Abstract
Purpose of review
Central nervous system (CNS) tuberculosis is the most devastating form of tuberculosis (TB), with mortality and or neurological sequelae in over half of individuals. We reviewed original research and systematic reviews published since 1 January 2019 for new developments in CNS TB pathophysiology, diagnosis, management, and prognosis.
Recent findings
Insight in the pathophysiology is increasing steadily since the landmark studies in 1933, focussing on granuloma type classification, the relevance of the M. tuberculosis bacterial burden, and the wide range of immunological responses. Although Xpert/RIF has been recommended by the WHO for extrapulmonary TB diagnosis, culture is still needed to increase the sensitivity of TB meningitis diagnosis. Sequential MRIs can improve understanding of neurological deficits at baseline and during treatment. Pharmacokinetic/pharmacodynamic modelling suggests that higher doses of rifampicin and isoniazid in TB meningitis could improve survival.
Summary
Recent studies in the field of CNS-TB have largely focussed on TB meningitis. The outcome may improve by optimising treatment dosing. This needs to be confirmed in clinical trials. Due to the important role of inflammation, these trials should be used as the platform to study the inflammatory and metabolomic responses. This could improve understanding of the biology of this disease and improve patient outlook by enabling individualised host-directed therapy.
Keywords: Tuberculosis, central nervous system, granuloma, Xpert/Rif, treatment optimisation
INTRODUCTION
A quarter of the world’s population is infected by Mycobacterium tuberculosis, and 5-10% of infected individuals develop tuberculosis (TB) disease during their lifetime. Central nervous system (CNS) tuberculosis occurs in 1% of all tuberculosis (TB) cases and is the most devastating form of TB. Given the 10 million new TB patients annually, approximately 100.000 new CNS TB cases would have occurred in 2019(1). HIV infection is a known risk factor for developing extrapulmonary TB, including CNS TB. Therefore, in areas with poor HIV control, the incidence of CNS TB is still high. In 2019, multidrug-resistant tuberculosis (MDR-TB) comprised approximately 3.3% of new TB cases (1). Unfortunately, surveillance data on drug-resistance in CNS TB is lacking. We searched PubMed for the NCIB MeSH term ‘tuberculosis, central nervous system’ which refers to meningeal tuberculosis (TBM), intracranial tuberculoma and tuberculous abscess(2, 3); and spinal cord tuberculosis(3, 4), among research papers and systematic reviews published between the 1st of January 2019 and30th November 2020. We screened 293 articles from which we distilled this review with an update on the diagnosis, pathophysiology, treatment, and prognosis of CNS tuberculosis.
TUBERCULOUS MENINGITIS
Pathophysiology.—
Tuberculous meningitis (TBM) is the most lethal and most well-studied disease entity among CNS TB. The landmark studies by Rich and McCordock in 1933 led to the “Rich focus theory”, postulating that, following the hematogenous spread of the bacilli, foci are formed for years in many places including the spinal cord, cerebral parenchyma, or cerebral cortex. Later rupture leads to the spread of the bacilli into the subarachnoid space, resulting in tuberculous meningitis (TBM). In 2005, Donald and Schoeman revisited this theory and suggested that the miliary dissemination may directly play a role, especially in paediatric TBM(3). Using molecular biology and immunohistochemistry, Zaharie and van Furth further divide granulomas into necrotizing, necrotizing gummatous, and necrotizing abscess types and postulate that they represent different stages of granuloma formation (2)**. To gain further insight in TBM, future research will need to look in more detail into the specific roles of microglia, neurons, and astrocytes and their respective production of inflammatory mediators(5), and influence on the blood-brain and blood-CSF barriers, as reviewed by Davis et al.(6), in which new models are needed(7). The compartmentalisation of the inflammatory response was illustrated by the increased activation of neutrophils, monocytes, and lymphocytes in the lumbar cerebrospinal fluid (CSF) of HIV-uninfected TB meningitis patients(8)*. In paediatric TBM, transcriptomics revealed that ventricular CSF, closer to the brain parenchyma than lumbar CSF, was indeed enriched for signals associated with neuronal damage(9). Compartmentalisation of the immune response is also found in the immune reconstitution inflammatory syndrome (IRIS) that can occur in HIV-infected TBM patients after initiation of antiretroviral therapy(10).
The genetic diversity of Mycobacterium tuberculosis lineage affects the disease phenotype, including the virulence, transmission, reactivation, reinfection, and treatment response. Euro-American lineage was found predominantly in 83 TBM patients in South Africa; lineage mixed infections detected in those samples indicating the rapid progression of the disease and inadequate response therapy(11).
