Abstract
Evidence on COVID-19 vaccine efficacy/effectiveness (VE) in preventing asymptomatic SARS-CoV-2 infections is needed to guide public health recommendations for vaccinated people. We report interim results of a living systematic review. We identified a total of 30 studies that investigated VE against symptomatic and/or asymptomatic infection. In fully vaccinated individuals, VE against symptomatic and asymptomatic infections was 80–90% in nearly all studies. Fully vaccinated persons are less likely to become infected and contribute to transmission.
Key words: SARS-CoV-2, systematic review, vaccine effectiveness, vaccination, COVID-19
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection plays a key role in the containment of the coronavirus disease (COVID-19) pandemic. All vaccines approved by the European Medical Agency (EMA) at the time of writing demonstrated high vaccine efficacy/effectiveness (VE) against severe COVID-19. With vaccination programmes being implemented in most European countries, it becomes urgent to assess the extent to which these vaccines are also able to prevent symptomatic and asymptomatic infections to guide public health recommendations and develop strategies for fully vaccinated people.
In December 2020, the Robert Koch Institute (RKI), in collaboration with the National Immunisation Technical Advisory Groups (NITAGs) network coordinated by the European Centre for Disease Prevention and Control (ECDC) initiated a living systematic review on the VE and safety of European Union (EU)-licensed COVID-19 vaccines (PROSPERO registration: CRD42020208935). In this paper, only efficacy and effectiveness data but not those on safety will be covered. In detail, we report the interim results of the review focusing on two research questions:
What is the efficacy/effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infections (irrespective of whether those infected were symptomatic or asymptomatic)?
What is the efficacy/effectiveness of COVID-19 vaccines in preventing asymptomatic SARS-CoV-2 infections?
Literature search
This living systematic review follows the recommendations of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guideline (Supplement Part S1). Monthly searches were done and results were immediately incorporated into the evidence base. We considered studies of any design as long as they had a comparison group that investigated VE against SARS-CoV-2 infection and/or asymptomatic SARS-CoV-2 infection after vaccination with an EMA-approved COVID-19 vaccine (see Supplement Part S2 for complete population intervention comparison outcomes (PICO) questions). No restrictions were made regarding publication language or status. The review started on 1 January 2021. The end date of this interim analysis was 14 May 2021.
We used an internal COVID-19 literature database constructed by the RKI library to search for relevant studies. This database covers PubMed, Embase (including Medline) and the preprint servers ArRvix, BioRxiv, ChemRxiv, MedRxiv, Preprints.org, ResearchSquare and Social Science Research Network (SSRN) (see Supplement Part S3 for search strategy). In addition, we hand-searched the websites of the ECDC, the United States (US) Centers for Disease Control and Prevention, the Public Health Agency of Canada and Public Health England for additional studies and reports. Potentially relevant publications were screened at title/abstract and full-text level by two independent investigators for eligibility. Disagreements were resolved by discussion. From the identified studies, data were extracted as described in the PROSPERO protocol and summarised in tabular form. For randomised controlled trials (RCTs), risk of bias was assessed using the Cochrane Risk of Bias tool-2 (RoB-2) [1]. To non-randomised studies, ROBINS-I was applied [2].
Due to heterogeneity of study design, time point of analysis, vaccine used, population and settings, we did not perform a meta-analysis.
Study screening
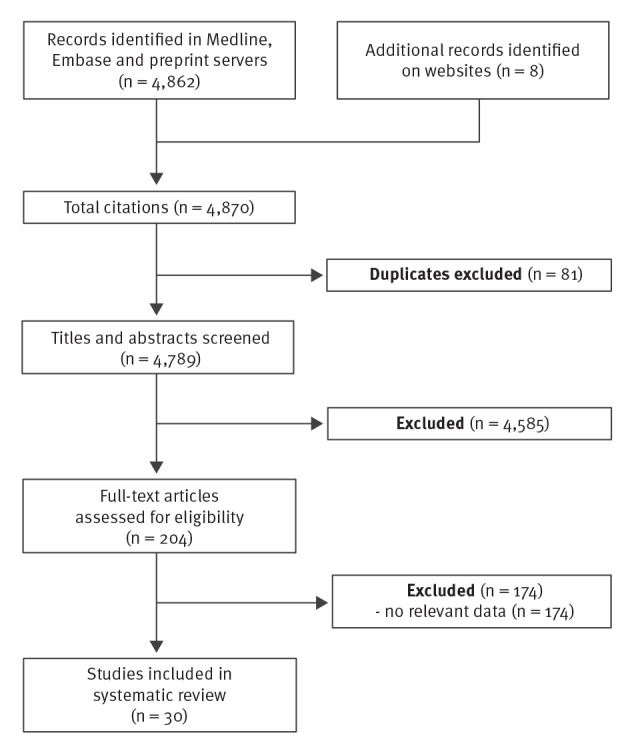
By 14 May 2021 (the date of last search), we identified and screened a total of 4,870 entries. After screening 204 full-text articles, 30 studies were included (Figure) [3-32]. Twenty-six studies reported on infections (irrespective of whether they were symptomatic or not), including three studies that reported both outcomes, and four additional studies reported exclusively on asymptomatic infections.
