Abstract
Background:
Chronic alcohol consumption is associated with a compromised innate and adaptive immune response to infectious disease. Mucosa-associated invariant T (MAIT) cells play a critical role in antibacterial host defense. However, whether alcohol-associated deficits in innate and adaptive immune responses are mediated by alterations in MAIT cells remains unclear.
Methods:
To investigate the impact of alcohol on MAIT cells, mice were treated with binge-on-chronic alcohol for 10 days and sacrificed at day 11. MAIT cells in the barrier organs (lung, liver and intestine) were characterized by flow cytometry. Two additional sets of animals were used to examine the involvement of gut microbiota on alcohol-induced MAIT cell changes. 1) Cecal microbiota from alcohol-fed (AF) mice were adoptive transferred into antibiotics-pretreated mice; 2) AF mice were treated with antibiotics during the experiment. MAIT cells in the barrier organs were measured via flow cytometry.
Results:
Binge-on-chronic alcohol feeding led to a significant reduction in the abundance of MAIT cells in the barrier tissues. However, CD69 expression on tissue-associated MAIT cells was increased in AF mice compared to pair-fed (PF) mice. The expression of Th1 cytokines and the corresponding transcriptional factor was tissue specific with downregulation in the intestine, but increased in the lung and liver in alcohol-fed animals. Transplantation of fecal microbiota from AF mice resulted in a MAIT cell profile aligned to that of AF mice donor. Antibiotic treatment abolished the MAIT cell differences between AF and PF animals.
Conclusion:
MAIT cells in the intestine, liver, and lung are perturbed by alcohol use, and these changes are, partially attributable to alcohol-associated dysbiosis. MAIT cell dysfunction may contribute to alcohol-induced, innate and adaptive immunity and consequently end-organ pathophysiology.
Keywords: Mucosa-associated invariant T cells, alcohol, dysbiosis, gut microbiota, fecal transplantation, antibiotics
INTRODUCTION
Alcohol use disorder (AUD) affects 15.1 million US adults and is associated with more severe medical illness (Fernandezsola et al., 1995, de Roux et al., 2006). A high prevalence of pneumonia and other infections, with subsequent poor clinical outcomes, are found in alcohol drinkers, as both innate and adaptive immune cells are compromised by alcohol use(Greenberg et al., 1999, Mandrekar et al., 2004, Mason et al., 2004, Gonzalez-Quintela et al., 2008, Samuelson et al., 2017). The mammalian intestinal tract is inhabited by trillions of microorganisms, which have a fundamental role in the induction, training, and function of the host immune system (Amoroso et al., 2020, Belkaid and Hand, 2014). Normally, the fetal gastrointestinal tract is believed to be sterile and the immune system at birth is relatively immature, which is characterized by blunted inflammatory cytokine production and a skewed T and B cell response after infection (Siegrist, 2001, PrabhuDas et al., 2011). Invariant natural killer T (iNKT) cells from germ-free (GF) mice show a less mature phenotype and lower activation by antigens than the normal control mice (Wingender et al., 2012). Further, antigen-activated CD8+ T cells from GF mice exhibit no transition to a memory phenotype, due to the shortage of microbiota-derived short chain fatty acids (Bachem et al., 2019). In GF mice, smaller Peyer’s patches and a reduced abundance of CD4+ T cells and IgA+ plasma cells in the gastrointestinal tract have been reported (Mazmanian et al., 2005, Smith et al., 2007).
Mucosa-associated invariant T (MAIT) cells have recently been recognized as participants in host defense against infection. MAIT cells recognize bacterial ligands in association with nonclassical MHC molecules. They are enriched in mucosa-associated tissues, such as intestine, lung and liver. In humans, MAIT cells are the most abundant T-cell subpopulation, representing as high as 10% of T cells in the intestine, lung and peripheral blood. Strikingly, MAIT cells account for 10%–45% of human hepatic T cells (Bolte and Rehermann, 2018, Dusseaux et al., 2011). MAIT cell development is highly reliant on gut microbiota. For example, GF mice have limited numbers of MAIT cells (Treiner et al., 2003), but the MAIT cell population can be expanded by recolonization with commensal microbes (Legoux et al., 2019, Le Bourhis et al., 2010). Antibiotics reduce MAIT cell numbers and IL-17A+ MAIT cells in mice, and normal microbiota transplantation into antibiotic-treated mice reverses these changes (Dumas et al., 2018). Further, changes in bacterial metabolism impacts MAIT cell activation and function (Mendler et al., 2020, Schmaler et al., 2018). The ability of MAIT cells to control bacterial and viral infections has recently been demonstrated (Georgel et al., 2011, Meierovics et al., 2013, Howson et al., 2015, Varelias et al., 2018, Wang et al., 2018). During Legionella longbeachae pulmonary infection, MAIT cells are activated in a MHC-related molecule I (MR1)-dependent manner and adoptive transfer of MAIT cells into immunodeficient Rag2−/−γC−/− mice protects mice from lethal Legionella infection (Wang et al., 2018). Similar to infection with Legionella, adoptive transfer of MAIT cells into MAIT cell deficient mice (MR1−/−) or immunodeficient Rag2−/−γC−/− mice ameliorates weight loss and mortality due to severe (H1N1) influenza infection (van Wilgenburg et al., 2018). The protective role of MAIT cells at the early stage of infection is through 1) the release of Th1 and Th17 cytokines (Gold et al., 2010, Le Bourhis et al., 2010), which prime and activate innate immune cells, like dendritic cells and macrophages, and recruit adaptive immune cells (Meierovics and Cowley, 2016, Hartmann et al., 2018), and 2) the release of cytotoxic products, such as granzyme B, perforin, and CD107 (Kurioka et al., 2015, Zinser et al., 2018), which are cytotoxic to infected cells, and modulate antibody production (Bennett et al., 2017).
MAIT cell depletion and dysfunction has been reported in patients with chronic liver disease (Le Bourhis et al., 2010, Dumas et al., 2018). Riva et al., found that circulating MAIT cell numbers were significantly reduced and functionally defective in patients with alcoholic liver disease (ALD). Specifically, resident MAIT cells are constitutively activated in patients with ALD, but exhibit an impaired response to bacterial challenge (Riva et al., 2018). Similarly, Li et al., found a reduction in the total number of blood MAIT cells, with a corresponding increased in activation in patients with alcoholic hepatitis when comparing to healthy controls. The effects of alcohol consumption on MAIT cells were not fully reversed by a one year alcohol abstinence (Li et al., 2019). Outside of these studies, there are limited data on MAIT cells in the context of alcohol-associated disease and few preclinical model confirmatory publications. As alcohol consumption induces gut dysbiosis and MAIT cell development is dependent on the gut flora, we hypothesized that alcohol-mediated dysbiosis, reduces the prevalence and dysregulates the function of MAIT cell in the intestinal tract and in distal organs.
MATERIAL AND METHODS
Mice
C57BL/6 mice (female, 10-12-week-old with body weight ~20 g) were obtained from Charles River (Wilmington, MA) and maintained in the specific-pathogen-free facility at LSUHSC. Mice were housed in filter-topped cages (5 mice/cage), fed sterile water and diet, and kept under standard environment conditions (25 °C, 56 % moisture, day/night 12 hour shifts). All experiments were reviewed and approved by the LSUHSC Institutional Animal Care and Use Committee.
Binge-on-chronic alcohol administration
Mice were acclimated to Lieber-DeCarli liquid control diet (Bioserv, Flemington, NJ) for 5 days. Afterwards, animals were randomized into alcohol-fed (AF) (Lieber-DeCarli ethanol liquid diet) and pair-fed (PF) groups (control diet) (n=10 /group). AF mice were fed with ethanol liquid diet (5.0 %, vol/vol) for 10 days and received 4 g/kg body weight ethanol by gavage at day 5 and day 10. Blood alcohol concentrations averaged 200 mg/dL before the binge and reached ~400 mg/dl 6 h-post binge (Samuelson et al., 2017). The amount of control-liquid diet for PF mice was adjusted daily according to the food intake of AF mice during the experiment. Mice were sacrificed 24 hours following the last binge ethanol administration.
