosphate (ALP) activity and deposition of bone matrix minerals in the stromal cell line ST2 cells that are undergoing osteoblast differentiation (58). miR-204 target RUNX2 is an essential transcription factor for osteoblastic differentiation and is upregulated in SMCs undergoing trans-differentiation (57,68,69). SMC-specific deletion of RUNX2 alleviates vascular calcification (70). Cui and colleagues (57) observed a stepwise decrease of miR-204 expression along with increase of bone matrix mineral deposition, ALP activity, osteocalcin secretion, and upregulation of RUNX2 in cultured VSMCs treated with β-glycerophosphate. This calcification process could be attenuated or exacerbated by miR-204 mimic or inhibitor transfection (Figure 4). Moreover, miR-204 overexpression reverses artery calcification and RUNX2 protein expression induced by vitamin D3 in mice in vivo (57).
2.3. Myocardial Ischemia/Reperfusion Injury
Myocardial infarction (MI) occurs when the coronary blood flow is occluded, preventing sufficient oxygen supply to the downstream myocardium. Paradoxically, reperfusion of the ischemic region contributes to the post-MI myocardial damage. The role of several miRNAs, including miR-204, in the cardiac ischemia/reperfusion (I/R) injury has been reported (Figure 4).
Autophagy is a regulated mechanism for removing unnecessary or dysfunctional cell components, which is essential for cellular homeostasis and has been implicated in I/R injury of cardiomyocyte (71,72). LC3 (microtubule-associated protein 1 light chain3) contributes to autophagosome formation. LC3-I is converted to LC3-II during autophagosome formation. Downregulation of miR-204 and upregulation of LC3-II were reported in a rat myocardial IR model (73). Yu et al. reported that AK139328, a lncRNA, sponged miR-204-3p and silencing of AK139328 increased miR-204-3p abundance, inhibited autophagy in myocardium, and attenuated myocardial I/R injury in a mouse model (74).
Apoptosis is importantly involved in the I/R injury of myocardium. (75,76). In a mouse model of I/R injury induced by coronary artery occlusion, lncRNA HIFIA-AS1 (hypoxia-inducible factor 1α-antisense RNA1) and SOCS2 (suppressor of cytokine signaling 2), a regulator of apoptosis, were elevated, whereas miR-204 and cardiac function were reduced (77). Overexpression of miR-204 or silencing of HIF1A-AS1 was reported to inhibit cell apoptosis, and HIF1A-AS1 might sponge miR-204 and elevate SOCS2 expression (77). Another lncRNA KCNQ1OT1 (KCNQ opposite strand/ antisense transcript 1) was found to be upregulated during I/R injury and might sponge miR-204, leading to the elevation of galectin-3 (LGALS3) and apoptotic factors (78).
2.4. Endothelial Dysfunction
Endothelial dysfunction, including impaired endothelium-dependent vasodilation, is a clinically relevant and mechanistically important intermediate phenotype in cardiovascular disease (79). Various factors may contribute to the development of endothelial dysfunction, such as decreased endothelial nitric oxide synthase (eNOS), lack of eNOS substrate or cofactors, and NO degradation by reactive oxygen species (ROS) (80). Vikram et al. (81) reported vascular miR-204 expression, promoted by gut microbiome, impaired vasodilation through downregulation of Sirt1 (Figure 4). Sirt1, a class III histone deacetylase, promotes endothelium-dependent vasorelaxation by deacetylating eNOS and stimulating endothelial NO production (82,83). Overexpression of miR-204 impaired endothelial-dependent vasorelaxation, which was rescued by Sirt1 reconstitution (81). Similarly, Kassan et al. (41) reported that endoplasmic reticulum (ER) stress-induced upregulation of miR-204 mediated impairment of endothelial-dependent vasorelaxation due to downregulation of Sirt1. On the other hand, another study by Kassan and colleagues (42) showed that Sirt1 suppressed ER stress and preserved endothelium-dependent vasorelaxation by downregulating miR-204. These findings suggest the presence of a feed forward relation in which reduction of vascular Sirt1 by miR-204 leads to amplification of miR-204 that exacerbates endothelial dysfunction by further downregulating Sirt1.