Figure.
PRISMA flowchart of the living systematic review on efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection
COVID-19: coronavirus disease; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; PRISMA: Preferred Reporting Items for Systematic Review and Meta-Analysis.
Date of last search: 14 May 2021.
Prevention of infection
Of the 30 studies, 26 investigated the efficacy/effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infections, based on reports of number of symptomatic and asymptomatic PCR-positive individuals [3-18,22-31] (Table 1). Studies were conducted in eight different countries (Denmark (n=1), Israel (n=4), Italy (n=1), Qatar (n=1), Spain (n=2), Sweden (n=1), UK (n=8), US (n=7)) and one study was multi-centric [22]. They included between 463 and 2,183,000 participants aged 16–99 years. Two studies were RCTs, 19 studies had a cohort design and five were case–control studies, including two with test-negative design. In 12 studies, the effectiveness of Comirnaty (BionTech, Mainz, Germany/Pfizer, Puurs, Belgium) was evaluated. Two studies investigated COVID-19 Vaccine Janssen (Janssen-Cilag International, Beerse, Belgium), one study studied Vaxzevria (AstraZeneca/Oxford, Oxford, United Kingdom (UK)), and 11 studies investigated more than one vaccine.
Table 1. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection (symptomatic and asymptomatic), 1 January–14 May 2021 (n = 26).
| Study | Country | Study design | Study population (n) | Age | Circulating variant | Vaccine | Time point of analysis after vaccine dose | Adjusted vaccine efficacy/effectiveness (95% CI) |
|
|---|---|---|---|---|---|---|---|---|---|
| After dose 1 | After dose 2 | ||||||||
| Abu-Raddad [23]; 10 May 2021 | Qatar | Case–control study (test-negative design) | General population (cases: n = 35,979; controls: n = 35,979) | Adults (median: 32 years) | Alpha (B.1.1.7); Beta (B.1.351) | Comirnaty | After dose 1; ≥ 14 days after dose 2 |
Alpha: 29.5% (22.9–35.5) Beta: 16.9% (10.4–23.0) |
Alpha: 89.5% (85.9–92.3) Beta: 75.0% (70.5–78.9) |
| Amit [3]; 18 Feb 2021 | Israel | Retrospective cohort study | HCW (n = 9,109) | Adults | Not reported | Comirnaty | 15–21 days after dose 1; 22–28 days after dose 1; 6 days after dose 2 |
65% (43–68) 75% (72–84) (including some persons vaccinated with two doses) |
Not reported |
| Andrejko [4]; 10 Apr 2021a | US | Case–control study (test-negative design) | Population-based (325 cases; 320 controls) |
≥ 18 years | Not reported | Comirnaty, Moderna | 8–14 days after dose 1 or dose 2; ≥ 15 days after dose 1 or 2 |
66.3% (− 68.7–93.3) 58.9% (− 9.7–84.5%) |
78.4% (23.2–94.3) 85.7% (67.2–93.9) |
| Björk [24]; 21 Apr 2021 |
Sweden | Cohort study | Population-based (n = 805,741) | 18–64 years | Not reported | Comirnaty | > 14 days after dose 1; > 7 days after dose 2 |
42% (14–63) | 86% (72–94) |
| Britton [5]; 19 Mar 2021a | US | Retrospective cohort study | LTCF inhabitants (n = 463) | Not reported | Not reported | Comirnaty | > 14 days after dose 1 | 63% (33–79) | Not reported |
| Chodick [6]; 29 Jan 2021a | Israel | Retrospective cohort study | Insurance members (n = 503,875) |
