Abstract
Background.
Sleep deprivation (SD) is an antidepressant intervention with multiple administration formats that has been investigated primarily with uncontrolled clinical trials and qualitative reviews of the literature. The validity and applicability of these findings to the treatment of bipolar depression (BPD) is uncertain.
Methods.
A PRISMA-based systematic review of the literature and meta-analysis were conducted to determine the efficacy of SD in the treatment of BPD and to identify moderator variables that influence response rate.
Results.
From a sample of 15 studies covering 384 patients, the overall, mean response rate to SD was 47.6% (CI 36.0%, 59.5%). This response rate compared post-SD to pre-SD depression scores, and not to a placebo control condition.
Of several potential moderating variables examined, the use of adjunctive pharmacotherapy achieved statistical significance with response rates of 59.4% [CI 48.5, 69.5] for patients using adjunctive medication vs 27.4% [CI 17.8, 39.8] for patients not using adjunctive medication.
Conclusions.
This meta-analysis of SD in the treatment of BPD found an overall, response rate of almost 50%, reinforcing earlier estimates of efficacy. The use of adjunctive pharmacotherapy had a statistically significant moderating effect on SD response suggesting that clinical practice should routinely pair these interventions. These findings provide a higher level of evidence supporting the use of SD, especially when used with medication, and should inform future management guidelines for the treatment of BPD.
Keywords: Chronobiology, Circadian Rhythm, Bipolar Disorder, Sleep, Meta Analysis
Introduction
Depression dominates the clinical presentation of bipolar disorders, occupying the vast majority of the syndromal and subsyndromal affective burden of this illness.1 In addition to the symptoms themselves, bipolar depression (BPD) is associated with cognitive impairment, occupational disability, medical morbidity and suicide.2 The treatment options for BPD are limited by suboptimal efficacy, pharmacologic side effects, mood destabilization, and delayed therapeutic response.3 Although pharmacotherapies are the predominant modality of treatment, alternative biological interventions are also available. Chronotherapeutic treatments are a set of interventions that are thought to act on the biological clock. In psychiatry, these interventions are used primarily in the management of affective illness.4
The acute deprivation of sleep to generate an antidepressant response is a chronotherapeutic treatment that has been used for almost fifty years.5 Clinical research on this intervention has explored its use across the depressive spectrum, evaluating its efficacy in unipolar, bipolar and treatment-resistant depressive states. Multiple treatment formats have been employed with variation in the number, timing, and duration of sleep deprivation (SD) cycles administered. Additional complexity pertains to whether SD is used as a monotherapy or as part of a composite intervention including other chronotherapeutic (typically bright light therapy and/or sleep phase advance) or psychopharmacologic components. (This paper will use SD to refer to all treatments that use SD, whether as a monotherapy or when combined with other chronotherapeutic or pharmacologic therapies.) The result of this five decades of clinical research is a small, highly heterogeneous literature with significant variation in diagnostic cohorts, procedural protocols, study design and quality. This literature has been summarized in several earlier, descriptive reviews, typically quoting cumulative acute antidepressant response rates between 40 and 60%.6–8 The validity of these estimates and their relevance for the treatment of BPD is called into question by several points.
First, most of the clinical research on treatments utilizing SD has consisted of non-randomized, uncontrolled trials. A meta-analysis of SD for all depressive subtypes done in 2017 by Boland et al found only six randomized studies out of a total of 66 included reports.9 A more recent systematic review of SD for the treatment of BPD obtained a total of 21 studies, only two of which were controlled, and only one, a randomized controlled trial.10 This low level of research evidence mandates caution in interpreting overall response rates from this outcome literature. A meta-analysis of studies specifically related to BPD could evaluate, clarify, and potentially provide greater empiric support for these initial response estimates.
Second, the earlier descriptive summaries of SD pooled reports across the diagnostic spectrum of depressive disorders. Boland et al.’s9 meta-analysis of SD in the treatment of depression repeated this aggregation of data for both unipolar and bipolar depressives. Further, while earlier reports had suggested greater antidepressant response in bipolar compared to unipolar depression11, Boland et al. reported inferior response in the BPD cohort.9 A meta-analysis of research done only on bipolar depressives could clarify these discrepant findings.
A final factor limiting the applicability of earlier meta-analyses is their restrictive eligibility criteria. Boland et al.9 included only studies in which SD was used without other chronotherapies. Although understandable as an effort to discern the antidepressant efficacy of SD per se, in current practice SD is almost always employed as part of a chronotherapeutic package that typically includes bright light therapy and may or may not also include sleep phase advance.12,13 In the only other meta-analysis of SD in the acute treatment of BPD, Ramirez-Mahaluf et al.14 included only studies in which SD was used along with adjunctive medication. Although valuable as representing more common practice, this design makes it difficult to separate the effects of SD from those of medication. In both meta-analyses, use of restrictive inclusion criteria led to examining only particular, limited ways of implementing SD. There remains a significant need to examine the efficacy of the full scope of SD in BPD, with or without adjunctive medications and whether-or-not as part of a composite chronotherapeutic intervention. In addition to providing a better assessment of the global efficacy of this intervention, such a design will allow statistical comparisons of the efficacy of SD with and without these additional treatments.
This paper has two aims: First, to conduct a meta-analysis of the research literature on SD in the treatment of BPD, either as a monotherapy or as part of a composite intervention. Second, to identify moderator variables that influence the response to SD in this set of studies.
