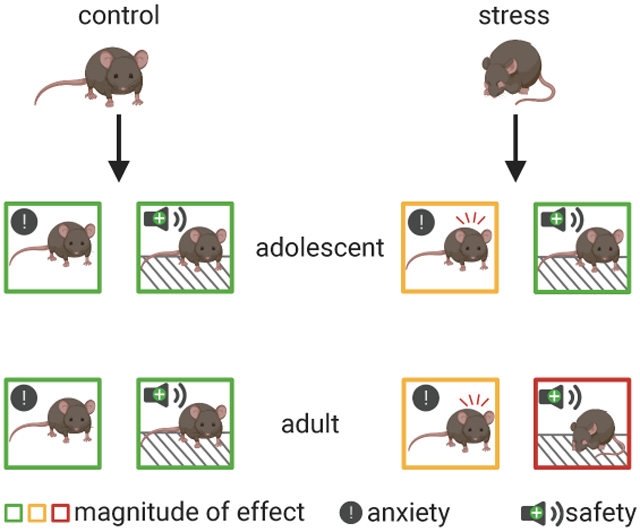
Abstract
Anxiety disorders are highly prevalent across the lifespan, although diagnoses peak early in adolescence. As a method for inhibiting fear, safety signals have the potential to augment conventional treatments for anxiety. However, the ability to acquire and use safety signals during adolescence remains unclear. Moreover, the impact of stress on safety learning has received surprisingly little attention given that stress is a major factor preceding anxiety onset. In this study, mice were trained in a discriminative conditioning protocol to facilitate safety learning and were tested for fear inhibition using a conditioned safety signal. Next, independent groups of mice were exposed to chronic unpredictable stress (CUS) conditions between postnatal day 22 and 28, followed by tests for anxiety-like phenotypes or fear inhibition using a safety signal, performed either 24 hours or five weeks following CUS. Pre-adolescent CUS reduced weight in adolescence and this effect endured into adulthood. CUS also increased specific anxiety-like behaviors in adolescence that were unique from the increase in anxiety observed in adulthood. Despite increased anxiety-like behaviors, adolescents were able to learn about and effectively use safety signals to inhibit fear. In contrast, adults that experienced CUS showed a subtle increase in anxiety but had impaired safety signal learning and usage. Together, these findings indicate that pre-adolescent stress has immediate and enduring effects on anxiety-like behaviors but impairs the capacity for conditioned inhibition only following incubation.
Keywords: Adolescence, safety learning, conditioned inhibition, stress, fear, anxiety
Graphical Abstract
1. Introduction
Fear responses facilitate self-preservation by increasing vigilance and helping an animal avoid potential danger. However, the inability to regulate fear responses can be maladaptive when it prevents the animal from engaging in other goal-directed activities. Altered fear regulation is a key feature of anxiety disorders. Notably, a peak in anxiety disorder diagnoses occurs during early adolescence and earlier onset has been associated with increased symptom severity and comorbidities later in life [1]. Prevalence of anxiety disorders is estimated to be as high as 30% [2], [3], with an even greater number of youths experiencing sub-diagnostic symptoms [4]. Unfortunately, conventional behavioral and pharmacological treatments have limited long-term efficacy for a notable percentage of the patient population [5], [6].
One of the most common components of treatment for anxiety disorders is exposure therapy, which is based on principles of fear extinction, an associative learning process by which a stimulus previously associated with an aversive outcome develops a secondary ‘safe’ association. Notably, the ‘safe’ memory does not overwrite the initial fear association, but rather masks its expression through associative competition, leaving open the possibility for the fear response to return [7]. Numerous studies have highlighted adolescent-specific difficulties with extinction learning [8]-[10]. Furthermore, the associative competition that manifests from extinction learning has been implicated as a key reason for adolescent fear inhibition failures [8].
As an alternative means to regulate fear that avoids issues related to associative competition, safety signal learning has emerged as an area of great promise [11]. Safety signals are stimuli that explicitly represent safety by indicating the absence of an aversive outcome. Importantly, safety signals are separate from a fear stimulus and can attenuate fear responding through a process known as conditioned inhibition [12], highlighting the clinical potential for using safety signals to augment existing treatments for anxiety. For example, safety signals have been used to attenuate depression-like phenotypes in mice, exerting behavioral changes similar to those observed after pharmacological intervention [13]. In addition, the neural circuitry underlying fear inhibition using a safety signal differs from that engaged by extinction [14], [15], highlighting the potential for use in patient populations where conventional treatments are less effective, as is commonly the case during adolescence. While adolescent mice exhibit diminished fear regulation following extinction [10], adolescent mice can successfully differentiate between fear and safety signals as measured by freezing levels [16]. Yet, the extent to which safety signals can directly inhibit fear during adolescence has not been established.
Developmental exposure to stress can greatly impact subsequent neurodevelopment and behavior on a long-term scale [17]-[21]. Moreover, human and rodent studies alike suggest that early life stress imparts a lifelong susceptibility to anxiety and depression [22]-[25]. Yet most animal studies investigating safety learning use stress-naive subjects, making it difficult to determine the impact of stress on the capacity for safety signals to directly inhibit fear responding (i.e., conditioned inhibition). Addressing this gap in the literature will be critical for establishing the potential clinical value of safety signals, as many individuals suffering from psychiatric disease have had significant exposure to stress prior to entering treatment. A recent study found that one-day repeated foot shock stress led to impaired extinction but did not impact the ability to use a safety signal to inhibit fear in adult rats [26]. These findings provide initial support for the intact efficacy of safety signals following stress. However, additional work is necessary to establish the impact of chronic stress exposure on the regulation of fear responses by safety signals, particularly during adolescence.
The chronic unpredictable stress (CUS) model in rodents is thought to mimic the uncontrollable and unpredictable nature of stress often experienced by humans. Given the strong evidence for chronic stress-induced alterations in brain and behavioral functioning, we predicted that exposure to CUS during the transition from late childhood to adolescence would disrupt safety learning and fear inhibition both immediately (i.e., during adolescence) and long-term (i.e., during adulthood). We adapted a CUS paradigm for use in pre-adolescent mice that has previously been used in adolescents [27] and adults alike [28] to induce anxiety- and depression-like behaviors. Previous literature has suggested that the transition into and throughout adolescence is a period of dynamic development and thus a sensitive period for adverse experience [18], [19], [29]. In line with this, adolescent mice appear to be more susceptible to a shorter bout of CUS than adults [27]. Here, we tested the sufficiency of a seven day CUS exposure confined to pre-adolescence (postnatal day, PND22-28) for inducing immediate and enduring increases in anxiety-like behaviors. In independent cohorts of mice, we then investigated the effect of prior stress exposure on adolescent and adult safety learning and conditioned inhibition. These experiments add to a growing body of literature regarding how timing and duration of stress impact the subsequent capacity for fear regulation.
2. Material and Methods
2.1. Subjects
Pregnant C57BL/6N mice (Charles River Laboratories) arrived at embryonic day 12. On PND21, weaned males were mixed across litters and group-housed in cohorts of 3-5 per cage. Cages were randomly assigned to control or experimental conditions. To validate safety learning protocols, cages of mice were assigned to undergo either summation or retardation tests for conditioned inhibition during either adolescence or adulthood. Separate cages of mice were assigned to undergo CUS exposure or serve as controls during the pre-adolescent period. Cages of control or CUS mice were then assigned to undergo either anxiety testing (open field, elevated plus maze, marble burying, and novelty-suppressed feeding) or safety learning protocols, during either adolescence or adulthood (Figure 2a). For experiments involving CUS, to ensure that any weight differences observed following stress were not due to baseline differences in weight, mice were weighed at the time of weaning and cages assigned to the control condition were weight-matched to cages assigned to the CUS condition. Mice were group housed throughout the study (with the exception of a single overnight isolation stress for CUS mice, described in Section 2.3) and maintained on a 12-hour light/dark cycle at 18-22°C with food (LabDiet, PicoLab Rodent Diet 20) and water ad libitum. All behavioral tests were conducted during the light cycle. Experiments were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and protocols were approved by the Weill Medical College of Cornell University’s Institutional Animal Care and Use Committee.
Figure 2. Experimental design for CUS-related studies.
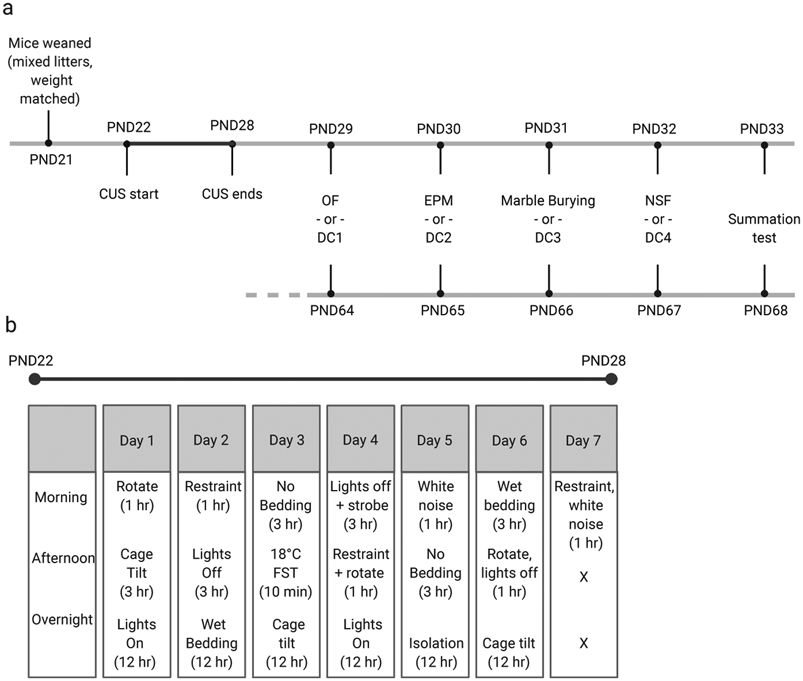
Schematic of the experimental schedule for the four groups: adolescent anxiety, adolescent safety, adult anxiety, and adult safety (a). Schematic of the chronic unpredictable stress (CUS) paradigm used (b). CUS was administered for seven days from PND22 to PND28. The first day of stress was always 1-hour rotation, cage tilt, and lights on overnight. For days 2-6, an assortment of three different stressors described in Section 2.3 were used. The final stress exposure on day 7 was always 1-hour restraint stress plus white noise, which ended 24 hours before any behavior was initiated. On PND29, cages of control or CUS mice were assigned to undergo either anxiety testing (open field (OF), elevated plus maze (EPM), marble burying, and novelty-suppressed feeding (NSF)) or safety learning protocols, during either adolescence or adulthood. DC1-4 = discriminative conditioning sessions 1-4.
