Abstract
Introduction
Patients with lung cancer (LC) are susceptible to severe outcomes from COVID-19. This study evaluated disruption to care of patients with LC during the COVID-19 pandemic.
Methods
The COVID-19 and Cancer Outcomes Study (CCOS) is a prospective cohort study comprised of patients with a current or past history of hematological or solid malignancies with outpatient visits between March 2 and March 6, 2020, at two academic cancer centers in the Northeastern United States (US). Data was collected for the three months prior to the index week (baseline period) and the following three months (pandemic period).
Results
313 of 2365 patients had LC, 1578 had other solid tumors, and 474 had hematological malignancies. Patients with LC were not at increased risk of COVID-19 diagnosis compared to patients with other solid or hematological malignancies. When comparing data from the pandemic period to the baseline period, patients with LC were more likely to have a decrease in in-person visits compared to patients with other solid tumors (aOR 1.94; 95% CI, 1.46–2.58), but without an increase in telehealth visits (aOR 1.13; 95% CI 0.85–1.50). Patients with LC were more likely to experience pandemic-related treatment delays than patients with other solid tumors (aOR 1.80; 95% CI 1.13–2.80) and were more likely to experience imaging/diagnostic procedure delays than patients with other solid tumors (aOR 2.59; 95% CI, 1.46–4.47) and hematological malignancies (aOR 2.01; 95% CI, 1.02–3.93). Among patients on systemic therapy, patients with LC were also at increased risk for decreased in-person visits and increased treatment delays compared to those with other solid tumors.
Discussion
Patients with LC experienced increased cancer care disruption compared to patients with other malignancies during the early phase of the COVID-19 pandemic. Focused efforts to ensure continuity of care for this patient population are warranted.
Keywords: Lung cancer, COVID-19, Cancer care, Continuity of care
1. Introduction
Patients with cancer, specifically lung cancer (LC) are at markedly higher risk of severe outcomes from COVID-19 compared to the general population [1], [2], [3], [4]. This population is particularly susceptible to COVID-19 given their older age, smoking history, and pre-existing cardiopulmonary comorbidities. In many occurrences, as we previously reported [5], the pandemic has resulted in significant disruption in cancer care, including decreased in-person visits, increased telehealth visits, and delays in treatment. The goal of this study was to evaluate the disruption to care of patients with LC during the COVID-19 pandemic.
2. Methods
The COVID-19 and Cancer Outcomes Study (CCOS) is a multicenter prospective cohort study comprised of adult patients with a current or past history of hematological malignancy or invasive solid tumor who participated in an outpatient medical oncology visit on the index week between March 2 and March 6, 2020, at the Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai in New York, New York (MSSM), and the Dana-Farber Cancer Institute in Boston, Massachusetts (DFCI). Data was abstracted manually by medical record review, and an electronic data capture platform was used to collect patient-, cancer-, and treatment-related variables during the 3 months prior to the index week (from December 2, 2019, referred to as the baseline period) and 3-month follow-up (until June 6, 2020, referred to as the pandemic period). Electronic data capture platform was used to ensure real-time data validation, and lead investigators conducted periodic quality checks to ensure consistency and accuracy in data collection.
The primary objective of this study was to examine the impact of COVID-19 on the delivery of cancer care among patients with LC as measured by the number of in-person outpatient visits, telehealth visits, and pandemic-related delays of oncologic care as recorded in the electronic medical record (EMR). Pandemic-related delays were defined as any postponement in treatment, imaging, or diagnostic procedures due to COVID-19 infection, or provider/patient decision to limit patient’s healthcare exposure as specifically documented in the EMR (further details provided in Supplementary Methods). Patients with either non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC) were included.
To evaluate the correlates of changes in cancer care delivery, multivariable logistic regression models, with listwise deletion for missing data, that included the following independent variables were computed: self-reported race and ethnicity (non-Hispanic White, Hispanic, non-Hispanic Black, or Other), cancer center (DFCI or MSSM), cancer status (metastatic/active, localized/stable, or no evaluable disease [NED]/complete remission, with the former referring to solid malignancies and the latter hematologic malignancies), and tumor type (LC, other solid tumor, or hematologic malignancies). For numbers of visits, per-patient differences in the numbers of visits in the pandemic period compared to the baseline period were used as the dichotomized dependent variables (any increase in telehealth visits and any decrease in in-person visits). Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were reported. For all logistic regression models, LC was used as the reference tumor type and the inverse of the model aOR and 95% CI were reported for all models. Median follow-up was determined using the inverse Kaplan-Meier method. All tests were two-tailed and considered statistically significant for p < 0.05. All analyses were done in the R statistical environment (v3.6.1).