Methods and Materials
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines for systematic reviews and meta‐analyses.15
Eligibility Criteria
Article inclusion criteria were as follows: peer‐reviewed, English‐language publication, adult patients (18 years or older) with a diagnosis of any form of bipolar disorder, treatment for depression with sleep deprivation or wake therapy (both referred to in this paper as sleep deprivation, SD), and treatment response tracked objectively.
Search Strategy
We completed two rounds of comprehensive searches with the same search strategies employed in PubMed, Embase, CENTRAL(Cochrane), and PsycINFO. The first search round had an end date of July 06, 2018, and the second search round covered literature published between July 01, 2018 and February 10, 2020. The following is a sample of the search strategy we used for PubMed: (((“bipolar and related disorders”[MeSH Terms] OR manic[tiab] OR mania[tiab] OR bipolar[tiab] OR affective illness*[tiab] OR affective disorder*[tiab]) AND (“sleep deprivation”[MeSH Terms] OR sleep deprivation[tiab] OR wake sleep[tiab] OR sleep wake[tiab] OR wake therapy[tiab] OR cycle modification[tiab] OR sleep interrupt*[tiab] OR sleep restriction[tiab] OR “circadian rhythm”[MeSH Terms] OR circadian[tiab]) AND (“Chronotherapy”[Mesh] OR “Sleep Phase Chronotherapy”[MeSH Terms] OR treatment*[tiab] OR therap*[tiab] OR chronotherap*[tiab]))).
Data Collection and Extraction
The search yielded a total of 2851 records for the first round and 247 records for the second round (Figure 1). All articles generated by searches were initially screened by the first author (J.G.) for eligibility based on their title and abstract. After reviewing the abstracts, 94 articles were eligible for further review, which was conducted by all authors. Studies were then excluded because: 1) the study did not report outcome results for bipolar depressed patients separate from those for unipolar depressed patients (34 studies); 2) the study sample overlapped with that of a previous study (28 studies); 3) the study did not report data on the number of responders immediately after sleep deprivation treatment (13 studies); 4) we were unable to determine if the study sample overlapped with a previously reported sample (4 studies); or 5) the study did not use an objective method to measure depression outcome (3 studies).
Figure 1.
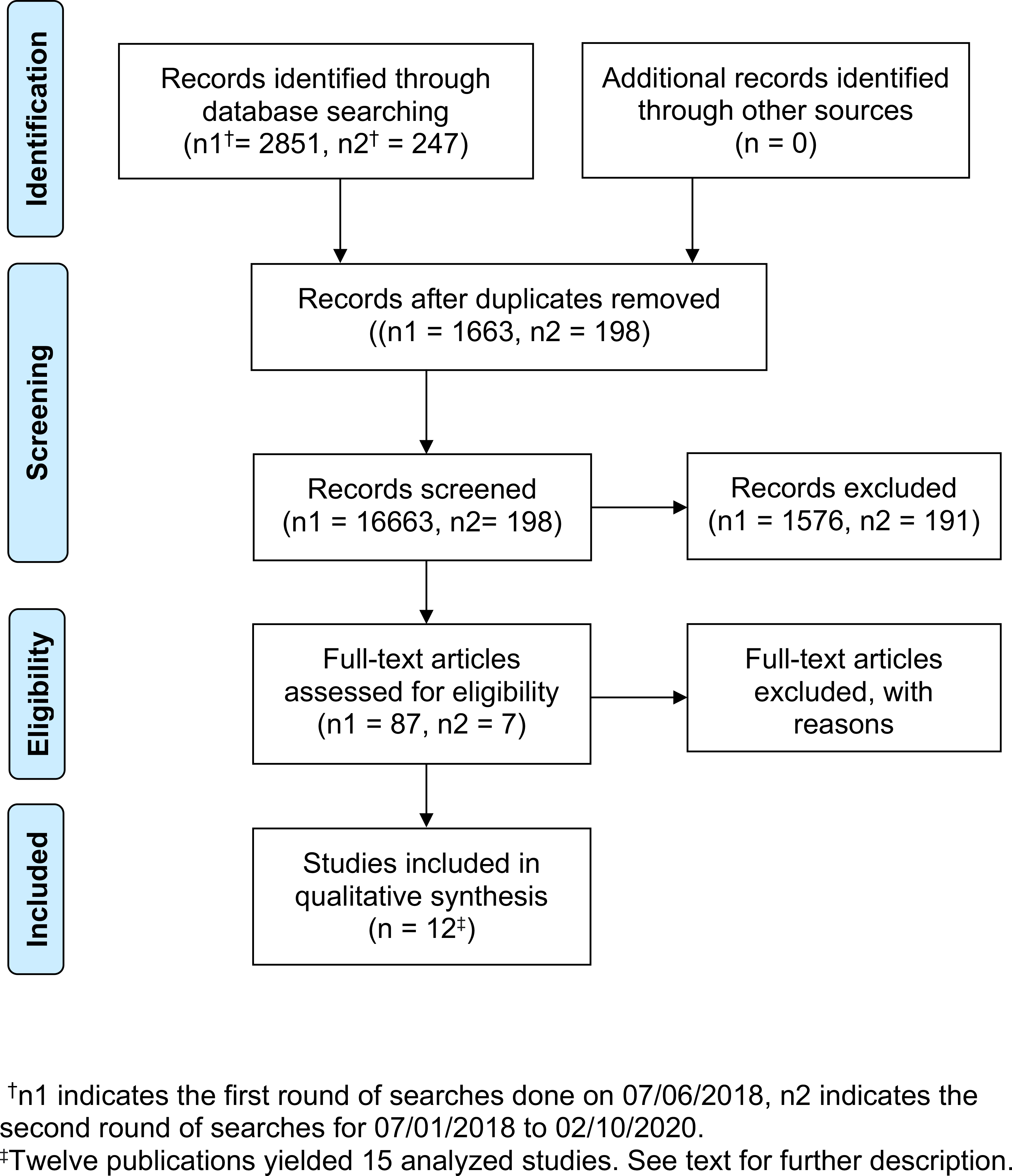
PRISMA Flow Diagram for Meta-Analysis of Sleep Deprivation Studies
The level of evidence of all studies per the 2009 National Health and Mental Research Council criteria of Australia was case study, even for the one randomized controlled trial13 for which only the active, single treatment arm was included to be comparable to data from the other studies. Consequently, the Jadad rating scale,10 which requires a randomized clinical trial, could not be used to assess risk of bias.
