Abstract
Background
Anterior uveitis (AU) is the most frequent extra-articular feature of axial spondyloarthritis (axSpA). We aimed to assess and compare the incidence of AU in axSpA patients treated with anti-TNF or anti-IL17A.
Methods
We systematically reviewed PubMed, EMBase, and Cochrane from inception to May 3, 2020, and searched for placebo-controlled and head-to-head randomized controlled trials (RCTs) assessing anti-TNF monoclonal antibodies (mAb) or soluble receptor fusion protein or anti-IL17A in patients with axSpA according to ASAS criteria and reporting safety data on AU. Data were extracted following a predefined protocol. We did pairwise and network meta-analyses for the primary outcome of AU flares (relapse or de novo) incidence and estimated summary odds ratios (ORs). We assessed the quality of evidence using the Cochrane risk-of-bias 2.0 tool. We ranked treatments according to their effectiveness in preventing AU flare using the P-score.
Results
We identified 752 citations and included 33 RCTs, comprising 4544 treated patients (anti-TNF mAb 2101, etanercept [ETN] 699, anti-IL17A 1744) and 2497 placebo-receiving patients. Incidence of uveitis was lower with anti-TNF mAb versus placebo (OR = 0.46; CI 95% [0.24; 0.90]) and versus anti-IL17A (OR = 0.34; CI 95% [0.12; 0.92]. According to the P-score, the ranking from the most to the least preventive treatment of uveitis flare was as follows: anti-TNF mAb, ETN, placebo, and anti-IL17A.
Conclusion
In RCTs assessing anti-TNF and anti-IL17A in axSpA, incident uveitis are rare events. However, this network meta-analysis demonstrates that anti-TNF mAb are associated with a lower incidence of uveitis compared to placebo and anti-IL17A.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13075-021-02549-0.
Keywords: Axial spondyloarthritis, Meta-analysis, Anti-TNF, Anti-IL17A, Uveitis
Background
Axial spondyloarthritis [axSpA] is a chronic inflammatory disease that mainly affects the axial skeleton. AxSpA can be classified into two subgroups: ankylosing spondylitis [AS] and non-radiographic axSpA [nr-axSpA] [1, 2]. The most frequent extra-articular manifestation associated with axSpA is acute anterior uveitis [AAU] which occurs in 23% – 33% of patients with AS [3, 4], and in 16% of patients with nr-axSpA [3].
Biologics therapy has dramatically changed the management of axSpA and its associated extra-articular manifestations. Two classes of biologics have demonstrated their efficacy for the treatment of axSpA: the tumor necrosis factor inhibitors [anti-TNF] including four anti-TNF monoclonal antibodies [mAb], infliximab [IFX], adalimumab [ADA], golimumab [GOL], and certolizumab [CTZ], and one soluble receptor fusion, etanercept [ETN], and the interleukin-17A inhibitors [anti-IL17A] including secukinumab [SCK] and ixekizumab [IXE].
The efficacy of biologics on axSpA-associated AAU is less well known. Among anti-TNF mAb, ADA has been shown to significantly reduce the risk of non-infectious uveitic flare, associated or not with axSpA, in two placebo-controlled RCTs [5, 6]. Several observational studies and post-hoc analysis from placebo-controlled studies focused on axSpA-associated AAU and showed that the incidence of AAU decreases after treatment with anti-TNF mAb compared to the incidence before treatment [7–13]. These studies mainly involved IFX and ADA. However, a meta-analysis of 8 RCTs concluded that anti-TNF mAb were not associated with fewer AAU flares, including relapses and new onset, than placebo [14].
The effect of receptor fusion proteins including ETN on AAU associated with axSpA is even more unclear. The before/after treatment rates of AAU in 1365 patients with AS from the Swedish biologics register concluded to a significant increase with ETN [9], also other observational studies did not observe any difference [7, 15]. At the opposite, Wu et al. reported a preventive effect of ETN for flares [relapse or new onset] of uveitis in AS patients in their meta-analysis [14].
Data is scarce on anti-IL17A on axSpA-associated AAU or HLA-B27-associated AAU. Two randomized, double-blinded, placebo-controlled studies assessing the efficacy of SCK in non-infectious non-Behcet’s uveitis showed no difference in the recurrence rate or in reducing intraocular inflammation versus placebo [16]. A pooled data analysis of three RCTs reviewed 794 patients with AS, of whom 135 had uveitis history, and reported an exposure-adjusted incidence rate of 0.03 per 100 patients-years [17]. The same type of pooled analysis of the COAST-V and COAST-W studies was performed on data from 641 patients with AS who received at least one injection of ixekizumab [18]. Across both studies, 20.1% of patients had a history of AAU, and 20 reported AAU flare, 15 of those had a prior history of AAU. The exposure-adjusted rate of AAU was 3.9 per patients-years.
Thus, association between biologic in axSpA and AU manifestations remains poorly defined. Therefore, we aimed to conduct a systematic review and a pairwise and a network meta-analysis to help clinical practice by comparing different biologics on their protecting effect on AU flares.
Methods
Data sources and search strategy
A systematic search of the literature was conducted in MEDLINE [via PubMed], EMBase, and the Cochrane Library from inception to May 3, 2020.
We used the following search strategy in PubMed: [“spondylitis, ankylosing” [MeSH Terms] OR spondylart*[tiab] OR spondyloa*[tiab]] AND [“infliximab” [MeSH Terms] OR “certolizumab pegol” [MeSH Terms] OR “etanercept” [MeSH Terms] OR “adalimumab” [MeSH Terms] OR “golimumab” [tw] OR “secukinumab” [tw] OR “ixekizumab” [tw] OR “infliximab” [tw] OR “certolizumab” [tw] OR “etanercept” [tw] OR “adalimumab” [tw]] AND “randomized”] and the following search strategy in EMBase: [“ankylosing spondylitis”/exp OR “axial spondylarthritis”/exp OR “spondylarthritis”/exp] AND [“etanercept”/exp OR “infliximab”/exp OR “adalimumab”/exp OR “golimumab”/exp OR “certolizumab pegol”/exp OR certolizumab OR “secukinumab”/exp OR “ixekizumab”/exp] AND [“randomized controlled trial”/de].