Gut microbiota adoptive transfer
Microbiota transplantation was performed as previously described (Samuelson et al., 2017). In brief, mice were maintained on Lieber-DeCarli control liquid diet and treated (oral gavage) with a cocktail of antibiotics (0.25 mg ampicillin, 0.25 mg gentamicin, 0.25 mg neomycin, 0.25 mg metronidazole and 0.125 mg vancomycin) daily for 2 weeks (100 μL/day). The cecal material collected from PF and AF mice was homogenized (1:5 wt/vol) in sterile PBS containing 0.5% L-cysteine. And the fecal slurry was obtained via centrifugation (80 g × 10 min, 4 °C) to remove large organic matter and either used immediately or stored at −80 °C. After antibiotic treatment, mice were randomly divided into two groups to receive cecal microbiota via gavage (200 μL/does) from either AF or PF mice donors on days 3, 6, and 9 (n=10/group). One week of recolonization is sufficient to induce immune modulation and homeostasis in germ-free mice (Dimitriu et al., 2013). Three days after the last dose of microbiota gavage mice were sacrificed.
Binge-on-chronic alcohol exposure in antibiotic-treated mice
Mice were treated with 100 μL of the antibiotic cocktail (as described above) daily for 1 week with free access to Lieber-DeCarli control liquid diet. Mice were then randomized to AF and PF groups (n=10 /group). Antibiotics were continued on an every other day schedule throughout the experiment. Mice were sacrificed 24 h following the last binge.
Fecal bacterial density enumeration
Fecal samples were homogenized (1:50 wt/vol) in sterile saline, followed by centrifugation at 150 g × 5 min at 4 °C. The supernatant was centrifuged at 10000 g for 10 min to pellet the bacteria. Pelleted bacteria were washed and diluted to a cell density of 103 cells/mL, and incubated with SYTO BC dye (Invitrogen, Eugene, Oregon) for 5 minutes. Bacterial density was determined by flow cytometry with calibration using counting beads (Invitrogen, Eugene, Oregon).
Microbiota DNA sequencing and data analysis
Cecal bacterial DNA was extracted using the QIAamp Fast DNA Stool Mini Kit (Qiagen, Germany) following the manufacturer’s instructions with slight modification. Beads beating was included to increase bacterial DNA yield. 16S rRNA gene libraries were prepared and sequenced by the Louisiana State University School of Medicine Microbial Genomics Resource Group (http://metagenomics.lsuhsc.edu/mgrg), as we have previously published (Samuelson et al., 2016). Sequencing data trimming and analysis were performed in R with the following packages: DADA2 v1.10.0, Phyloseq v1.26.0, DESeq2 v1.20.0, and vegan v2.3-5. Package PICRUSt2 (v2.2.0) was applied to predict the microbial community functions and STAMP (v2.1.3) was used to visualize the results with statistical significance estimated using calculated by Welch’s t-test. 16S rRNA gene sequence data (accession number PRJNA641400) were deposited in the National Center for Biotechnology Information Sequence Read Archive.
Immune cell preparation and phenotyping
To prepare single cell suspension, livers were mechanically dissociated with C-tubes (Miltenyi Biotec, North Rhine-Westphalia, Germany). Lungs were cut into small pieces and incubated with 200 U/mL DNase (Sigma-Aldrich, St. Louis, MO) and 1.0 mg/mL Collagenase Type I (Gibco/Thermo fisher Scientific) at 37 °C for 30 min. Cells were then filtered (70 μm) and washed with PBS/2% FBS (Gibco/ Thermo Fisher Scientific). Liver lymphocytes were isolated by 40% Percoll (GE Healthcare, Uppsala, Sweden) gradient centrifugation (2000 rpm × 10 min without brake, room temperature). Red blood cells were lysed from lung and liver preparations for 5 min at 37 °C. Lymphocytes were isolated from the lamina propria of the entire small intestine, as previously described (Goodyear et al., 2014). Briefly, the intestine was cleaned and cut into approximately 3 cm lengths. Removal of intestinal mucosa was performed by incubation on a shaker in HBSS containing 5 mM DTT (Thermo Fisher Scientific), 100 IU/mL penicillin/streptomycin and 2% FBS for 20 min at 37 °C. Dissociation of epithelial cells was performed by incubation with HBSS with 5 mM EDTA, 100 IU/mL penicillin/streptomycin (Gibco/Thermo Fisher Scientific) and 2% FBS for 15 min at 37 °C. This step was repeated three times. Afterwards, samples were washed by HBSS+ 10 mM HEPES and digested with HBSS containing 0.2 U/mL Librase™ (Roche Diagnostics, Indianapolis, IN) and 200 U/mL DNase (Sigma-Aldrich, St. Louis, MO) on a shaker for 30 min at 37 °C. Immune cells were pelleted and washed in PBS+2% FBS.
Mouse MAIT cells were identified by flow cytometry using the following panel: Live+CD45+CD3+ 5-OP-RU MR1 tetramer+TCRβ+ and 6-FP MR1 tetramer+ was used as negative control for MAIT cell identification (Koay et al., 2019). Human MAIT cells were identified as live+CD3+Va7.2 TCR+CD161++. We measured (1) activation markers (CD69/CD38); (2) intracellular cytokines/cytotoxicity markers (IFN-γ/TNF-α/Granzyme B); and (3) transcriptional factors (PLZF/T-bet). Detailed information of the antibodies used is provided in Table S1. To block non-specific staining, mouse lymphocytes were pretreated with TruStain FcX Anti-mouse CD16/32 antibody (BioLegend, San Diego, CA) and then incubated with surface staining cocktails in staining buffer for 30 min at 4 °C. Cells were permeabilized and stained with intracellular antibodies against transcriptional factors and cytokines. To measure cell apoptosis, treated-human PBMCs were stained with fixable viability dye eFluor 780 (Invitrogen) for 30 min at 4 °C, followed by surface antibody staining for 20 min at 4 °C. After that, cells were washed with Annexin V binding buffer (BioLegend) and stained with Annexin V-PerCP/Cyanine 5.5 (BioLegend) for 15 min. Cells were analyzed on a LSRII flow cytometer (BD Biosciences) and data analysis was performed with the FlowJo software v10 (Tree Star, Ashland, OR).
Serum iFABP ELISA
Blood was collected via cardiac puncture into BD serum separator tubes (BD Biosciences, San Jose, CA). The serum fraction was obtained by centrifugation (2,000 g × 10 min, 4 °C). Serum iFABP level was measured by ELISA following manufacturer’s instruction (LifeSpan BioSciences, Seattle, WA).
Serum alanine aminotransferase (ALT) analysis
100 μL tail vein blood was collected 6 h post the last binge and serum ALT level was measured with Alanine Transaminase Colorimetric Activity Assay kit (Cayman, MI, USA).
Hepatic histopathology
To determine the lipid accumulation in the liver, Oil Red O staining was performed by the Louisiana State University Health Science Center Morphology and Imaging Core (https://www.medschool.lsuhsc.edu/mic/). Briefly, frozen liver tissues were cut into 10 μm thick sections then stained with Oil Red O (Sigma), counterstained with hematoxylin (Sigma), and visualized with light microscope at 100x magnification. Lipid density in the stained sections was quantified as described previously (Bergheim et al., 2006).