Diagnosis.—
A high index of clinical suspicion is paramount in the diagnosis of TBM. Progress has been made in molecular testing. Recently, WHO recommends Xpert MTB/RIF assay (Cepheid, Sunnyvale, USA), and Xpert MTB/RIF Ultra are used as initial tests for the diagnosis of pulmonary and extrapulmonary TB and rifampicin resistance in adults and children(1). However, both have a low performance in diagnosing TBM with a sensitivity of 47.2% (95% CI 34.4-60.3); 39.6% (95% CI 27.6-53.1) and specificity of 100% (95% CI 92-100) and of 100% (95% CI 92.6-100) against uniform case definition(12). A large systematic review of 1381 cases of confirmed TBM and 5712 non-TBM controls by Pormohammad et al. confirmed the overall estimates of commercial nucleic acid amplification (NAA) sensitivity and specificity of 82% (95% CI 75%-87%) and 99% (95% CI, 98-99%) respectively against combined reference standard (CRS) (clinical criteria plus one or more of the followings: Ziehl Neelsen (ZN), culture, or CSF-positive NAA test(13)*. This is in line with another large study by Heemskerk et.al that found the sensitivities against culture of ZN stain, modified ZN, and Xpert MTB/Rif were 66.4%, 67.5%, and 72.3%, respectively(14). Isoniazid-resistance can be detected both by cartridge-based technologies or phenotypic drug susceptibility testing (DST). The WHO recommends line probe assay to detect the resistance to fluoroquinolones and second-line injectable agents (1) and is now evaluating the use of the new Xpert XDR-TB cartridge.
CSF and urine lipoarabinomannan (LAM) are very specific yet had low sensitivity for TBM diagnosis in a cohort of mainly HIV-infected TBM patients in Zambia(15). Circumstantial evidence may be provided by chest radiography, which identifies pulmonary abnormalities in 30-50% of patients with tuberculous meningitis(16, 17). Brain imaging may be improved by three-dimensional (3D) magnetization-prepared rapid gradient-echo (MP-RAGE) Magnetic Resonance Imaging (MRI) which has improved sensitivity due to its use of thin slices (1 mm), thereby detecting more brain lesions(18*). Fluid attenuated inversion-recovery (FLAIR) imaging, diffusion-weighted imaging (DWI), and apparent diffusion coefficient (ADC) might help in detecting brain infarction(19). More than half of TBM patients are reported to have either meningeal enhancement (14%-81%), mainly at the basal cistern and Sylvian fissure, hydrocephalus (40%-56%), tuberculoma (40%-77%), mainly of the miliary type characterized by diffuse small granulomas in the subcortical white matter, or brain infarction (27%-60%)(18*, 20), as illustrated in figure 1. Brain infarction is mainly found in HIV-infected patients(21). Obstructing exudates can also be seen at contrast imaging at the foramen of Monro and 4th ventricle(19), which usually relates to non-communicating hydrocephalus.
Figure.1. Tuberculous meningitis imaging.
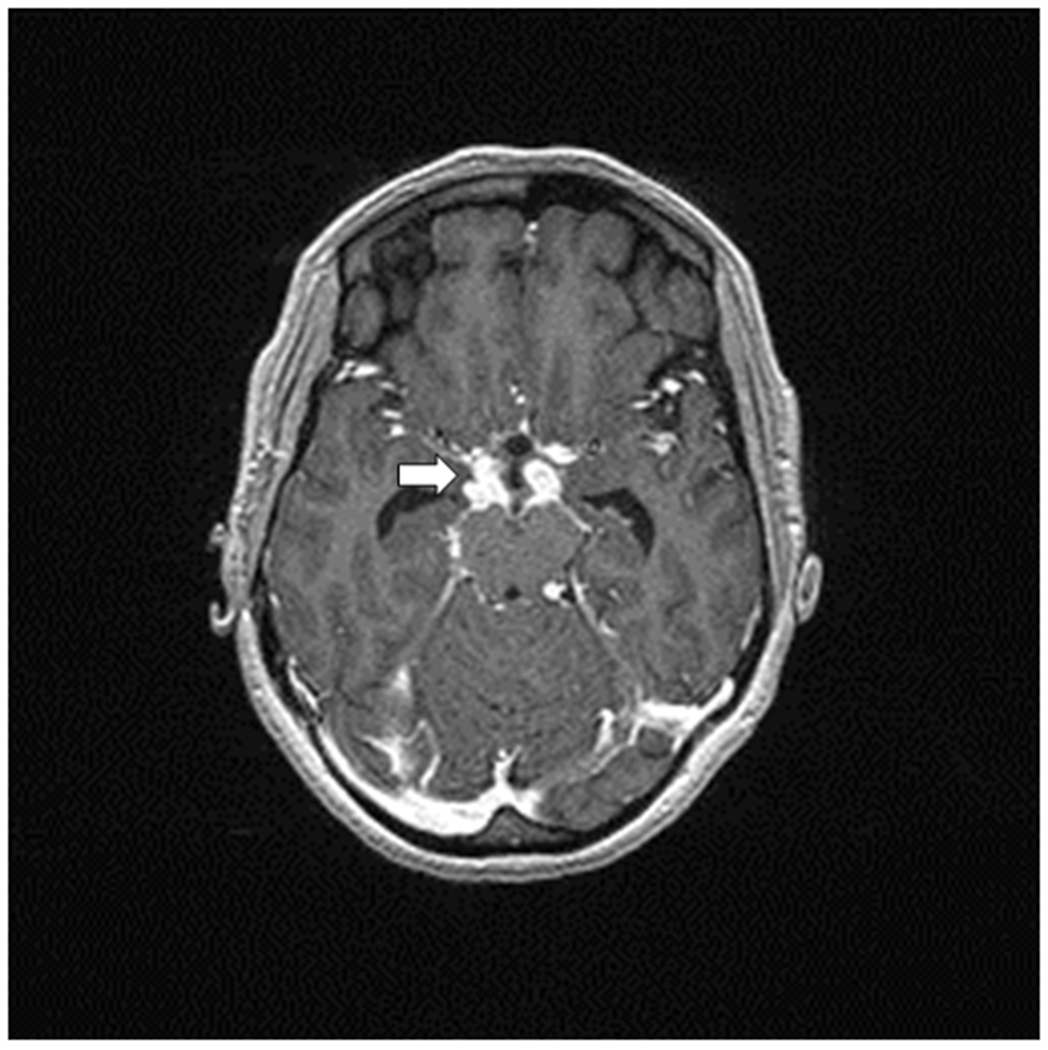
Axial spin-echo T2 weighted MR image shows meningeal enhancement at suprasellar and both middle cerebral artery cistern. Enlargement of lateral ventricles indicating a hydrocephalus.