Our search concerned articles published in English. A hand search was also performed. Finally, we collected data from electronic abstract databases of the annual scientific meetings of the EUropean League Against Rheumatism Rheumatology congress and the American College of Rheumatology from 2016 to 2019.
Study selection
Two authors [DR, MB] independently determined the eligibility of the studies after reading title, keywords and abstract. Discrepancy was resolved by consensus.
We pre-specified the target population, interventions, comparators, outcome measures of interest, and timing, following the PICOTs framework.
Inclusion criteria for full text were: 1) RCT published in English before May 3, 2020; 2) comparing the efficacy of any anti-TNF or anti-IL17A versus a comparator (placebo or another active treatment); 3) in a study population of patients with axSpA according to the Assessment of SpondyloArthritis International Society (ASAS) [19] or modified New York (mNY) criteria [20]; 4) with data provided on the number of AU occurring during the controlled period in the safety chapter.
We applied the following exclusion criteria in a sequential order: (1) duplicates (between 2 electronic databases, or in a same electronic database but between 2 different journals), (2) language not English, (3) off topic, (4) design other than RCT, (5) population (not axSpA, or patients under the age of 18, or wrong classification criteria), (6) inadequate comparison, and (7) inadequate safety data reporting (adverse events had to be noticed during the controlled period, separately from the open-label extension).
We considered all five currently available anti-TNF (ADA, ETN, IFX, GOL, CTZ) and two anti-IL17A (SCK, IXE). We included open-labeled controlled studies only if they included an initial double-blind period with detailed safety analysis during this period.
Data extraction and study quality assessment
Two investigators (DM, MB) independently extracted all data using a standardized spreadsheet and assessed the quality of evidence using the Cochrane risk-of-bias (RiOB) 2.0 tool. Discrepancy was resolved by consensus. For each article, we collected according a pre-specified strategy the following information: age and gender, disease symptoms duration, percentage of history of uveitis, concomitant use of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), corticosteroids or NSAIDs at baseline, study design, inclusion criteria, dosage and schedule of the treatment, duration of the RCT and/or of the double-blind period, and sample size. For all extracted data, a central value (mean or median) and variability (standard deviation or interquartile range) were collected.
Outcomes
Our primary outcome was the AU flares incidence, including relapses and new onsets. We collected the number of AU for each clinical trial and each group of treatment and control, by taking into account the safety population. We took into account the terms “uveitis” and “iritis” for AU events. For the studies including a double-blind period and an open-labeled period, only the data from the double-blind period were extracted.
Statistical analyses
Pairwise meta-analysis
All pairwise meta-analyses were performed using the inverse variance approach, which assumes a fixed effect model, to determine the weight given to each study [21], with a direct comparison of the risk of AAU events with each biologic treatment, categorized by anti-TNF mAb, ETN, anti-IL17A and placebo. We also conducted supplementary subgroups analysis: a) According to the axSpA phenotype: RCTs with only patients with AS according to the mNY criteria versus RCTs including both nr-axSpA and AS patients according to the ASAS criteria; b) According to the disease duration: RCTs with only early axSpA, i.e. < 5 years since onset of symptoms, versus RCTs with non-early axSpA; c) According to the trial quality: RCTs with overall low risk of bias using the Cochrane RiOB 2.0 Tool versus RCTs with moderate or high risk of bias and d) According to the focus on AAU: RCTs with mentioned AAU history versus RCTs without detailed information on AAU history.
We measured heterogeneity across studies using the Cochran’s Q test, and I2 statistic, with higher values reflecting increasing heterogeneity. We assessed publication bias by examining funnel plots and using the Egger’s regression asymmetry test.
The statistical analysis was performed with Comprehensive Meta-Analysis Version 3 [22].
Network meta-analysis
We also conducted a network meta-analysis to perform an adjusted indirect comparison of the investigational treatment arms categorized in 3 different groups of treatment (anti-TNF mAb, ETN and anti-IL17A). The results of these meta-analyses are the odds ratio (OR) of AU events between treatments (i.e., anti-TNF mAb, ETN and anti-IL17A and placebo) with their 95% confidence interval and the statistical significance level of the comparison. The homogeneity and consistency assumption was based on a generalized Cochran’s Q statistic for multivariate meta-analysis. We ranked the efficacy of the 3 different groups of treatment and placebo, using P-scores that measure the mean extent of certainty that a treatment is better than the competing treatment. In our study, the higher the P-score, the more effective the treatment was in preventing AU flares. The assessment of publication bias was made using funnel plots in this multiple treatment comparison.
The statistical analysis was performed using R statistical packages (version 3.2.4) and the meta-library, Netmeta [20].
Results
Eligible studies
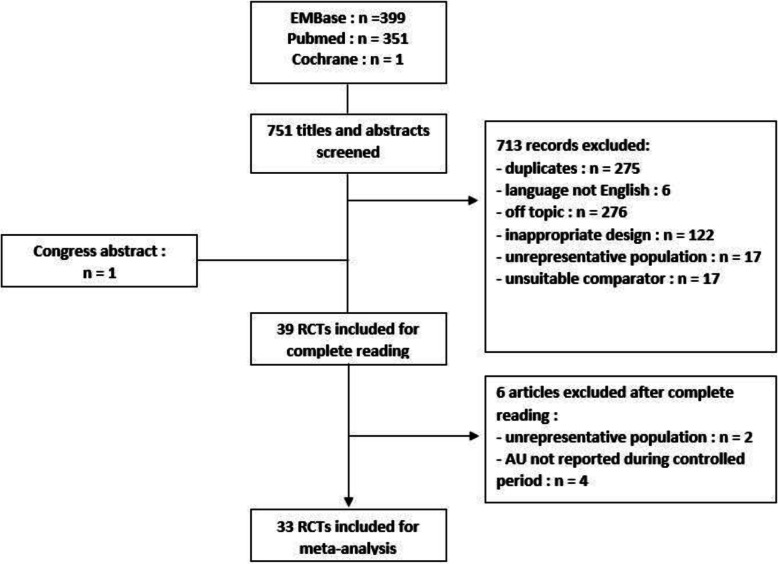
We identified 751 citations after search in the 3 databases plus one more after search in the congress abstract databases (Fig. 1). After reading titles and abstracts, we excluded 713 abstracts, mainly because of duplicates or off topic studies. After the complete reading, we excluded 6 articles: 4 because AU were not described during the controlled period and 2 for an unrepresentative population. Finally, we included 33 RCTs (Figs. 1 and 2), allowing 35 comparisons, comprising 4544 patients with axSpA treated with a biologic treatment and 2497 treated with placebo.