Quantitative PCR for bacterial 16S rRNA in peripheral blood
DNA was isolated from 100 μL of whole blood using the QIAamp DNA Blood Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol with the addition of a bead-beating step. The quantitative PCR was performed with StrataGene MX 3000P (StrataGene, La Jolla, CA) with TaqMan® Fast Advanced Master Mix (Thermo Fisher Scientific, Waltham, MA). The sequences of the bacterial universal primers and probe were forward primer: 5’-ACTCCTRCGGGAGGCAGCAG-3’; reverse primer 5’-GGACTACCVGGGTATCTAAT-3’; and the probe: 5’-6-FAM-TKACCGCGGCTGCTGGCAC-BHQ1-3’. The TaqMan PCR protocol was as follows: 1 cycle at 50 °C for 2 min; 1 cycle at 95 °C for 20 s, 42 two step cycles at 95 °C for 3 s, followed by 60 °C for 30 s. The bacterial DNA copies was calculated based on standard curve.
Microbiota riboflavin production measurement
Microbiota riboflavin production was measured based on the colorimetric method described elsewhere (Lin et al., 2014). Collected cecal microbiota from PF and AF mice were cultured with autoclaved Gifu anaerobic medium broth (HiMedia Laboratories, PA) for 48 h. Culture samples were centrifuged (16,000 g × 5 min, 4 °C) to remove the cells and the supernatants diluted with 0.05 M NaOH to the linear range of the spectrophotometer. Riboflavin concentrations were determined by spectrophotometer at 444 nm (standard curve: y=0.0048x+0.0404, R2=0.9986).
Preparation of fixed fecal bacteria
Fecal microbiota from PF and AF mice were counted with flow cytometry, fixed with 4% formaldehyde (BD Biosciences, Oxford, UK) for 10 min at room temperature, extensively washed in sterile PBS, and then preserved at −20 °C.
Ex vivo MAIT cell stimulation assay
Cryopreserved PBMCs from healthy donors were defrosted, counted and cultured overnight (37 °C, 5% CO2) in round-bottom 96-well plates (106 cells/well) in RPMI 1640 (Gibco/ Thermo Fisher Scientific) supplemented with 10% heat-inactivated FBS and 100 IU/mL penicillin/streptomycin. Cell recovery and viability were greater than 90%. Cells were stimulated for 4 h with (1) fixed mice fecal microbiota (10 bacteria per cell (BpC)); (2) mouse serum (10%); or (3) increasing concentrations of ethanol (50–100 mM). Brefeldin A (Sigma-Aldrich) was added to cultures used for intracellular flow cytometry 4 h before cell harvest (10 μg/mL).
Statistical analysis
Data are presented as mean ± SD and analyzed with GraphPad Prism 8 (GraphPad Software, La Jolla, CA). Two-group statistical difference was evaluated with Mann-Whitney U-test. Statistical significance is indicated as: * p<0.05; ** p< 0.01, *** p < 0.001, **** p<0.0001.
RESULTS
Binge-on-chronic alcohol feeding alters MAIT cell numbers and phenotype in a tissue-specific manner
No significant differences in daily food intake or body weight were observed between alcohol-fed (AF) and pair-fed (PF) groups (Figure 1A & Figure S1A-B). AF mice had higher serum ALT level (p<0.01) (Figure S1C), and more hepatic lipid accumulation than PF mice (p<0.05) (Figure S1D-E).
Figure 1:
Binge-on chronic alcohol reduces MAIT cell number in the intestine, lung and liver. (A) Experimental design of binge-on chronic alcohol exposure on mice. (B) Gating strategy for mouse MAIT cell identification by flow cytometry. (C) MAIT cell count in alcohol drinking mice (counted by flow cytometry and adjusted by counting beads). PF, pair-fed; AF, alcohol-fed. Scatter plots and bar shows the means±SD. * p<0.05, ** p<0.01, *** p<0.001 by Mann-Whitney U test.
MAIT cells were gated as shown in Figure 1B. In the intestine, lung and liver, the average number of MAIT cell in AF mice was ~ 50% the number found in PF mice (p<0.05) (Figure 1C). CD69 is a classical activation marker of lymphocytes due to its rapid expression on cell surface upon stimulation (Gonzalez-Amaro et al., 2013). The percentage of MAIT cells expressing CD69 and the CD69 MFI were increased in the intestine and lung (p<0.05) of AF mice. No statistically significant difference in the abundance of CD69+ MAIT cells was detected in the liver of between the two groups (Figure 2A). The frequency of MAIT cells expressing the cytokines IFN-γ and TNF-α was lower in AF mice (p<0.05) than in PF mice. The expression of Th1 cytokines was higher in the liver and lung of AF mice (p<0.05) (Figure 2B). The transcriptional factor PLZF is vital for MAIT cell maturation (Koay et al., 2016). Compared to PF mice, there was a lower percentage of PLZF+ intestinal MAIT cells in AF mice (p<0.05), while no significant changes were found in the liver and lung (Figure 2C). Consistent with the changes seen for Th1 cytokines, the expression of T-bet, a regulator of Th1 cytokine production, was lower in the intestine, and higher in the lung and liver of AF mice (p<0.05, Figure 2C).
Figure 2:
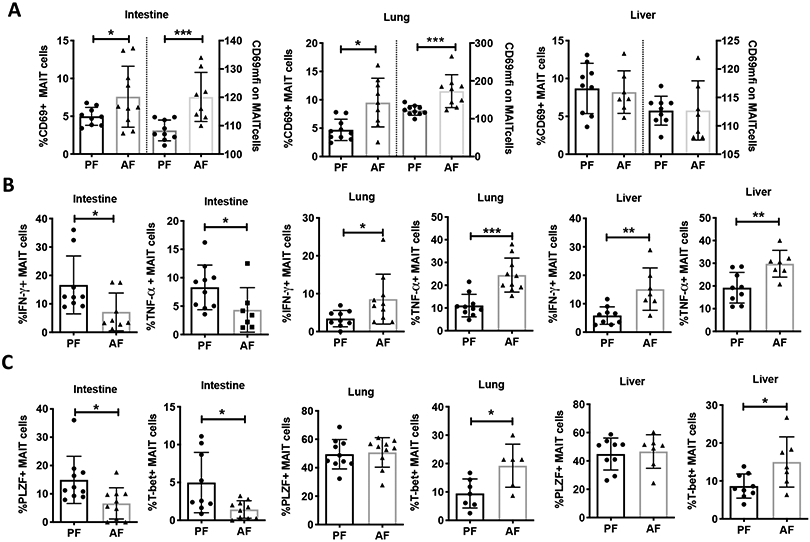
Binge-on chronic alcohol alters MAIT cell activation, and expression of cytokines and transcriptional factors. (A) MAIT cell expression of activation marker CD69 and CD69 mean fluorescent intensity (MFI) (B) Th1 cytokine expression (IFN-γ and TNF-a), and (C) frequency of transcriptional factor (PLZF and T-bet) expression on MAIT cells isolated from intestine, lung, and liver of pair fed (PF) and alcohol-fed (AF) mice. MFI: mean fluorescence intensity. Scatter plots and bar shows the means±SD. * p<0.05, ** p<0.01, *** p<0.001 by Mann-Whitney U-test.
Binge-on-chronic alcohol feeding shifts gut microbiota diversity and functional capacity
Considering the close relationship between the gut microbiota and MAIT cells, we characterized gut microbiota diversity and function in AF mice. Species richness of cecal microbiota was assessed using Observed_OTUs, Chao1 and ACE diversity measures. AF mice had no significant changes in species richness compared to PF mice (Figure 3A). However, the β-diversity based on Bray-Curtis dissimilarity, showed that samples from AF group clustered away from the PF group (p = 0.007), suggesting that alcohol exposure altered the gut microbiota structure (Figure 3B). PICRUSt2 was used to predict the functional capacity of the intestinal microbiota. Principle component analysis (PCA) showed that samples from the two experimental groups clustered separately with principle component 1 (PC1) accounting for 62.6% of the observed variance (Figure 3C), indicating that global cecal microbiota functional capacity was shifted dramatically by alcohol consumption. Specifically, alcohol feeding lowered the inferred capacity for purine ribonucleoside degradation by the intestinal microbiota, suggesting lower riboflavin synthesis capacity of intestinal microbiota in AF mice (Figure 3D). We examined the riboflavin production capacity of the gut microbiota obtained from AF or PF mice using an ex vivo fermentation approach. The production of riboflavin by the microbiota isolated from AF mice was significantly lower than from PF mice following 48 h of fermentation (p<0.05, Figure 3E).