2.5. Hypertension
Hypertension (systolic blood pressure ≥ 130 mm Hg or a diastolic blood pressure ≥ 80 mm Hg) is estimated to affect nearly half of adults in the US and increases the risk of cardiovascular diseases and stroke (84). Several miRNAs have been reported to be involved in the pathogenesis of hypertension (85–88). A recent study indicated that miR-204 might promote the development of hypertension (Figure 4) (89). Uninephrectomized mice treat with angiotensin II and a 4% NaCl diet typically exhibit a sustained increase of mean arterial pressure of 40–50 mmHg within two weeks. Global miR-204 gene knockout (Mir204−/−) substantially attenuated the development of hypertension in this model, reducing mean arterial pressure by up to 30 mmHg compared to wild-type mice (89). In SS.13BN26 rats, a congenic model derived from the Dahl salt-sensitive (SS) rat and exhibiting mild salt sensitivity, miR-204-5p knockdown did not significantly change mean arterial pressure but appeared to increase pulse pressure (89).
The mechanism by which miR-204 influences blood pressure is unknown. miR-204-induced endothelial dysfunction, discussed in the preceding section, may contribute to hypertension. In addition to the vasculature, other organ systems such as the kidney and the nervous system also participate in the regulation of blood pressure and the development of hypertension. miR-204-5p is down-regulated in kidney biopsy specimens from patients with hypertension as well as in kidneys of SS rats fed a high-salt diet (89). miR-204 is upregulated in the hippocampus in aging mice and induces senescence-like phenotype in matured neurons (90). It remains to be investigated whether or how miR-204 influences blood pressure through vascular, renal, or neural mechanisms.
3. miR-204 in Renal Disease
3.1. Chronic Kidney Disease
Chronic kidney disease (CKD) is a serious health problem that affects around 13% of the US population (91). The increasing prevalence of CKD is associated with the expansion of patients with diabetes and hypertension, which are the two leading causes of CKD and end-stage renal disease (ESRD) (92,93). Several miRNAs are specifically enriched in the kidney (94,95), and the importance of several miRNAs in the development of CKD has been demonstrated (14,15). The development of CKD involves both glomeruli and tubulointerstitial regions, and miRNAs exhibit expression changes specific to glomeruli and tubules in different types of kidney pathology (96).
Rudnicki et al. (97) performed miRNA and mRNA profiling in renal biopsies of 43 patients with various glomerular disease and showed that miR-204 was downregulated in the progressive CKD subjects compared to the stable cases. miR-204-5p exhibited the largest downregulation among all miRNAs detected in a small RNA deep-sequencing analysis of human kidney biopsy specimens from patients diagnosed with hypertensive nephrosclerosis (87). Subsequent analyses confirmed the downregulation of miR-204-5p in kidney biopsy specimens from additional patients with hypertension, hypertensive nephrosclerosis, or diabetic nephropathy, as well as in kidneys of SS rats fed a high-salt diet (89). Knockdown of miR-204-5p in SS.13BN26 rats led to substantial pathological changes in the kidney, and global knockout of Mir204 gene in a hypertensive mouse model and knockdown of miR-204-5p in a diabetic mouse model resulted in exacerbated albuminuria and renal pathologies (89). The protective role of miR-204 in chronic renal injury was associated with suppression of miR-204-5p target gene SHP2 and the STAT3 pathway. Components of the SHP2-Scr-STAT3 pathway is involved in the pathogenesis of renal interstitial fibrosis (98–100) and the regulation of renal vasculature (101).
3.2. Acute Kidney Injury
Acute kidney injury (AKI) is a syndrome that displays rapid loss of kidney function and may result from a variety of insults, such as renal I/R injury and nephrotoxins (102). AKI has a high mortality rate, and AKI that appears to have recovered may cause the development of CKD, promote progression of pre-exiting CKD, and lead to higher risk of ESRD (103–106). In addition, AKI is an independent risk factor for the development of cardiovascular diseases and related mortality (103,107). Several miRNAs, such as miR-21 and miR-489, are involved in the development of AKI (108,109).
Chen et al. showed that miR-204 was the most substantially reduced miRNA in a mouse I/R model of AKI induced by unilaterally clamping of the renal artery (110). miR-204 may target specific protein 1 (SP1) and suppress epithelial-mesenchymal transition (EMT), and hypoxia-induced EMT could be partially reversed by miR-204 overexpression (110). Overexpression of miR-204-5p and blockage of the Fas/FasL pathway by targeting FasL may attenuate renal I/R injury in mice (111). Yi et al. (112) observed that lncRNA NEAT1 was increased in serum samples of patients with sepsis, a common cause of AKI, and associated with the severity of AKI. miR-204 expression was negatively corelated with NEAT1, and overexpression of miR-204 suppressed NEAT1 and alleviated LPS-induced cell injury in rat mesangial cells that may mediated by the NF-κB signaling pathway (112).