Fig. 1.
Study selection process (flowchart). RCT, randomized controlled trial; AU, anterior uveitis
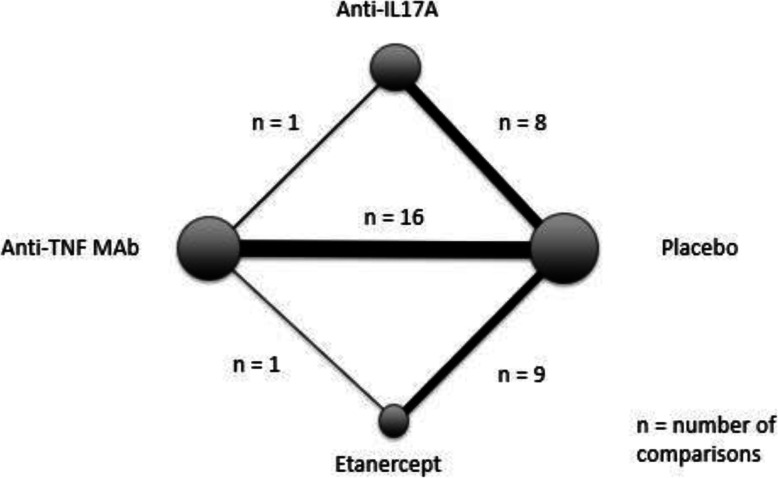
Fig. 2.
Network configuration of eligible comparisons. Nodes represent each intervention with a size proportional to the number of patients receiving treatment. Lines represent direct comparisons; the more patients involved in comparison, the thicker the line. TNF, tumor necrosis factor; mAb, monoclonal antibody; ETN, etanercept; IL17A, interleukin-17A
Study characteristics
The Additional file 1 provides detailed characteristics of the 33 RCTs included in the analyses. A comparison of a biologic treatment versus placebo was performed in 32 placebo-controlled RCTs [23–55], including one RCT comparing 2 biologic treatments, i.e. IXE and ADA, versus placebo [26], and in a head-to-head RCT comparing IFX versus ETN [39]. Anti-TNF mAb were assessed in 17 RCTs (ADA: 4 [23–26]; CTZ: 2 [27, 28]; GOL: 4 [29–32]; IFX: 7 [33–39]), ETN was assessed in 10 RCTs [39–48] and anti-IL17A were assessed in 8 RCTs (SCZ: 5 [49–53]; IXE: 3 [26, 54, 55]. The mean duration of the controlled period was 22.7 weeks ±18.5 (SD), median: 16 weeks (range: 6–104 weeks).
According to the Cochrane RiOB 2.0 tool, 16 RCTs had a low risk of bias, 17 RCTs presented some concerns, and none had a high risk of bias (Additional file 2).
Characteristics of the patients with axSpA
The main characteristics of the intention-to-treat (ITT) population are summarized in Table 1. Because our objective was to collect the AU flares, we included in the analysis the safety populations. A total of 7041 patients were included in the analysis, of whom 2497 received a placebo, 2101 were treated with an anti-TNF mAb, 699 with ETN, and 1744 with an anti-IL17A, with a follow-up of 3264 patient years. The total cumulative exposure under active treatment was 2265 patient years.
Table 1.
AAU Flare reported in the included RCTs
| Study (ref) | Treatment | Placebo | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Study duration (weeks) | Patients (n) | Uveitis (n) | Incidence (per 100 PY) | Patients (n) | Uveitis (n) | Incidence (per 100 PY) | |
| Van der Heijde D et al. 2006 [23] | ADA | 24 | 208 | 0 | 0.00 | 107 | 0 | 0.00 |
| Sieper J et al. 2012 [24] | ADA | 12 | 95 | 0 | 0.00 | 97 | 0 | 0.00 |
| Huang F et al. 2013 [25] | ADA | 12 | 229 | 0 | 0.00 | 115 | 0 | 0.00 |
| Landewé R et al. 2013 [27] | CTZ | 24 | 218 | 2 | 1.98 | 107 | 3 | 6.07 |
| Deodhar A et al. 2019 [28] | CTZ | 52 | 159 | 4 | 2.51 | 158 | 8 | 5.06 |
| Inman R et al. 2008 [29] | GOL | 24 | 278 | 0 | 0.00 | 77 | 0 | 0.00 |
| Deodhar A et al. 2017 [32] | GOL | 16 | 105 | 0 | 0.00 | 103 | 2 | 6.31 |
| Sieper J et al. 2015 [31] | GOL | 16 | 97 | 0 | 0.00 | 100 | 0 | 0.00 |
| Bao C et al. 2014 [30] | GOL | 24 | 169 | 1 | 1.28 | 44 | 0 | 0.00 |
| Van der Heijde D et al. 2005 [34] | IFX | 18 | 202 | 0 | 0.00 | 75 | 0 | 0.00 |
| Barkham N et al. 2009 [36] | IFX | 16 | 20 | 0 | 0.00 | 20 | 0 | 0.00 |
| Inman R et al. 2010 [37] | IFX | 12 | 39 | 0 | 0.00 | 37 | 0 | 0.00 |