Figure 3:
Impact of binge-on chronic alcohol on gut microbiota diversity and function. (A) α-diversity of cecal microbiota from mice with/without alcohol exposure. Observed_OTUs, Chao1 and ACE are estimators of microbiota species richness. (B) β-diversity of cecal microbiota based on sample-wise Bray-Curtis dissimilarity distance. The significance between the PF and AF group was calculated with pairwise ADONIS2 (permutation=10000, p=0.007). Red circles were samples from AF group, and blue triangles were samples from PF group. Principal component (PC) value indicates the percent variation showed by the PC according to the linear correlation between sample microbiota taxonomy and identity. PC1 and PC2 showed 61.4% of the total difference between the two groups. (C) Principle component analysis (PCA) of the predicted functions of cecal microbiota estimated by package PICRUSt2 in R. AF samples clustered separately from the PF, and PC1 and PC2 showed 78% of total difference. Orange squares represent samples from PF mice, and blue circles represent samples from AF mice. (D) Fold change of the bacterial metabolic pathway of purine ribonucleotides degradation. (E) Concentration of riboflavin released in the culture broth by cecal microbiota after 48 h fermentation with sterile Gifu anaerobic medium. Scatter plots and bar shows the means±SD. * p<0.05 by Mann-Whitney U-test.
Alcohol-associated intestinal dysbiosis alters MAIT cells ex vivo and in vivo.
To further examine the effect of alcohol-associated intestinal dysbiosis on MAIT cells, healthy human PBMCs were incubated with fixed cecal microbiota from AF and PF mice for 4 h. Human MAIT cells were identified as live CD3+Vα7.2 TCR+CD161++ as shown in Figure 4A. The number of viable MAIT cells was lower following culture with fixed cecal microbiota from AF compared to those from PF mice (p<0.05, Figure 4B). We then examined the activation and function of CD8+ MAIT cells, as CD8+ MAIT cell is the main sub-phenotype of MAIT cells in humans and accounts for 80-90% of total population of circulating MAIT cells. The frequency of granzyme B+, IFN-γ+, CD38+ MAIT cells, as well as the expression of CD38 (MFI) were increased in CD8+ MAIT cells following culture with fixed cecal microbiota from AF compared to those from PF mice (p<0.05, Figure 4C-D).
Figure 4:
Alcohol dysbiosis incubates with human PBMCs (Bpc=10) for 4 h causing MAIT cell changes in vitro . (A) Representative flow cytometric pseudocolour plots of MAIT cell identification and the distribution of CD4/CD8 receptors on MAIT cells (B) MAIT cell number in human PBMCs after 4 h incubation. (C) The expression of CD38 and CD38 MFI on CD8+ MAIT cells. CD8+ cell is about 80-90% of total MAIT cells in healthy human PBMCs. (D) The expression of granzyme B and IFN-γ on CD8+ MAIT cells after alcohol dysbiosis stimulation. Bpc: bacteria per cell. Scatter plots and bar shows the means±SD. * p<0.05 and ** <0.01 by Mann-Whitney U-test.
To confirm the effect of alcohol dysbiosis on MAIT cells in vivo, cecal microbiota collected from alcohol drinking mice were adoptively transplanted into alcohol-naïve recipient mice, which had been treated with an antibiotic cocktail for 2 weeks (Figure 5A). Fecal bacteria density was reduced to 10% after 2-week antibiotic treatment and recovered to the pre-antibiotic level following 3 doses of microbiota (Figure S2A). At the end of the experiment, body weights were similar in mice recolonized with PF microbiota and AF microbiota (Figure S2B). Mice receiving AF microbiota had a significantly different microbiota community structure compared to mice receiving PF microbiota (p<0.05, Figure S2C), indicating that microbiota adoptive transfer successfully changed the recipients’ gut microbiota between the two groups. Mice recolonized with AF microbiota had a lower number of pulmonary and hepatic MAIT cells than mice recolonized with PF microbiota (p<0.05), while no difference was found in the number of intestinal MAIT cells (Figure 5B). The frequency of intestinal, pulmonary and hepatic CD69+ MAIT cells was increased in AF microbiota recolonized mice (p<0.05, Figure 5C). The frequency of IFN-γ+ MAIT cells was lower in the intestinal lamina propria, but higher in the lung and liver of mice recolonized with AF microbiota compared to mice recolonized with PF microbiota (p<0.05). Additionally, the frequency of TNF-a+ MAIT cells was increased in the lung of mice recolonized with AF microbiota (p<0.05, Figure 5D). Similar to the changes in the Th1 cytokine IFN-γ, the frequency of T-bet+ MAIT cells was reduced in intestine and increased in the lung and liver of AF microbiota recolonized mice (p<0.05). In addition, the frequency of PLZF+ MAIT cells was reduced in liver (p<0.05), but no differences were seen in the intestine and lung of AF microbiota recolonized mice (Figure 5E).
Figure 5:

Alcohol dysbiosis replicates the effect of alcohol feeding on MAIT cells in alcohol naïve mice. (A) Experimental design of mouse to mouse gut microbiota adoptive transfer. After 14 days’ antibiotics treatment, mice received 3 doses of cecal microbiota by gavage. Donor microbiota was collected from binge-on chronic alcohol experiment, and recipients were fed with Lieber-Decarli control liquid diet. (B) MAIT cell number in mice tissues after microbiota recolonization. PF microbiota: mice recolonized with cecal microbiota from pair-fed mice; AF microbiota: mice recolonized with cecal microbiota from alcohol drinking mice. (C) MAIT cell expression of CD69 and CD69 MFI in mice tissues. (D) MAIT cell expression of cytokine IFN-γ and TNF-a in microbiota reconstituted mice. (E) MAIT cell expression of transcriptional factors PLZF and T-bet in tissues. Scatter plots and bar shows the means±SD. * p<0.05 and ** p<0.01 by Mann-Whitney U-test.
Antibiotics abolish the effects of alcohol on MAIT cells
To further verify the role of the gut microbiota on MAIT cell frequency, activation and function, an antibiotic cocktail was used to deplete host gut microbiota (Figure 6A). No statistic differences were seen in body weight or diet intake between the groups (Figure S3A-B). Antibiotics reduced fecal microbiota density by 10-fold (Figure S3C). No significant differences were found in the number of MAIT cells in the intestine, lung and liver of PF mice treated with antibiotics (PF+Abx) compared to AF mice treated with antibiotics (AF+Abx) (Figure 6B). Similarly, no statistical differences were observed in the frequency of CD69+, cytokine+ or transcriptional factors+ MAIT cells between PF+Abx and AF+Abx in any of the tissues examined (Figure 6C-E).
Figure 6:

Binge-on chronic alcohol has limited effects on MAIT cell number, activation, cytokines and transcriptional factor expression in antibiotic-treated mice. (A) Schematic of mice exposure to antibiotics and binge-on chronic alcohol. (B) MAIT cell number, (C) the frequency of CD69 and CD69 MFI on MAIT cells, (D) MAIT cell expression of cytokine IFN-γ and TNF-a, (E) MAIT cell expression of transcriptional factors PLZF and T-bet in the intestine, lung and liver in mice exposed to antibiotics plus binge-on chronic alcohol. PF+Abx, mice exposed to antibiotics and liquid control diet; AF+Abx, mice exposed to and antibiotics and binge-on chronic alcohol. Scatter plots and bar shows the means±SD. Statistical difference was calculated by Mann-Whitney U-test between the two groups.
To investigate the direct effect of alcohol alone on MAIT cells, we incubated healthy human PBMCs with increasing concentration of ethanol (0, 50, and 100 mM) in vitro for 4 h. MAIT cell number, activation (CD38+) and effector production (granzyme B and IFN-γ) were not affected by ethanol (Figure S4), suggesting that alcohol alone is insufficient to perturb MAIT cells.