One author (S.C.) extracted data using a standard data table (Table 1). If a publication reported on groups of individuals receiving different types of sleep deprivation or different diagnoses or medication status, the groups were treated as separate studies. Thus, although there were 12 publications, there were 15 studies used for the meta-analyses.13,16–26 All authors together reviewed each study to verify its eligibility and the coding of its characteristics. Publication authors were contacted by e-mail if relevant study information (e.g., treatment response, diagnostic group) was not reported or if clarification was needed.
Table 1.
Data Table for Meta-Analysis of Sleep Deprivation Studies in Bipolar Disorder
| Study | N | BP | Interventions | # TSD | Adjunct Treatment | Response Rate | Definition of Response |
|---|---|---|---|---|---|---|---|
| Benedetti et al., 199916† | 20 | BP I | TSD x 3 with presence and ongoing lithium | 3 | lithium | 14/20 | HDRS score <8 at day 10 |
| Benedetti et al., 199916† | 20 | BP I | TSD x 3 with absence of long-term lithium treatment lithium and no psychotropic treatment | 3 | drug-free | 5/20 | HDRS score <8 at day 10 |
| Benedetti et al., 2001b17 | 13 | BP Depression | TSD x 3 with PBO | 3 | drug-free | 5/13 | MADRAS < 6 at day 7 |
| Benedetti et al., 2001b17 | 14 | BP Depression | TSD x 3 with amineptine | 3 | amineptine | 1/14 | MADRAS < 6 at day 7 |
| Benedetti et al., 200518 | 60 | BP I | TSD x 3 + LT (400 lux green light x 30 min at 3 am on TSD night and between 0800 h to 0900 h after recovery sleep) + antidepressants and lithium salts | 3 | LT, antidepressants and lithium salts | 35/60 | ≥ 50% reduction of HDRS between day 1 and day 7 |
| Benedetti et al., 201419 | 143 | BP Depression | TSD x 3 + 30 min LT at 0300 h during each of 3 TSD nights and in the morning during, and for 2 wks after, the wk of TSD. All subjects either started or continued lithium during TSD and LT | 3 | LT + lithium | 99/143 | ≥50% reduction in HDRS-NOW after one wk |
| Kurczewska et al., 201920 | 10 | BP Depression | TSD x 1 + 3d SPA; 1 or more antidepressants and mood-stabilizers | 1 | SPA + medication | 5/10 | ≥ 50% reduction of HDRS on day 14 |
| Larsen et al., 197621 | 3 | BP Depression | TSD x 1 | 1 | drug-free | 2/3 | ≥ 3-point divergence in pre vs. post treatment score |
| Papadimitriou et al.,199322 | 7 | BP Depression | TSD x 2 per wk for 4 wks | 8 | drug-free | 2/7 | ≥50% reduction on HRSD |
| Sikkens et al., 201923 | 10 | BP Depression | TSD x 3 + LT x 10 + continue lithium | 3 | LT + Lithium | 3/10 | ≥ 50% reduction of IDS-C on day 14 |
| Smeraldi et al., 199924 | 20 | BP I | TSD x 3 with PBO | 3 | drug-free | 3/20 | HDRS < 8 at day 10 |
| Smeraldi et al., 199924 | 20 | BP I | TSD x 3 with pindolol | 3 | pindolol | 15/20 | HDRS < 8 at day 10 |
| Souetre et al., 198725 | 5 | BP Depression | Late PSD x1 (sleep from 2300 h to 0200 h) + 13d SPA | 1 | SPA + drug-free | 1/5 | HDRS ≥ 50% reduction or HDRS <8 after 10 days of drug withdrawal |
| Trautmann et al., 201826 | 7 | BP I = 6, BP II = 1 |
TSD x 1 + SPA; medication TAU |
1 | SPA + TAU | 5/7 | ≤ 2 on CGI |
| Wu et al., 200913 | 32 | BP Depression | CAT group: TSD x 1 + LT (3d, 2h 5000lux) and SPA (3 nights) and medication TAU | 1 | SPA + TAU | 19/32 | ≥ 50% reduction of HDRS at day 7 |
Abbreviations
BP: Bipolar
CAT: Chronotherapeutic Augmentation
CGI: Clinical Global Impression
HAM-D, HDRS: Hamilton Depression Rating Scale
HDRS-NOW: Modified 21-item HDRS
HRSD: Hamilton Rating Scale for Depression
IDS-C: Inventory of Depressive Symptomology- Clinical interview
LT: Light Therapy
MADRAS: The Montgomery-Åsberg Depression Rating Scale
PBO: Placebo
PSD: Partial Sleep Deprivation
SPA: Sleep Phase Advance
TAU: Treatment as Usual
TSD: Total Sleep Deprivation
All had 7-day run-in period, with HAM-D score >18 at the end. TSD period ended on day 10 (4 days after treatment).