≥ 16 years, mean: 59.7 years (SD: 14.7) | Not reported | Comirnaty | > 13 days after dose 1 | 51.4% (− 7.2–78) | Not reported |
| Corchado-Garcia [25]; 30 Apr 2021 |
US | Retrospective cohort study | Mayo Clinics health system records (vaccinated: 2,195; unvaccinated: 21,950) | ≥ 18 years | Not reported | COVID-19 vaccine Janssen | > 15 days after dose | 76.7% (30.3–95.3) | Not applicable |
| Dagan [7]; 15 Apr 2021 | Israel | Matched case–control study | Insurance members (n = 1,193,236) |
≥ 16 years, median: 45 years | 80% Alpha (B.1.1.7) | Comirnaty | ≥ 7 days after dose 2 | 46% (40–51) | 92% (88–95) |
| EMA Assessment report COVID-19 Vaccine Janssen [22]; 11 Mar 2021 |
Multi-centre (incl. US, Brazil, South Africa) | RCT (phase 3-licensure trial) | Vaccine group (n = 19,306); placebo group (n = 19,178) | ≥ 18 years | Beta (B.1.351); Zeta (P.2); D614G-carrying ‘WT/ref’ | COVID-19 vaccine Janssen | 14 days after vaccination | 67.2% (56.86–75.26) | Not applicable |
| Emary [8]; 30 Mar 2021 | UK | RCT | Randomised population, 66% working in health and social care settings (vaccinated n = 4,244; control: n = 4,290) |
≥ 18 years | Alpha (B.1.1.7); Non-Alpha | Vaxzevria | ≥ 15 days after dose 2 | Not reported | Non-Alpha: 77.3% (65.4–85.0); Alpha: 61.7% (36.7–76.9) |
| Fabiani [26]; 29 Apr 2021 |
Italy | Retrospective cohort study | HCW (n = 6,423) | Mean: 47.1 years (SD: 10.8 years) | Not reported | Comirnaty | 14–21 days after dose 1; ≥ 7 days after dose 2 |
84% (40–96) | 95% (62–99) |
| Glampson [9]; 10 Apr 2021a | UK | Retrospective cohort study | Population-based (n = 2,183,939; (n = 389,587 vaccinated) | ≥ 16 years | Alpha (B.1.1.7) | Comirnaty; Vaxzevria | 28 days after dose 1 | Vaxzevria: 74% (HR: 0.26 (0.19–0.35) Comirnaty: 78% (HR: 0.22 (0.18–0.27)) |
Not reported |
| Guijarro [10]; 26 Mar 2021a |
Spain | Cohort study | HCW: n = 2,590 (cf.d with average population: n = 170,513) | Not reported | Not reported | Comirnaty | 2–4 weeks after dose 1; 7 days after dose 2 |
63% incidence reduction (cf.d with average population) | 99% incidence reduction (cf.d with average population) |
| Haas [11]; 24 Mar 2021 | Israel | Cohort study | Surveillance data (national); n = 202,684 SARS-CoV-2 infections; n = 102,012 non-vaccinated | ≥ 15 years | 94.5% Alpha (B.1.1.7) | Comirnaty | ≥ 7 days after dose 2; ≥ 14 days after dose 2 |
Not reported | 95.3% (94.9–95.7) 96.5% (96.3–96.8) |
| Hall [12]; 22 Feb 2021 | UK | Cohort study | HCW without previous SARS-CoV-2 infection (n = 23,324) | Median: 46.1 years (IQR: 36.0–54.1) | Alpha (B.1.1.7) | Comirnaty | 21 days after dose 1; 7 days after dose 2 |
72% (58–86) | 86% (76–97) |
| Lumley [13]; 12 Mar 2021 | UK | Cohort study | HCW (n = 13,109) | Median: 39 years (range: 30–50) | Alpha (B.1.1.7 ) | Comirnaty; Vaxzevria | > 14 days after dose 1 and dose 2 | 64% (aIRR = 0.36 (0.26–0.50)) |
90% (aIRR = 0.10 (0.02–0.38)) |
| Mason [27]; 22 Apr 2021a |
UK | Matched case–control study | Population (n = 170,226) | 80–83 years | Alpha (B.1.1.7) | Comirnaty | 21 to 27 days after dose 1; 35–41 days after dose 1 and 7 days after dose 2 |
55.2% (40.8 - 66.8) 70.1% (55.1–80.1) (including persons vaccinated with two doses |
Not reported |
| Menni [28]; 27 Apr 2021 |