| Marzo-Ortega H et al. 2005 [35] | IFX | 30 | 28 | 1 | 6.19 | 14 | 0 | 0.00 |
| Sieper J et al. 2012 [38] | IFX | 28 | 105 | 0 | 0.00 | 52 | 0 | 0.00 |
| Braun J et al. 2002 [33] | IFX | 12 | 34 | 1 | 12.74 | 35 | 3 | 37.14 |
| Gorman JD et al. 2002 [40] | ETN | 16 | 20 | 0 | 0.00 | 20 | 0 | 0.00 |
| Davis JC et al. 2003 [41] | ETN | 24 | 138 | 3 | 4.71 | 139 | 8 | 12.47 |
| Brandt J et al. 2003 [42] | ETN | 6 | 14 | 0 | 0.00 | 16 | 0 | 0.00 |
| Calin A et al. 2004 [43] | ETN | 12 | 45 | 0 | 0.00 | 39 | 0 | 0.00 |
| Van der Heijde D et al. 2006 [44] | ETN | 12 | 305 | 0 | 0.00 | 51 | 1 | 8.49 |
| Barkham N et al. 2010 [45] | ETN | 12 | 15 | 0 | 0.00 | 17 | 0 | 0.00 |
| Dougados M et al. 2011 [46] | ETN | 12 | 39 | 0 | 0.00 | 43 | 0 | 0.00 |
| Dougados M et al. 2014 [48] | ETN | 8 | 42 | 0 | 0.00 | 48 | 0 | 0.00 |
| Dougados M et al. 2014 [47] | ETN | 12 | 106 | 0 | 0.00 | 109 | 0 | 0.00 |
| Deodhar A et al. 2019 [54] | IXE | 16 | 212 | 5 | 7.66 | 104 | 0 | 0.00 |
| Van der Heijde D et al. 2018 [26] | IXE | 16 | 164 | 1 | 1.98 | 86 | 0 | 0.00 |
| Deodhar A et al. 2019 [55] | IXE | 52 | 198 | 3 | 2.09 | 104 | 2 | 1.92 |
| Baeten D et al. 2013 [49] | SEC | 28 | 24 | 0 | 0.00 | 6 | 0 | 0.00 |
| Baeten D et al. 2015 [50] | SEC | 16 | 394 | 7 | 5.77 | 196 | 2 | 3.31 |
| Pavelka K et al. 2017 [51] | SEC | 16 | 150 | 0 | 0.00 | 75 | 0 | 0.00 |
| Kivitz A et al. 2018 [52] | SEC | 16 | 233 | 0 | 0.00 | 117 | 0 | 0.00 |
| Deodhar A et al. 2019 [53] | SEC | 52 | 369 | 7 | 1.21 | 186 | 2 | 1.07 |
| Giardina A et al. 2010 [39] | IFX | 104 | 25 | 1 | 2.00 | - | - | - |
| ETN | 25 | 2 | 4.00 | - | - | - | ||
The different treatment groups were similar in terms of gender, age, duration of symptoms, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) at baseline, concomitant csDMARD, non-steroidal anti-inflammatory drugs (NSAIDs), and corticosteroids intake. AU and inflammatory bowel disease (IBD) history were less frequent in the ETN and anti-IL17A groups than in the anti-TNF mAb.
Annual Incidence of AU
A total of 69 AU flares (de novo or relapses) was reported during the controlled periods: 31 among placebo-treated patients, 10 among anti-TNF mAb-treated patients (CTZ 6, IFX 3, and GOL 1), 5 among ETN-treated patients, and 23 among anti-IL17A-treated patients (SCK 14 and IXE 9) (Table 1). Crude annual incidence of AU was 1.06%, 2.14%, 2.11%, and 3.10%, in the anti-TNF mAb, ETN, anti-IL17A, and placebo groups, respectively.
Pairwise meta-analysis
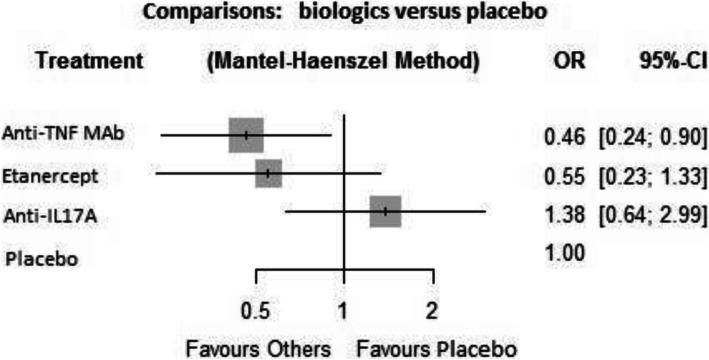
The AU incidence reported in patients with axSpA treated with anti-TNF mAb was significantly lower than with placebo (OR = 0.499, CI 95% [0.256–0.973] p = 0.041). There was no significant difference in AU incidence between ETN (OR = 0.499, CI 95% [0.198–1,259] p = 0.141) or anti-IL17A (OR = 1,345, CI 95% [0.465–3,886] p = 0.585) and placebo (Fig. 3a–c). No publication bias is suggested according to the Egger’s regression test (p = 0.308) for each category of biologic treatment.
Fig. 3.