Data Analyses
The meta-analyses were conducted using Comprehensive Meta-Analysis 3.0 software.27 Because of wide variation in study methods, we used a random model, in which the overall mean response rate is the estimated true average of response rate across the (theoretical) population of studies using various methodologies. The 95% confidence interval provides information on the precision of this estimate (not the variability in response rates). We analyzed the response rate (N responders/N treated) using untransformed rates; results with logistically transformed rates were highly similar. Heterogeneity was assessed using Q and I2 statistics. Publication bias was examined visually using funnel plots.9,28
Results
Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for search and selection of studies. Fifteen studies were eligible for inclusion in the meta-analysis. Two of these were obtained through contacting the authors directly for their pre- and post-treatment outcome data.20,23 Notably, one study25 used late partial sleep deprivation (PSD) exposure with 3h time-in-bed (2300 h-0200 h) instead of total sleep deprivation (TSD); we decided to include this study in the analysis since this duration of PSD has been shown to be equivalent to TSD in terms of therapeutic response.9
Meta-Analyses: Primary Outcomes
Table 1 shows the key characteristics of each of the final included studies. Figure 2 shows results of the meta-analysis: response rate, 95% CI and weight for each study. The overall mean response rate [CI] was 47.6% [36.0, 59.5]. Consistent with the random effects model, there was a significant amount of heterogeneity in response rates beyond that attributable to sampling error (Q(14) = 47.005, p < 0.001): about 70% of the variance in observed response rates reflected variance in true rates rather than variance from sampling error (I2 = 70.216).
Figure 2.
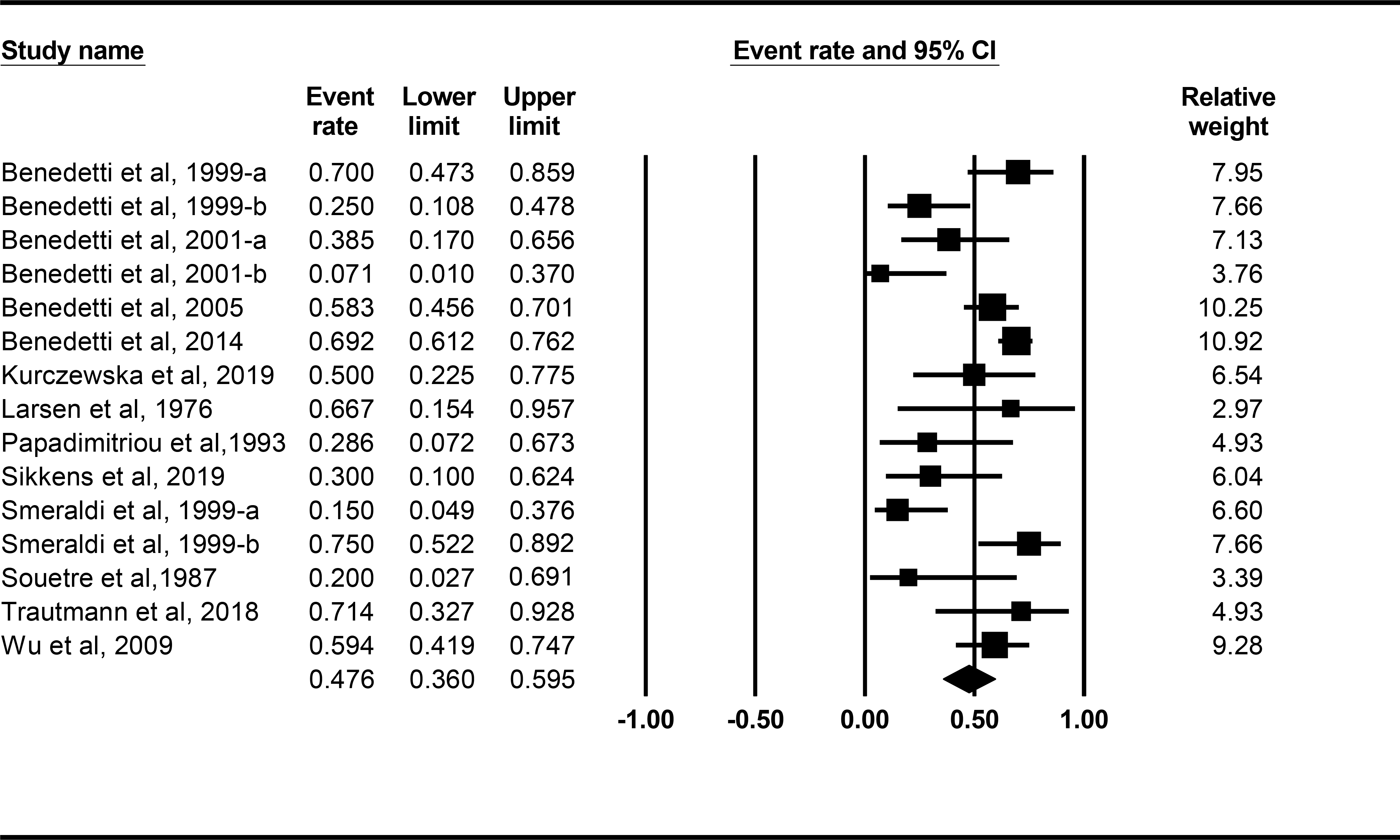
Forest plots from meta-analysis for response rate, 95% CI and weight for each study. Lines represent 95% CI.