Treatment.—
Evidence from preclinical, clinical, and modelling studies support the use of high-dose rifampicin. The exposure-response relationships for rifampicin have been studied in Indonesia and Vietnam. The largest trial thus far, randomising 817 patients, did not demonstrate a survival benefit of a 15 mg/kg/day over 10 mg/kg/day rifampicin in the first 8 weeks of treatment(22*), except for the possible benefit in patients with isoniazid-resistance M. tuberculosis strains(23). Could it be that 15mg/kg/day is still not high enough?. A modelling analysis based on three clinical trials on high-dose rifampicin in Indonesia indicated that high rifampicin exposure may substantially decrease the risk of death and that optimum doses may be as high as 30 mg/kg(24**). From the same study, the pharmacokinetic and pharmacodynamic analysis found that low isoniazid exposure was linked to death, and that was associated with rapid acetylators(22*). Duration of anti-tuberculosis treatment in TBM is 9-12 months according to WHO recommendation(25). An alternative approach to suboptimal antibiotic exposure in the site of infection other than increasing the dose is by using a new antibiotic with better penetration to the brain. Linezolid is now under investigation in South Africa(26) and Indonesia (ClinicalTrials.gov Identifier: NCT03927313 and NCT03537495). Second-line antituberculotic agents with good brain penetration include levofloxacin, moxifloxacin, ethionamide or protionamide, and cycloserine with 70-90% human CSF penetrance(27). The current recommendation from WHO that includes a five-drug regimen for pulmonary TB(1, 28) still needs to be evaluated in MDR central nervous TB patients. Of interest might be that delamanid, which has poor human and rabbit CSF concentrations, seems to accumulate in rabbit brain tissue and therefore regained a possible role in treating MDR CNS TB(29).
Dexamethasone can improve survival mainly in HIV-negative TBM patients during the early stage I of the disease. Trials are now underway to evaluate the role of adjunctive dexamethasone on the 12-month survival and neurological outcomes in HIV-infected TBM patients (ClinicalTrials.gov Identifier: NCT03092817). Of large interest is that aspirin has also been investigated as adjunctive treatment in a phase 2 trial that used an 81 mg and 1000 mg aspirin dose for the first 60 days of treatment, both doses were found to be safe. Planned subgroup analysis indicated a reduction of the combined endpoints of infarction and deaths by day 60 which occurred in 10.7% of the 1000mg aspirin, 14.8% of the 81 mg aspirin, and 34% of the placebo group. This correlates with dose-dependent inhibition of CSF thromboxane A2 and upregulation of pre-resolvins(30).
Neurocritical care of TBM includes control of high intracranial pressure resulted from hydrocephalus, severe ischaemic stroke, seizures, fever, impaired ventilation, and hyponatremia(31). Hyponatremia in TBM requires closely monitored treatment, with careful administration of saline or oral salt. Fludrocortisone might be beneficial in some refractory cases(32).
Prognosis.—
Mortality of TBM patients is associated consistently with HIV-coinfection, drug resistance and a lower level of consciousness at hospital presentation, in different populations. In a new model using time-updated data, Glasgow Coma Scale reliably predicts patient outcome(33). Studies on genomics and metabolomics are increasingly performed recently and have the potential of identifying survival predicting biomarkers and eventually development of host-directed therapy. Higher CSF αβ T cells, NK cells(8*), and several CSF lipid, so-called pro-resolving, mediators were also associated with better survival(34). High pre-treatment M. tuberculosis load as measured by GeneXpert, was associated with higher disease severity, CSF neutrophil count, and the occurrence of new neurological events, but did not predict mortality(35*). Paradoxical response in HIV-negative TBM does not adversely impact mortality as much as in HIV-infected TBM patients(10, 18).
Summarising, thus far, corticosteroids remain the only trial-supported host-direct therapy that improves survival of TBM patients(36), including those with CNS-TB-IRIS(10), but new pathophysiological insights provide hopes for new precision medicine in the near future(37).