2.2. Safety Learning Protocols
2.2.1. Discriminative Conditioning
Discriminative conditioning was carried out in standard conditioning chambers (Med Associates) as previously described [15]. In the chamber, ambient light was provided through an LED Stimulus Light (50 lux; 18-cm above the grid floor). The chamber was scented with peppermint (1/1000 in 70% ethanol). Each day, for four consecutive days, mice were acclimated to the chamber for two minutes prior to any stimulus exposures then presented with intermixed trials of fear or safety cues on a variable intertrial interval (ITI; 30-90 seconds) schedule. The cues were distinct tones (2.9 and 12.5 kHz, counterbalanced) played at 80dB for 20 seconds. For a subset of mice (Section 3.1) an additional 22.1 kHz tone was also included. Mice were exposed to two of the three tones during discriminative conditioning and the third tone served as a novel cue during the subsequent summation test (Section 2.2.2). Co-terminating with fear cue presentations, delivery of a 1-s 0.5mA foot shock served as the aversive unconditioned stimulus. Mice were exposed to two presentations of the fear cue and 30 presentations of the safety cue each day, with trial order varied daily. A subset of mice (Section 3.2) experienced parallel presentations of the 2.9 or 12.5 kHz tones but in the absence of the foot shock. These mice served as yoked stimulus controls in subsequent retardation tests. Mice remained in the conditioning chamber for one minute after the final stimulus presentation before being returned to their home cages. Each discriminative conditioning session and all subsequent test sessions were recorded and analyzed using Video Freeze ® software (Med Associates).
2.2.2. Summation Test for Conditioned Inhibition
A summation test (applicable to mice in Sections 3.1, 3.4, and 3.6) took place 24 hours after the final discriminative conditioning session. A novel context (“Context B”) was used in order to isolate cue-elicited behavioral responses from residual contextual fear. The test context was differentiated using a black acrylic A-frame contextual insert, a white acrylic floor cover, and chevron print wallpaper on the back wall. Ambient light was provided throughout the session through a light box (125 lux; ceiling-mounted 52-cm above the floor). The chamber was scented with (−)-Limonene, 92% (1/1000 in 70% ethanol). Following a two-minute acclimation period to the testing chamber mice were presented with one trial each of the fear cue, the safety cue, a novel cue, a simultaneous presentation of the fear and safety cues together (i.e., safety compound) and a simultaneous presentation of the fear and novel cues together (i.e., a novel compound), all in the absence of shock.
To hone in on the ability of a safety cue to inhibit fear, mice in CUS groups and their control counterparts underwent a modified summation test. Following a two-minute acclimation period to the testing chamber mice were presented with intermixed trials of fear cues, safety cues, and simultaneous presentations of both cues together (i.e., the safety compound). Three of each stimulus type were presented to mice in the absence of shock pseudorandomly (i.e., no stimuli were repeated two trials in a row). For both variations of the summation test, cue presentations lasted for 20 seconds with an ITI of 60 seconds. After the final tone presentation, mice remained in the chamber for one minute before being returned to their home cages.
2.2.3. Retardation Test for Conditioned Inhibition
A retardation test (applicable to mice in Section 3.2) took place 24 hours after the final session of discriminative conditioning. In addition to mice with standard discriminative conditioning (“Safety”) and yoked stimulus controls (“Stimulus”), a third group of mice that had remained in the homecage was used (“Naive”). The retardation test took place in Context B (see details in Section 2.2.2), but with the floor cover removed to expose the grid floor for foot shock. Following a two-minute acclimation period to the testing chamber mice were presented with the safety cue (or corresponding yoked cue) co-terminating with a foot shock (1-s, 0.5mA) on a partial reinforcement schedule with randomly alternating presentations of shocked and un-shocked cues, four of each. Cue presentations lasted for 20 seconds with a 60 second ITI. After the final tone presentation, mice remained in the chamber for one minute before being returned to their home cages.
2.3. Chronic Unpredictable Stress (CUS)
CUS was administered from PND22 to PND28 (Figure 2). The CUS paradigm was adapted from previous studies [27], [28] with minor changes noted below. In brief, mice were exposed to three random, mild, and unpredictable stressors per day, including morning (1-3 hours), afternoon (1-3 hours), and overnight (12 hours) exposures. Stressors included: cage rotation, light off in the day, light on overnight, isolation, restraint stress, cold water swim (18°C, 10 minutes), cage tilt, wet bedding, no bedding, soiled rat bedding, white noise, stroboscopic light, and cage change. Because the subjects were recently weaned pre-adolescent mice, food deprivation and cold stress were excluded as stressors. The first day of stress was always 1-hour rotation, cage tilt, and lights on overnight, and the final stress exposure was always 1-hour restraint stress plus white noise. The final stress ended 24 hours before any behavior was initiated. Control mice remained in the colony and were handled twice between PND22-28 for cage changes and tail markings. All mice were weighed 24 hours after the last stress exposure (PND29). Following CUS, mice were returned to the colony room until testing began. Adolescent behavioral testing began on PND29, 24 hours after the final stressor was administered on PND28. For adult behavioral experiments, mice were returned to the colony and handled weekly for cage changes and tail markings, otherwise remaining undisturbed until testing began on PND64.
2.4. Tests for Anxiety-like Phenotypes
2.4.1. Open Field (OF)
Locomotor activity and anxiety-like phenotypes were measured in a plexiglass test apparatus (41cm2) and recorded for 10 minutes. At the start, each mouse was placed into the same corner of the arena under 100 lux. Activity was recorded and analyzed using Ethovision 5.1 (Noldus). The arena was sectioned in Ethovision to include a 26cm2 square in the center. Total distance traveled and time spent in the center and edges of the apparatus were quantified using the center point of the mouse.
2.4.2. Elevated Plus Maze (EPM)
Each mouse was placed on the same location of an open arm and allowed to explore the apparatus for 5 minutes. Testing occurred under 100 lux and activity was recorded and analyzed using Ethovision 5.1 (Noldus). Time spent in the open and closed arms and transition zones was measured using the center point of the mouse. Time in transition zones was calculated as the time spent in the middle of the apparatus plus time spent in the first 3.4 cm of the open arms.
2.4.3. Marble Burying
The marble burying test was run as previously described [27], [30]. Briefly, mice were placed in a small cage (27 x 17 x 12 cm) with 5-cm deep aspen chip bedding, on top of which 20 marbles were equally distributed. Mice were placed in the cage for 10 (adults) or 30 (adolescents) minutes. These test times were chosen based on data from pilot experiments that found these durations were sufficient for stress-naive mice to bury ~50% of marbles (data not shown). Testing occurred under 50 lux. Pictures were taken before and after testing for later analysis in ImageJ. A marble was considered buried if 75% of the marble was under bedding, determined by individual ROI pixel intensity for each exposed marble relative to pixel intensity of an uncovered marble set prior to testing.
2.4.4. Novelty-Suppressed Feeding (NSF)
Prior to testing, mice were food deprived for 12 hours (adolescents) or 18 hours (adults). A shorter deprivation period was used for adolescents given that adolescent mice are still rapidly growing. Accordingly, while adult mice were given a maximum of 10 minutes (600 sec) to feed, adolescents were given a maximum of 5 minutes (300 sec) to feed. On test day, mice were placed in the corner of an arena (41 x 27 x 16.5 cm) containing a layer of fresh bedding and a single food pellet placed in the center of the arena under 100 lux. Latency to feed was recorded. Mice that failed to feed in the predetermined time limit were censored but not excluded from the main analysis (see [31]). In a secondary analysis, mice that failed to feed in the predetermined time limit were excluded from the analysis to investigate differences in latency to feed in those mice that do feed. Homecage food intake over a 15-minute period after the test (i.e., homecage feeding) was measured as a feeding control. In order to measure individual homecage feeding, only one mouse was placed in the homecage at a time while cagemates were placed in a holding cage.
2.5. Statistics
Multifactor Analysis of Variance (ANOVAs) with the Greenhouse-Geisser correction for non-spherical data (when applicable) were used to evaluate weight gain, discriminative conditioning, contextual fear, and summation and retardation tests. For weight gain, Condition (control, CUS) served as a between-subjects measure and Age (PND21, PND29, and when applicable: PND64) served as a repeated measure. For discriminative conditioning, Stimulus Type (fear, safety) served as a within-subjects measure and Session (discriminative conditioning, DC1-4) served as a repeated measure. For contextual fear, Session (DC1-4) served as a repeated measure. For summation test data, Stimulus Type (fear, safety, safety compound, and when applicable: novel compound, novel) served as a within-subjects measure. For experiments involving CUS, Condition (control, CUS) was also included as a between-subjects measure in analysis of discriminative conditioning, contextual fear, and summation test data. To analyze retardation test data, Experience (safety, stimulus, naive) served as a between-subjects measure and Trial (1-8) served as a repeated measure. Significant interactions were decomposed using post-hoc Bonferroni-corrected multiple comparisons. Unpaired, two-tailed t-tests were used to analyze group differences in the OF, EPM, marble burying test, and homecage feeding. Given the lack of normal distribution in latency to feed data during the NSF test, we used the Kaplan-Meier survival analysis and Mantel-Cox log rank test to evaluate group differences [31]. In all safety learning protocols freezing was defined as the absence of visible movement except that required for respiration. Video Freeze ® (Med Associates) was set at a motion threshold of 18 Units for automatic scoring. For all cued fear analyses the percentage time spent freezing was calculated by dividing the cumulative time freezing during the cue by the cue duration (20s) then averaged across like-cues. For analysis of retardation test data, freezing was evaluated relative to levels attained at the end of discriminative conditioning to account for baseline differences in freezing between groups following exposure to (safety group), or absence of (stimulus group) foot shocks. Freezing data from naive mice was not adjusted. To examine contextual fear, freezing was quantified during 40 seconds of the chamber acclimation period (the initial 20s in the chamber and the 20s preceding the first tone) on each day of discriminative conditioning. For experiments involving CUS, planned comparisons were employed to evaluate hypotheses related to discriminative conditioning and summation test data. Specifically, to test the hypothesis that CUS would delay fear and safety discrimination, a planned comparison was used to establish the number of days required to reach significant discrimination during discriminative conditioning. In addition, to test the hypothesis that CUS would disrupt conditioned inhibition, a planned comparison was used to examine relative levels of freezing to fear, safety, and safety compound cues during the summation test. Across all analyses, differences were considered significant for P values less than 0.05. Generalized eta squared was reported where appropriate as a measure of effect size. Statistical analyses were performed in R Studio.