Serum from AF mice increases MAIT cell activation and apoptosis
Serum level of intestinal fatty acid binding protein (iFABP) is a biomarker of intestinal epithelial damage (Adriaanse et al., 2013). AF mice had higher level of circulating iFABP and bacterial 16S rRNA gene copies than PF mice (p<0.01) (Figure 7A). Consistent with previous publications, alcohol exposure increases gut barrier damage, resulting in increased bacterial translocation (Samuelson et al., 2017, Leclercq et al., 2014b). Similar to the animal results, the number of MAIT cells was reduced following culture with serum from AF mice for 4 h, compared to serum from PF mice (p<0.05). AF serum exposure also increased the frequency of CD38+ CD8+ MAIT cells, and the expression of CD38 (MFI) on CD8+MAIT cells (p<0.05) (Figure 7C). Further, AF serum increased the frequency of granzyme B+ and IFN-γ+ MAIT cells (p<0.01, Figure 7D).
Figure 7:

Alcohol increases gut epithelial injury and AF mice serum stimulates blood MAIT cells. Human PBMCs were incubated for 4 h with mice serum (10%, v/v) collected from binge-on chronic alcohol experiment. (A) Serum level of iFABP and circulating bacterial 16S rRNA gene copies in binge-on chronic alcohol exposed mice. (B) MAIT cell number in PBMCs after 4 h incubation. (C) Expression of CD38 and CD38 MFI on CD8+MAIT cells. (D) Production of Granzyme B and IFN-γ by CD8+ MAIT cells. iFABP, intestinal fatty acid binding protein. Scatter plots and bar shows the means±SD. * p<0.05, ** <0.01, and *** p<0.001 by Mann-Whitney U-test.
As a lower number of MAIT cells was observed after incubation with serum from AF mice, we examined whether AF serum promotes MAIT cell apoptosis. After incubation with serum from AF and PF mice, MAIT cells were stained with Annexin V and viability dye to identify cell apoptosis (Figure 8A). Comparing with PF mice, serum from AF mice increased MAIT cell early apoptosis (p<0.05) and no difference was found for late apoptosis and necroptosis between the two groups (Figure 8B). Strikingly, higher frequency of live cells was found in adaptive T cells (CD4+ and CD8+ T cells) than innate T cells (MAIT cells), suggesting that MAIT cells were more susceptible to serum induced cell apoptosis (Figure 8D).
Figure 8:
Serum from alcohol-fed mice promotes MAIT cell apoptosis. The frequency of early (Annexin V+Live/dead−), late apoptosis (Annexin V+Live/dead+) and necroptosis (Annexin V-Live/dead+) was measured in MAIT cells, CD4+ and CD8+ T cells by flow cytometry. (A) Representative flow cytometry dot plots of frequency of apoptotic cells (early + late apoptosis and necroptosis) among MAIT cells after co-culture with PF and AF serum for 4 h. (B) Fold change (AF vs. PF) of the frequency of apoptotic cells. (C) Representative dot plots of the frequency of apoptotic cells among MAIT cells, CD4+ and CD8+ T cell. (D) Percentage of live cells among MAIT cells, CD4+ and CD8+ T cells. Scatter plots and bar shows the means±SD. * p<0.05, ** <0.01, and **** p<0.0001 by Mann-Whitney U-test.
DISCUSSION
MAIT cells, a subset of T cells, are enriched in mucosa-associated tissues and function as innate-like immune cells for defense against invading microorganisms. MAIT cells are activated by antigen presenting cells through MR1-mediated presentation of microbial metabolite-riboflavin (vitamin B2) synthesis precursor derivate (Georgel et al., 2011, Meierovics et al., 2013, van Wilgenburg et al., 2018). Alcohol use disorder is known to dysregulate host immune cells, such as adaptive T cells (Ewald and Shao, 1993), B cells (Gonzalez-Quintela et al., 2008) and innate dendritic cells (Mandrekar et al., 2004). However, the role of alcohol on MAIT cell abundance, activation and function remains unclear. Our work describes for the first time the characterization of MAIT cell number, activation and function in animals following a binge-on-chronic alcohol exposure. Further, we demonstrate that alcohol-associated dysbiosis is mechanistic in alcohol disrupted MAIT cell function. To our knowledge, this is the first study to investigate the impact of alcohol on MAIT cells in the absence of associated disease (e.g., cirrhosis).
MAIT cell counts were significantly reduced in the intestine, lung and liver of mice following binge-on-chronic alcohol exposure, as well as in human PBMCs exposed to either serum or cecal microbiota from AF mice. Additionally, MAIT cells were hyperactivated by alcohol in mice and by exposure to mouse serum/microbiota in vitro. There are several possible mechanisms by which alcohol might reduce the number of MAIT cells in mice. The first possible mechanism is reduced capacity of riboflavin production by the intestinal microbiota following alcohol consumption. Germ-free mice have limited number of MAIT cells, while MAIT cell population expansion is observed after reconstituting germ-free mice with commensal bacteria with conserved genes for riboflavin biosynthesis. Notably, MAIT cell level is positively related with the capacity of riboflavin synthesis (Le Bourhis et al., 2010).
Another possible mechanism responsible for MAIT cell decline in tissues is activation induced cell death (AICD) in alcohol-fed mice. During chronic HIV infection, MAIT cells are hyperactivated and the loss of MAIT cells correlates with activation level (Leeansyah et al., 2013). Similarly, decreased MAIT cell frequency and increased activation is observed in patients with alcoholic hepatitis (Li et al., 2019). The number of circulating MAIT cells reduces gradually during the progress from adulthood to old age (>60-year-old) and correlates with the increased apoptosis. Moreover, increased expression of CD69 is observed during the aging progress (Chen et al., 2019). We observed higher levels of activation in AF compared to PF mice. Serum from AF mice increased activation in human circulating MAIT cells and led to early apoptosis. These results suggest that serum from AF mice has the capacity to activate MAIT cells, promoting cell apoptosis, which may explain the lower MAIT cell count in alcohol drinking mice. In both clinical and preclinical studies, excessive alcohol use impairs gut barrier integrity, resulting in increased translocation of gut bacteria, bacterial antigen and metabolites (Samuelson et al., 2017, Parlesak et al., 2000, Leclercq et al., 2014b). In this study, we found higher levels of circulating iFABP and bacterial gene copies in AF mice, suggesting more severe gut barrier damage and higher bacterial translocation. In alcohol exposed mice, MAIT cells can be activated in both MR1-dependent and MR1-independent pathways. Translocated bacteria with riboflavin synthesis capacity might activate MAIT cells via the classical MR1-dependent pathway. Conversely, other bacterial products (e.g., LPS, flagellin, peptidoglycan, dsRNA) activate monocytes and other immune cells to release cytokines, like IL-12, IL-15, and IL-18, which can activate MAIT cells in MR1-independent pathway (Chiba et al., 2018). In support of gut leak and MAIT cell activation relationship, we found that human MAIT cells incubated with serum and, potentially, translocated microbiota components or metabolites, from AF mice exhibited increased activation compared to serum from PF mice. This suggests that increased gut permeability contributes to MAIT cell hyperactivation and cell loss through apoptosis.
While several mechanistic pathways could be responsible for the observed changes in MAIT cells following alcohol consumption, we found no evidence to support a direct effect of ethanol exposure. Specifically, alcohol exposure in vivo after depletion of the gut microbiota and in vitro exposure of PBMCs to ethanol had no effect on MAIT cell number or activation, consistent with previously published results, showing that ethanol alone has a negligible effect on human MAIT cell number and activation (Riva et al., 2018). These findings suggest that AICD and reduced riboflavin synthesis capacity, associated with the gut microbiota, contribute to lower numbers of MAIT cells in alcohol-fed mice.