TUBERCULOMA AND TUBERCULOUS ABSCESS
Tuberculomas are round and encapsulated space-occupying lesions that have developed from a parenchymal granuloma that do not rupture into the subarachnoid space during maturation. Pathologically they are characterized as a granulomatous lesion consisting of epithelioid cells and Langerhans giant cells, surrounded by lymphocytes cells, sometimes with central caseation(38, 39). Tuberculomas are reported in 12%-77% of TBM patients in different series depending on imaging techniques (17, 18, 40).
Diagnosis.—
Tuberculomas vary in their location, size, and the extent of the mass effect they infer on the adjacent area. Neuroradiologically, tuberculomas can be characterised by the size of the ring-like enhancement (2-3 mm for miliary types) and on the pathological state of its centre, which can be noncaseating, solidly caseating, or caseating with central liquefication(38, 39). Tuberculomas are frequently observed coincidently in patients with TBM. They can enlarge or newly develop during TB treatment as so-called a paradoxical reaction(18*).
Tuberculous abscesses are a rare manifestation of CNS TB, identified in only 2% in a series of 48 TBM patients with extensive imaging data from Bandung, Indonesia(18*). They appear mostly as large, multiloculated lesions with significant mass effect, and cavity formation with central pus(18, 38), more similar to pyogenic brain abscess than tuberculoma. On MRI the centre is T1-hypointense and T2-hyperintense with restricted diffusion and rim enhancement on post-contrast T1 W1. In a research setting, lactate and lipid peaks in the absence of significant amino acid peaks on MR spectroscopy are suggestive of a tuberculous rather than pyogenic brain abscess(38, 39).
Treatment.—
Tuberculomas are treated with anti-tuberculous drugs for 9-12 months based on expert opinion in most international guidelines, as thoroughly reviewed in(41). Additionally, corticosteroids are recommended in some guidelines for all patients (42*), and are unequivocally recommended for those patients that develop the so-called paradoxical responses. This entails clinical or radiological worsening several weeks after anti-tuberculous treatment is started(18*), and is thought to result from an aberrant immune response. In this situation, dexamethasone should be readministered and subsequently tapered. Adjunctive host-directed therapy using an anti-TNF-α treatment, or adjunctive interferon-γ or thalidomide has been applied in individual cases as reviewed in(41).
Prognosis.—
Occasionally the post-treatment improvement is not very rapid or even worsens in the follow-up neuroimaging despite corticosteroid re-administration. The longer periods of antibiotics potentially increase drug toxicity and worsening in neuroimaging findings does not necessarily correspond to treatment failure in a susceptible patient(41).
SPINAL TUBERCULOSIS
Spinal tuberculosis comprises more than half of the cases of skeletal TB, which itself accounts for 10-20% of all extrapulmonary TB cases. Due to the high worldwide incidence of roughly 10 million TB cases yearly, spinal tuberculosis thus still causes more than 100.000 cases annually. This manifestation of tuberculosis, first known as Pott’s disease, mainly affects the thoracic and lumbar anterior of vertebral bodies. Alternative manifestations are those of the spinal cord and nerve roots or intramedullary tuberculoma.
Pathophysiology.—
Spinal tuberculosis is predominantly caused by hematogenous spread of M. tuberculosis via paravertebral veins, destroying the anterior-inferior part of the vertebral body and subsequent spread under the anterior spinal ligament to an adjacent inferior vertebra. In adults, spondylitis is more common than spondylodiscitis, meaning that the vertebral discs are usually spared. This is because blood vessels of the human cartilage plate gradually disappear during growth and M. tuberculosis does not produce proteolytic enzymes that can cause damages to the intervertebral discs. Anterior involvement is mostly due to the spread of exudate or a paraspinal cold abscess under the ligaments and periosteum or to distant vertebral bodies via venous plexus of Batson(19, 43). There is a local immune response involving both pro-inflammatory and anti-inflammatory macrophage products that is, identified in patients with spinal TB in the area adjacent to the spinal tuberculous granuloma (44).
Diagnosis.—
The most commonly observed clinical signs and symptoms are back pain and vertebral deformity, which later may result in kyphosis due to vertebral fracture and collapse. Neurological deficits include radicular pain, cauda equina syndrome, or spinal cord symptoms i.e. motor deficit, sensory disorders, and bladder and bowel dysfunction. Patient may develop a neurological deficit gradually and occasionally with obvious signs of infection such as fever or malaise. A high index of clinical suspicion is the mainstay to the diagnosis of spinal TB. On biopsy, in addition to culture, molecular testing can be performed with Xpert/RIF sensitivity of 82%-95.6% and specificity of 96.2%-100% to detect spinal TB (45, 46). Material for detection of M. tuberculosis can be acquired via bone biopsy, with limited availability in resource-limited settings. Additionally, spinal MRI can show a meningeal enhancement with or without wedge-shaped bone destruction and paraspinal abscess as illustrated in figure 2.
Figure.2. Spinal tuberculosis/ Pott’s Disease.