Subgroup (Moderator) Analyses
Since our studies varied in methodology, we identified several methodological variables that potentially influenced the response rates and determined different response rates for each of the moderator conditions (Table 2). Response rates were significantly different (Q(1) = 13.899, p < 0.001; Table 2) based on whether patients were also on medications during TSD exposure (Figure 3). The mean [CI] response rate was 59.4% [48.5, 69.5] for the nine studies in which patients were on medication, but only 27.4% [17.8, 39.8] for the six studies in which patients were medication free. Figure 3 shows response rate, 95% CI and weight for each study using medication status as a moderator. The medication-free response rate, which included placebo responding, might be as low as 17.8%, compared to a low of 48.5% for the medication response rate. The medications used in the nine aforementioned studies were as follows: lithium (n = 3), lithium (or “mood stabilizer”) and antidepressant (n = 2), antidepressant (n = 1), pindolol (n = 1), and “treatment as usual” (n = 2). Given the heterogeneity of medications used, no conclusions can be drawn as to whether TSD as an adjunct to a specific medication enhances response rate.
Table 2.
Moderators of Response Rate
| Moderator | Mean Response Rate % [CI] | Test of Group Difference Q(1) |
|---|---|---|
| On Medication (n = 9) | 59.4 [48.5, 69.5] | 13.899, p < 0.001 |
| Off Medication (n = 6) | 27.4 [17.8, 39.8] | |
| Without Adjunct Chronotherapy (n = 8) | 38.7 [20.6, 60.6] | 2.493, p = 0.114 |
| With Adjunct Chronotherapy (n = 7) | 58.3 [47.7, 68.3] | |
| 1 TSD Exposure (n = 5)† | 56.8 [43.4, 69.3] | 1.176, p = 0.278 |
| 3 TSD Exposures (n = 9)‡ | 45.1 [29.9, 61.3] | |
| Response Percentage (n = 7) | 54.3 [42.2, 65.8] | 0.663, p = 0.415 |
| Response Score (n = 8) | 43.6 [23.8, 65.7] | |
| Bipolar I (n = 5) | 48.6 [27.5, 70.3] | 0.021, p = 0.884 |
| Bipolar I and II (n = 10) | 46.6 [32.0, 61.7] | |
| Non-Milan-based Study (n = 7) | 49.5 [36.2, 62.8] | 0.058, p = 0.810 |
| Milan-based Study (n = 8) | 46.8 [30.7, 63.6] |
One study used late PSD exposure with 3h time-in-bed (Souetre et al., 1987)
One study with 8 TSD exposures, a number deemed to be an outlier, was excluded (Papadimitriou et al., 1993)
Figure 3.
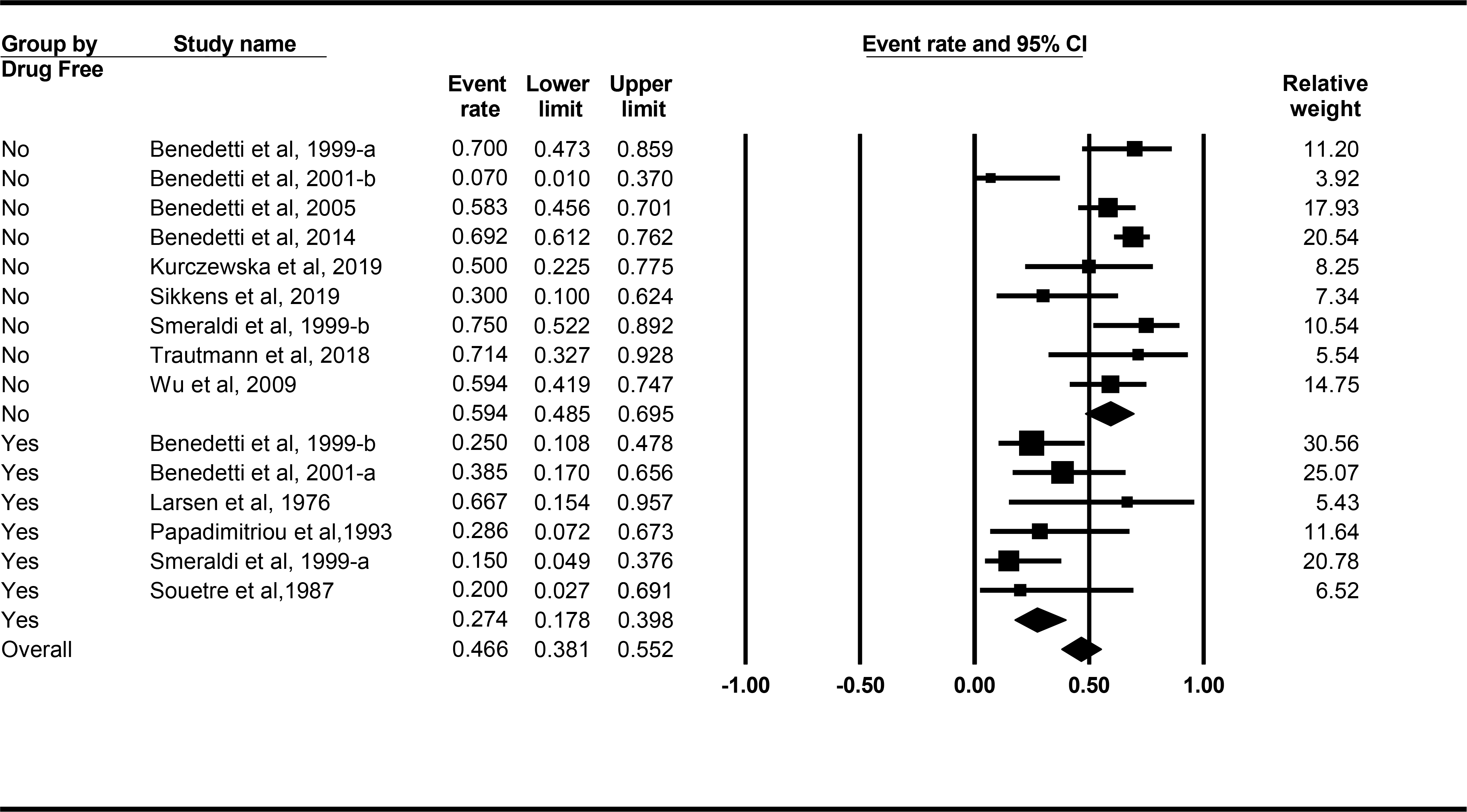
Forest plots from meta-analysis for response rate, 95% CI and weight for each study using medication treatment as a moderator of response rate. Lines represent 95% CI.