3. Results
3.1. Safety signals attenuate fear in adolescent and adult mice
While the safety learning protocols used in the present study have been shown to produce a safety signal capable of inhibiting fear in adult mice [15], it has yet to be established whether adolescents can successfully learn to use safety signals. Mice underwent discriminative conditioning beginning on either PND29 (adolescents, n = 13) or PND70 (adults, n = 12). Mice learned to discriminate between presentations of fear and safety cues across the four sessions of discriminative conditioning, freezing more to the fear than the safety cue (Figure 1a,c), supported by an interaction between Session and Stimulus Type in both adults (F(3, 33) = 17.37, p < 0.001, η2 = 0.20) and adolescents (F(3, 36) = 35.66, p < 0.001, η2 = 0.35). Both adolescent and adult mice exhibited increasing contextual fear across sessions (Figure 1a,c; adults: F(3, 33) = 8.10, p < 0.001, η2 = 0.27; adolescents: F(3, 36) = 7.81, p < 0.001, η2 = 0.25). Both ages exhibited an increase in contextual fear relative to a context-naive baseline (DC1) during DC2 (adults: padj = 0.036; adolescents: padj = 0.018) and DC3 (adults: padj = 0.012; adolescents: padj = 0.012). However, contextual fear attenuated back to baseline (DC1) levels by DC4 (adults: padj = 0.066; adolescents: padj = 0.084). Contextual fear did not differ between any other sessions (adults: padj from 0.180 to >0.999; adolescents: padj from 0.510 to >0.999).
Figure 1. Safety signals attenuate fear and retard new fear learning in adolescent and adult mice.
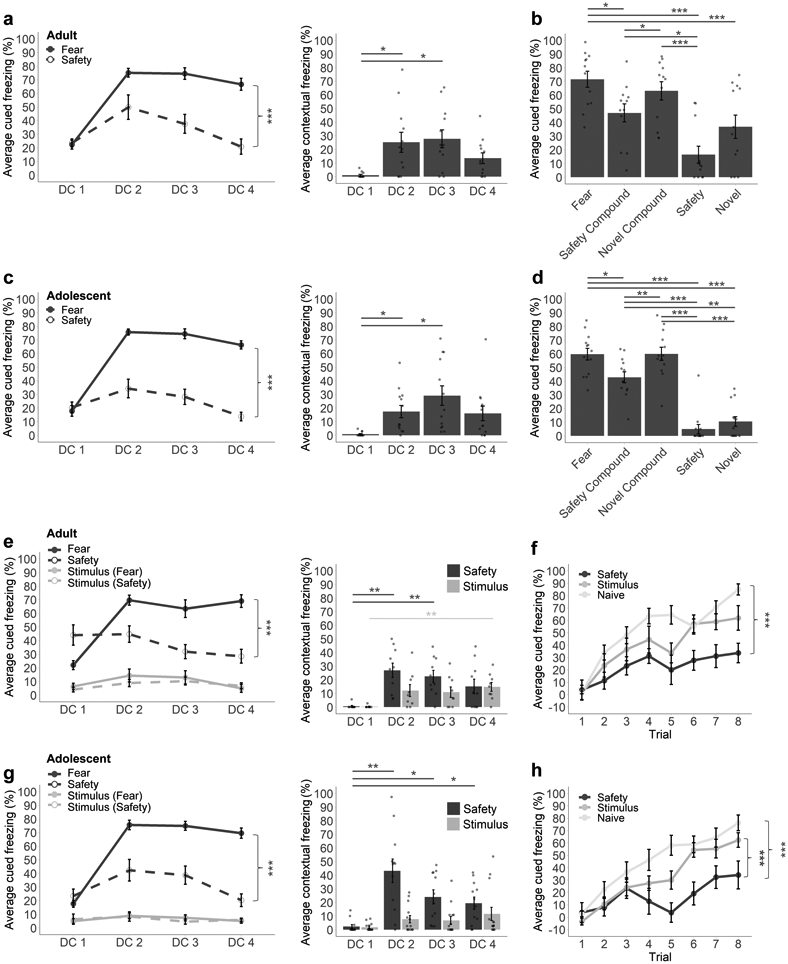
Average percentage of time spent freezing during fear and safety cues across discriminative conditioning sessions in adult (a, e, left) and adolescent (c, g, left) mice. Average percentage of time spent freezing during conditioning chamber acclimation (i.e., contextual fear) across discriminative conditioning sessions in adult (a, e, right) and adolescent (c, g, right) mice. Average percentage of time spent freezing during fear, safety compound, novel compound, safety, and novel cues during the summation test session in adult (b) and adolescent (d) mice. Average percentage of time spent freezing relative to discriminative conditioning during shock-paired presentations of the safety cue or corresponding yoked cue during the retardation test in adult (f) and adolescent (h) mice. Summation (a-d) n = 12 adult/13 adolescent; Retardation (e-h) n = 10 adult safety/9 adult stimulus/12 adult naive, 12 adolescent safety/14 adolescent stimulus/12 adolescent naive. DC1-4 = discriminative conditioning sessions 1-4. Multifactor ANOVA with Bonferroni corrected post-hoc test. Error bars represent the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Following discriminative conditioning, mice underwent a test session in which they were presented with one trial each of the fear cue, the safety cue, a novel cue, a simultaneous presentation of the fear and safety cues together (i.e., safety compound) and a simultaneous presentation of the fear and novel cues together (i.e., a novel compound), all in the absence of shock (Figure 1b,d). For both adolescent and adult mice there was a main effect of Stimulus Type (adults: F(4, 44) = 22.49, p < 0.001, η2 = 0.42; adolescents: F(4, 48) = 68.94, p < 0.001, η2 = 0.74). Decomposition of this effect in adult mice revealed that freezing was lowest during the safety cue, relative to the fear cue (padj < 0.001), safety compound (padj = 0.030), and novel compound (padj < 0.001). Freezing did not differ between the safety and novel cues (padj = 0.060). Freezing also did not differ between the novel cue and the safety compound (padj > 0.999) or novel compound (padj = 0.100), but was reduced during the novel cue relative to the fear cue (padj < 0.001). Importantly, mice froze less during the safety compound relative to fear cue (padj = 0.020), replicating our previous finding [15] that the safety cue is capable of inhibiting fear. Mice also froze less during the safety compound than the novel compound (padj = 0.030), while freezing did not differ between the fear cue and the novel compound (padj > 0.999), indicating that the capacity for fear inhibition is specific to the safety cue and reduced freezing during the safety compound does not reflect a generalization decrement due to novelty. In adolescent mice, freezing was again lowest during the safety cue, relative to the fear cue, safety compound, and novel compound (all padj < 0.001), while freezing did not differ between the safety and novel cues (padj > 0.999). Unlike adult mice, adolescent mice froze less during the novel cue relative to the safety compound (padj = 0.001) and novel compound (padj < 0.001), as well as the fear cue (padj < 0.001). As with adults, adolescent mice froze less during the safety compound relative to fear cue (padj = 0.020), providing the first evidence that adolescent mice can use safety cues to inhibit fear. Adolescent mice also froze less during the safety compound than the novel compound (padj = 0.002), but freezing did not differ between the fear cue and the novel compound (padj > 0.999), indicating that the capacity for fear inhibition is specific to the safety cue in adolescents as well as adults.
3.2. Safety learning retards fear learning in adolescent and adult mice
Mice underwent discriminative conditioning beginning on either PND29 (adolescents, n = 12) or PND70 (adults, n = 10). As expected based on the results presented in Section 3.1, both adolescent (Figure 1g) and adult (Figure 1e) mice learned to discriminate between presentations of fear and safety cues across sessions, freezing more to the fear than the safety cue (interaction between Session and Stimulus Type, adults: (F(3, 27) = 20.12, p < 0.001, η2 = 0.36; adolescents: (F(3, 33) = 27.12, p < 0.001, η2 = 0.28). Additional mice of each age (adolescents, n = 14; adults, n = 9) that experienced parallel presentations of the tones but no foot shock were included as stimulus controls. These mice showed no differences in responding to the two cues (adults: p from 0.060 to 0.559; adolescents: p from 0.459 to 0.840), showing that cue-shock pairings, rather than cue presentations alone, are required to induce freezing behavior in both adolescent and adult mice.
Both adolescent and adult mice exhibited increasing contextual fear across discriminative conditioning sessions (Figure 1e,g; adults: F(3, 27) = 7.31, p < 0.001, η2 = 0.37; adolescents: F(3, 33) = 10.56, p < 0.001, η2 = 0.39). Both ages exhibited an increase in contextual fear relative to DC1 during DC2 (adults: padj = 0.004; adolescents: padj = 0.002) and DC3 (adults: padj = 0.006; adolescents: padj = 0.012). Contextual fear attenuated back to baseline (DC1) levels by DC4 in adults (padj = 0.156), but not adolescents (padj = 0.012). Contextual fear did not differ between any other sessions (adults: all padj > 0.999; adolescents: padj from 0.090 to >0.999). Adult mice serving as stimulus controls showed mild elevations in freezing across sessions (F(3, 24) = 3.68, p = 0.026, η2 = 0.26), driven by a difference in freezing between DC1 and DC4 (padj = 0.012), although average contextual fear did not exceed 15% of the recording period and no other session comparisons reached significance (padj from 0.150 to >0.999), limiting the interpretation of this increase in freezing as contextual fear per se. Adolescent mice serving as stimulus controls did not show any differences in freezing across sessions (p = 0.076).