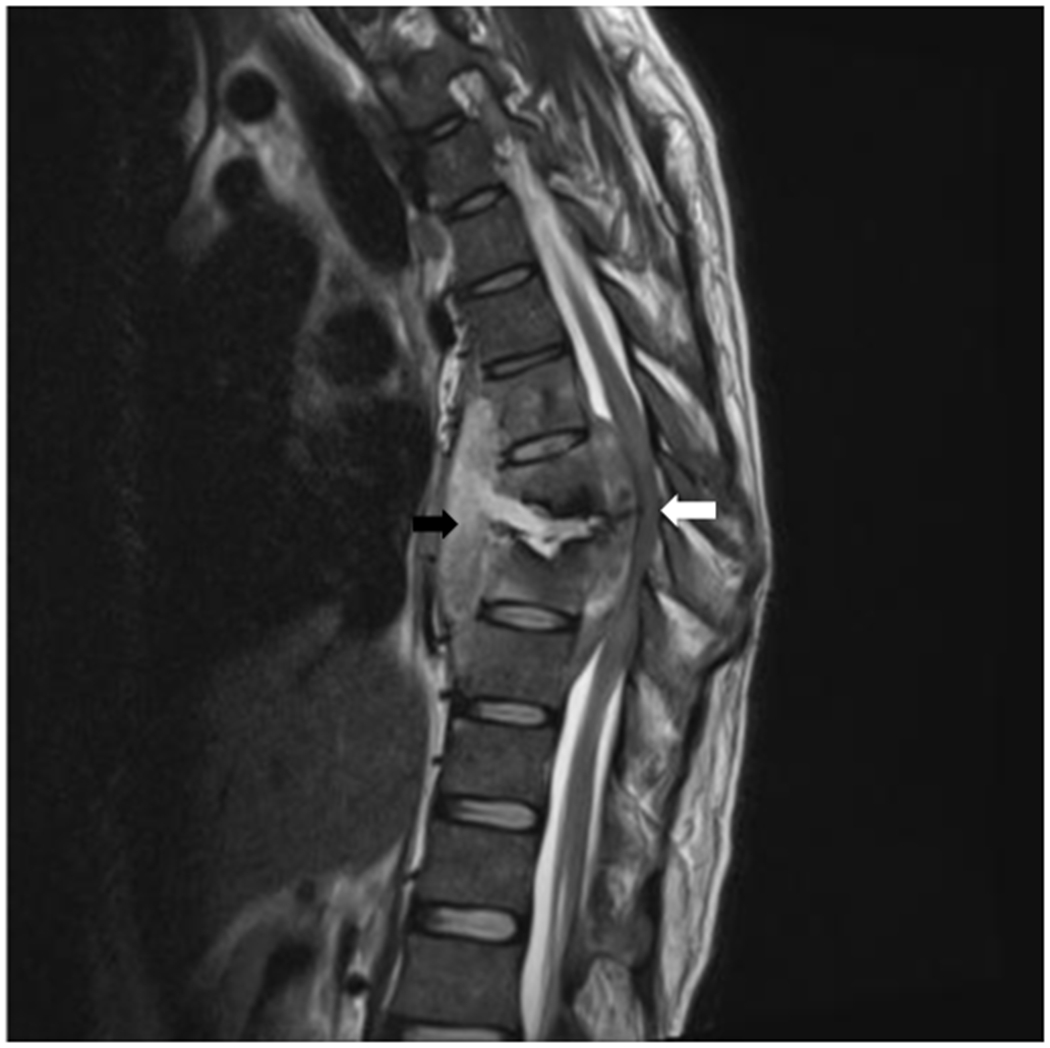
Sagittal spin-echo T2-weighted MR image shows tuberculous spondylitis of T6-T9 with wedge shaped bone deformity (black arrows) of vertebral body T6-T8 causing occlusion of spinal canal (white arrows) at level T7-T8 with pus collection (paravertebral abscess) at vertebral T6-T9 and leptomeningeal abscess at level T6-9.
Treatment.—
The treatment regimen for spinal TB, as much as for other extrapulmonary TB, is still based on the clinical trials in pulmonary TB. Of note, intermittent administration (i.e. thrice weekly) is not recommended for spinal TB due to the lack of data. Most international societies recommended daily therapy with a 6-9 months duration(47), but in clinical practice, treatment is often given longer, up to 24 months, if the clinical and radiological improvement is not satisfying. The administration of linezolid can be considered in selected MDR spinal TB but it should be taken into account that the drug exposure in the bone samples is only half of that in plasma(48). Surgery is not the mainstay of treatment, but decompression should be considered in cases with progressive neurological deficits(49). However, in uncomplicated cases, both conservative and surgical treatment give a similar effect on the functional outcome(50). In a small and observational study in spinal TB patients, local application of streptomycin intraoperatively to prevent surgical site infections has shortened the duration of hospitalization and antibiotic use (51).
Prognosis.—
Response to treatment is evaluated based on the improvement of pain, appetite, resolution of fever, and body weight gain with a serial decrease in inflammatory markers. Failure of wound healing within a few months of anti-TB treatment can suggest drug-resistance, immunodeficiency, or, rarely, paradoxical worsening. However, the healing process is highly variable among individuals. Evaluation of paraspinal abscess or spinal oedema by serial MRI is recommended. Complete resolution is rarely found within 8 months of treatment and anti-TB administration is therefore prolonged, but supporting evidence is needed.