Use of adjunctive chronotherapeutic treatment (sleep phase advance or light treatment) during TSD exposure also might be an important moderator of response rate; however, this effect failed to reach statistical significance (Q(1) = 2.493, p = 0.114; Table 2, Supplemental Figure 1): the mean [CI] response rate was 58.3% [47.7, 68.3] for the seven studies in which patients were using adjunctive chronotherapeutic treatment, but only 38.7% [20.6, 60.6] for the eight studies in which patients were not on such treatment. Again, the response rate for SD without adjunctive chronotherapy, which included placebo responding, might be as low as 20.6%, compared to a low of 47.7% for the response rate for SD with adjunctive chronotherapy.
None of the following moderator variables were significantly related to response rate (p > 0.27) (Table 2; Supplemental Figures 2–5): number of TSD exposures (1 vs 3; Q(1) = 1.176, p = 0.278), type of response criterion (percentage vs score; Q(1) = 0.663, p = 0.415), bipolar subtype (Bipolar I only vs Bipolar I and Bipolar II; Q(1) = 0.021, p = 0.884) or whether studies were from the Milan group, which conducted 8 of the 15 studies (53.3%) (Q(1) = 0.058, p = 0.810).
Supplemental Figure 6 shows the unadjusted funnel plot of standard errors by effect size estimates used to examine the possibility of publication bias — that not all completed studies are published and the publication selection process is not random. Visual inspection of the funnel plot indicated some asymmetry, suggesting a possible potential for publication bias, with the bottom of the plot showing a higher concentration of studies on the left compared to the right side of the mean effect size. This implies that smaller studies may have been more likely to be published if they have larger than average effects, possibly inflating the meta-analysis response rate estimate.
Discussion
SD is a therapeutic intervention with a long history of use as an antidepressant, significant variation in administration format, and efficacy supported by a low level of empirical evidence. Its use has been studied across the depressive spectrum but the applicability of these general findings to the treatment of BPD had not been specifically assessed. This paper reports on the first meta-analysis of SD in the acute treatment of BPD that has assessed the full range of treatment protocols used in this intervention. As such, it presents an assessment of the global efficacy of this antidepressant intervention for this diagnostic group.
With 15 studies covering 384 patients, the major findings of this comprehensive meta-analysis are as follows. The overall, mean response rate of BPD to SD was 47.6% (CI 36.0%, 59.5%). Because these were uncontrolled trials, this figure is not relative to a placebo response rate but presumably includes both SD-specific and placebo-based components. The placebo response might contain both general, non-specific elements that would be found in all clinical trials, such as the expectation of helpfulness or mobilization of hope, along with possible SD-specific factors including the use of an elaborate procedure with specific schedules and temporally prescribed activities. This response rate of 47% reinforces and strengthens earlier assertions of efficacy that were based on qualitative reviews of the literature6–8 or meta-analyses conducted on all depressive disorders.9 This meta-analytic endorsement provides new, level II evidence of efficacy of SD in the acute treatment of BPD and argues for its reclassification as a first line management option per ISBD/CANMAT guidelines.29
In addition, there was significant heterogeneity in response rates across the 15 studies. Of the potential moderating variables that may have contributed to this heterogeneity, only the presence or absence of adjunctive pharmacotherapy reached statistical significance, with the mean response rate of studies using adjunctive medication being more than twice that of studies not using adjunctive medication (59.4% vs. 27.4%). There also was a notable difference in the response rates between the seven studies that employed adjunctive chronotherapy (58.3%) and the eight studies that did not (38.7%), although this difference was not statistically significant, possibly due to the small sample size. Differences between other moderating variables subgroups were small and not statistically significant: number of TSD cycles administered (1 vs. 3), method of response measurement (percentage change vs criterion score), bipolar subtype (I vs. I and II), and the institutional site of the studies (Milan vs non-Milan).