3.3. Chronic Allograft Dysfunction
Kidney transplantation is a desired treatment option for patients with ESRD. Acute rejection and chronic allograft dysfunction (CAD) with interstitial fibrosis (IF) and tubular atrophy (TA) are major challenges in the management of transplant recipients (113). The current tests to monitor and predict graft functions are either inaccurate or highly invasive, such as kidney biopsy. miRNA signatures have been reported for kidney allografts with acute rejection (114,115). A study by Scian et al. (116) identified significant differences in the expression of 5 miRNAs (miR-142-3p, miR-204, miR-107, miR-211 and miR-32) in biopsy samples from patients with CAD-IF/TA compared to patients with normal allograft. Importantly, the differential expression of miR-204, miR-142-3p and miR-211 was also detected in urine samples in patients with CAD-IF/TA from an independent cohort. Using these three miRNAs, the authors were able to prospectively identify several kidney transplant patients with IF/TA-like miRNA alteration in parallel with the histological changes. It is noteworthy that the serum creatinine and eGFR in these patients were still in normal range. These findings suggest the urine miRNA signature may be used as a noninvasive biomarker for monitoring graft function and facilitate early intervention.
4. miR-204 target pathways
Several miR-204 target genes and the biological pathways that they are involved in have been discussed in the preceding sections. One could also examine reported miR-204 target genes in aggregate to gain a more systematic and comprehensive view of biological pathways that miR-204 could influence. We retrieved 62 human miR-204-5p target genes from an online resource miRTarBase (117). To avoid false-positive predictions of miRNA-target interactions, we only selected target genes that have been experimentally supported by reporter assay, western blot or real-time PCR. A KEGG pathway analysis of these 62 genes using DAVID (https://david.ncifcrf.gov/) reveals the enrichment of miR-204-5p targets for various cancer-related pathways such as colorectal cancer and pancreatic cancer. Targets of miR-204-5p are also enriched for pathways related to adherens junction, Hippo signaling, pluripotency of stem cells, and several other signaling pathways (Table 1).
Table 1.
miR-204-5p target genes are significantly enriched for several biological pathways.
| KEGG Pathway | Genes | Fold Enrichment | FDR |
|---|---|---|---|
| hsa04520:Adherens junction | CDC42, SMAD4, CDH1, SNAI1, SNAI2, TGFBR1, TGFBR2 | 16.5 | 0.0003 |
| hsa05200:Pathways in cancer | FZD1, CDC42, MAP2K1, SMAD4, CDH1, BCL2, DVL3, CXCR4, MMP9, TGFBR1, BIRC2, TGFBR2 | 5.1 | 0.0005 |
| hsa05161:Hepatitis B | MAP2K1, SMAD4, CREB1, BCL2, MMP9, TGFBR1, CREB5 | 8.1 | 0.0060 |
| hsa04390:Hippo signaling pathway | FZD1, SMAD4, CDH1, DVL3, SNAI2, TGFBR1, TGFBR2 | 7.8 | 0.0060 |
| hsa05202:Transcriptional misregulation in cancer | HOXA10, MEIS1, HMGA2, SIX1, MMP9, RUNX2, TGFBR2 | 7.0 | 0.0070 |
| hsa04725:Cholinergic synapse | MAP2K1, CREB1, BCL2, ITPR1, JAK2, CREB5 | 9.1 | 0.0070 |
| hsa05210:Colorectal cancer | MAP2K1, SMAD4, BCL2, TGFBR1, TGFBR2 | 13.5 | 0.0070 |
| hsa05212:Pancreatic cancer | CDC42, MAP2K1, SMAD4, TGFBR1, TGFBR2 | 12.9 | 0.0074 |
| hsa05206:MicroRNAs in cancer | MAP2K1, BCL2, HMGA2, VIM, EZR, SIRT1, MMP9, BCL2L2 | 4.7 | 0.0139 |
| hsa04550:Signaling pathways regulating pluripotency of stem cells | FZD1, MEIS1, MAP2K1, SMAD4, DVL3, JAK2 | 7.2 | 0.0139 |
| hsa04915:Estrogen signaling pathway | MAP2K1, CREB1, ITPR1, MMP9, CREB5 | 8.5 | 0.0259 |
| hsa04668:TNF signaling pathway | MAP2K1, CREB1, MMP9, BIRC2, CREB5 | 7.8 | 0.0315 |
| hsa04722:Neurotrophin signaling pathway | CDC42, NTRK2, MAP2K1, BDNF, BCL2 | 7.0 | 0.0439 |
| hsa05205:Proteoglycans in cancer | FZD1, CDC42, MAP2K1, ITPR1, EZR, MMP9 | 5.0 | 0.0467 |
Total of 62 human miR-204-5p target genes were retrieved from miRTarBase and enrichment of biological pathways analyzed. The targeting of these genes by miR-204-5p has been supported by strong experimental evidence as defined by miRTarBase, which includes evidence from reporter assay, Western blot, or real time PCR. FDR, false discovery rate.