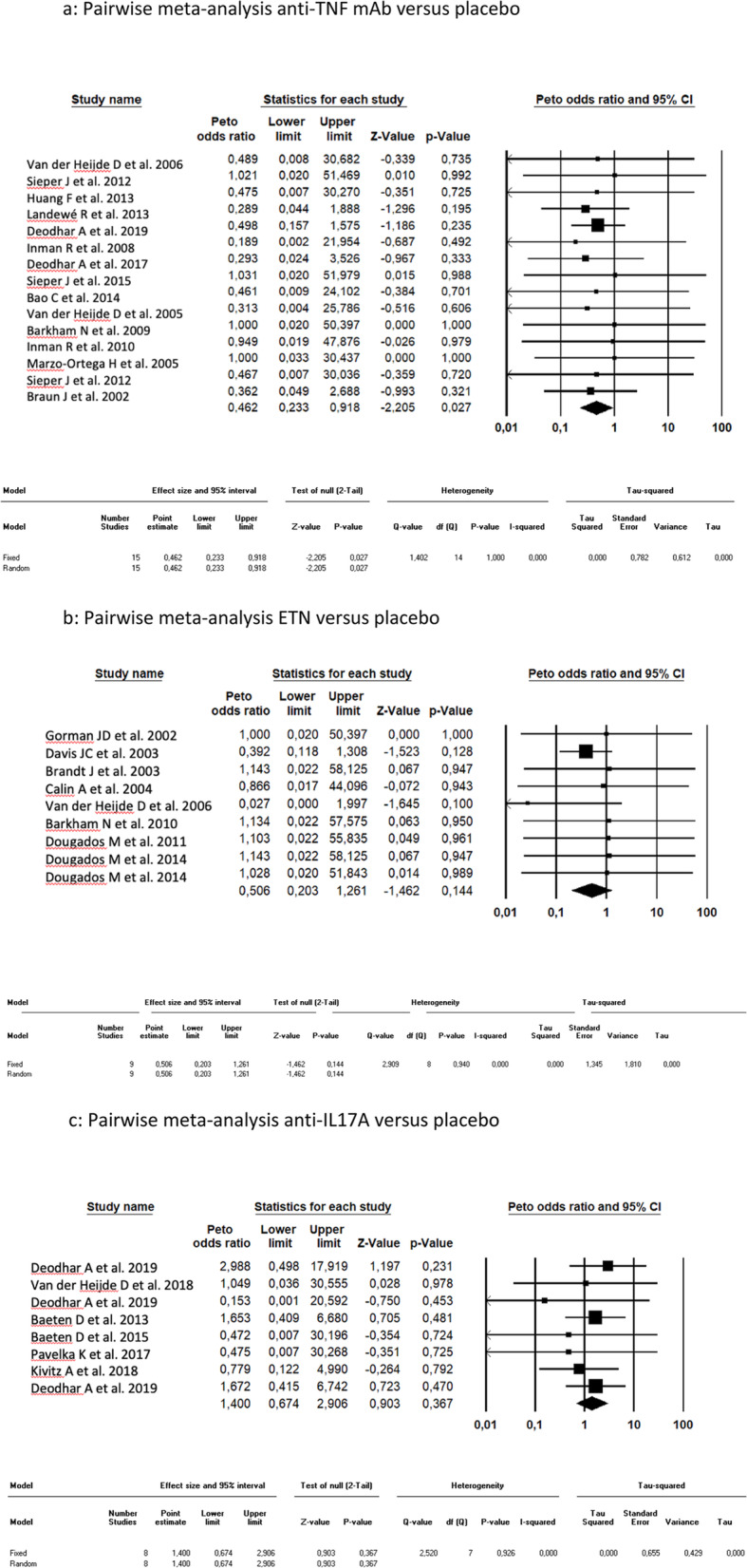
Pairwise meta-analysis. a Pairwise meta-analysis anti-TNF mAb versus placebo. b Pairwise meta-analysis ETN versus placebo. c Pairwise meta-analysis anti-IL17A versus placebo. TNF, tumor necrosis factor; mAb, monoclonal antibody; ETN, etanercept; IL17A, interleukin-17A; CI, confidence interval
Subgroup analyses following pre-specified criteria to compare the incidence of uveitis in each subgroup for each biologic versus placebo showed no significant differences according to axSpA phenotype, disease duration, risk of bias, or focus on AU history.
Network meta-analysis
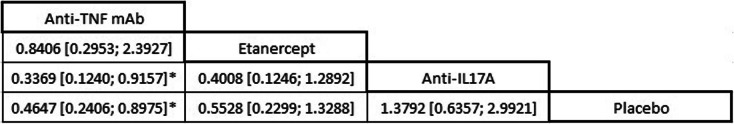
Incidence of AU flares was lower with anti-TNF mAb compared to placebo (OR = 0.46; IC 95% [0.24–0.90]) (Fig. 4). There was also a significant difference for a decreased incidence of AU with anti-TNF mAb compared to anti-IL17A (OR = 0.34; CI 95% [0.12–0.92]) (Table 2). The other comparisons between biologics or between biologics and placebo were not significant (Table 2). The Cochran’s Q test was 0.57 (p = 0.903) ascertaining the absence of heterogeneity/inconsistency between RCTs included.
Fig. 4.
Forest plots of network meta-analysis of all trials for AAU incidence. AAU, acute anterior uveitis; TNF, tumor necrosis factor; mAb, monoclonal antibody; IL17A, interleukin-17A; OR, odds ratio; CI, confidence interval
Table 2.
Comparison for the preventive effect on AAU flares (OR and 95% CI)
OR Odd-Ratio, CI Confidence Interval, AU Anterior Uveitis, TNF Tumor Necrosis Factor, mAb monoclonal antibody, IL17A interleukin-17A
*p < 0.05
P-scores that measure the mean extent of certainty that a treatment is better than the competing treatments were 0.86, 0.728, 0.274, and 0.137 in the anti-TNF mAb, ETN, placebo, and anti-IL17A groups, respectively. Ranking treatments by using P-scores suggested that incidence of AU was the lowest with anti-TNF mAb and the highest with anti-IL17A.
The examination of the funnel plot does not provide suspicion of an asymmetrical distribution of the points representing the studies.
Discussion
This study of 33 RCTs is, to our knowledge, the first network meta-analysis comparing incidence of AU in both anti-TNF, anti-IL17A, and placebo. Flares of AU were uncommon whatever the treatment with a total of 38 AU events reported under active treatment during controlled periods, for a total cumulative exposure under active treatment of 2265 patient years. Despite this low incidence, our results showed a significant protective effect on AU flares of anti-TNF mAb compared to placebo and compared to anti-IL17A.
The reduction of AAU incidence rate with anti-TNF mAb compared to placebo or before/after treatment has already been described in various observational studies for IFX, ADA and GOL [7–10, 12, 13]. However, unlike in our study, a previous pairwise meta-analysis did not report a protective effect of anti-TNF mAb on AAU flares versus placebo [OR: 0.43, 95% CI: 0.12–1.49, p = 0.18] [14]. This discrepancy can be explained by differences in the inclusion criteria. The Wu et al. pairwise meta-analysis selected RCTs including only patients with AS and RCTs with a follow-up > 12 weeks. When applying our inclusion criteria until February 2014 (limit of their meta-analysis research), we would have included 18 RCTs instead of the 8 RCTs included in their analysis.