Following discriminative conditioning, mice underwent a retardation test session in which they were presented with the safety cue (or corresponding yoked cue) co-terminating with a foot shock (Figure 1f,h). Within both age groups, mice exhibited a reduced rate of fear acquisition when the stimulus was previously trained as a safety cue relative to mice trained as stimulus controls, and naive mice with no previous training (naive adolescents, n = 12; adults, n = 12). This was supported by a significant interaction between Trial and Experience in both adults (F(14, 196) = 2.38, p = 0.004, η2 = 0.09) and adolescents (F(14, 245) = 2.83, p < 0.001, η2 = 0.09). To decompose this interaction and further investigate the rate of fear acquisition, the difference in freezing across the session (Trial 8 relative to Trial 1) was compared between the three groups. This analysis revealed an attenuated rate of fear acquisition in adult mice trained with the safety signal relative to mice with no previous training (padj < 0.001) but not mice trained as stimulus controls (padj = 0.15). Adolescent mice trained with the safety signal exhibited an attenuated rate of fear acquisition relative to mice with no previous training (padj = 0.006) as well as mice trained as stimulus controls (padj = 0.021). The rate of fear acquisition did not differ between mice trained as stimulus controls and mice with no previous training for either adults (padj = 0.066) or adolescents (padj > 0.999). Thus, previous training with a safety cue retards new fear learning in both adolescent and adult mice, indicative of the safety signal having acquired conditioned inhibitory properties during discriminative conditioning [32]. However, while safety learning in adolescent mice delays fear learning relative to both stimulus trained and naive controls, fear learning following safety learning in adult mice was delayed only relative to naive mice, suggesting that latent inhibition due to stimulus pre-exposure may partially explain the retardation effect in adults only.
3.3. Pre-adolescent stress reduces weight gain and increases anxiety-like behavior in adolescents
Starting on PND22, mice in the stress condition (n = 25) underwent seven days of CUS (Figure 2). Mice in the control condition (n = 24) remained in the homecage. Control and CUS mice were weighed again 24 hours after the last stress exposure (PND29). As expected, mice in the CUS condition gained significantly less weight compared to controls (Figure 3a), supported by an interaction between Age and Condition, F(1, 47) = 58.55, p < 0.001, η2 = 0.11, which was further decomposed to reveal that CUS mice weighed less than control mice at PND29 (padj < 0.001) but not PND21 (padj > 0.999).
Figure 3. Pre-adolescent stress reduces adolescent weight and increases adolescent anxiety-like behaviors.
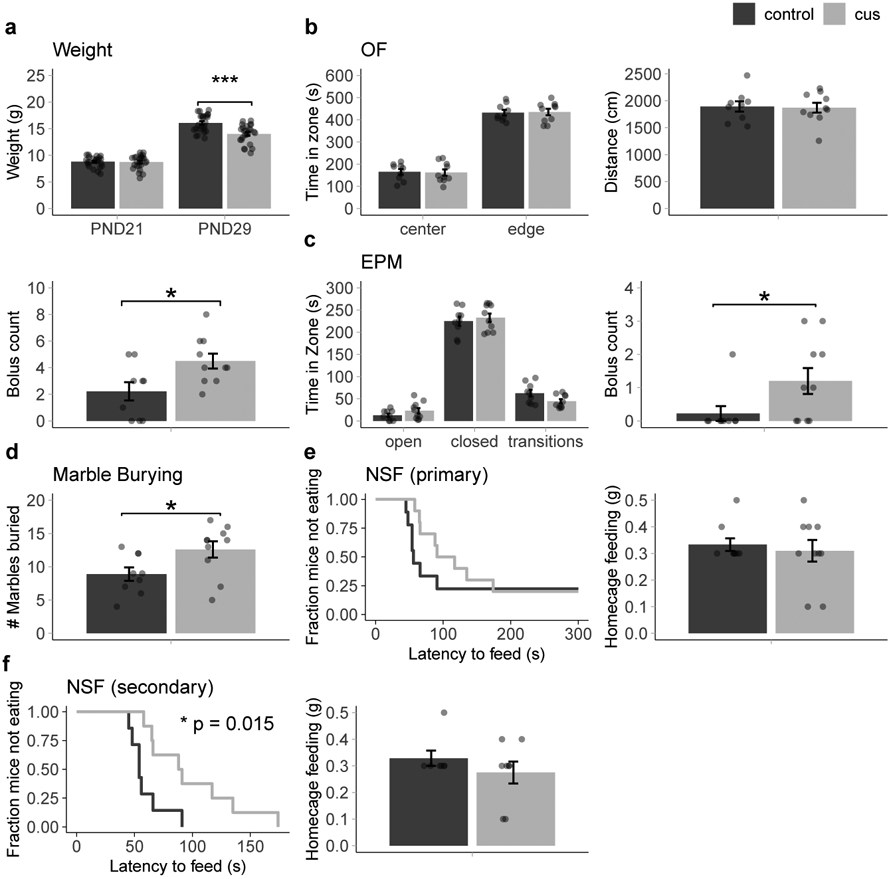
Weights from all mice that underwent adolescent behavior (a) (N = 24 control/25 CUS). Results from Open Field (OF) (b), Elevated Plus Maze (EPM) (c), marble burying (d), and Novelty Suppressed Feeding (NSF) tests (e, primary analysis and f, secondary analysis). OF, EPM, marble: n = 9 control/10 CUS; NSF: n = 9 control/10 CUS for primary analysis and 7 control/8 CUS for secondary analysis). CUS = chronic unpredictable stress. Multifactor ANOVA with Bonferroni corrected post-hoc test (a), unpaired, two-tailed t-tests (b-d), and Kaplan-Meier survival analysis with Mantel-Cox log-rank test (e-f). Error bars represent the mean ± SEM. *p < 0.05.***p < 0.001.
To determine whether seven days of CUS was sufficient to induce an anxiety-like phenotype, a subset of mice (n = 9 control, 10 CUS) received a battery of anxiety tests (Figure 2). In the OF test, there was no significant difference in time spent in center (p = 0.869) or edge (p = 0.882) zones or in distance traveled (p = 0.867); however, CUS mice produced more fecal boli than controls (t(17) = 2.59, p = 0.019)(Figure 3b). In the EPM, there were no significant differences in time spent in open arms (p = 0.186), closed arms (p = 0.570) or investigative transition zones (p = 0.051), but CUS mice produced more fecal boli (t(17) = 2.12, p = 0.049) than control mice (Figure 3c). In the marble burying test, CUS mice buried more marbles than controls (t(17) = 2.31, p = 0.034)(Figure 3d). Finally, in the NSF test, there were no differences in either latency to feed (p = 0.352) or homecage feeding (p = 0.515)(Figure 3e). Two control mice and two CUS mice were censored (but not excluded) from the analysis for failing to eat within the predetermined five-minute time limit, consistent with previous analysis parameters for this test [31]. In a secondary analysis that excluded, rather than censor, mice that failed to eat, CUS mice showed a significant increase in latency to feed (X2(1, N = 15) = 5.9, p = 0.015) with no difference in homecage feeding (p = 0.318)(Figure 3f). Overall, these results suggest that seven days of pre-adolescent CUS is sufficient to produce immediate anxiety-like behaviors.
3.4. Pre-adolescent stress does not impact adolescent safety learning
To examine the impact of pre-adolescent CUS on adolescent safety learning, mice (n = 15 control, 15 CUS) underwent discriminative conditioning beginning on PND29 (Figure 4a). Both groups similarly learned to discriminate between presentations of the fear and safety cues across sessions, freezing more to the fear cue than the safety cue. Analysis revealed significant interactions between Session and Stimulus Type, F(3, 84) = 85.56, p < 0.001, η2 = 0.41, and Session and Condition, F(3, 84) = 3.17, p = 0.028, η2 = 0.03). To determine the number of sessions required to attain significant discrimination, planned comparisons were performed between fear and safety cues for each session, by condition. These comparisons revealed that both groups of mice reached significant discrimination (i.e., greater freezing during the fear cue than the safety cue) during the second session (Control mice: DC1, t(14) = 0.99, padj > 0.999; DC2, t(14) = 6.44, padj < 0.001; DC3, t(14) = 10.70, padj < 0.001; DC4, t(14) = 8.92, padj < 0.001; CUS mice: DC1, t(14) = 1.40, padj = 0.740; DC2, t(14) = 7.52, padj < 0.001; DC3, t(14) = 11.30, padj < 0.001; DC4, t(14) = 11.70, padj < 0.001). Contrary to our prediction, these data indicate that pre-adolescent exposure to CUS does not impact adolescent discriminative conditioning.
Figure 4. Pre-adolescent stress does not impact adolescent safety learning or conditioned inhibition.
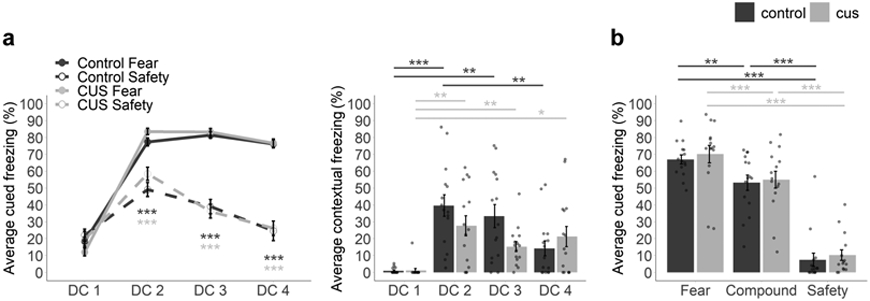
Average percentage of time spent freezing during fear and safety cues across discriminative conditioning sessions (a, left). Average percentage of time spent freezing during conditioning chamber acclimation (i.e., contextual fear) across discriminative conditioning sessions (a, right). Average percentage of time spent freezing during fear, compound, and safety cues during the test session (b). n = 15 control/15 CUS. CUS = chronic unpredictable stress. DC1-4 = discriminative conditioning sessions 1-4. Multifactor ANOVA with Bonferroni corrected post-hoc test (a, b) and planned comparisons to establish day of significant discrimination (a, left) and relative stimulus-induced freezing (b). Error bars represent the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Both control and CUS mice exhibited increasing contextual fear across discriminative conditioning sessions (Figure 4a), supported by a main effect of Session (F(3, 84) = 22.96, p < 0.001, η2 = 0.30). In addition, although contextual fear did not differ between control and CUS mice (no effect of Condition, p = 0.241) the dynamics of contextual fear expression differed across sessions (interaction between Session and Condition, F(3, 84) = 3.96, p = 0.011, η2 = 0.07). This interaction was decomposed to reveal that mice in both groups exhibited increased contextual fear relative to a context-naive baseline (DC1) during DC2 (Control: padj < 0.001, CUS: padj = 0.002) and DC3 (Control: padj = 0.002, CUS: padj = 0.004). However, while control mice attenuated back to baseline levels by DC4 (padj = 0.072), contextual fear persisted in CUS mice (padj = 0.030). Contextual fear did not differ between DC2 and DC3 (Control: padj > 0.999, CUS: padj = 0.102) or between DC3 and DC4 (Control: padj = 0.090, CUS: padj > 0.999) for either group and did not differ between DC2 and DC4 for CUS mice (padj > 0.999), but freezing was higher during DC2 than DC4 in control mice (padj = 0.003). Together, these data support modest differences in how adolescent contextual fear is acquired and regulated over time as a result of stress exposure.