Human and rodent studies reported that the excessive alcohol consumption changes gut microbiota composition, structure and metabolic function (Mutlu et al., 2012, Leclercq et al., 2014a, Gerard, 2015). In patients with AUDs, dysbiosis of colonic microbiota has been associated with an increased abundance of Enterobacteriaceae and Proteobacteria and a decrease in Bacteroidetes (Mutlu et al., 2012). The disrupted microbiota homeostasis caused by alcohol is associated with a broad range of alcohol-associated conditions (Duan et al., 2019, Zhou et al., 2019, Hillemacher et al., 2018, Samuelson et al., 2019). Germ-free mice humanized with intestinal microbiota from patients with severe alcoholic hepatitis (sAH) develop liver inflammation and gut barrier impairment, and a subsequent transfer of fecal samples from alcoholic patients without sAH prevents the development of liver disease (Llopis et al., 2016). In patients with alcohol dependence, the relative abundance of butyrate-producing species from the Clostridiales order is inversely associated with alcohol dependence, and the opportunist pathogen Enterobacteriaceae is linked to alcohol dependence as well (Dubinkina et al., 2017). Regardless of HIV status, mice recolonized with intestinal microbiota from humanized alcohol-fed mice, have impaired pulmonary host defense against staphylococcus pneumoniae infection (Samuelson et al., 2019). In this study, we show that binge-on chronic alcohol exposure alters the gut microbiota and show for the first time that alcohol-associated intestinal dysbiosis replicates many of the alcohol-mediated effects on MAIT function. Furthermore, antibiotic treatment abolished the effect of alcohol on MAIT cells in mice. Together these results suggest that alcohol associated changes in intestinal microbiota, rather than direct effects of ethanol, mediates changes to MAIT cell numbers and function.
There are several limitations to these studies. We used exclusively female mice in these studies in order to maintain gender consistency in adoptive transfer experiments. Sex is an important biological variable when studying the impact of alcohol (Alfonso-Loeches et al., 2013). In future studies, we will include both genders. Additionally, we utilized CD69 as our primary activation marker. However, CD69 expression on immune cells in tissue has pleotropic effects, including retention, metabolism and activation, reducing its specificity as an activation marker (Cibrian and Sanchez-Madrid, 2017). Future studies will expand the number of MAIT cell activation markers (Meierovics et al., 2013). Finally, we did not include tests of alcohol metabolites, which could affect MAIT cells even in the absence of a direct effect of ethanol.
To summarize, our data show that alcohol feeding induces MAIT cell loss, hyperactivation, and functional dysregulation in the intestine, lung and liver. Further, we demonstrate that the changes to the MAIT cell profile in alcohol-fed animals are mediated by the gut microbiota, as alcohol dysbiosis transplantation replicated the many of the effects of alcohol on MAIT cells in the absence of alcohol exposure. The reduction of MAIT cell abundance in the intestine, lung and liver was associated with lower gut microbiota riboflavin synthesis capacity, although other mechanisms may be involved. Bacteria and microbial products translocation due to epithelial damage may also contribute to MAIT cell hyperactivation. These data highlight the role that MAIT cells may play in host defense found in alcohol consuming individuals.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the National Institute of Health (NIH) tetramer core facility for providing the MR1 5-OP-RU tetramer (PE) and MR1 6-FP (PE) tetramer for mouse. We also want to thank the Porretta Constance, for assistance with flow cytometry.
This work was funded by National Institute of General Medical Science of the NIH, who funded the Louisiana Clinical and Translational Science Center grant (#U54- GM104940) and National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants (#P60-AA009803, #R21-AA027199, and #K99-AA026336).
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- ADRIAANSE MPM, TACK GJ, PASSOS VL, DAMOISEAUX JGMC, SCHREURS MWJ, VAN WIJCK K, RIEDL RG, MASCLEE AAM, BUURMAN WA, MULDER CJJ & VREUGDENHIL ACE 2013. Serum IFABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Alimentary Pharmacology & Therapeutics, 37, 482–490. [DOI] [PubMed] [Google Scholar]
- ALFONSO-LOECHES S, PASCUAL M & GUERRI C 2013. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology, 311, 27–34. [DOI] [PubMed] [Google Scholar]
- AMOROSO C, PERILLO F, STRATI F, FANTINI MC, CAPRIOLI F & FACCIOTTI F 2020. The Role of Gut Microbiota Biomodulators on Mucosal Immunity and Intestinal Inflammation. Cells, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACHEM A, MAKHLOUF C, BINGER KJ, DE SOUZA DP, TULL D, HOCHHEISER K, WHITNEY PG, FERNANDEZ-RUIZ D, DAHLING S, KASTEMULLER W, JONSSON J, GRESSIER E, LEW AM, PERDOMO C, KUPZ A, FIGGETT W, MACKAY F, OLESHANSKY M, RUSS BE, PARISH IA, KALLIES A, MCCONVILLE MJ, TUMER SJ, GEBHARDT T & BEDOUI S 2019. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8(+) T Cells. Immunity, 51, 285-+. [DOI] [PubMed] [Google Scholar]
- BELKAID Y & HAND TW 2014. Role of the microbiota in immunity and inflammation. Cell, 157, 121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT MS, TRIVEDI S, IYER AS, HALE JS & LEUNG DT 2017. Human mucosal-associated invariant T (MAIT) cells possess capacity for B cell help. J Leukoc Biol, 102, 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGHEIM I, GUO LP, DAVIS MA, LAMBERT JC, BEIER JI, DUVEAU I, LUYENDYK JP, ROTH RA & ARTEEL GE 2006. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology, 130, 2099–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTE FJ & REHERMANN B 2018. Mucosal-Associated Invariant T Cells in Chronic Inflammatory Liver Disease. Semin Liver Dis, 38, 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN PC, DENG WH, LI DD, ZENG T, HUANG L, WANG Q, WANG JL, ZHANG WG, YU XX, DUAN DM, WANG JL, XIA H, CHEN HB, HUANG W, LI JG, ZHANG DH, ZHONG XP & GAO JM 2019. Circulating Mucosal-Associated Invariant T Cells in a Large Cohort of Healthy Chinese Individuals From Newborn to Elderly. Frontiers in Immunology, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIBA A, MURAYAMA G & MIYAKE S 2018. Mucosal-Associated invariant T Cells in Autoimmune Diseases. Frontiers in Immunology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIBRIAN D & SANCHEZ-MADRID F 2017. CD69: from activation marker to metabolic gatekeeper. European Journal of Immunology, 47, 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE ROUX A, CAVALCANTI M, MARCOS MA, GARCIA E, EWIG S, MENSA J & TORRES A 2006. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest, 129, 1219–25. [DOI] [PubMed] [Google Scholar]