5. Summary and future directions
In summary, miR-204 exhibits a highly tissue-specific expression pattern, and miR-204 abundance is regulated by several transcriptional and post-transcriptional mechanisms. miR-204 has a well-established role in maintaining the normal function of the retinal pigment epithelium, suppressing tumors, and possibly influencing the development of osteoarthritis. Within cardiovascular and renal diseases, strong evidence supports a role for miR-204 in attenuating pulmonary arterial hypertension and hypertensive and diabetic renal injury while promoting hypertension and endothelial dysfunction. The clinical relevance of miR-204 in cardiovascular and renal disease is supported by dysregulation of miR-204 in specimens obtained from patients with these diseases.
The unequivocal role of miR-204 in disease development in a wide range of model systems, the significant evidence for its relevance to human disease, and the substantial abundance of miR-204 in relevant tissues make miR-204 a “high value” microRNA worthy of further investigation.
It will be highly valuable to examine how a defined cellular context confers specificity to the effect of miR-204. The effect of miR-204 on various disease processes sometimes appears to be contradictory. For example, miR-204 gene knockout in mice substantially attenuates hypertension but exacerbates hypertensive renal injury (89). These findings suggest a highly tissue-specific nature of the role of miR-204. It is possible that miR-204 targets different genes in different tissues depending on the cellular context. It is also possible that miR-204 targets the same genes, but these target genes have different functional implications in different tissues. Some investigators may see the apparent target promiscuity as an insurmountable challenge for microRNA research. However, many regulatory pathways are promiscuous but, like microRNAs, may achieve various degrees of regulatory specificity in a defined cellular context. Such complexity is ubiquitous in biology and underscores the critical importance of studying and understanding humans as molecular systems (28). Elucidating the highly tissue-specific nature of the role of miR-204 and the underlying mechanisms could help to correct misperceptions about microRNA biology and drive forward the field of microRNA research and, more broadly, molecular systems medicine research.
Several studies reviewed in this article suggest miR-204 could be developed as a diagnostic or prognostic biomarker or a therapeutic target or agent. Studies of large, well-defined patient cohorts will be necessary to assess the diagnostic or prognostic value of miR-204. As the effect of miR-204 is likely to be tissue-specific, it will be important to consider targeting specific tissues in the development of miR-204-based therapeutics for complex diseases, such as hypertension, renal disease, and other cardiovascular diseases. Significant advances have been made in targeting therapeutic oligonucleotides to specific tissues (118,119).
Sources of Funding
This work was supported by US National Institutes of Health grants HL121233 and HL149620 and the Advancing a Healthier Wisconsin Endowment. J.L. was supported by T32 HL134643 and the Cardiovascular Center’s A.O. Smith Fellowship Scholars Program.
Footnotes
Disclosure
None.