The findings regarding adjunctive medication use are noteworthy. Our results comport well with the conclusions of Ramirez-Mahaluf et al. who found significant differences in efficacy between TSD monotherapy vs TSD plus medications.14 The low response rates for TSD without adjunctive pharmacotherapy obtained in this meta-analysis (mean = 27.4% [17.8%, 39.8%]) raise an important question about whether TSD used alone is a viable therapeutic option. This result supports current practice standards that recommend the use of mood-stabilizing medication, especially lithium, for bipolar patients being treated with TSD.4 While the use of adjunctive chronotherapy did not reach statistical significance as a moderating variable, this result is possibly due to inadequate power, and thus should be taken simply as a lack of evidence for a difference in response rate between TSD with vs without this add-on therapy.
Confidence in the findings of this meta-analysis is limited by several factors. Conclusions about the true causal effect of SD are tempered by the analysis including only trials without controls (because of their near absence in the literature.) The power of the meta-analysis was relatively low due to the relatively small number of studies that met eligibility criteria (N = 15) and the small sample sizes of these studies (median N = 14, range 3 to 143); however, despite this, the main outcome variable of response rate was still statistically significant. The substantial heterogeneity in response rates (due at least in part to the variations in procedures of the studies), as well as the small number of studies and sample sizes, resulted in wide confidence intervals around estimates. The use of larger, controlled studies with standardized administration protocols would address these shortcomings. While the use of pharmacotherapy was a significant moderator of response, there were not enough studies using the same type of medication to determine whether some medications had more pronounced effects on efficacy than others. Finally, the funnel plot suggested some degree of publication bias, which is a limitation to this field of research in general.9
In addition to conducting placebo-controlled trials with larger, randomized samples and standardized administration formats, additional refinements could further advance this field of research. While lithium was the drug most frequently used in the current sample of studies, future trials comparing one SD-pharmacotherapy combination to another employing other first-line medications for BPD (e.g., SD plus lithium vs SD plus lamotrigine vs SD plus quetiapine) would be informative. In addition, identification of patient characteristics that may moderate outcome (e.g., chronobiological variables30,31) would enable a more customized, precision medicine approach to this treatment.
In conclusion, this meta-analysis demonstrated acute antidepressant response rates in BPD of almost 50%, thereby strengthening estimates from earlier qualitative reviews or extrapolations from meta-analyses conducted on patients with both unipolar and bipolar depression. This study also documented that response rates are higher when SD is accompanied by adjunctive pharmacotherapy. The results of this meta-analysis should inform future treatment guidelines for the acute management of BPD.
Supplementary Material
Summation.
In this first meta-analysis of sleep deprivation in the acute treatment of bipolar depression, which included all formats of the procedure, the overall response rate of almost 50% strengthens earlier efficacy estimates and supports reappraisal of the status of this intervention in treatment guidelines for this condition.
The only moderator variable that reached statistical significance was the use or lack of use of adjunctive pharmacotherapy. The difference in response rates on this variable (59.4 vs 27.4%) suggests that sleep deprivation should routinely be used with adjunctive pharmacotherapy.
Limitations.
The meta-analysis is limited by a relatively low number of eligible studies (15), sample sizes that were small, and a literature that was almost entirely composed of uncontrolled studies.
Acknowledgements
Partial support for this meta-analysis was provided by National Aeronautics and Space Administration (NASA) NNX14AN49G and grant 80NSSC20K0243 (to N.G.), and National Institutes of Health grant NIH R01DK117488 (to N.G.). J.F.G., S.C. and M.Y. received no funding support for this paper. None of the sponsors had any role in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Statement
None declared.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Judd LL, Schettler PJ, Akiskal HS, et al. Long-term symptomatic status of bipolar I vs. bipolar II disorders. International Journal of Neuropsychopharmacology. 2003;6(2):127–137. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Current Medical Research and Opinion. 2019:1–13. [DOI] [PubMed] [Google Scholar]
- 3.Frye MA, Prieto ML, Bobo WV, et al. Current landscape, unmet needs, and future directions for treatment of bipolar depression. J Affect Disord. 2014;169 Suppl 1:S17–23. [DOI] [PubMed] [Google Scholar]
- 4.Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for Affective Disorders: A Clinician’s Manual for Light And Wake Therapy. Karger; 2013. [DOI] [PubMed] [Google Scholar]
- 5.Pflug B, Tolle R. [Therapy of endogenous depressions using sleep deprivation. Practical and theoretical consequences]. Nervenarzt. 1971;42(3):117–124. [PubMed] [Google Scholar]