Following discriminative conditioning, mice underwent a summation test session in which they were presented with intermixed fear, safety, and safety compound cues (Figure 4b). Differential responding to fear, safety, and safety compound cues was apparent (main effect of Stimulus Type, degrees of freedom corrected using Greenhouse-Geisser estimates (ε = 0.79) to account for a violation of the assumption of sphericity, F(1.58, 44.24) = 214.41, p < 0.001, η2 = 0.73). However, pre-adolescent stress had no impact on conditioned responding (no effect of Condition, F(1, 28) = 0.29, p = 0.592, η2 = 0.01; no interaction, F(1.58, 44.24) = 0.03, p = 0.972, η2 = 0.00). To investigate hypotheses related to the capacity for conditioned inhibition, planned comparisons were performed to compare freezing levels between the three cues. As expected, mice froze the least during safety cues, relative to fear cues (Control, t(14) = 18.20, padj < 0.001; CUS, t(14) = 13.50, padj < 0.001) as well as safety compound cues (Control, t(14) = 8.61, padj < 0.001; CUS, t(14) = 8.68, padj < 0.001). Notably, freezing was also significantly lower during safety compound cues than fear cues (i.e., conditioned inhibition; Control, t(14) = 3.63, padj = 0.009; CUS, t(14) = 5.06, padj < 0.001). In sum, contrary to our prediction, pre-adolescent exposure to CUS does not impact the ability to use a safety signal to attenuate fear during adolescence.
3.5. Pre-adolescent stress reduces weight gain and produces mild anxiety-like behavior in adults
To examine the enduring effects of pre-adolescent CUS on adult anxiety-like behaviors, mice underwent the same procedures described above (Figure 2). Mice were handled weekly but otherwise remained undisturbed until adult testing began. On PND64, prior to behavioral testing, mice were weighed for a third time. CUS mice (n = 21) weighed significantly less at PND29 compared to controls (n = 21; Figure 5a), replicating our findings from Section 3.3 and Figure 3a. Interestingly, CUS mice continued to show significant weight differences at PND64 (Figure 5a), suggesting these mice did not fully recover from the CUS-induced reductions in weight gain (interaction between the Age and Condition, degrees of freedom corrected using Greenhouse-Geisser estimates (ε = 0.87) to account for a violation of the assumption of sphericity, F(1.74, 69.6) = 14.44, p < 0.001, η2 = 0.108). This interaction was decomposed to reveal that CUS mice weighed less than control mice at PND29 (padj < 0.001) and PND64 (padj = 0.016) but not PND21 (padj > 0.999).
Figure 5. Pre-adolescent stress reduces adult weight and leads to mild increases in adult anxiety-like behaviors.
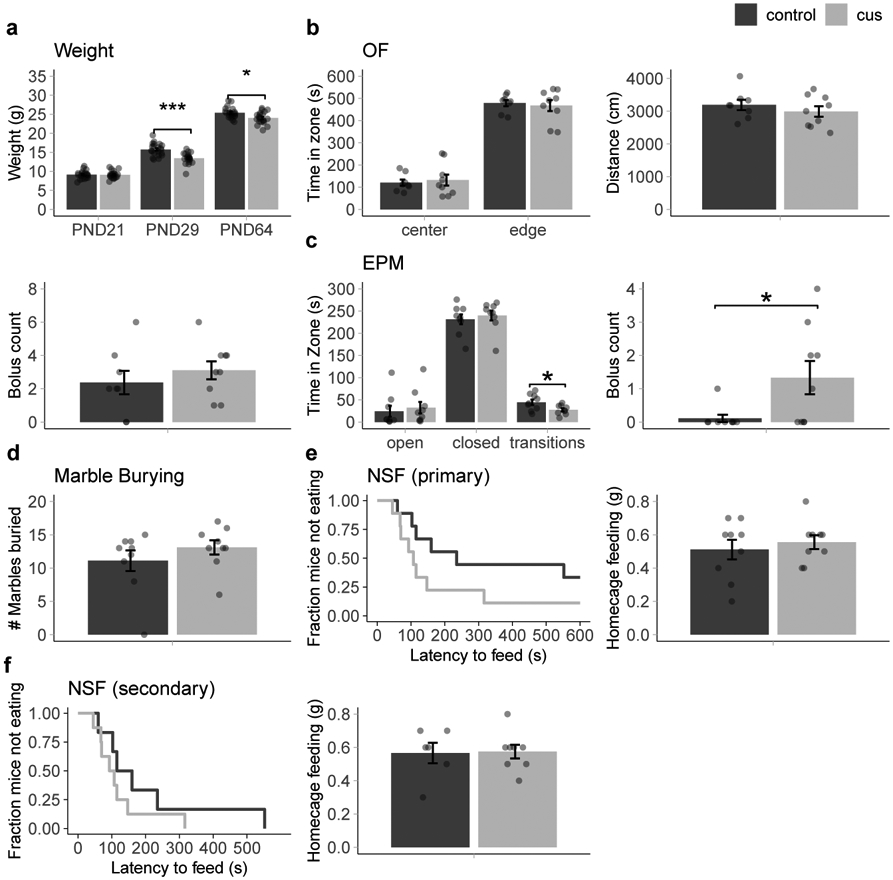
Weights from all mice that underwent adult behavior (a) (N = 21/group). Results from Open Field (OF) (b), Elevated Plus Maze (EPM) (c), marble burying (d), and Novelty Suppressed Feeding (NSF) tests (e, primary analysis and f, secondary analysis). OF, n = 8 control/9 CUS; EPM and marble, n = 9 control/9 CUS; NSF, n = 9 control/9 CUS for primary analysis and 6 control/8 CUS for secondary analysis. CUS = chronic unpredictable stress. Multifactor ANOVA with Bonferroni corrected post-hoc test (a), unpaired, two-tailed t-tests (b-d), and Kaplan-Meier survival analysis with Mantel-Cox log-rank test (e-f). Error bars represent the mean ± SEM. *p < 0.05, ***p < 0.001.
A subset of these mice (n = 9 control, 9 CUS) underwent the same anxiety tests described above starting at PND64 (Figure 2a). In the OF test, there were no differences between control and CUS mice in time spent in center (p = 0.693) or edge (p = 0.705) zones, distance traveled (p = 0.386), or in number of fecal boli produced (p = 0.414)(Figure 5b). One mouse was excluded from the control condition during the OF test due to video failure. In the EPM, there were no differences in time spent in open arms (p = 0.655) or in closed arms (p = 0.581); however, CUS mice spent less time in investigative transition zones (t(16) = 2.38, p = 0.03), and produced more fecal boli (t(18) = 2.39, p = 0.03) compared to controls (Figure 5c). In the marble burying test, pre-adolescent CUS did not affect the number of marbles buried (Figure 5d; p = 0.304). Finally, in the NSF test, pre-adolescent CUS did not affect adult latency to feed (p = 0.127) or homecage feeding (p = 0.545)(Figure 5e). Three control mice and one CUS mouse were censored (but not excluded) from the analysis for failing to eat within the predetermined 10-minute time limit, consistent with previous analysis parameters for this test [31]. A secondary analysis that excluded, rather than censor, mice that failed to eat, also did not show any differences in latency to feed (p = 0.257) or homecage feeding (p = 0.909)(Figure 5f). These results suggest that seven days of pre-adolescent stress causes long-lasting effects on weight and mild effects on baseline anxiety-like behaviors.
3.6. Pre-adolescent stress delays discriminative conditioning and disrupts conditioned inhibition in adults
To examine the enduring effects of pre-adolescent CUS on adult safety learning, a subset of mice underwent discriminative conditioning beginning on PND64 (n = 12 control, 12 CUS). Both groups similarly learned to discriminate between presentations of the fear and safety cues across sessions, freezing more to the fear cue than the safety cue (Figure 6a), supported by an interaction between Session and Stimulus Type (F(3, 66) = 34.60, p < 0.001, η2 = 0.32). To determine the number of sessions required to attain significant discrimination, planned comparisons were performed between fear and safety cues for each session, by group. While control mice reached significant levels of discrimination during the second conditioning session (DC1, t(11) = 0.74, padj > 0.999; DC2, t(11) = 3.01, padj = 0.047; DC3, t(11) = 8.07, padj < 0.001; DC4, t(11) = 18.30, padj < 0.001), CUS mice did not discriminate until the third conditioning session (DC1, t(11) = 0.41, padj > 0.999; DC2, t(11) = 1.38, padj = 0.784; DC3, t(11) = 3.53, padj = 0.019; DC4, t(11) = 7.51, padj < 0.001). Thus, although pre-adolescent exposure to CUS does not impact adolescent discriminative conditioning, transient delays in safety learning emerge during adulthood.
Figure 6. Pre-adolescent stress delays safety learning and disrupts adult conditioned inhibition.