References
- 1.Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res 2019;47:D155–D162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005;120:15–20. [DOI] [PubMed] [Google Scholar]
- 3.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009;19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 2011;12:99–110. [DOI] [PubMed] [Google Scholar]
- 5.Ipsaro JJ, Joshua-Tor L. From guide to target: molecular insights into eukaryotic RNA-interference machinery. Nat Struct Mol Biol 2015;22:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu W, San Lucas A, Wang Z, Liu Y. Identifying microRNA targets in different gene regions. BMC Bioinformatics 2014;15 Suppl 7:S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman JJ, Legesse-Miller A, Coller HA. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc Natl Acad Sci U S A 2008;105:14879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mladinov D, Liu Y, Mattson DL, Liang M. MicroRNAs contribute to the maintenance of cell-type-specific physiological characteristics: miR-192 targets Na+/K+-ATPase beta1. Nucleic Acids Res 2013;41:1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hata A Functions of microRNAs in cardiovascular biology and disease. Annu Rev Physiol 2013;75:69–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang M MicroRNA: a new entrance to the broad paradigm of systems molecular medicine. Physiol Genomics 2009;38:113–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med 2008;358:1370–80. [DOI] [PubMed] [Google Scholar]
- 12.Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation 2010;121:1022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol 2009;6:419–29. [DOI] [PubMed] [Google Scholar]
- 14.Trionfini P, Benigni A. MicroRNAs as Master Regulators of Glomerular Function in Health and Disease. J Am Soc Nephrol 2017;28:1686–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang M, Liu Y, Mladinov D et al. MicroRNA: a new frontier in kidney and blood pressure research. Am J Physiol Renal Physiol 2009;297:F553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikhaylova O, Stratton Y, Hall D et al. VHL-regulated MiR-204 suppresses tumor growth through inhibition of LC3B-mediated autophagy in renal clear cell carcinoma. Cancer Cell 2012;21:532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall DP, Cost NG, Hegde S et al. TRPM3 and miR-204 establish a regulatory circuit that controls oncogenic autophagy in clear cell renal cell carcinoma. Cancer Cell 2014;26:738–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C, Miyagishima KJ, Dong L et al. Regulation of phagolysosomal activity by miR-204 critically influences structure and function of retinal pigment epithelium/retina. Hum Mol Genet 2019;28:3355–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conte I, Hadfield KD, Barbato S et al. MiR-204 is responsible for inherited retinal dystrophy associated with ocular coloboma. Proc Natl Acad Sci U S A 2015;112:E3236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J, Zhao L, Fan Y et al. The microRNAs miR-204 and miR-211 maintain joint homeostasis and protect against osteoarthritis progression. Nat Commun 2019;10:2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang D, Shin J, Cho Y et al. Stress-activated miR-204 governs senescent phenotypes of chondrocytes to promote osteoarthritis development. Sci Transl Med 2019;11. [DOI] [PubMed] [Google Scholar]
- 22.Shiels A TRPM3_miR-204: a complex locus for eye development and disease. Hum Genomics 2020;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludwig N, Leidinger P, Becker K et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res 2016;44:3865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Rie D, Abugessaisa I, Alam T et al. An integrated expression atlas of miRNAs and their promoters in human and mouse. Nat Biotechnol 2017;35:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith A, Calley J, Mathur S et al. The Rat microRNA body atlas; Evaluation of the microRNA content of rat organs through deep sequencing and characterization of pancreas enriched miRNAs as biomarkers of pancreatic toxicity in the rat and dog. BMC Genomics 2016;17:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kriegel AJ, Liu Y, Liu P et al. Characteristics of microRNAs enriched in specific cell types and primary tissue types in solid organs. Physiol Genomics 2013;45:1144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imam JS, Plyler JR, Bansal H et al. Genomic loss of tumor suppressor miRNA-204 promotes cancer cell migration and invasion by activating AKT/mTOR/Rac1 signaling and actin reorganization. PLoS One 2012;7:e52397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang M Epigenetic Mechanisms and Hypertension. Hypertension 2018;72:1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 1983;301:89–92. [DOI] [PubMed] [Google Scholar]
- 30.Jones PA, Baylin SB. The epigenomics of cancer. Cell 2007;128:683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell 2010;19:698–711. [DOI] [PubMed] [Google Scholar]