Conclusion
Central nervous system TB remains a global problem, especially in countries with a high TB burden and poor HIV control. Diagnosis is still not easy despite the availability of new commercial molecular tests. Trial-supported treatment is yet lacking for tuberculoma or spinal TB but increasingly becomes available for TB meningitis.
Key Points.
Central nervous system (CNS) tuberculosis (TB) causes significant mortality and disability in countries with a high TB prevalence and poor HIV control.
A high level of clinical suspicion is warranted for early diagnosis, and current diagnostic tools lack sufficient satisfactory sensitivity to rule out CNS TB.
Sequential MRIs improve understanding of neurological deficits at baseline and during treatment.
Pharmacodynamic/pharmacokinetic studies recommend that increased rifampicin and isoniazid doses may improve the outcome of TB meningitis.
Research on TB meningitis has recently gained impetus, which is also much needed for studies on CNS tuberculoma and spinal TB.
Financial support and sponsorship
SD, ARG and AvL are supported by the National Institute of Health for the “Using Tryptophan Metabolism and Response to Corticosteroids to Define New Therapeutic Targets for Tuberculosis Meningitis: Integration of Large Scale Clinical, Metabolomic, and Genomic Data” project [R01AI145781]. ARG is are supported for research in TB meningitis by the Medical Research Council UK, High Dose Oral Rifampicin to Improve Survival from Adult TB Meningitis - (HARVEST) Trial [MR/S004963/1].
Footnotes
Conflict of interest
None
REFERENCES
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.WHO. Global Tuberculosis Report 2020. [Google Scholar]
- 2.**.Zaharie SD, Franken DJ, van der Kuip M, van Elsland S, de Bakker BS, Hagoort J, et al. The immunological architecture of granulomatous inflammation in central nervous system tuberculosis. Tuberculosis (Edinb). 2020;125:102016. [DOI] [PubMed] [Google Scholar]; This article presents the new possible mechanism of granuloma formation in TBM by performing a sophisticated immunohistochemistry technique in a large number of brain specimens (439 post-mortem and 24 biopsy-derived brain) from cohort of 84 patients. Study on the brain specimens is not largely available in some countries where TBM cases mostly present due to the ethical matter.
- 3.Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK. Central nervous system tuberculosis: pathogenesis and clinical aspects. Clin Microbiol Rev. 2008;21(2):243–61, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59(3):167–87. [DOI] [PubMed] [Google Scholar]
- 5.Koeken VACM, Ganiem AR, Dian S, Ruslami R, Chaidir L, Netea MG, et al. Cerebrospinal fluid IL-1β is elevated in tuberculous meningitis patients but not associated with mortality. Tuberculosis (Edinb). 2020;126:102019. [DOI] [PubMed] [Google Scholar]
- 6.Davis AG, Rohlwink UK, Proust A, Figaji AA, Wilkinson RJ. The pathogenesis of tuberculous meningitis. J Leukoc Biol. 2019;105(2):267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter FR, Gilpin TE, Herbath M, Deli MA, Sandor M, Fabry Z. A Novel In Vitro Mouse Model to Study Mycobacterium tuberculosis Dissemination Across Brain Vessels: A Combination Granuloma and Blood-Brain Barrier Mouse Model. Curr Protoc Immunol. 2020;130(1):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.*.van Laarhoven A, Dian S, van Dorp S, Purnama F, Koeken VACM, Diandini E, et al. Immune cell characteristics and cytokine responses in adult HIV-negative tuberculous meningitis: an observational cohort study. Sci Rep. 2019;9(1):884. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study presents an important finding about immunological response during TBM infection.
- 9.Rohlwink UK, Figaji A, Wilkinson KA, Horswell S, Sesay AK, Deffur A, et al. Tuberculous meningitis in children is characterized by compartmentalized immune responses and neural excitotoxicity. Nat Commun. 2019;10(1):3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinn CM, Poplin V, Kasibante J, Yuquimpo K, Gakuru J, Cresswell FV, et al. Tuberculosis IRIS: Pathogenesis, Presentation, and Management across the Spectrum of Disease. Life (Basel). 2020;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Leeuwen LM, Versteegen P, Zaharie SD, van Elsland SL, Jordaan A, Streicher EM, et al. Bacterial Genotyping of Central Nervous System Tuberculosis in South Africa: Heterogenic Mycobacterium tuberculosis Infection and Predominance of Lineage 4. J Clin Microbiol. 2019;57(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan J, Thu DDA, Phu NH, Dung VTM, Quang TP, Nghia HDT, et al. Xpert MTB/RIF Ultra versus Xpert MTB/RIF for the diagnosis of tuberculous meningitis: a prospective, randomised, diagnostic accuracy study. Lancet Infect Dis. 2020;20(3):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.*.Pormohammad A, Nasiri MJ, McHugh TD, Riahi SM, Bahr NC. A Systematic Review and Meta-analysis of the Diagnostic Accuracy of Nucleic Acid Amplification Tests for Tuberculous Meningitis. J Clin Microbiol. 2019;57(6). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the latest systematic review and meta-analysis about NAA test for TBM, the highest level of evidence in research.