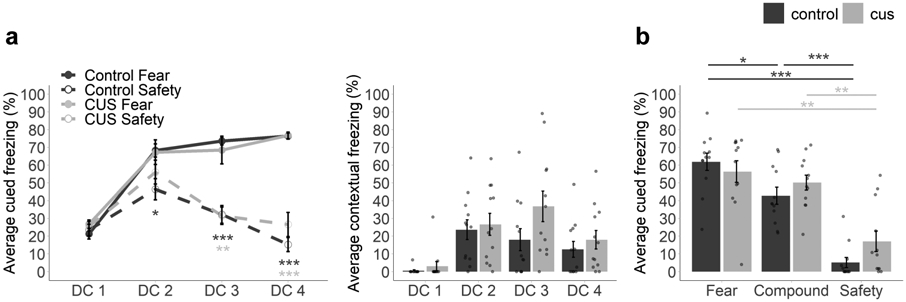
Average percentage of time spent freezing during fear and safety cues across discriminative conditioning sessions (a, left). Average percentage of time spent freezing during conditioning chamber acclimation (i.e., contextual fear) across discriminative conditioning sessions (a, right). Average percentage of time spent freezing during fear, compound, and safety cues during the test session (b). n = 12 control/12 CUS. CUS = chronic unpredictable stress. DC1-4 = discriminative conditioning sessions 1-4. Multifactor ANOVA with Bonferroni corrected post-hoc test (a, b) and planned comparisons to establish day of significant discrimination (a, left) and relative stimulus-induced freezing (b). Error bars represent the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Examination of adult contextual (Figure 6a) revealed an increase in contextual fear across discriminative conditioning sessions (main effect of Session, F(3, 66) = 11.93, p < 0.001, η2 = 0.24). However, pre-adolescent CUS had no impact on contextual fear (no effect of Condition, p = 0.149; no interaction between Condition and Session, p = 0.285).
During the summation test (Figure 6b), both groups showed differences in freezing to fear, safety, and safety compound cues (main effect of Stimulus Type, F(2, 44) = 54.28, p < 0.001, η2 = 0.61), although no group differences were observed for conditioned responding (no effect of Condition, p = 0.273; no interaction, p = 0.178). As with adolescent mice (Section 3.4), planned comparisons were performed to investigate hypotheses related to safety learning and the capacity for conditioned inhibition. Mice froze the least during safety cues, relative to fear cues (Control, t(11) = 15.10, padj < 0.001; CUS, t(11) = 3.86, padj = 0.009) as well as compound cues (Control, t(11) = 6.98, padj < 0.001; CUS, t(11) = 4.61, padj = 0.002). However, while control mice exhibited less freezing during safety compound cues than fear cues (i.e., conditioned inhibition; t(11) = 2.94, padj = 0.039), freezing was comparable in CUS mice (t(11) = 1.08, padj = 0.909). In sum, in line with our prediction, but contrary to our findings in adolescent mice, pre-adolescent exposure to CUS disrupts the ability to use a safety signal to attenuate fear during adulthood.
4. Discussion
In this study, we first validated a safety learning protocol to determine whether adolescent mice can acquire a safety signal capable of inhibiting fear. Our findings show that adolescent mice are adept at discriminating between fear and safety cues, exhibiting a discrimination profile comparable to adult mice. Importantly, this is the first report, to our knowledge, demonstrating that adolescent mice are capable of using a safety signal to attenuate fear. By showing that fear inhibition was specific to the safety signal (i.e., did not occur in the presence of a novel stimulus) and that previous safety learning retards new fear learning, we confirmed that the safety signal acts as a conditioned inhibitor of fear. Previous literature has suggested that adolescents experience difficulties regulating behavior under a variety of affectively charged circumstances [33]-[37]. Our findings suggest a divergence in which safety signals, given their explicit value as an indicator of safety, effectively facilitate behavioral inhibition. Additional studies directly comparing adolescent conditioned inhibition of fear using safety signals relative to adults are necessary to fully elucidate the development of inhibitory control.
Substantial research has highlighted adverse behavioral outcomes, as well as an increased susceptibility to psychiatric disease, that can occur following early life stress [22]-[25]. In light of this, our study aimed to establish the efficacy of safety signals as a means to inhibit fear in both stress-naive and stress-exposed populations. Although prior stress exposure causes an increase in some measures of anxiety during adolescence, mice can effectively acquire and use a safety signal to inhibit fear responding. In contrast, while adults with prior stress exposure manifest fewer anxiety-like behaviors, they exhibit delayed safety learning and fail to inhibit fear in the presence of a safety signal.
We applied a battery of measures to assess anxiety-related behaviors. Among them, reduced weight gain has been shown to be a robust marker of the impact of CUS in adolescent and adult rodents [38], [39]. Additional tests were chosen to assay different manifestations of anxiety-like behavior, including locomotion and exploratory behavior (OF), approach/avoidance behavior (EPM), repetitive behaviors or defensive probe burying (marble burying), and hyponeophagia (NSF). We found that stress exposure during a transient window of pre-adolescent development reduced weight gain and increased some anxiety-like behaviors during early adolescence. While we did not observe any effects of CUS on zone preference or locomotion in the OF or EPM, we observed a significant increase in fecal boli from CUS mice in both tests, suggesting higher baseline levels of anxiety in CUS mice (e.g., [40] but see [41]). Using a variety of stress models, others have shown that peri-pubertal stress either decreases anxiety-like behaviors in the OF and EPM and increases novelty-seeking [42], [43], or has no effect on anxiety-like behaviors in the EPM compared to controls when tested in adolescence [44]. Thus, while adolescents exhibit an increase in repetitive behaviors like defensive burying, they may be temporarily protected from stress-induced deficits in exploratory behaviors (OFT and EPM). The observed protection is specific to adolescence as rodents that experience pre-adolescent and adolescent stress show increased anxiety-like behavior in adulthood [25], [27], [42]. Similarly, we found that pre-adolescent stress caused long-lasting reductions in weight and increased anxiety-like behaviors in the EPM in adulthood. Although there were no differences in time spent in open or closed arms, control mice spent more time in investigative transition zones, which can be interpreted as shifts in risk assessment. In contrast, we observed no effect of pre-adolescent CUS on adult OF, marble burying, or NSF behaviors. These findings are in line with a previous report that 14 days of stress from PND21-35 was sufficient to reduce weight at PND120, but did not impact OF behaviors [29]. In conclusion, one week of pre-adolescent stress has immediate and enduring effects on weight gain, a short-term protection from reductions in exploratory behavior, and a short-term susceptibility to increased defensive burying and hyponeophagia.
Surprisingly, despite reductions in weight gain and increases in some anxiety-like behaviors, previously stressed adolescent mice had comparable safety learning and conditioned inhibition compared to controls. In contrast, pre-adolescent stress disrupts the ability to use a safety signal to attenuate fear during adulthood. The differential effects of pre-adolescent CUS on adolescent and adult behavior may be mediated by a variety of factors. For example, the nature of the effects of stress likely depends on the type of stressor(s) used [45]. Homotypic stressors can become predictable and elicit a habituated or attenuated response following repeated exposures (see [46]). Furthermore, chronic but predictable mild stress has also been shown to decrease depression and anxiety-related behaviors and confer protection to future stress in adolescents and adults [47], [48]. Conversely, unpredictable stress increases depression and anxiety-related behaviors [13] and does not induce habituation of the corticosterone response to a subsequent bout of restraint stress [49].
Another factor that may influence the impacts of stress exposure on later anxiety and behavioral performance is the precise age at which stress exposure occurs. Yohn & Blendy (2017) [27] found that adolescent stress led to an anxiety-like phenotype on multiple behavioral measures 30 days later, but that adult stress had no impact on later anxiety. Similarly, exposure to mild foot shock stress through fear conditioning during adolescence disrupted adult conditioned inhibition [50], yet exposure to mild unpredictable stress only during adulthood left conditioned inhibition intact [13]. Importantly, the duration of time between stress and behavioral tests could also influence the interpretation of these findings, as well as our present results. For example, one lab found that a single, high-intensity foot shock increased sensitized fear responding and led to generalized avoidance behavior 28 days, but not 24 hours, after the stress exposure [51], [52]. One major link between stress and subsequent anxiety may be the disruption of neurodevelopmental processes that occurs during the incubation period [53]. Indeed, the heightened plasticity that sets the foundation for rapid neural change during development also leaves developing circuits susceptible to environmental impacts. As such, stress exposure during a peri-adolescent window is most likely to alter the maturational trajectories of brain circuitry related to fear regulation, which are rapidly developing during early adolescence and throughout the incubation period observed in our study [54], [55].
Interestingly, the absence of detrimental effects of stress on adolescent safety learning suggest that ongoing brain development may confer temporary protection that overrides the expression of aberrant fear responding to facilitate developmental goals. Of particular note regarding peri-adolescent development of fear circuitry is a temporary surge in connectivity between subcortical regions, including the amygdala and ventral hippocampus, and the prefrontal cortex [56], as well as continued functional integration of these circuits [57], [58] as the brain approaches maturity. The same circuits are implicated in adult expression and regulation of fear [15], [59]-[64]. Transient increases in synaptic integration in these circuits may therefore enable safety learning in adolescents, while the deleterious effects of pre-adolescent CUS exposure may create lasting impairments in safety learning by disrupting circuit maturation [65], [66].
Although interpretation of the present study has been limited to preclinical studies, our findings emphasize the importance of considering both age and prior experience when determining the appropriate course of clinical treatment [67]-[69]. Our findings suggest it is worth exploring in clinical studies whether stressed adolescents might benefit from using safety signals, as a novel way to mitigate fear when other methods (e.g., fear extinction [8]-[10]) are otherwise limited and ineffective. In contrast, the clinical application of safety signals for adults with a history of stress exposure may not be as simple. Even so, perhaps with additional training or pharmacological intervention, adults may reap the same fear inhibition benefits. The combination of an antidepressant and cognitive behavioral therapy has been shown to be superior to either treatment alone for anxiety and PTSD [4], [70]. Future research may show similar validation for the integration of pharmacotherapy and safety learning to serve as a promising avenue for treatment.
One limitation of our study is age differences in normative performance during anxiety tests. Therefore, specific parameters like test duration and food deprivation time were adapted to accommodate these performance differences. While we cannot say for certain that these testing changes do not contribute to the observed age differences in anxiety-related phenotypes following CUS, we believe age-specific parameters are needed when analyzing within-age effects of CUS. While using the same testing parameters across ages works for some tests (e.g. OF or safety learning), it may produce scale attenuation effects for other tests (e.g. marble burying and NSF) and occlude within-age effects. For example, extending marble burying time for adults risks hitting a ceiling effect, whereas reducing marble burying time for adolescents risks no marbles being buried. In future research, different considerations may be necessary for across-age comparisons.