UK | Cohort study | Users of the COVID Symptom study app (vaccinated: n = 103,622; unvaccinated: n = 464,356) | 16–99 years; Comirnaty: 54.5 years (SD: 14.3); Vaxzevria: 60.8 years (SD: 13.5); unvaccinated: 49.4 years (SD: 14.6) | Not reported | Comirnaty; Vaxzevria | Comirnaty: 45–59 days after dose 1; Vaxzevria: 21–44 days after dose 1 |
Comirnaty: 72% (63–79); Vaxzevria: 60% (49–68) |
Not reported |
| Monge [14]; 15 Apr 2021a | Spain | Retrospective cohort study | LTCF inhabitants (n = 299,209) |
Mean: 85.9 years | Not reported | 99.8% Comirnaty | 15–21 days after dose 1; ≥ 7 days after dose 2 |
51.0% (49.7–52.3) | 81.2% (80.2–82) |
| Moustsen-Helms [15]; 9 Mar 2021a | Denmark | Retrospective cohort study | LTCF inhabitants (n = 39,040); HCW (n = 331,039) |
LTCF median: 84 years (IQR: 77–90); HCW median: 47 years (IQR: 36–57) |
Not reported | LTCF: > 99% Comirnaty HCW: 89% Comirnaty |
> 14 days after dose 1; > 7 days after dose 2 |
HCW: 17% (4–28), LTCF inhabitants: 21% (− 11–44) |
HCW: 90% (82–95) LTCF inhabitants: 64% (14–84) |
| Pawlowski [16]; 27 Feb 2021a | US | Matched case–control study | 62,138 persons tested at Mayo Clinics | ≥ 18 years | Not reported | Comirnaty; Moderna | > 36 days after dose 1; 1–2 weeks after dose 2 | 83.4% (60.2–94.3) | 88.7% (68.4–97.1%) |
| Pritchard [29]; 23 Apr 2021a |
UK | Prospective cohort study | Population-based (n = 373,402) | ≥ 16 years | Alpha (B.1.1.7); Non-Alpha | Comirnaty; Vaxzevria | ≥ 21 days after dose 1 and dose 2 (only Comirnaty) | Alpha: 66% (OR: 0.34 (0.28–0.41)) Non-Alpha: 71% (OR: 0.29 (0.16–0.51)) |
Alpha: 78% (OR: 0.22; (0.15–0.32)) Non-Alpha: 82% (OR: 0.18 (0.06–0.51)) |
| Shrotri [17]; 7 Apr 2021a | UK | Cohort study | LTCF inhabitants (n = 10,412) |
Mean: 86 years | Mainly Alpha (B.1.1.7) | Comirnaty (33%); Vaxzevria (67%) | 35–48 days after dose 1 | 62% (23–81) (HR: 0.38 (0.19–0.77)) Comirnaty: 65% (HR: 0.35 (0.17–0.71) Vaxzevria: 68% (HR 0.32 (0.15–0.66)) |
Not reported |
| Swift [30]; 26 Apr 2021a |
US | Retrospective cohort study | HCW at Mayo Clinics (n = 71,152) | Median: 41 years | Not reported | Comirnaty; Moderna | > 14 days after dose 1 and ≤ 14 days from dose 2; > 14 days after dose 2 |
Comirnaty: 78.1% (71.1–82.0) Moderna: 91.2% (80.6- 96.1) |
Comirnaty: 96.8% (95.3–97.8) Moderna: 98.6% (90.1–99.8) |
| Tang [31]; 6 May 2021 |
US | Cohort study | HCW (n = 5,217) | Adults | Not reported | Comirnaty | ≥ 12 days after dose 1 and before dose 2; ≥ 7 days after dose 2 |
58% (IRR: 0.42 (0.26–0.70)) | 96% (IRR: 0.04 (0.02–0.09)) |
| Thompson [18]; 2 Apr 2021 |
US | Prospective cohort study | HCW, first responders, other essential and frontline workers (n = 3,950) | ≥ 18 years | Not reported | Comirnaty (62.7%); Moderna (29.6%); unknown mRNA vaccine (7.7%) | ≥ 14 days after dose 1 and dose 2 | 80% (59–90) | 90% (68–97) |
aIRR: adjusted incidence rate ratio; CI: confidence interval; COVID-19: coronavirus disease; HCW: healthcare workers; HR: hazard ratio; IQR: interquartile range; IRR: incidence rate ratio; LTCF: long-term care facility; RCT: randomised controlled trial; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SD: standard deviation; UK: United Kingdom; US: United States.
a Preprint.