- 14.Heemskerk AD, Donovan J, Thu DDA, Marais S, Chaidir L, Dung VTM, et al. Improving the microbiological diagnosis of tuberculous meningitis: A prospective, international, multicentre comparison of conventional and modified Ziehl-Neelsen stain, GeneXpert, and culture of cerebrospinal fluid. J Infect. 2018;77(6):509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiqi OK, Birbeck GL, Ghebremichael M, Mubanga E, Love S, Buback C, et al. Prospective Cohort Study on Performance of Cerebrospinal Fluid (CSF) Xpert MTB/RIF, CSF Lipoarabinomannan (LAM) Lateral Flow Assay (LFA), and Urine LAM LFA for Diagnosis of Tuberculous Meningitis in Zambia. J Clin Microbiol. 2019;57(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhasin H, Goyal M, Sharma S. Advances in the Diagnosis and Management of Tubercular Meningitis in Children. Indian J Pediatr. 2020;87(1):26–33. [DOI] [PubMed] [Google Scholar]
- 17.García-Grimshaw M, Gutiérrez-Manjarrez FA, Navarro-Álvarez S, González-Duarte A. Clinical, Imaging, and Laboratory Characteristics of Adult Mexican Patients with Tuberculous Meningitis: A Retrospective Cohort Study. J Epidemiol Glob Health. 2020;10(1):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.*.Dian S, Hermawan R, van Laarhoven A, Immaculata S, Achmad TH, Ruslami R, et al. Brain MRI findings in relation to clinical characteristics and outcome of tuberculous meningitis. PLoS One. 2020;15(11):e0241974. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article systematically evaluates paired neuroimaging results from 48 subjects within 2 months of anti-tuberculosis treatment using 3D MP-RAGE MRI sequence given a more detail reading.
- 19.Rodriguez-Takeuchi SY, Renjifo ME, Medina FJ. Extrapulmonary Tuberculosis: Pathophysiology and Imaging Findings. Radiographics. 2019;39(7):2023–37. [DOI] [PubMed] [Google Scholar]
- 20.Azeemuddin M, Alvi A, Sayani R, Khan MK, Farooq S, Beg MA, et al. Neuroimaging Findings in Tuberculosis: A Single-Center Experience in 559 Cases. J Neuroimaging. 2019;29(5):657–68. [DOI] [PubMed] [Google Scholar]
- 21.Di Napoli A, Cristofaro M, Romano A, Pianura E, Papale G, Di Stefano F, et al. Central Nervous System involvement in tuberculosis: An MRI study considering differences between patients with and without Human Immunodeficiency Virus 1 infection. J Neuroradiol. 2020;47(5):334–8. [DOI] [PubMed] [Google Scholar]
- 22.*.Ding J, Thuy Thuong Thuong N, Pham TV, Heemskerk D, Pouplin T, Tran CTH, et al. Pharmacokinetics and Pharmacodynamics of Intensive Antituberculosis Treatment of Tuberculous Meningitis. Clin Pharmacol Ther. 2020;107(4):1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article evaluates the pharmacokinetic/pharmacodynamic of high dose rifampicin and isoniazid from a clinical trial with large number of sample size.
- 23.Heemskerk AD, Bang ND, Mai NT, Chau TT, Phu NH, Loc PP, et al. Intensified Antituberculosis Therapy in Adults with Tuberculous Meningitis. N Engl J Med. 2016;374(2):124–34. [DOI] [PubMed] [Google Scholar]
- 24.**.Svensson EM, Dian S, Te Brake L, Ganiem AR, Yunivita V, van Laarhoven A, et al. Model-Based Meta-analysis of Rifampicin Exposure and Mortality in Indonesian Tuberculous Meningitis Trials. Clin Infect Dis. 2020;71(8):1817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article evaluates the pharmacodynamic and pharmacokinetic of high dose rifampicin up to 35 mg/kg using a robust mathematical modelling from 3 different clinical trials data. To date there is no clinical trial of TBM patients using rifampicin higher than 35 mg/kg.