- 32.Ying Z, Li Y, Wu J et al. Loss of miR-204 expression enhances glioma migration and stem cell-like phenotype. Cancer Res 2013;73:990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi L, Zhang B, Sun X et al. MiR-204 inhibits human NSCLC metastasis through suppression of NUAK1. Br J Cancer 2014;111:2316–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osumi N, Shinohara H, Numayama-Tsuruta K, Maekawa M. Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells 2008;26:1663–72. [DOI] [PubMed] [Google Scholar]
- 35.Shaham O, Menuchin Y, Farhy C, Ashery-Padan R. Pax6: a multi-level regulator of ocular development. Prog Retin Eye Res 2012;31:351–76. [DOI] [PubMed] [Google Scholar]
- 36.Shaham O, Gueta K, Mor E et al. Pax6 regulates gene expression in the vertebrate lens through miR-204. PLoS Genet 2013;9:e1003357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Courboulin A, Paulin R, Giguere NJ et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 2011;208:535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulin R, Meloche J, Jacob MH, Bisserier M, Courboulin A, Bonnet S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 2011;301:H1798–809. [DOI] [PubMed] [Google Scholar]
- 39.Sacconi A, Biagioni F, Canu V et al. miR-204 targets Bcl-2 expression and enhances responsiveness of gastric cancer. Cell Death Dis 2012;3:e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bao W, Wang HH, Tian FJ et al. A TrkB-STAT3-miR-204-5p regulatory circuitry controls proliferation and invasion of endometrial carcinoma cells. Mol Cancer 2013;12:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kassan M, Vikram A, Li Q et al. MicroRNA-204 promotes vascular endoplasmic reticulum stress and endothelial dysfunction by targeting Sirtuin1. Sci Rep 2017;7:9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kassan M, Vikram A, Kim YR et al. Sirtuin1 protects endothelial Caveolin-1 expression and preserves endothelial function via suppressing miR-204 and endoplasmic reticulum stress. Sci Rep 2017;7:42265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng J, Wei M, Shi R et al. miR-204-5p/Six1 feedback loop promotes epithelial-mesenchymal transition in breast cancer. Tumour Biol 2016;37:2729–35. [DOI] [PubMed] [Google Scholar]
- 44.Kong Y, Lu Z, Liu P et al. Long Noncoding RNA: Genomics and Relevance to Physiology. Compr Physiol 2019;9:933–946. [DOI] [PubMed] [Google Scholar]
- 45.Yu C, Li L, Xie F et al. LncRNA TUG1 sponges miR-204-5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification. Cardiovasc Res 2018;114:168–179. [DOI] [PubMed] [Google Scholar]
- 46.Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol 2008;44:14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrell NW, Adnot S, Archer SL et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 2007;28:23–42, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou G, Chen T, Raj JU. MicroRNAs in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2015;52:139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caruso P, MacLean MR, Khanin R et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol 2010;30:716–23. [DOI] [PubMed] [Google Scholar]
- 51.Estephan LE, Genuardi MV, Kosanovich CM et al. Distinct plasma gradients of microRNA-204 in the pulmonary circulation of patients suffering from WHO Groups I and II pulmonary hypertension. Pulm Circ 2019;9:2045894019840646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu G, Thielen LA, Chen J et al. Serum miR-204 is an early biomarker of type 1 diabetes-associated pancreatic beta-cell loss. Am J Physiol Endocrinol Metab 2019;317:E723–E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong WK, Knowles JA, Morse JH. Bone morphogenetic protein receptor type II C-terminus interacts with c-Src: implication for a role in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2005;33:438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brock M, Trenkmann M, Gay RE et al. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res 2009;104:1184–91. [DOI] [PubMed] [Google Scholar]
- 55.Bonnet S, Rochefort G, Sutendra G et al. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A 2007;104:11418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonnet S, Michelakis ED, Porter CJ et al. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 2006;113:2630–41. [DOI] [PubMed] [Google Scholar]
- 57.Cui RR, Li SJ, Liu LJ et al. MicroRNA-204 regulates vascular smooth muscle cell calcification in vitro and in vivo. Cardiovasc Res 2012;96:320–9. [DOI] [PubMed] [Google Scholar]
- 58.Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells 2010;28:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee SH, Che X, Jeong JH et al. Runx2 protein stabilizes hypoxia-inducible factor-1alpha through competition with von Hippel-Lindau protein (pVHL) and stimulates angiogenesis in growth plate hypertrophic chondrocytes. J Biol Chem 2012;287:14760–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon TG, Zhao X, Yang Q et al. Physical and functional interactions between Runx2 and HIF-1alpha induce vascular endothelial growth factor gene expression. J Cell Biochem 2011;112:3582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Gise A, Archer SL, Maclean MR, Hansmann G. The first Keystone Symposia Conference on pulmonary vascular isease and right ventricular dysfunction: Current concepts and future therapies. Pulm Circ 2013;3:275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Potus F, Graydon C, Provencher S, Bonnet S. Vascular remodeling process in pulmonary arterial hypertension, with focus on miR-204 and miR-126 (2013 Grover Conference series). Pulm Circ 2014;4:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation 1992;86:64–70. [DOI] [PubMed] [Google Scholar]