The same pairwise meta-analysis [14] suggested a protective effect on AAU flares of ETN compared to placebo, while we did not find any significant difference between the ETN and placebo groups. Our results are confirmed by the conclusions of two randomized placebo-controlled clinical trials where etanercept did not show superiority to placebo in preventing flare rates of uveitis [56, 57]. As well, the analysis of a large US claims database aimed at comparing the risk of developing uveitis in patients initiating anti-TNF in patients with AS [58] showed that adalimumab and infliximab were associated with a lower incidence of uveitis episodes than etanercept: 2.4% for adalimumab, 3.2% for infliximab and 4.5% for etanercept.
While our results showed that anti-IL17A do not have the same protective effect against AU flares as anti-TNF mAb, they are also reassuring with regard to a possible deleterious effect of anti-IL17 without significant difference between anti-IL17 and placebo (OR = 1.38 [CI 95% 0.63–2.99]). Among anti-IL17A, the incidence rate was 1.69 per 100 PY with SCK and 3.47 per 100 PY with IXE. Incidence of AU with anti-IL17A in axSpA has recently been published in a pooled analysis of 3 RCTs [53] assessing SCK with an incidence rate of 1.4 per 100 PY, close to our results.
In the pairwise meta-analysis, subgroup analyses did not show any interaction of axSpA phenotype, disease duration, or AU history reporting on AU incidence under anti-TNF MAb, ETN, or anti-IL17A. In other words, we did not find difference between AS and nr-axSpA, between recent and non-recent ax-SpA and between patients with or without AU history.
The methodological quality of this meta-analysis relies on a double lecture aiming at limiting the risk of errors in selecting studies and extracting data. We obtained 33 homogeneous RCTs, with no publication bias according to funnel plots. This broad selection of RCTs has provided us a total cumulative exposure of 3264 PY. However, despite the number of RCTs included, few comparisons between two active treatments or between an active treatment and placebo are not significant. The absence of statistical difference between different biologic therapies may reflect a real lack of clinical effect but can also be due to a lack of power. This lack of power results from the choice to perform a network meta-analysis imposed to limit extraction of data to the controlled periods. The median duration of placebo (or active)-controlled period was 16 weeks, which limits the risk of rare events such as AU.
Conclusion
In RCTs assessing treatments in axSpA, incident AU are rare events.
This network meta-analysis demonstrates that anti-TNF mAb are associated with a lower incidence of AU flare compared to placebo and to anti-IL17A. The incidence of AU was not increased with anti-IL17A or ETN compared to placebo.
Supplementary Information
Additional file 1. Characteristics of randomized controlled trials included in this meta-analysis.
Additional file 2. Risk of bias in included RCTs (Cochrane risk of bias 2.0 tool).
Acknowledgements
We are grateful to the professors and organizers of SMART (Seminars for Meta-Analysis in RheumaTology) for their useful advices. We thank AbbVie who provided logistic support in the organization of sessions about the implementation of meta-analysis and remained independent of the subject choice, study design, collection, analysis, and interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication. The study was not financially support by AbbVie.
Authors’ contributions
Conception and design of the work: TP, PL, LB. Article selection process: DR, MB. Data collection: DR, MB. Data analysis: LB. Data interpretation: DR, MB, LB, PL, TP. Drafting the article: DR, MB, TP. Critical revision of the article: DR, MB, LB, PL, TP. Final approval of the version to be published: DR, MB, LB, PL, TP.
Funding
No funding source to declare
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
D.R., M.B., and L.B. have no conflict of interest to declare; P.L. have received research grants or lecture fees from Abbvie, Amgen, Lilly, MSD, and Pfizer; and T.P. have received research grants or lecture fees from Abbvie, Amgen, Biogen, Fresenius-Kabi, Lilly, MSD, Novartis, Pfizer, Sandoz, and UCB.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Damien Roche and Martin Badard contributed equally to this work.
References
- 1.Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73–84. doi: 10.1016/S0140-6736(16)31591-4. [DOI] [PubMed] [Google Scholar]
- 2.Garg N, van den Bosch F, Deodhar A. The concept of spondyloarthritis: where are we now? Best Pract Res Clin Rheumatol. 2014;28:663–672. doi: 10.1016/j.berh.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 3.de Winter JJ, van Mens LJ, van der Heijde D, Landewé R, Baeten DL. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther. 2016;18:196. doi: 10.1186/s13075-016-1093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeboulon N, Dougados M, Gossec L. Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis. 2008;67:955–959. doi: 10.1136/ard.2007.075754. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe GJ, Dick AD, Brézin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375:932–943. doi: 10.1056/NEJMoa1509852. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen QD, Merrill PT, Jaffe GJ, Dick AD, Kurup SK, Sheppard J, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet. 2016;388:1183–1192. doi: 10.1016/S0140-6736(16)31339-3. [DOI] [PubMed] [Google Scholar]
- 7.Guignard S, Gossec L, Salliot C, Ruyssen-Witrand A, Luc M, Duclos M, et al. Efficacy of tumour necrosis factor blockers in reducing uveitis flares in patients with spondylarthropathy: a retrospective study. Ann Rheum Dis. 2006;65:1631–1634. doi: 10.1136/ard.2006.052092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun J, Baraliakos X, Listing J, Sieper J. Decreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti-tumor necrosis factor agents infliximab and etanercept. Arthritis Rheum. 2005;52:2447–2451. doi: 10.1002/art.21197. [DOI] [PubMed] [Google Scholar]