- DIMITRIU PA, BOYCE G, SAMARAKOON A, HARTMANN M, JOHNSON P & MOHN WW 2013. Temporal stability of the mouse gut microbiota in relation to innate and adaptive immunity. Environmental Microbiology Reports, 5, 200–210. [DOI] [PubMed] [Google Scholar]
- DUAN Y, LLORENTE C, LANG S, BRANDL K, CHU HK, JIANG L, WHITE RC, CLARKE TH, NGUYEN K, TORRALBA M, SHAO Y, LIU JY, HERNANDEZ-MORALES A, LESSOR L, RAHMAN IR, MIYAMOTO Y, LY M, GAO B, SUN WZ, KIESEL R, HUTMACHER F, LEE S, VENTURA-COTS M, BOSQUES-PADILLA F, VERNA EC, ABRALDES JG, BROWN RS, VARGAS V, ALTAMIRANO J, CABALLERIA J, SHAWCROSS DL, HO SB, LOUVET A, LUCEY MR, MATHURIN P, GARCIA-TSAO G, BATALLER R, TU XM, ECKMANN L, VAN DER DONK WA, YOUNG R, LAWLEY TD, STARKEL P, PRIDE D, FOUTS DE & SCHNABL B 2019. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature, 575, 505-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBINKINA VB, TYAKHT AV, ODINTSOVA VY, YARYGIN KS, KOVARSKY BA, PAVLENKO AV, ISCHENKO DS, POPENKO AS, ALEXEEV DG, TARASKINA AY, NASYROVA RF, KRUPITSKY EM, SHALIKIANI NV, BAKULIN IG, SHCHERBAKOV PL, SKORODUMOVA LO, LARIN AK, KOSTRYUKOVA ES, ABDULKHAKOV RA, ABDULKHAKOV SR, MALANIN SY, ISMAGILOVA RK, GRIGORYEVA TV, ILINA EN & GOVORUN VM 2017. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMAS A, CORRAL D, COLOM A, LEVILLAIN F, PEIXOTO A, HUDRISIER D, POQUET Y & NEYROLLES O 2018. The Host Microbiota Contributes to Early Protection Against Lung Colonization by Mycobacterium tuberculosis. Front Immunol, 9, 2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUSSEAUX M, MARTIN E, SERRIARI N, PEGUILLET I, PREMEL V, LOUIS D, MILDER M, LE BOURHIS L, SOUDAIS C, TREINER E & LANTZ O 2011. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood, 117, 1250–9. [DOI] [PubMed] [Google Scholar]
- EWALD SJ & SHAO H 1993. Ethanol increases apoptotic cell death of thymocytes in vitro. Alcohol Clin Exp Res, 17, 359–65. [DOI] [PubMed] [Google Scholar]
- FERNANDEZSOLA J, JUNQUE A, ESTRUCH R, MONFORTE R, TORRES A & URBANOMARQUEZ A 1995. High Alcohol Intake as a Risk and Prognostic Factor for Community-Acquired Pneumonia. Archives of Internal Medicine, 155, 1649–1654. [DOI] [PubMed] [Google Scholar]
- GEORGEL P, RADOSAVLJEVIC M, MACQUIN C & BAHRAM S 2011. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol, 48, 769–75. [DOI] [PubMed] [Google Scholar]
- GERARD P 2015. Microbiota, alcohol and liver. Correspondances En Metabolismes Hormones Diabetes Et Nutrition, 19, 142–144. [Google Scholar]
- GOLD MC, CERRI S, SMYK-PEARSON S, CANSLER ME, VOGT TM, DELEPINE J, WINATA E, SWARBRICK GM, CHUA WJ, YU YY, LANTZ O, COOK MS, NULL MD, JACOBY DB, HARRIFF MJ, LEWINSOHN DA, HANSEN TH & LEWINSOHN DM 2010. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol, 8, e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZALEZ-AMARO R, CORTES JR, SANCHEZ-MADRID F & MARTIN P 2013. Is CD69 an effective brake to control inflammatory diseases? Trends in Molecular Medicine, 19, 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZALEZ-QUINTELA A, ALENDE R, GUDE F, CAMPOS J, REY J, MEIJIDE LM, FERNANDEZ-MERINO C & VIDAL C 2008. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clinical and Experimental Immunology, 151, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODYEAR AW, KUMAR A, DOW S & RYAN EP 2014. Optimization of murine small intestine leukocyte isolation for global immune phenotype analysis. Journal of Immunological Methods, 405, 97–108. [DOI] [PubMed] [Google Scholar]
- GREENBERG SS, ZHAO X, HUA L, WANG JF, NELSON S & OUYANG J 1999. Ethanol inhibits lung clearance of Pseudomonas aeruginosa by a neutrophil and nitric oxide-dependent mechanism, in vivo. Alcohol Clin Exp Res, 23, 735–44. [PubMed] [Google Scholar]
- HARTMANN N, HARRIFF MJ, MCMURTREY CP, HILDEBRAND WH, LEWINSOHN DM & KRONENBERG M 2018. Role of MAIT cells in pulmonary bacterial infection. Mol Immunol, 101, 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLEMACHER T, BACHMANN O, KAHL KG & FRIELING H 2018. Alcohol, microbiome, and their effect on psychiatric disorders. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 85, 105–115. [DOI] [PubMed] [Google Scholar]
- HOWSON LJ, SALIO M & CERUNDOLO V 2015. MR1-Restricted Mucosal-Associated Invariant T Cells and Their Activation during Infectious Diseases. Front Immunol, 6, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOAY HF, GHERARDIN NA, ENDERS A, LOH L, MACKAY LK, ALMEIDA CF, RUSS BE, NOLD-PETRY CA, NOLD MF, BEDOUI S, CHEN Z, CORBETT AJ, ECKLE SB, MEEHAN B, D’UDEKEM Y, KONSTANTINOV IE, LAPPAS M, LIU L, GOODNOW CC, FAIRLIE DP, ROSSJOHN J, CHONG MM, KEDZIERSKA K, BERZINS SP, BELZ GT, MCCLUSKEY J, ULDRICH AP, GODFREY DI & PELLICCI DG 2016. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol, 17, 1300–1311. [DOI] [PubMed] [Google Scholar]
- KOAY HF, GHERARDIN NA, XU C, SENEVIRATNA R, ZHAO Z, CHEN Z, FAIRLIE DP, MCCLUSKEY J, PELLICCI DG, ULDRICH AP & GODFREY DI 2019. Diverse MR1-restricted T cells in mice and humans. Nat Commun, 10, 2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIOKA A, USSHER JE, COSGROVE C, CLOUGH C, FERGUSSON JR, SMITH K, KANG YH, WALKER LJ, HANSEN TH, WILLBERG CB & KLENERMAN P 2015. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol, 8, 429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE BOURHIS L, MARTIN E, PEGUILLET I, GUIHOT A, FROUX N, CORE M, LEVY E, DUSSEAUX M, MEYSSONNIER V, PREMEL V, NGO C, RITEAU B, DUBAN L, ROBERT D, HUANG S, ROTTMAN M, SOUDAIS C & LANTZ O 2010. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol, 11, 701–8. [DOI] [PubMed] [Google Scholar]
- LECLERCQ S, MATAMOROS S, CANI PD, NEYRINCK AM, JAMAR F, STARKEL P, WINDEY K, TREMAROLI V, BACKHED F, VERBEKE K, DE TIMARY P & DELZENNE NM 2014a. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proceedings of the National Academy of Sciences of the United States of America, 111, E4485–E4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECLERCQ S, MATAMOROS S, CANI PD, NEYRINCK AM, JAMAR F, STARKEL P, WINDEY K, TREMAROLI V, BACKHED F, VERBEKE K, DE TIMARY P & DELZENNE NM 2014b. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A, 111, E4485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEEANSYAH E, GANESH A, QUIGLEY MF, SONNERBORG A, ANDERSSON J, HUNT PW, SOMSOUK M, DEEKS SG, MARTIN JN, MOLL M, SHACKLETT BL & SANDBERG JK 2013. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood, 121, 1124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGOUX F, BELLET D, DAVIAUD C, EL MORR Y, DARBOIS A, NIORT K, PROCOPIO E, SALOU M, GILET J, RYFFEL B, BALVAY A, FOUSSIER A, SARKIS M, EL MARJOU A, SCHMIDT F, RABOT S & LANTZ O 2019. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science, 366, 494–499. [DOI] [PubMed] [Google Scholar]