One-dose efficacy/effectiveness was investigated in 24 studies (Table 1) and estimates ranged from 16.9% to 91.2%, with the majority of estimates ranging between 60% and 70%. The VE was lower in older (e.g. long-term care facility inhabitants) than in younger participants (e.g. healthcare workers). However, age-related effects could not be assessed in a number of studies since subgroup data were not reported. Vaccine type and study design did not appear to have an impact on VE estimates.
In 17 of 26 studies, VE was reported after the second dose. Estimates ranged between 61.7% and 98.6%. One study found an incidence reduction of 99% [10]. The majority of estimates ranged from 80% to 90%. Again, VE estimates were not affected by participant age, vaccine type and/or study design (see Supplement Part S4 for exact definitions of outcomes).
Prevention of asymptomatic infection
Seven of 30 studies investigated VE against asymptomatic SARS-CoV-2 infections [11,19-22,31,32] (Table 2). With the exception of one study that had a multicentre design with study centres in the US, Brazil and South Africa, the remainder were performed in single centres in Israel (n = 3), the UK (n = 1) and the US (n = 2). Studies included between 5,217 and more than 300,000 participants, with three of them including healthcare workers only. Only the multicentre study investigating COVID-19 vaccine Janssen was an RCT [22]. The other studies had a cohort design and investigated Comirnaty or Comirnaty and COVID-19 vaccine Moderna using either hospital, insurance or surveillance data. In five of these six studies, VE against asymptomatic infection after one dose of Comirnaty or COVID-19 vaccine Moderna ranged from 36% to 79%. Five cohort studies also analysed VE against asymptomatic infection after a second dose and reported VE estimates between 80% and 94%. For the single-dose regimen of COVID-19 vaccine Janssen, VE against asymptomatic infections was 74% in the RCT [22].
Table 2. Efficacy and effectiveness of COVID-19 vaccines against asymptomatic SARS-CoV-2 infection, 1 January–14 May 2021 (n = 7).
| Study | Country | Study design | Study population (n) | Age | Circulating variant | Vaccine | Time point of analysis after vaccine dose | Adjusted vaccine efficacy/effectiveness (95% CI) |
|
|---|---|---|---|---|---|---|---|---|---|
| After dose 1 | After dose 2 | ||||||||
| Angel [32]; 6 May2021 |
Israel | Retrospective cohort study | HCW (n = 6,710) | Mean (SD) all: 44.3 (12.5) years; vaccinated: 44.8 (12.5) years; unvaccinated: 40.7 (11.7) years | Not reported | Comirnaty | 7-28 days after dose 1; > 7 days after dose 2; > 21 days after dose 2 |
36% (IRR: 0.64; 0.31–1.51) | 86% (IRR: 0.14 (0.07–0.31)) 94% (IRR: 0.02 (0–0.06)) |
| EMA Assessment report COVID-19 vaccine Janssen [22]; 11 Mar 2021 |
Multicentre (incl. US, Brazil, South Africa) | RCT (phase 3-licensure trial) | Vaccine group (n = 19,306); placebo group (n = 19,178) | ≥ 18 years | Beta (B.1.351) (); Zeta (P.2); D614G-carrying ‘WT/ref’ | COVID-19 vaccine Janssen | > 28 days after vaccination | 74% (27.9–92.4) | Not applicable |
| Haas [11]; 24 Mar 2021 | Israel | Cohort study | Surveillance data (national); n = 202,684 SARS-CoV-2 infections; n = 102,012 non-vaccinated | ≥ 16 years | Alpha (B.1.1.7) (94.5%) | Comirnaty | ≥ 7 days after dose 2; ≥ 14 days after dose 2 |
Not reported | 91.5% (90.7–92.2) 93.8% (93.3–94.2) |
| Jones [21]; 8 Apr 2021 |
UK | Retrospective cohort study | HCW (n = 8,776) |
Adults | Mainly Alpha (B.1.1.7 ) | Comirnaty | ≥ 12 days after dose 1 | 75%a | Not reported |
| Tande [19]; 10 Mar 2021b |
US | Retrospective cohort study | Population-based; patients at Mayo Clinics (n = 39,156) | ≥ 18 years, Mean: 54,2 years (SD 19.7) | Not reported | Comirnaty; Moderna | > 10 days after dose 1; > 0 days after dose 2 |
79% (RR: 0.21 (0.12–0.37)) Comirnaty only: 79% (RR = 0.21 (0.11–0.38)) |
80% (RR: 0.20 (0.09–0.44)) Comirnaty only: 80% (RR: 0.20 (0.09–0.44)) |
| Tang [31]; 6 May2021 |
US | Cohort study | HCW (n = 5,217) | Adults | Not reported | Comirnaty | ≥ 12 days after dose 1 and before dose 2; ≥ 7 days after dose 2 |
42% (IRR: 0.58 (0.30–1.13)) | 90% (IRR: 0.10 (0.04–0.22)) |
| Zacay [20]; 3 Mar 2021b |
Israel | Retrospective cohort study | Insurance members (n = 6,286) |
≥ 16 years | Mainly Alpha, (B.1.1.7) also Beta (B.1.351 ) | Comirnaty | ≥ 14 days after dose 1; ≥ 7 days after dose 2 |
61% (49–71) | 89% (82–94) |
CI: confidence interval; COVID-19: coronavirus disease; HCW: healthcare workers; IRR: incidence rate ratio; RCT: randomised controlled trial; RR: risk ratio; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SD: standard deviation; UK: United Kingdom; US: United States.