- 9.Lie E, Lindström U, Zverkova-Sandström T, Olsen IC, Forsblad-d'Elia H, Askling J, et al. Tumour necrosis factor inhibitor treatment and occurrence of anterior uveitis in ankylosing spondylitis: results from the Swedish biologics register. Ann Rheum Dis. 2017;76:1515–1521. doi: 10.1136/annrheumdis-2016-210931. [DOI] [PubMed] [Google Scholar]
- 10.van Denderen JC, Visman IM, Nurmohamed MT, Suttorp-Schulten MSA, van der Horst-Bruinsma IE. Adalimumab significantly reduces the recurrence rate of anterior uveitis in patients with ankylosing spondylitis. J Rheumatol. 2014;41:1843–1848. doi: 10.3899/jrheum.131289. [DOI] [PubMed] [Google Scholar]
- 11.Rudwaleit M, Rosenbaum JT, Landewe R, Marzo-Ortega H, Sieper J, Van Der Heijde D, et al. Observed incidence of uveitis following Certolizumab Pegol treatment in patients with axial Spondyloarthritis. Arthritis Care Res. 2016;68:838–844. doi: 10.1002/acr.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faez S, Lobo A-M, Sobrin L, Papaliodis GN. Treatment of seronegative spondyloarthropathy-associated uveitis with golimumab: retrospective case series. Clin Exp Ophthalmol. 2014;42:392–395. doi: 10.1111/ceo.12207. [DOI] [PubMed] [Google Scholar]
- 13.van Bentum RE, Heslinga SC, Nurmohamed MT, Gerards AH, Griep EN, Koehorst CBJM, et al. Reduced occurrence rate of acute anterior uveitis in Ankylosing spondylitis treated with Golimumab - the GO-EASY study. J Rheumatol. 2019;46:153–159. doi: 10.3899/jrheum.180312. [DOI] [PubMed] [Google Scholar]
- 14.Wu D, Guo Y-Y, Xu N-N, Zhao S, Hou L-X, Jiao T, et al. Efficacy of anti–tumor necrosis factor therapy for extra-articular manifestations in patients with ankylosing spondylitis: a meta–analysis. BMC Musculoskelet Disord. 2015;16:19. doi: 10.1186/s12891-015-0489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma SM, Jackson D. Uveitis and spondyloarthropathies. Best Pract Res Clin Rheumatol. 2017;31:846–862. doi: 10.1016/j.berh.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013;120:777–787. doi: 10.1016/j.ophtha.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Deodhar AA, Miceli Richard C, Baraliakos X, Marzo-Ortega H, Gladman DD, Blanco R, et al. Incidence of uveitis in Secukinumab-treated patients with Ankylosing spondylitis: pooled data analysis from three phase 3 studies. ACR Open Rheumatol. 2020;2:294–299. doi: 10.1002/acr2.11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartzman S, Deodhar A, Kronbergs A, Santisteban S, Garces S, Sandoval D, et al. Inflammatory bowel disease and anterior uveitis in patients treated with Ixekizumab for radiographic axial Spondyloarthritis: results from two phase 3 studies through 52 weeks [abstract]. Arthritis Rheum. 2019;71(suppl 10).
- 19.Rudwaleit M, Van Der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of assessment of SpondyloArthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 20.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 21.Mathes T, Kuss O. A comparison of methods for meta-analysis of a small number of studies with binary outcomes. Res Synth Methods. 2018;9:366–381. doi: 10.1002/jrsm.1296. [DOI] [PubMed] [Google Scholar]
- 22.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis version 3. Englewood: Biostat; 2013. [Google Scholar]
- 23.van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BAC, Braun J, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:2136–2146. doi: 10.1002/art.21913. [DOI] [PubMed] [Google Scholar]
- 24.Sieper J, Van Der Heijde D, Dougados M, Mease PJ, Maksymowych WP, Brown MA, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1) Ann Rheum Dis. 2013;72:815–822. doi: 10.1136/annrheumdis-2012-201766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang F, Gu J, Zhu P, Bao C, Xu J, Xu H, et al. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann Rheum Dis. 2014;73:587–594. doi: 10.1136/annrheumdis-2012-202533. [DOI] [PubMed] [Google Scholar]
- 26.van der Heijde D, Cheng-Chung Wei J, Dougados M, Mease P, Deodhar A, Maksymowych WP, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441–2451. doi: 10.1016/S0140-6736(18)31946-9. [DOI] [PubMed] [Google Scholar]
- 27.Landewe R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis. 2014;73:39–47. doi: 10.1136/annrheumdis-2013-204231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deodhar A, Gensler LS, Kay J, Maksymowych WP, Haroon N, Landewé R, et al. A fifty-two-week, randomized, placebo-controlled trial of Certolizumab Pegol in nonradiographic axial Spondyloarthritis. Arthritis Rheum. 2019;71:1101–1111. doi: 10.1002/art.40866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inman RD, Davis JC, Heijde D, Diekman L, Sieper J, Kim SI, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58:3402–3412. doi: 10.1002/art.23969. [DOI] [PubMed] [Google Scholar]
- 30.Bao C, Huang F, Khan MA, Fei K, Wu Z, Han C, et al. Safety and efficacy of golimumab in Chinese patients with active ankylosing spondylitis: 1-year results of a multicentre, randomized, double-blind, placebo-controlled phase III trial. Rheumatology (Oxford) 2014;53:1654–1663. doi: 10.1093/rheumatology/keu132. [DOI] [PubMed] [Google Scholar]
- 31.Sieper J, Van Der Heijde D, Dougados M, Maksymowych WP, Scott BB, Boice JA, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheum. 2015;67:2702–2712. doi: 10.1002/art.39257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deodhar A, Reveille JD, Harrison DD, Kim L, Lo K-H, Leu JH, et al. Safety and efficacy of Golimumab administered intravenously in adults with Ankylosing spondylitis: results through week 28 of the GO-ALIVE study. J Rheumatol. 2018;45:341–348. doi: 10.3899/jrheum.170487. [DOI] [PubMed] [Google Scholar]
- 33.Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet. 2002;359:1187–1193. doi: 10.1016/S0140-6736(02)08215-6. [DOI] [PubMed] [Google Scholar]
- 34.van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT) Arthritis Rheum. 2005;52:582–591. doi: 10.1002/art.20852. [DOI] [PubMed] [Google Scholar]
- 35.Marzo-Ortega H, McGonagle D, Jarrett S, Haugeberg G, Hensor E, O'Connor P, et al. Infliximab in combination with methotrexate in active ankylosing spondylitis: a clinical and imaging study. Ann Rheum Dis. 2005;64:1568–1575. doi: 10.1136/ard.2004.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barkham N, Keen HI, Coates LC, O'Connor P, Hensor E, Fraser AD, et al. Clinical and imaging efficacy of infliximab in HLA-B27-positive patients with magnetic resonance imaging-determined early sacroiliitis. Arthritis Rheum. 2009;60:946–954. doi: 10.1002/art.24408. [DOI] [PubMed] [Google Scholar]