- 25.WHO. Treatment of Tuberculosis guidelines. Geneva, Switzerland.2010. [Google Scholar]
- 26.Seddon JA, Tugume L, Solomons R, Prasad K, Bahr NC, Consortium TMIR. The current global situation for tuberculous meningitis: epidemiology, diagnostics, treatment and outcomes. Wellcome Open Res. 2019;4:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis A, Meintjes G, Wilkinson RJ. Treatment of Tuberculous Meningitis and Its Complications in Adults. Curr Treat Options Neurol. 2018;20(3):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Switzerland: WHO; 2019. [PubMed] [Google Scholar]
- 29.Tucker EW, Pieterse L, Zimmerman MD, Udwadia ZF, Peloquin CA, Gler MT, et al. Delamanid Central Nervous System Pharmacokinetics in Tuberculous Meningitis in Rabbits and Humans. Antimicrob Agents Chemother. 2019;63(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mai NT, Dobbs N, Phu NH, Colas RA, Thao LT, Thuong NT, et al. A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donovan J, Figaji A, Imran D, Phu NH, Rohlwink U, Thwaites GE. The neurocritical care of tuberculous meningitis. Lancet Neurol. 2019;18(8):771–83. [DOI] [PubMed] [Google Scholar]
- 32.Misra UK, Kalita J, Consortium TMIR. Mechanism, spectrum, consequences and management of hyponatremia in tuberculous meningitis. Wellcome Open Res. 2019;4:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thao LTP, Wolbers M, Heemskerk AD, Thi Hoang Mai N, Thi Minh Ha D, Thi Hong Chau T, et al. Dynamic Prediction of Death in Patients With Tuberculous Meningitis Using Time-updated Glasgow Coma Scale and Plasma Sodium Measurements. Clin Infect Dis. 2020;70(5):827–34. [DOI] [PubMed] [Google Scholar]
- 34.Colas RA, Nhat LTH, Thuong NTT, Gómez EA, Ly L, Thanh HH, et al. Proresolving mediator profiles in cerebrospinal fluid are linked with disease severity and outcome in adults with tuberculous meningitis. FASEB J. 2019;33(11):13028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.*.Thuong NTT, Vinh DN, Hai HT, Thu DDA, Nhat LTH, Heemskerk D, et al. Pretreatment Cerebrospinal Fluid Bacterial Load Correlates With Inflammatory Response and Predicts Neurological Events During Tuberculous Meningitis Treatment. J Infect Dis. 2019;219(6):986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article found a crucial finding about role of bacterial load and host inflammatory response in TBM outcome.
- 36.Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741–51. [DOI] [PubMed] [Google Scholar]
- 37.Lange C, Aarnoutse R, Chesov D, van Crevel R, Gillespie SH, Grobbel HP, et al. Perspective for Precision Medicine for Tuberculosis. Front Immunol. 2020;11:566608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salvador GLO, Basso ACN, Barbieri PP, Leitao CA, Teixeira BCA, Neto AC. Central nervous system and spinal cord tuberculosis: Revisiting an important disease. Clin Imaging. 2020;69:158–68. [DOI] [PubMed] [Google Scholar]
- 39.Schaller MA, Wicke F, Foerch C, Weidauer S. Central Nervous System Tuberculosis : Etiology, Clinical Manifestations and Neuroradiological Features. Clin Neuroradiol. 2019;29(1):3–18. [DOI] [PubMed] [Google Scholar]
- 40.Maheswari EU, Bhoopathy RM, Bhanu K, Anandan H. Clinical Spectrum of Central Nervous System Tuberculosis and the Efficacy of Revised National Tuberculosis Control Program in its Management. J Neurosci Rural Pract. 2019;10(1):71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marais S, Van Toorn R, Chow FC, Manesh A, Siddiqi OK, Figaji A, et al. Management of intracranial tuberculous mass lesions: how long should we treat for? Wellcome Open Res. 2019;4:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.*.Suárez I, Gruell H, Heyckendorf J, Fünger S, Lichtenstein T, Jung N, et al. Intensified adjunctive corticosteroid therapy for CNS tuberculomas. Infection. 2020;48(2):289–93. [DOI] [PubMed] [Google Scholar]; This article evaluates the use of corticosteroid in CNS tuberculomas. Although this is not a randomized controlled trial study, the result is important provided that research on CNS tuberculoma is scarce, and that this particular study has a large sample size.
- 43.Lee KY. Comparison of pyogenic spondylitis and tuberculous spondylitis. Asian Spine J. 2014;8(2):216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Shang X, Qi X, Ba D, Lv J, Zhou X, et al. Clinical Significance of M1/M2 Macrophages and Related Cytokines in Patients with Spinal Tuberculosis. Dis Markers. 2020;2020:2509454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu Y, Kong Y, Ye J, Wang A. Performance of conventional histopathology and GeneXpert MTB/RIF in the diagnosis of spinal tuberculosis from bone specimens: A prospective clinical study. Clin Biochem. 2020;85:33–7. [DOI] [PubMed] [Google Scholar]
- 46.Solanki AM, Basu S, Biswas A, Roy S, Banta A. Sensitivity and Specificity of Gene Xpert in the Diagnosis of Spinal Tuberculosis: A Prospective Controlled Clinical Study. Global Spine J. 2020;10(5):553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandita A, Madhuripan N, Pandita S, Hurtado RM. Challenges and controversies in the treatment of spinal tuberculosis. J Clin Tuberc Other Mycobact Dis. 2020;19:100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Dong W, Lan T, Fan J, Qin S, Guo A. Distribution of Linezolid in Tuberculosis Lesions in Patients with Spinal Multidrug-Resistant Tuberculosis. Antimicrob Agents Chemother. 2020;64(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rathod TN, Sathe AH, Marathe NA. I’s Never Too Late: Neurological Outcome of Delayed Decompression in Tuberculosis of Spine. Global Spine J. 2020:2192568220922209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yong LN, Ahmedy F, Yin KN, Engkasan JP. Functional Outcomes in Spinal Tuberculosis: A Review of the Literature. Asian Spine J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahuja K, Yadav G, Sudhakar PV, Kandwal P. Role of local streptomycin in prevention of surgical site infection in TB spine. Eur J Orthop Surg Traumatol. 2020;30(4):701–6. [DOI] [PubMed] [Google Scholar]