aOwn calculation.
bPreprint.
Risk of bias
Risk of bias was low in one RCT [22]. In the other RCT [8], we detected some concerns due to different vaccine dosages being analysed. Of the 28 non-randomised studies, risk of bias was critical in four studies which did not adjust for confounders and reported unadjusted estimates. In a further two non-randomised studies, risk of bias was considered to be serious because adjustment for confounders was inappropriate. Besides one study with unclear risk of bias, all remaining studies had moderate risk of bias, mainly due to possible residual confounding (see Supplement Part S5 for details).
Discussion
These interim results of a living systematic review show that after completed course the EMA-approved COVID-19 vaccines have a VE of 80% to 90% in preventing SARS-CoV2 infections, including asymptomatic ones. We found some indication that VE estimates are not reduced in cases infected with variant of concern (VOC) Alpha (Phylogenetic Assignment of Named Global Outbreak (Pango) lineage designation B.1.1.7), however these results should be interpreted with caution. VE against infection can also be regarded as an indicator of how well a vaccine prevents transmission. In addition, studies suggest that persons who become SARS-CoV-2-positive despite vaccination had a shorter duration of virus shedding and lower viral load [8].
Some of our methodological limitations stem from the rapidly changing publication landscape of COVID-19 vaccine studies. In particular, non-randomised studies on real-world effectiveness are continuously and frequently published on preprint servers. Although we systematically searched seven preprint servers, additional studies could have been published on other servers or websites that we did not capture. Moreover, it has to be considered that these studies did not undergo peer review and should therefore be considered with caution. A recent study reported that some final journal publications of COVID-19 studies differ to a certain extent from versions which were previously published on pre-print servers [33]. A further limitation is the small number of countries where the studies were performed, including the possibility that some studies, in particular those from Israel and the US, might have analysed partly overlapping study populations. The majority of studies included were conducted in persons vaccinated with Comirnaty. At the time point of data cut for this interim analysis, only limited information was available on VOCs other than Alpha. Meanwhile, some studies have been published indicating reduced effectiveness against infections with VOC Delta for Comirnaty and Vaxzevria, whereas effectiveness against hospitalisation was unchanged, as compared to VOC Alpha [34,35].
Conclusion
Results of this living systematic review imply that COVID-19 vaccines are highly effective in preventing SARS-CoV-2 infections, including those which are asymptomatic. From a public health perspective, it can be concluded that fully vaccinated persons might in some instances still become PCR-positive for SARS-CoV-2 but only play a minor role in the transmission of SARS-CoV-2.
Acknowledgements
The authors would like to thank the members of the EU/EEA NITAG Collaboration Working Group 3 for valuable comments on the PICO questions and review protocol.
Supplementary Data
Conflict of interest: None declared.
Authors’ contributions: TH conceived the study, was second reviewer, and drafted the manuscript. JK and SVB were primary reviewers and contributed to the manuscript. SR, WKS and AP were second reviewers and contributed to the manuscript. SS provided input into the interpretation of the results. OW held general oversight of the conducted work and revised the manuscript. All authors contributed to the interpretation of the data and provided important intellectual content to the manuscript.