- 37.Inman RD, Maksymowych WP, Group CS A double-blind, placebo-controlled trial of low dose infliximab in ankylosing spondylitis. J Rheumatol. 2010;37:1203–1210. doi: 10.3899/jrheum.091042. [DOI] [PubMed] [Google Scholar]
- 38.Sieper J, Lenaerts J, Wollenhaupt J, Rudwaleit M, Mazurov VI, Myasoutova L, et al. Efficacy and safety of infliximab plus naproxen versus naproxen alone in patients with early, active axial spondyloarthritis: results from the double-blind, placebo-controlled INFAST study, part 1. Ann Rheum Dis. 2014;73:101–107. doi: 10.1136/annrheumdis-2012-203201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giardina AR, Ferrante A, Ciccia F, Impastato R, Miceli MC, Principato A, et al. A 2-year comparative open label randomized study of efficacy and safety of etanercept and infliximab in patients with ankylosing spondylitis. Rheumatol Int. 2010;30:1437–1440. doi: 10.1007/s00296-009-1157-3. [DOI] [PubMed] [Google Scholar]
- 40.Gorman JD, Sack KE, Davis JC. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002;346:1349–1356. doi: 10.1056/NEJMoa012664. [DOI] [PubMed] [Google Scholar]
- 41.Davis JC, Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg DO, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 2003;48:3230–3236. doi: 10.1002/art.11325. [DOI] [PubMed] [Google Scholar]
- 42.Brandt J, Khariouzov A, Listing J, Haibel H, Sörensen H, Grassnickel L, et al. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum. 2003;48:1667–1675. doi: 10.1002/art.11017. [DOI] [PubMed] [Google Scholar]
- 43.Calin A, Dijkmans BAC, Emery P, Hakala M, Kalden J, Leirisalo-Repo M, et al. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis. 2004;63:1594–1600. doi: 10.1136/ard.2004.020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Heijde D, Da Silva JC, Dougados M, Geher P, van der Horst-Bruinsma I, Juanola X, et al. Etanercept 50 mg once weekly is as effective as 25 mg twice weekly in patients with ankylosing spondylitis. Ann Rheum Dis. 2006;65:1572–1577. doi: 10.1136/ard.2006.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barkham N, Coates LC, Keen H, Hensor E, Fraser A, Redmond A, et al. Double-blind placebo-controlled trial of etanercept in the prevention of work disability in ankylosing spondylitis. Ann Rheum Dis. 2010;69:1926–1928. doi: 10.1136/ard.2009.121327. [DOI] [PubMed] [Google Scholar]
- 46.Dougados M, Braun J, Szanto S, Combe B, Elbaz M, Geher P, et al. Efficacy of etanercept on rheumatic signs and pulmonary function tests in advanced ankylosing spondylitis: results of a randomised double-blind placebo-controlled study (SPINE) Ann Rheum Dis. 2011;70:799–804. doi: 10.1136/ard.2010.139261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dougados M, Van Der Heijde D, Sieper J, Braun J, Maksymowych WP, Citera G, et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2014;66:2091–2102. doi: 10.1002/art.38721. [DOI] [PubMed] [Google Scholar]
- 48.Dougados M, Wood E, Combe B, Schaeverbeke T, Miceli-Richard C, Berenbaum F, et al. Evaluation of the nonsteroidal anti-inflammatory drug-sparing effect of etanercept in axial spondyloarthritis: results of the multicenter, randomized, double-blind, placebo-controlled SPARSE study. Arthritis Res Ther. 2014;16:481. doi: 10.1186/s13075-014-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, Van Der Heijde D, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:1705–1713. doi: 10.1016/S0140-6736(13)61134-4. [DOI] [PubMed] [Google Scholar]
- 50.Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17A inhibitor, in Ankylosing spondylitis. N Engl J Med. 2015;373:2534–2548. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 51.Pavelka K, Kivitz A, Dokoupilova E, Blanco R, Maradiaga M, Tahir H, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285. doi: 10.1186/s13075-017-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kivitz AJ, Wagner U, Dokoupilova E, Supronik J, Martin R, Talloczy Z, et al. Efficacy and safety of Secukinumab 150 mg with and without loading regimen in Ankylosing spondylitis: 104-week results from MEASURE 4 study. Rheumatol Ther. 2018;5:447–462. doi: 10.1007/s40744-018-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deodhar A, Blanco R, Dokoupilova E, Hall S, Kameda H, Kivitz AJ, et al. Secukinumab improves signs and symptoms of non-radiographic axial spondyloarthritis: primary results of a randomized controlled phase III study. Arthritis Rheum. 2020. [DOI] [PMC free article] [PubMed]
- 54.Deodhar A, Poddubnyy D, Pacheco-Tena C, Salvarani C, Lespessailles É, Rahman P, et al. Efficacy and safety of Ixekizumab in the treatment of radiographic axial Spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheum. 2019;71:599–611. doi: 10.1002/art.40753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deodhar A, Van Der Heijde D, Gensler LS, Kim T-H, Maksymowych WP, Østergaard M, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet. 2020;395:53–64. doi: 10.1016/S0140-6736(19)32971-X. [DOI] [PubMed] [Google Scholar]
- 56.Foster CS, Tufail F, Waheed NK, Chu D, Miserocchi E, Baltatzis S, et al. Efficacy of etanercept in preventing relapse of uveitis controlled by methotrexate. Arch Ophthalmol. 2003;121:437–440. doi: 10.1001/archopht.121.4.437. [DOI] [PubMed] [Google Scholar]
- 57.Smith JA, Thompson DJS, Whitcup SM, Suhler E, Clarke G, Smith S, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum. 2005;53:18–23. doi: 10.1002/art.20904. [DOI] [PubMed] [Google Scholar]
- 58.Wendling D, Joshi A, Reilly P, Jalundhwala YJ, Mittal M, Bao Y. Comparing the risk of developing uveitis in patients initiating anti-tumor necrosis factor therapy for ankylosing spondylitis: an analysis of a large US claims database. Curr Med Res Opin. 2014;30:2515–2521. doi: 10.1185/03007995.2014.969368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Characteristics of randomized controlled trials included in this meta-analysis.
Additional file 2. Risk of bias in included RCTs (Cochrane risk of bias 2.0 tool).
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.