- LI W, LIN EL, LIANGPUNSAKUL S, LAN J, CHALASANI S, RANE S, PURI P, KAMATH PS, SANYAL AJ, SHAH VH, RADAEVA S, CRABB DW, CHALASANI N & YU Q 2019. Alcohol Abstinence Does Not Fully Reverse Abnormalities of Mucosal-Associated Invariant T Cells in the Blood of Patients With Alcoholic Hepatitis. Clin Transl Gastroenterol, 10, e00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN Z, XU Z, LI Y, WANG Z, CHEN T & ZHAO X 2014. Metabolic engineering of Escherichia coli for the production of riboflavin. Microb Cell Fact, 13, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOPIS M, CASSARD AM, WRZOSEK L, BOSCHAT L, BRUNEAU A, FERRERE G, PUCHOIS V, MARTIN JC, LEPAGE P, LE ROY T, LEFEVRE L, LANGELIER B, CAILLEUX F, GONZALEZ-CASTRO AM, RABOT S, GAUDIN F, AGOSTINI H, PREVOT S, BERREBI D, CIOCAN D, JOUSSE C, NAVEAU S, GERARD P & PERLEMUTER G 2016. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut, 65, 830–9. [DOI] [PubMed] [Google Scholar]
- MANDREKAR P, CATALANO D, DOLGANIUC A, KODYS K & SZABO G 2004. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol, 173, 3398–407. [DOI] [PubMed] [Google Scholar]
- MASON CM, DOBARD E, ZHANG P & NELSON S 2004. Alcohol exacerbates murine pulmonary tuberculosis. Infect Immun, 72, 2556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZMANIAN SK, LIU CH, TZIANABOS AO & KASPER DL 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell, 122, 107–118. [DOI] [PubMed] [Google Scholar]
- MEIEROVICS A, YANKELEVICH WJ & COWLEY SC 2013. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci U S A, 110, E3119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEIEROVICS AI & COWLEY SC 2016. MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection. J Exp Med, 213, 2793–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDLER A, GEIER F, HAANGE SB, PIERZCHALSKI A, KRAUSE JL, NIJENHUIS I, FROMENT J, JEHMLICH N, BERGER U, ACKERMANN G, ROLLE-KAMPCZYK U, VON BERGEN M & HERBERTH G 2020. Mucosal-associated invariant T-Cell (MAIT) activation is altered by chlorpyrifos- and glyphosate-treated commensal gut bacteria. Journal of Immunotoxicology, 17, 10–20. [DOI] [PubMed] [Google Scholar]
- MUTLU EA, GILLEVET PM, RANGWALA H, SIKAROODI M, NAQVI A, ENGEN PA, KWASNY M, LAU CK & KESHAVARZIAN A 2012. Colonic microbiome is altered in alcoholism. American Journal of Physiology-Gastrointestinal and Liver Physiology, 302, G966–G978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARLESAK A, SCHAFER C, SCHUTZ T, BODE JC & BODE C 2000. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol, 32, 742–7. [DOI] [PubMed] [Google Scholar]
- PRABHUDAS M, ADKINS B, GANS H, KING C, LEVY O, RAMILO O & SIEGRIST CA 2011. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol, 12, 189–94. [DOI] [PubMed] [Google Scholar]
- RIVA A, PATEL V, KURIOKA A, JEFFERY HC, WRIGHT G, TARFF S, SHAWCROSS D, RYAN JM, EVANS A, AZARIAN S, BAJAJ JS, FAGAN A, PATEL V, MEHTA K, LOPEZ C, SIMONOVA M, KATZAROV K, HADZHIOLOVA T, PAVLOVA S, WENDON JA, OO YH, KLENERMAN P, WILLIAMS R & CHOKSHI S 2018. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut, 67, 918–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMUELSON DR, DE LA RUA NM, CHARLES TP, RUAN S, TAYLOR CM, BLANCHARD EE, LUO M, RAMSAY AJ, SHELLITO JE & WELSH DA 2016. Oral Immunization of Mice with Live Pneumocystis murina Protects against Pneumocystis Pneumonia. J Immunol, 196, 2655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMUELSON DR, SHELLITO JE, MAFFEI VJ, TAGUE ED, CAMPAGNA SR, BLANCHARD EE, LUO M, TAYLOR CM, RONIS MJJ, MOLINA PE & WELSH DA 2017. Alcohol-associated intestinal dysbiosis impairs pulmonary host defense against Klebsiella pneumoniae. Plos Pathogens, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMUELSON DR, SIGGINS RW, RUAN SB, AMEDEE AM, SUN JS, ZHU QK, MARASCO WA, TAYLOR CM, LUO M, WELSH DA & SHELLITO JE 2019. Alcohol consumption increases susceptibility to pneumococcal pneumonia in a humanized murine HIV model mediated by intestinal dysbiosis. Alcohol, 80, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMALER M, COLONE A, SPAGNUOLO J, ZIMMERMANN M, LEPORE M, KALINICHENKO A, BHATIA S, COTTIER F, RUTISHAUSER T, PAVELKA N, EGLI A, AZZALI E, PIERONI M, COSTANTINO G, HRUZ P, SAUER U, MORI L & DE LIBERO G 2018. Modulation of bacterial metabolism by the microenvironment controls MAIT cell stimulation. Mucosal Immunol, 11, 1060–1070. [DOI] [PubMed] [Google Scholar]
- SIEGRIST CA 2001. Neonatal and early life vaccinology. Vaccine, 19, 3331–46. [DOI] [PubMed] [Google Scholar]
- SMITH K, MCCOY KD & MACPHERSON AJ 2007. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Seminars in Immunology, 19, 59–69. [DOI] [PubMed] [Google Scholar]
- TREINER E, DUBAN L, BAHRAM S, RADOSAVLJEVIC M, WANNER V, TILLOY F, AFFATICATI P, GILFILLAN S & LANTZ O 2003. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature, 422, 164–9. [DOI] [PubMed] [Google Scholar]
- VAN WILGENBURG B, LOH L, CHEN Z, PEDIONGCO TJ, WANG H, SHI M, ZHAO Z, KOUTSAKOS M, NÜSSING S, SANT S, WANG Z, D’SOUZA C, ALMEIDA CF, KOSTENKO L, ECKLE SBG, MEEHAN BS, GODFREY DI, READING PC, CORBETT AJ, MCCLUSKEY J, KLENERMAN P, KEDZIERSKA K & HINKS TSC 2018. MAIT cells contribute to protection against lethal influenza infection in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARELIAS A, BUNTING MD, ORMEROD KL, KOYAMA M, OLVER SD, STRAUBE J, KUNS RD, ROBB RJ, HENDEN AS, COOPER L, LACHNER N, GARTLAN KH, LANTZ O, KJER-NIELSEN L, MAK JY, FAIRLIE DP, CLOUSTON AD, MCCLUSKEY J, ROSSJOHN J, LANE SW, HUGENHOLTZ P & HILL GR 2018. Recipient mucosal-associated invariant T cells control GVHD within the colon. J Clin Invest, 128, 1919–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H, D’SOUZA C, LIM XY, KOSTENKO L, PEDIONGCO TJ, ECKLE SBG, MEEHAN BS, SHI M, WANG N, LI S, LIU L, MAK JYW, FAIRLIE DP, IWAKURA Y, GUNNERSEN JM, STENT AW, GODFREY DI, ROSSJOHN J, WESTALL GP, KJER-NIELSEN L, STRUGNELL RA, MCCLUSKEY J, CORBETT AJ, HINKS TSC & CHEN Z 2018. MAIT cells protect against pulmonary Legionella longbeachae infection. Nat Commun, 9, 3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINGENDER G, HISS M, ENGEL I, PEUKERT K, LEY K, HALLER H, KRONENBERG M & VON VIETINGHOFF S 2012. Neutrophilic granulocytes modulate invariant NKT cell function in mice and humans. J Immunol, 188, 3000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU R, FAN X & SCHNABL B 2019. Role of the intestinal microbiome in liver fibrosis development and new treatment strategies. Transl Res, 209, 22–38. [DOI] [PubMed] [Google Scholar]
- ZINSER ME, HIGHTON AJ, KURIOKA A, KRONSTEINER B, HAGEL J, LENG TQ, MARCHI E, PHETSOUPHANH C, WILLBERG CB, DUNACHIE SJ & KLENERMAN P 2018. Human MAIT cells show metabolic quiescence with rapid glucose-dependent upregulation of granzyme B upon stimulation. Immunology and Cell Biology, 96, 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