Additionally, individual differences may exist in the efficacy of different tones to serve as a conditioned inhibitor. Tone frequency had no impact on dynamics of safety learning or safety cue usage between CUS and control mice (an analysis conducted to rule out the possibility of perceptual impacts of pre-adolescent CUS exposure for responding to the two auditory frequencies used to designate either fear or safety cues, data separated by frequency not shown). However, collapsing across groups, freezing was higher during the compound cue, though not the fear or safety cue, in mice trained with a 2.9 kHz fear cue relative to mice trained with a 12.5 kHz fear cue. Future work should examine the specific parameters that determine the relative success of a safety signal to facilitate fear regulation.
As a final, but important note, our study was conducted entirely in males, but marked sex differences exist in the maturational trajectories of fear circuitry [55]. Others have begun to examine how stress might differentially impact female and male fear and anxiety behavior (e.g., [43], [71]). Therefore, additional research in this area that considers sex as well as the impact of stress at different ages (i.e., early life stress) [72]-[75] will be highly informative for understanding and treating anxiety disorders across the lifespan.
5. Conclusion
In all, the present study confirms that early life adversity has immediate and enduring effects on behavior and highlights the importance of considering age and previous experience when investigating affective learning and behavior. Our findings in adults provide insight into the utility of safety signals for adult fear regulation. While safety signals can be an effective means to inhibit fear for some adults, those with a history of stress exposure may require additional safety signal training or pharmacological aids to overcome persistent fear. In a surprising contrast, despite increases in anxiety-like behavior, adolescents with a history of stress are adept at using safety signals to inhibit fear. As a method for directly inhibiting fear, safety signals have gained traction in recent years and will likely continue to make waves for their potential value in augmenting conventional treatments for anxiety. Adding to this foundation, our findings highlight great initial promise for the efficacy of using safety signals and leveraging processes of conditioned inhibition for adolescent populations that exhibit otherwise limited fear regulation.
Acknowledgments
This work was supported by the National Institutes of Mental Health (NIMH) Pathway to Independence Award (K99MH119320) to H.C.M., the National Institutes of Health (NIH) National Center for Advancing Translational Science (TL1TR002386) to D.M.G., NIH R01 (NS052819, MH123154) to F.S.L., Pritzker Neuropsychiatric Disorders Research Consortium Award to F.S.L., the NewYork-Presbyterian Youth Anxiety Center (F.S.L.), and the DeWitt-Wallace Fund of the New York Community Trust (F.S.L. and H.C.M.). We gratefully acknowledge Dr. Nicole Ferrara for useful comments and suggestions on the manuscript. Graphical abstract and Figure 2 created with BioRender.com.
References
- [1].Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, and Walters EE, “Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication,” Arch. Gen. Psychiatry, vol. 62, no. 6, pp. 593–602, June. 2005, doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- [2].Beesdo-Baum K and Knappe S, “Developmental Epidemiology of Anxiety Disorders,” Child Adolesc. Psychiatr. Clin. N. Am, vol. 21, no. 3, pp. 457–478, July. 2012, doi: 10.1016/j.chc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- [3].Merikangas KR et al. , “Lifetime Prevalence of Mental Disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A),” J. Am. Acad. Child Adolesc. Psychiatry, vol. 49, no. 10, pp. 980–989, October. 2010, doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Walkup JT et al. , “Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety,” N. Engl. J. Med, vol. 359, no. 26, pp. 2753–2766, December. 2008, doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ginsburg GS et al. , “Results From the Child/Adolescent Anxiety Multimodal Extended Long-Term Study (CAMELS): Primary Anxiety Outcomes,” J. Am. Acad. Child Adolesc. Psychiatry, vol. 57, no. 7, pp. 471–480, July. 2018, doi: 10.1016/j.jaac.2018.03.017. [DOI] [PubMed] [Google Scholar]
- [6].James AA, Soler A, and Weatherall RR, “Cognitive behavioural therapy for anxiety disorders in children and adolescents,” Cochrane Database Syst. Rev, no. 4, 2005, doi: 10.1002/14651858.CD004690.pub2. [DOI] [PubMed] [Google Scholar]
- [7].Bouton ME, “Context, ambiguity, and unlearning: sources of relapse after behavioral extinction,” Biol. Psychiatry, vol. 52, no. 10, pp. 976–986, November. 2002, doi: 10.1016/S0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- [8].Baker KD and Richardson R, “Forming competing fear learning and extinction memories in adolescence makes fear difficult to inhibit,” Learn. Mem, vol. 22, no. 11, pp. 537–543, November. 2015, doi: 10.1101/lm.039487.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McCallum J, Kim JH, and Richardson R, “Impaired Extinction Retention in Adolescent Rats: Effects of D-Cycloserine,” Neuropsychopharmacology, vol. 35, no. 10, pp. 2134–2142, September. 2010, doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pattwell SS et al. , “Altered fear learning across development in both mouse and human,” Proc. Natl. Acad. Sci, vol. 109, no. 40, pp. 16318–16323, October. 2012, doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Odriozola P and Gee DG, “Learning about safety: Conditioned inhibition as a novel approach to fear reduction targeting the developing brain,” Am. J. Psychiatry, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Christianson JP, Fernando AB, Kazama AM, Jovanovic T, Ostroff LE, and Sangha S, “Inhibition of fear by learned safety signals: a mini-symposium review,” J Neurosci, vol. 32, no. 41, pp. 14118–24, October. 2012, doi: 10.1523/JNEUROSCI.3340-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, and Kandel ER, “An Animal Model of a Behavioral Intervention for Depression,” Neuron, vol. 60, no. 1, pp. 149–161, October. 2008, doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Foilb AR and Christianson JP, “11 - Brain Mechanisms for Learning and Using Safety Signals,” in Neurobiology of Abnormal Emotion and Motivated Behaviors, Sangha S and Foti D, Eds. Academic Press, 2018, pp. 204–222. [Google Scholar]
- [15].Meyer HC, Odriozola P et al. , “Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans,” Proc. Natl. Acad. Sci, December. 2019, doi: 10.1073/pnas.1910481116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ito W, Pan B-X, Yang C, Thakur S, and Morozov A, “Enhanced generalization of auditory conditioned fear in juvenile mice,” Learn. Mem, vol. 16, no. 3, pp. 187–192, March. 2009, doi: 10.1101/lm.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Anda RF et al. , “The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology,” Eur. Arch. Psychiatry Clin. Neurosci, vol. 256, no. 3, pp. 174–186, April. 2006, doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hollis F, Isgor C, and Kabbaj M, “The consequences of adolescent chronic unpredictable stress exposure on brain and behavior,” Neuroscience, vol. 249, pp. 232–241, September. 2013, doi: 10.1016/j.neuroscience.2012.09.018. [DOI] [PubMed] [Google Scholar]
- [19].Lupien SJ, McEwen BS, Gunnar MR, and Heim C, “Effects of stress throughout the lifespan on the brain, behaviour and cognition,” Nat. Rev. Neurosci, vol. 10, no. 6, pp. 434–445, June. 2009, doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- [20].Opendak M, Gould E, and Sullivan R, “Early life adversity during the infant sensitive period for attachment: Programming of behavioral neurobiology of threat processing and social behavior,” Dev. Cogn. Neurosci, vol. 25, pp. 145–159, 2017, doi: 10.1016/j.dcn.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Short AK and Baram TZ, “Early-life adversity and neurological disease: age-old questions and novel answers,” Nat. Rev. Neurol, vol. 15, no. 11, pp. 657–669, 2019, doi: 10.1038/s41582-019-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Heim C and Nemeroff CB, “The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies,” Biol. Psychiatry, vol. 49, no. 12, pp. 1023–1039, June. 2001, doi: 10.1016/S0006-3223(01)01157-X. [DOI] [PubMed] [Google Scholar]
- [23].Peña CJ et al. , “Early life stress alters transcriptomic patterning across reward circuitry in male and female mice,” Nat. Commun, vol. 10, no. 1, Art. no. 1, November. 2019, doi: 10.1038/s41467-019-13085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tsoory M, Cohen H, and Richter-Levin G, “Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood,” Eur. Neuropsychopharmacol, vol. 17, no. 4, pp. 245–256, March. 2007, doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- [25].Tsoory M and Richter-Levin G, “Learning under stress in the adult rat is differentially affected by ‘juvenile’ or ‘adolescent’ stress,” Int. J. Neuropsychopharmacol, vol. 9, no. 6, pp. 713–728, 2006, doi: 10.1017/S1461145705006255. [DOI] [PubMed] [Google Scholar]
- [26].Woon EP, Seibert TA, Urbanczyk PJ, Ng KH, and Sangha S, “Differential effects of prior stress on conditioned inhibition of fear and fear extinction,” Behav. Brain Res, vol. 381, p. 112414, March. 2020, doi: 10.1016/j.bbr.2019.112414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yohn NL and Blendy JA, “Adolescent Chronic Unpredictable Stress Exposure Is a Sensitive Window for Long-Term Changes in Adult Behavior in Mice,” Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol, vol. 42, no. 8, pp. 1670–1678, July. 2017, doi: 10.1038/npp.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wohleb ES, Terwilliger R, Duman CH, and Duman RS, “Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior,” Biol. Psychiatry, vol. 83, no. 1, pp. 38–49, January. 2018, doi: 10.1016/j.biopsych.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hynes TJ et al. , “Early life adversity potentiates expression of addiction-related traits,” Prog. Neuropsychopharmacol. Biol. Psychiatry, vol. 87, pp. 56–67, December. 2018, doi: 10.1016/j.pnpbp.2017.09.005. [DOI] [PubMed] [Google Scholar]
- [30].Lee BG, Anastasia A, Hempstead BL, Lee FS, and Blendy JA, “Effects of the BDNF Val66Met Polymorphism on Anxiety-Like Behavior Following Nicotine Withdrawal in Mice,” Nicotine Tob. Res, vol. 17, no. 12, pp. 1428–1435, December. 2015, doi: 10.1093/ntr/ntv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Samuels BA and Hen R, “Novelty-Suppressed Feeding in the Mouse,” in Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests, Volume II, Gould TD, Ed. Totowa, NJ: Humana Press, 2011, pp. 107–121. [Google Scholar]