- 64.Beadenkopf WG, Daoud AS, Love BM. Calcification in the Coronary Arteries and Its Relationship to Arteriosclerosis and Myocardial Infarction. Am J Roentgenol Radium Ther Nucl Med 1964;92:865–71. [PubMed] [Google Scholar]
- 65.Loecker TH, Schwartz RS, Cotta CW, Hickman JR Jr. Fluoroscopic coronary artery calcification and associated coronary disease in asymptomatic young men. J Am Coll Cardiol 1992;19:1167–72. [DOI] [PubMed] [Google Scholar]
- 66.Bostrom K, Watson KE, Stanford WP, Demer LL. Atherosclerotic calcification: relation to developmental osteogenesis. Am J Cardiol 1995;75:88B–91B. [DOI] [PubMed] [Google Scholar]
- 67.Liu W, Zhang Y, Yu CM et al. Current understanding of coronary artery calcification. J Geriatr Cardiol 2015;12:668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naik V, Leaf EM, Hu JH et al. Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study. Cardiovasc Res 2012;94:545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen N, Naik V, Speer MY. Diabetes mellitus accelerates cartilaginous metaplasia and calcification in atherosclerotic vessels of LDLr mutant mice. Cardiovasc Pathol 2013;22:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin ME, Chen TM, Wallingford MC et al. Runx2 deletion in smooth muscle cells inhibits vascular osteochondrogenesis and calcification but not atherosclerotic lesion formation. Cardiovasc Res 2016;112:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 2003;301:1387–91. [DOI] [PubMed] [Google Scholar]
- 72.Stromhaug PE, Klionsky DJ. Approaching the molecular mechanism of autophagy. Traffic 2001;2:524–31. [DOI] [PubMed] [Google Scholar]
- 73.Xiao J, Zhu X, He B et al. MiR-204 regulates cardiomyocyte autophagy induced by ischemia-reperfusion through LC3-II. J Biomed Sci 2011;18:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu SY, Dong B, Fang ZF, Hu XQ, Tang L, Zhou SH. Knockdown of lncRNA AK139328 alleviates myocardial ischaemia/reperfusion injury in diabetic mice via modulating miR-204-3p and inhibiting autophagy. J Cell Mol Med 2018;22:4886–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hofstra L, Liem IH, Dumont EA et al. Visualisation of cell death in vivo in patients with acute myocardial infarction. Lancet 2000;356:209–12. [DOI] [PubMed] [Google Scholar]
- 76.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 1994;94:1621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xue X, Luo L. LncRNA HIF1A-AS1 contributes to ventricular remodeling after myocardial ischemia/reperfusion injury by adsorption of microRNA-204 to regulating SOCS2 expression. Cell Cycle 2019;18:2465–2480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Rong J, Pan H, He J et al. Long non-coding RNA KCNQ1OT1/microRNA-204–5p/LGALS3 axis regulates myocardial ischemia/reperfusion injury in mice. Cell Signal 2020;66:109441. [DOI] [PubMed] [Google Scholar]
- 79.Widlansky ME, Gokce N, Keaney JF Jr., Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 2003;42:1149–60. [DOI] [PubMed] [Google Scholar]
- 80.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 2000;87:840–4. [DOI] [PubMed] [Google Scholar]
- 81.Vikram A, Kim YR, Kumar S et al. Vascular microRNA-204 is remotely governed by the microbiome and impairs endothelium-dependent vasorelaxation by downregulating Sirtuin1. Nat Commun 2016;7:12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mattagajasingh I, Kim CS, Naqvi A et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 2007;104:14855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang QJ, Wang Z, Chen HZ et al. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res 2008;80:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian Z, Liang M. Renal metabolism and hypertension. Nat Commun 2021;12:963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sethupathy P, Borel C, Gagnebin M et al. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3’ untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes. Am J Hum Genet 2007;81:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baker MA, Wang F, Liu Y et al. MiR-192-5p in the Kidney Protects Against the Development of Hypertension. Hypertension 2019;73:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, Usa K, Wang F et al. MicroRNA-214-3p in the Kidney Contributes to the Development of Hypertension. J Am Soc Nephrol 2018;29:2518–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jusic A, Devaux Y, Action EU-CC. Noncoding RNAs in Hypertension. Hypertension 2019;74:477–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng Y, Wang D, Wang F et al. Endogenous miR-204 Protects the Kidney against Chronic Injury in Hypertension and Diabetes. J Am Soc Nephrol 2020;31:1539–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohammed CP, Rhee H, Phee BK et al. miR-204 downregulates EphB2 in aging mouse hippocampal neurons. Aging Cell 2016;15:380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coresh J, Selvin E, Stevens LA et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- 92.Atkins RC. The changing patterns of chronic kidney disease: the need to develop strategies for prevention relevant to different regions and countries. Kidney Int Suppl 2005:S83–5. [DOI] [PubMed] [Google Scholar]