- 6.Wu J, Bunney W. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. American Journal of Psychiatry. 1990;147(1):14–21. [DOI] [PubMed] [Google Scholar]
- 7.Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go? Biological Psychiatry. 1999;46(4):445–453. [DOI] [PubMed] [Google Scholar]
- 8.Hemmeter U-M, Hemmeter-Spernal J, Krieg J-C. Sleep deprivation in depression. Expert Review of Neurotherapeutics. 2010;10(7):1101–1115. [DOI] [PubMed] [Google Scholar]
- 9.Boland EM, Rao H, Dinges DF, et al. Meta-Analysis of the Antidepressant Effects of Acute Sleep Deprivation. J Clin Psychiatry. 2017:e1020–e1034. [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb JF, Benedetti F, Geoffroy PA, et al. The chronotherapeutic treatment of bipolar disorders: A systematic review and practice recommendations from the ISBD task force on chronotherapy and chronobiology. Bipolar Disord. 2019;21(8):741–773. [DOI] [PubMed] [Google Scholar]
- 11.Barbini B, Colombo C, Benedetti F, Campori E, Bellodi L, Smeraldi E. The unipolar-bipolar dichotomy and the response to sleep deprivation. Psychiatry Research. 1998;79(1):43–50. [DOI] [PubMed] [Google Scholar]
- 12.Benedetti F Antidepressant chronotherapeutics for bipolar depression. Dialogues Clin Neurosci. 2012;14(4):401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu JC, Kelsoe JR, Schachat C, et al. Rapid and Sustained Antidepressant Response with Sleep Deprivation and Chronotherapy in Bipolar Disorder. Biological Psychiatry. 2009;66(3):298–301. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez-Mahaluf JP, Rozas-Serri E, Ivanovic-Zuvic F, Risco L, Vöhringer PA. Effectiveness of Sleep Deprivation in Treating Acute Bipolar Depression as Augmentation Strategy: A Systematic Review and Meta-Analysis. Frontiers in Psychiatry. 2020;11(70). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benedetti F, Colombo C, Barbini B, Campori E, Smeraldi E. Ongoing lithium treatment prevents relapse after total sleep deprivation. Journal of Clinical Psychopharmacology. 1999;19(3):240–245. [DOI] [PubMed] [Google Scholar]
- 17.Benedetti F, Campori E, Barbini B, Fulgosi MC, Colombo C. Dopaminergic augmentation of sleep deprivation effects in bipolar depression. Psychiatry Research. 2001;104(3):239–246. [DOI] [PubMed] [Google Scholar]
- 18.Benedetti F, Barbini B, Fulgosi MC, et al. Combined total sleep deprivation and light therapy in the treatment of drug-resistant bipolar depression: acute response and long-term remission rates. Journal of Clinical Psychiatry. 2005;66(12):1535–1540. [DOI] [PubMed] [Google Scholar]
- 19.Benedetti F, Riccaboni R, Locatelli C, Poletti S, Dallaspezia S, Colombo C. Rapid treatment response of suicidal symptoms to lithium, sleep deprivation, and light therapy (chronotherapeutics) in drug-resistant bipolar depression. J Clin Psychiatry. 2014;75(2):133–140. [DOI] [PubMed] [Google Scholar]
- 20.Kurczewska E, Ferensztajn-Rochowiak E, Jasińska-Mikołajczyk A, Chłopocka-Woźniak M, Rybakowski JK. Augmentation of Pharmacotherapy by Sleep Deprivation with Sleep Phase Advance in Treatment-Resistant Depression. Pharmacopsychiatry. 2019;52(4):186–192. [DOI] [PubMed] [Google Scholar]
- 21.Larsen JK, Lindberg ML, Skovgaard B. Sleep deprivation as treatment for endogenous depression. Acta Psychiatrica Scandinavica. 1976;54(3):167–173. [DOI] [PubMed] [Google Scholar]
- 22.Papadimitriou GN, Christodoulou GN, Katsouyanni K, Stefanis CN. Therapy and prevention of affective illness by total sleep deprivation. J Affect Disord. 1993;27(2):107–116. [DOI] [PubMed] [Google Scholar]
- 23.Sikkens D, Riemersma - Van der Lek RF, Meesters Y, Schoevers RA, Haarman BCM. Combined sleep deprivation and light therapy: Clinical treatment outcomes in patients with complex unipolar and bipolar depression. Journal of Affective Disorders. 2019;246((Sikkens D.; Riemersma - Van der Lek R.F.; Meesters Y.; Schoevers R.A.; Haarman B.C.M., b.c.m.haarman@rug.nl) University of Groningen, University Medical Center Groningen, Department of Psychiatry, CC44,P.O. Box 30.001, 9700 RB, Groningen, Netherlands):727–730. [DOI] [PubMed] [Google Scholar]
- 24.Smeraldi E, Benedetti F, Barbini B, Campori E, Colombo C. Sustained Antidepressant Effect of Sleep Deprivation Combined with Pindolol in Bipolar Depression: A Placebo-Controlled Trial. Neuropsychopharmacology. 1999;20(4):380–385. [DOI] [PubMed] [Google Scholar]
- 25.Souetre E, Salvati E, Pringuey D, Plasse Y, Savelli M, Darcourt G. Antidepressant effects of the sleep/wake cycle phase advance : Preliminary report. Journal of Affective Disorders. 1987;12(1):41–46. [DOI] [PubMed] [Google Scholar]
- 26.Trautmann N, Foo JC, Frank J, et al. Response to therapeutic sleep deprivation: a naturalistic study of clinical and genetic factors and post-treatment depressive symptom trajectory. Neuropsychopharmacology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borenstein M, Hedges L, Higgins J, & Rothstein H Comprehensive Meta-Analysis Version 3. Englewood, NJ: Biostat; 2013. [Google Scholar]
- 28.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 29.Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disorders. 2018:n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dallaspezia S, Suzuki M, Clara L, Colombo C, Benedetti F. Chronotype influences response to antidepressant chronotherapeutics in bipolar patients. Chronobiology international. 2018;35(9):1319–1325. [DOI] [PubMed] [Google Scholar]
- 31.Romo-Nava F, Blom TJ, Cuellar-Barboza AB, et al. Evening chronotype as a discrete clinical subphenotype in bipolar disorder. Journal of Affective Disorders. 2020;266:556–562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