References
- 1. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 2. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397(10277):875-7. 10.1016/S0140-6736(21)00448-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrejko K, Pry J, Myers JF, Jewell NP, Openshaw J, Watt J, et al. Early evidence of COVID-19 vaccine effectiveness within the general population of California. medRxiv. 2021.04.08.21255135. 10.1101/2021.04.08.21255135 [DOI]
- 5. Britton A, Jacobs Slifka KM, Edens C, Nanduri SA, Bart SM, Shang N, et al. Effectiveness of the Pfizer-BioNTech COVID-19 vaccine among residents of two skilled nursing facilities experiencing COVID-19 outbreaks—Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(11):396-401. 10.15585/mmwr.mm7011e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chodick G, Tene L, Patalon T, Gazit S, Tov AB, Cohen D, et al. The effectiveness of the first dose of BNT162b2 vaccine in reducing SARS-CoV-2 infection 13-24 days after immunization: real-world evidence. medRxiv. 2021:2021.01.27.21250612. 10.2139/ssrn.3769977 [DOI]
- 7. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412-23. 10.1056/NEJMoa2101765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397(10282):1351-62. 10.1016/S0140-6736(21)00628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glampson B, Brittain J, Kaura A, Mulla A, Mercuri L, Brett S, et al. North West London Covid-19 vaccination programme: real-world evidence for vaccine uptake and effectiveness. medRxiv. 2021.04.08.21254580. 10.1101/2021.04.08.21254580 [DOI] [PMC free article] [PubMed]
- 10.Guijarro C, Galán I, Martínez-Ponce D, Pérez-Fernández E, José Goyanes M, Castilla V, et al. Dramatic drop of new SARS-CoV-2 infections among health care workers after the first dose of the BNT162b2 mRNA Covid-19 vaccine. medRxiv. 2021.03.24.21254238. 10.1101/2021.03.24.21254238 [DOI] [PMC free article] [PubMed]
- 11.Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Nationwide vaccination campaign with BNT162b2 in Israel demonstrates high vaccine effectiveness and marked declines in incidence of SARS-CoV-2 infections and COVID-19 cases, hospitalizations, and deaths. SSRN. 2021. Available from: https://ssrn.com/abstract=3811387 [DOI] [PMC free article] [PubMed]
- 12.Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, et al. Effectiveness of BNT162b2 mRNA vaccine against infection and COVID-19 vaccine coverage in healthcare workers in England, multicentre prospective cohort study (the SIREN Study). SSRN. 2021. Available from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3790399 [DOI] [PMC free article] [PubMed]
- 13.Lumley SF, Rodger G, Constantinides B, Sanderson N, Chau KK, Street TL, et al. An observational cohort study on the incidence of SARS-CoV-2 infection and B.1.1.7 variant infection in healthcare workers by antibody and vaccination status. medRxiv. 2021.03.09.21253218. 10.1101/2021.03.09.21253218 [DOI] [PMC free article] [PubMed]
- 14.Monge S, Olmedo C, Alejos B, Lapeña MF, Sierra MJ, Limia A. Direct and indirect effectiveness of mRNA vaccination against SARS-CoV-2 infection in long-term care facilities in Spain. medRxiv. 2021.04.08.21255055. 10.1101/2021.04.08.21255055 [DOI] [PMC free article] [PubMed]
- 15.Moustsen-Helms IR, Emborg H-D, Nielsen J, Nielsen KF, Krause TG, Molbak K, et al. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA Covid-19 vaccine in long-term care facility residents and healthcare workers–a Danish cohort study. MedRxiv. 2021.03.08.21252200. 10.1101/2021.03.08.21252200 [DOI]
- 16.Pawlowski C, Lenehan P, Puranik A, Agarwal V, Venkatakrishnan A, Niesen MJ, et al. FDA-authorized COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. MedRxiv. 2021.02.15.21251623. 10.1101/2021.02.15.21251623 [DOI] [PMC free article] [PubMed]
- 17.Shrotri M, Krutikov M, Palmer T, Giddings R, Azmi B, Subbarao S, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021;S1473-3099(21)00289-9 [DOI] [PMC free article] [PubMed]