- [32].Rescorla RA, “Pavlovian conditioned inhibition,” Psychol. Bull, vol. 72, no. 2, pp. 77–94, 1969, doi: 10.1037/h0027760. [DOI] [Google Scholar]
- [33].Somerville LH, Hare T, and Casey BJ, “Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents,” J. Cogn. Neurosci, vol. 23, no. 9, pp. 2123–2134, September. 2011, doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cohen AO et al. , “When Is an Adolescent an Adult? Assessing Cognitive Control in Emotional and Nonemotional Contexts,” Psychol. Sci, vol. 27, no. 4, pp. 549–562, April. 2016, doi: 10.1177/0956797615627625. [DOI] [PubMed] [Google Scholar]
- [35].Cohen-Gilbert JE and Thomas KM, “Inhibitory control during emotional distraction across adolescence and early adulthood,” Child Dev., vol. 84, no. 6, pp. 1954–1966, December. 2013, doi: 10.1111/cdev.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Aïte A, Cassotti M, Linzarini A, Osmont A, Houdé O, and Borst G, “Adolescents’ inhibitory control: keep it cool or lose control,” Dev. Sci, vol. 21, no. 1, 2018, doi: 10.1111/desc.12491. [DOI] [PubMed] [Google Scholar]
- [37].Meyer HC and Bucci DJ, “Setting the occasion for adolescent inhibitory control,” Neurobiol. Learn. Mem, vol. 143, pp. 8–17, September. 2017, doi: 10.1016/j.nlm.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sequeira-Cordero A, Salas-Bastos A, Fornaguera J, and Brenes JC, “Behavioural characterisation of chronic unpredictable stress based on ethologically relevant paradigms in rats,” Sci. Rep, vol. 9, no. 1, Art. no. 1, November. 2019, doi: 10.1038/s41598-019-53624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Monteiro S, Roque S, de Sá-Calçada D, Sousa N, Correia-Neves M, and Cerqueira JJ, “An Efficient Chronic Unpredictable Stress Protocol to Induce Stress-Related Responses in C57BL/6 Mice,” Front. Psychiatry, vol. 6, 2015, doi: 10.3389/fpsyt.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Carola V, D’Olimpio F, Brunamonti E, Mangia F, and Renzi P, “Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice,” Behav. Brain Res, vol. 134, no. 1–2, pp. 49–57, August. 2002, doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- [41].Archer J, “Tests for emotionality in rats and mice: A review,” Anim. Behav, vol. 21, no. 2, pp. 205–235, 1973, doi: 10.1016/S0003-3472(73)80065-X. [DOI] [PubMed] [Google Scholar]
- [42].Jacobson-Pick S and Richter-Levin G, “Differential impact of juvenile stress and corticosterone in juvenility and in adulthood, in male and female rats,” Behav. Brain Res, vol. 214, no. 2, pp. 268–276, December. 2010, doi: 10.1016/j.bbr.2010.05.036. [DOI] [PubMed] [Google Scholar]
- [43].Toledo-Rodriguez M and Sandi C, “Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat,” Neural Plast., vol. 2007, p. 71203, 2007, doi: 10.1155/2007/71203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McCormick CM, Smith C, and Mathews IZ, “Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats,” Behav. Brain Res, vol. 187, no. 2, pp. 228–238, March. 2008, doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- [45].Anisman H and Matheson K, “Stress, depression, and anhedonia: caveats concerning animal models,” Neurosci. Biobehav. Rev, vol. 29, no. 4–5, pp. 525–546, 2005, doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- [46].Grissom N and Bhatnagar S, “Habituation to repeated stress: get used to it.,” Neurobiol. Learn. Mem, vol. 92, no. 2, pp. 215–224, September. 2009, doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Parihar VK, Hattiangady B, Kuruba R, Shuai B, and Shetty AK, “Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory,” Mol. Psychiatry, vol. 16, no. 2, Art. no. 2, February. 2011, doi: 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Suo L et al. , “Predictable Chronic Mild Stress in Adolescence Increases Resilience in Adulthood,” Neuropsychopharmacology, vol. 38, no. 8, Art. no. 8, July. 2013, doi: 10.1038/npp.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Magariños AM and McEwen BS, “Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors,” Neuroscience, vol. 69, no. 1, pp. 83–88, November. 1995, doi: 10.1016/0306-4522(95)00256-I. [DOI] [PubMed] [Google Scholar]
- [50].Müller I, Brinkman AL, Sowinski EM, and Sangha S, “Adolescent conditioning affects rate of adult fear, safety and reward learning during discriminative conditioning,” Sci. Rep, vol. 8, no. 1, p. 17315, November. 2018, doi: 10.1038/s41598-018-35678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Siegmund A and Wotjak CT, “A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear,” J. Psychiatr. Res, vol. 41, no. 10, pp. 848–860, November. 2007, doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- [52].Pamplona FA, Henes K, Micale V, Mauch CP, Takahashi RN, and Wotjak CT, “Prolonged fear incubation leads to generalized avoidance behavior in mice,” J. Psychiatr. Res, vol. 45, no. 3, pp. 354–360, March. 2011, doi: 10.1016/j.jpsychires.2010.06.015. [DOI] [PubMed] [Google Scholar]
- [53].Romeo RD, “The impact of stress on the structure of the adolescent brain: Implications for adolescent mental health,” Brain Res., vol. 1654, pp. 185–191, January. 2017, doi: 10.1016/j.brainres.2016.03.021. [DOI] [PubMed] [Google Scholar]
- [54].Gee DG et al. , “Neurocognitive Development of Motivated Behavior: Dynamic Changes across Childhood and Adolescence,” J. Neurosci, vol. 38, no. 44, pp. 9433–9445, October. 2018, doi: 10.1523/JNEUROSCI.1674-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gerhard DM, Meyer HC, and Lee FS, “An adolescent sensitive period for threat responding: impacts of stress and sex,” Biol. Psychiatry, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pattwell SS et al. , “Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories,” Nat. Commun, vol. 7, p. 11475, May 2016, doi: 10.1038/ncomms11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Arruda-Carvalho M, Wu W-C, Cummings KA, and Clem RL, “Optogenetic Examination of Prefrontal-Amygdala Synaptic Development,” J. Neurosci, vol. 37, no. 11, pp. 2976–2985, March. 2017, doi: 10.1523/JNEUROSCI.3097-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gee DG et al. , “A Developmental Shift from Positive to Negative Connectivity in Human Amygdala–Prefrontal Circuitry,” J. Neurosci, vol. 33, no. 10, pp. 4584–4593, March. 2013, doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Foilb AR, Flyer-Adams JG, Maier SF, and Christianson JP, “Posterior insular cortex is necessary for conditioned inhibition of fear,” Neurobiol. Learn. Mem, vol. 134, pp. 317–327, October. 2016, doi: 10.1016/j.nlm.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kreutzmann JC, Jovanovic T, and Fendt M, “Infralimbic cortex activity is required for the expression but not the acquisition of conditioned safety,” Psychopharmacology (Berl.), May 2020, doi: 10.1007/s00213-020-05527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ostroff LE, Cain CK, Bedont J, Monfils MH, and LeDoux JE, “Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala,” Proc. Natl. Acad. Sci, vol. 107, no. 20, pp. 9418–9423, May 2010, doi: 10.1073/pnas.0913384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sangha S, Robinson PD, Greba Q, Davies DA, and Howland JG, “Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices,” Neuropsychopharmacology, vol. 39, no. 10, pp. 2405–2413, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sarlitto MC, Foilb AR, and Christianson JP, “Inactivation of the Ventrolateral Orbitofrontal Cortex Impairs Flexible Use of Safety Signals,” Neuroscience, March. 2018, doi: 10.1016/j.neuroscience.2018.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sangha S, Chadick JZ, and Janak PH, “Safety Encoding in the Basal Amygdala,” J. Neurosci, vol. 33, no. 9, pp. 3744–3751, February. 2013, doi: 10.1523/JNEUROSCI.3302-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gerhard DM, Wohleb ES, and Duman RS, “Emerging Treatment Mechanisms for Depression: Focus on Glutamate and Synaptic Plasticity,” Drug Discov. Today, vol. 21, no. 3, pp. 454–464, March. 2016, doi: 10.1016/j.drudis.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Liston C and Gan W-B, “Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo,” Proc. Natl. Acad. Sci, vol. 108, no. 38, pp. 16074–16079, September. 2011, doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gee DG and Kribakaran S, “Developmental Differences in Neural Responding to Threat and Safety: Implications for Treating Youths With Anxiety,” Am. J. Psychiatry, vol. 177, no. 5, pp. 378–380, May 2020, doi: 10.1176/appi.ajp.2020.20020225. [DOI] [PubMed] [Google Scholar]
- [68].McLaughlin KA, DeCross SN, Jovanovic T, and Tottenham N, “Mechanisms linking childhood adversity with psychopathology: Learning as an intervention target,” Behav. Res. Ther, vol. 118, pp. 101–109, 2019, doi: 10.1016/j.brat.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Parsons RG, “Behavioral and neural mechanisms by which prior experience impacts subsequent learning,” Neurobiol. Learn. Mem, vol. 154, pp. 22–29, October. 2018, doi: 10.1016/j.nlm.2017.11.008. [DOI] [PubMed] [Google Scholar]
- [70].Schneier FR et al. , “Combined prolonged exposure therapy and paroxetine for PTSD related to the World Trade Center attack: a randomized controlled trial,” Am. J. Psychiatry, vol. 169, no. 1, pp. 80–88, January. 2012, doi: 10.1176/appi.ajp.2011.11020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Peyrot C, Brouillard A, Morand-Beaulieu S, and Marin M-F, “A review on how stress modulates fear conditioning: Let’s not forget the role of sex and sex hormones,” Behav. Res. Ther, vol. 129, p. 103615, June. 2020, doi: 10.1016/j.brat.2020.103615. [DOI] [PubMed] [Google Scholar]
- [72].Baram TZ et al. , “Fragmentation and unpredictability of early-life experience in mental disorders,” Am. J. Psychiatry, vol. 169, no. 9, pp. 907–915, September. 2012, doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, and Casey BJ, “Early-life stress has persistent effects on amygdala function and development in mice and humans,” Proc. Natl. Acad. Sci. U. S. A, vol. 110, no. 45, pp. 18274–18278, November. 2013, doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sarro EC, Sullivan RM, and Barr G, “Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA,” Neuroscience, vol. 258, pp. 147–161, January. 2014, doi: 10.1016/j.neuroscience.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Syed SA and Nemeroff CB, “Early Life Stress, Mood, and Anxiety Disorders,” Chronic Stress, vol. 1, April. 2017, doi: 10.1177/2470547017694461. [DOI] [PMC free article] [PubMed] [Google Scholar]