- 93.Jha V, Garcia-Garcia G. Global kidney disease - Authors’ reply. Lancet 2013;382:1244. [DOI] [PubMed] [Google Scholar]
- 94.Tian Z, Greene AS, Pietrusz JL, Matus IR, Liang M. MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatic analysis. Genome Res 2008;18:404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun Y, Koo S, White N et al. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res 2004;32:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baker MA, Davis SJ, Liu P et al. Tissue-Specific MicroRNA Expression Patterns in Four Types of Kidney Disease. J Am Soc Nephrol 2017;28:2985–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rudnicki M, Perco P, B DH et al. Renal microRNA- and RNA-profiles in progressive chronic kidney disease. Eur J Clin Invest 2016;46:213–26. [DOI] [PubMed] [Google Scholar]
- 98.Pang M, Ma L, Gong R et al. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 2010;78:257–68. [DOI] [PubMed] [Google Scholar]
- 99.Yan Y, Ma L, Zhou X et al. Src inhibition blocks renal interstitial fibroblast activation and ameliorates renal fibrosis. Kidney Int 2016;89:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bienaime F, Muorah M, Yammine L et al. Stat3 Controls Tubulointerstitial Communication during CKD. J Am Soc Nephrol 2016;27:3690–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens 2013;22:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 2012;380:756–66. [DOI] [PubMed] [Google Scholar]
- 103.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012;82:516–24. [DOI] [PubMed] [Google Scholar]
- 104.Ishani A, Xue JL, Himmelfarb J et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 2009;20:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012;81:442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wald R, Quinn RR, Luo J et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 2009;302:1179–85. [DOI] [PubMed] [Google Scholar]
- 107.James MT, Ghali WA, Knudtson ML et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation 2011;123:409–16. [DOI] [PubMed] [Google Scholar]
- 108.Xu X, Kriegel AJ, Liu Y et al. Delayed ischemic preconditioning contributes to renal protection by upregulation of miR-21. Kidney Int 2012;82:1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei Q, Liu Y, Liu P et al. MicroRNA-489 Induction by Hypoxia-Inducible Factor-1 Protects against Ischemic Kidney Injury. J Am Soc Nephrol 2016;27:2784–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen SJ, Wu P, Sun LJ et al. miR-204 regulates epithelial-mesenchymal transition by targeting SP1 in the tubular epithelial cells after acute kidney injury induced by ischemia-reperfusion. Oncol Rep 2017;37:1148–1158. [DOI] [PubMed] [Google Scholar]
- 111.Zhu Y, Yin X, Li J, Zhang L. Overexpression of microRNA-204–5p alleviates renal ischemia-reperfusion injury in mice through blockage of Fas/FasL pathway. Exp Cell Res 2019;381:208–214. [DOI] [PubMed] [Google Scholar]
- 112.Chen Y, Qiu J, Chen B et al. Long non-coding RNA NEAT1 plays an important role in sepsis-induced acute kidney injury by targeting miR-204 and modulating the NF-kappaB pathway. Int Immunopharmacol 2018;59:252–260. [DOI] [PubMed] [Google Scholar]
- 113.Racusen LC, Solez K, Colvin RB et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999;55:713–23. [DOI] [PubMed] [Google Scholar]
- 114.Anglicheau D, Sharma VK, Ding R et al. MicroRNA expression profiles predictive of human renal allograft status. Proc Natl Acad Sci U S A 2009;106:5330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sui W, Dai Y, Huang Y, Lan H, Yan Q, Huang H. Microarray analysis of MicroRNA expression in acute rejection after renal transplantation. Transpl Immunol 2008;19:81–5. [DOI] [PubMed] [Google Scholar]
- 116.Scian MJ, Maluf DG, David KG et al. MicroRNA profiles in allograft tissues and paired urines associate with chronic allograft dysfunction with IF/TA. Am J Transplant 2011;11:2110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang HY, Lin YC, Li J et al. miRTarBase 2020: updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res 2020;48:D148–D154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Seth PP, Tanowitz M, Bennett CF. Selective tissue targeting of synthetic nucleic acid drugs. J Clin Invest 2019;129:915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov 2020;19:673–694. [DOI] [PMC free article] [PubMed] [Google Scholar]