- 18. Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(13):495-500. 10.15585/mmwr.mm7013e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tande AJ, Pollock BD, Shah ND, Farrugia G, Virk A, Swift M, et al. Impact of the COVID-19 vaccine on asymptomatic infection among patients undergoing pre-procedural COVID-19 molecular screening. Clin Infect Dis. 2021;ciab229. 10.1093/cid/ciab229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heymann AD, Zacay G, Shasha D, Bareket R, Kadim I, Sikron FH, et al. BNT162b2 vaccine effectiveness in preventing asymptomatic infection with SARS-CoV-2 virus: a nationwide historical cohort study. SSRN. 2021. Available from: https://ssrn.com/abstract=3796868 [DOI] [PMC free article] [PubMed]
- 21. Jones NK, Rivett L, Seaman S, Samworth RJ, Warne B, Workman C, et al. Single-dose BNT162b2 vaccine protects against asymptomatic SARS-CoV-2 infection. eLife. 2021;10:10:e68808. 10.7554/eLife.68808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Medicines Agency (EMA). Assessment report - COVID-19 Vaccine Janssen. Amsterdam: EMA. 2021. Available from: https://www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccine-janssen-epar-public-assessment-report_en.pdf
- 23. Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for COVID-19 Vaccination . Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385(2):187-9. 10.1056/NEJMc2104974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjork J, Inghammar M, Moghaddassi M, Rasmussen M, Malmqvist U, Kahn F. Effectiveness of the BNT162b2 vaccine in preventing COVID-19 in the working age population-first results from a cohort study in southern Sweden. medRxiv. 2021.04.20.21254636. 10.1101/2021.04.20.21254636 [DOI] [PMC free article] [PubMed]
- 25.Corchado-Garcia J, Hughes T, Cristea-Platon T, Lenehan P, Pawlowski C, Bade S, et al. Real-world effectiveness of Ad26. COV2. S adenoviral vector vaccine for COVID-19. SSRN. 2021. Available from: https://ssrn.com/abstract=3835737 [DOI] [PMC free article] [PubMed]
- 26. Fabiani M, Ramigni M, Gobbetto V, Mateo-Urdiales A, Pezzotti P, Piovesan C. Effectiveness of the Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso province, Veneto region, Italy, 27 December 2020 to 24 March 2021. Euro Surveill. 2021;26(17):2100420. 10.2807/1560-7917.ES.2021.26.17.2100420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason TF, Whitston M, Hodgson J, Watkinson RE, Lau Y-S, Abdulrazeg O, et al. Effects of BNT162b2 mRNA vaccine on Covid-19 infection and hospitalisation among older people: matched case control study for England. medRxiv. 2021. 04.19.21255461. 10.1101/2021.04.19.21255461 [DOI] [PMC free article] [PubMed]
- 28. Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939-49. 10.1016/S1473-3099(21)00224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pritchard E, Matthews PC, Stoesser N, Eyre DW, Gethings O, Vihta K-D, et al. Impact of vaccination on SARS-CoV-2 cases in the community: a population-based study using the UK’s COVID-19 infection survey. medRxiv. 2021.04.22.21255913. 10.1101/2021.04.22.21255913 [DOI]
- 30. Swift MD, Breeher LE, Tande AJ, Tommaso CP, Hainy CM, Chu H, et al. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin Infect Dis. 2021;ciab361. 10.1093/cid/ciab361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang L, Hijano DR, Gaur AH, Geiger TL, Neufeld EJ, Hoffman JM, et al. Asymptomatic and symptomatic SARS-CoV-2 infections after BNT162b2 vaccination in a routinely screened workforce. JAMA. 2021;325(24):2500-2. 10.1001/jama.2021.6564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457-65. 10.1001/jama.2021.7152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bero L, Lawrence R, Leslie L, Chiu K, McDonald S, Page M, et al. Comparison of preprints and final journal publications from COVID-19 studies: discrepancies in results reporting and spin in interpretation. medRxiv. 2021. 04.12.21255329. 10.1101/2021.04.12.21255329 [DOI] [PMC free article] [PubMed]
- 34. Sheikh A, McMenamin J, Taylor B, Robertson C, Public Health Scotland and the EAVE II Collaborators . SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461- 2462. 10.1016/S0140-6736(21)01358-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stowe J, Andrews N, Gower C, Gallagher E, Utsi L, Simmons R. Effectiveness of COVID-19 vaccines against hospital admission with the Delta (B. 1.617. 2) variant. Public Health England. 2021. Available from: https://media.tghn.org/articles/Effectiveness_of_COVID-19_vaccines_against_hospital_admission_with_the_Delta_B._G6gnnqJ.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