3. Results
The overall cohort included 2365 patients, of whom 313 (13.2%) had LC, 1578 (66.7%) had other solid tumors, and 474 (20.0%) had hematological malignancies (baseline characteristics summarized in Table 1 ). In terms of tumor status, 205 (65.5%) patients with LC had metastatic disease, 88 (28.1%) had local or locally advanced disease, and 15 (4.8%) had no evidence of disease. At the time of the index visit, 209 (66.8%) patients were receiving systemic therapy with a chemotherapy-containing regimen (28.1%, which included chemotherapy-immune checkpoint inhibitor combinations), targeted therapy (20.8%), or immune checkpoint inhibitor alone (12.3%), or other (2.6%).
Table 1.
Baseline characteristics of overall cohort.
| Lung Cancer (n = 313) | Other Solid Tumor (n = 1578) | Hematological Malignancy (n = 474) | |
|---|---|---|---|
| Age | |||
| Mean (median) | 66.6 (22–93) | 64.0 (20–101) | 63.1 (21–92) |
| Gender | |||
| Female | 187 (59.7%) | 676 (42.8%) | 224 (47.3%) |
| Male | 126 (40.3%) | 902 (57.2%) | 250 (52.7%) |
| Site | |||
| MSSM | 103 (32.9%) | 946 (59.9%) | 454 (95.8%) |
| DFCI | 210 (67.1%) | 632 (40.1%) | 20 (4.2%) |
| Race/Ethnicity | |||
| Non-Hispanic White | 205 (65.5%) | 899 (57.0%) | 242 (51.1%) |
| Non-Hispanic Black | 28 (8.9%) | 198 (12.5%) | 73 (15.4%) |
| Hispanic | 25 (8.0%) | 194 (12.3%) | 78 (16.5%) |
| Other | 24 (7.7%) | 114 (7.2%) | 29 (6.1%) |
| Unknown | 31 (9.9%) | 173 (11.0%) | 52 (11.0%) |
| Tobacco Use | |||
| Current/prior use | 226 (72.2%) | 707 (44.8%) | 176 (37.1%) |
| Tumor Status | |||
| CR or NED | 15 (4.8%) | 327 (20.7%) | 146 (30.8%) |
| Local, LA, or stable | 88 (28.1%) | 590 (37.4%) | 137 (28.9%) |
| Metastatic or active | 205 (65.5%) | 623 (39.5%) | 188 (39.7%) |
| Other or unknown | 5 (1.6%) | 38 (2.4%) | 3 (0.6%) |
| Receiving Systemic Therapy at Index Visit | |||
| Yes | 209 (66.8%) | 999 (63.3%) | 276 (58.2%) |
CR: complete response; NED: no evidence of disease; LA: locally advanced.
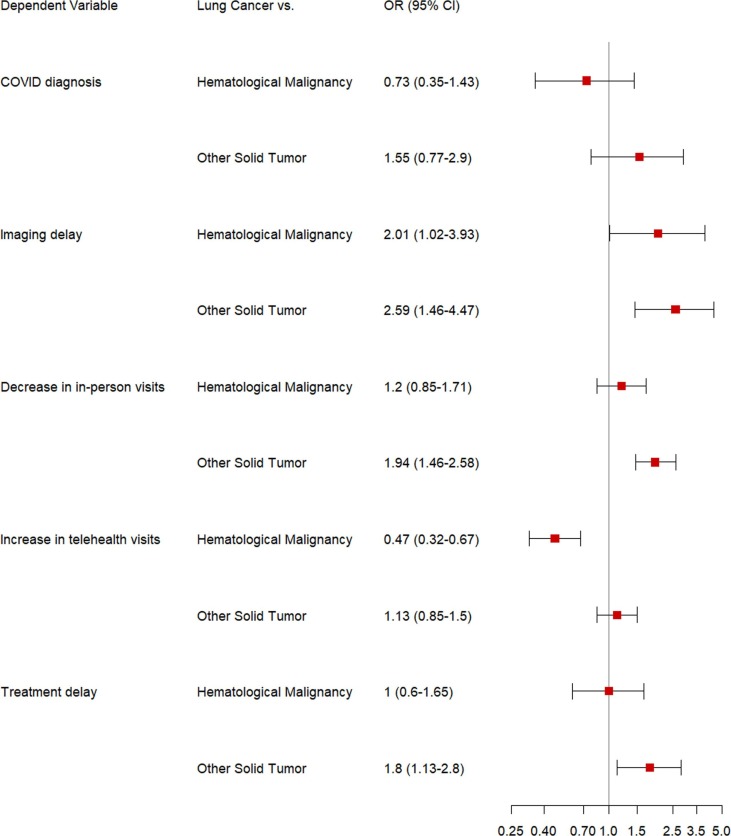
At a median follow-up of 84 days (inter-quartile range 56–90 days), 13 patients with LC (4.1%) had developed COVID-19 (vs. other solid tumors: 63 [4.0%] and hematological malignancies: 52 [11.0%]). The adjusted odds ratios using patients with LC as the comparator indicated that patients with LC were not at increased risk of COVID-19 diagnosis compared to patients with other solid tumor types (aOR 1.55; 95% CI, 0.77–2.90) and hematological malignancies (aOR 0.73; 95% CI, 0.35–1.43) ( Fig. 1 ).
Fig. 1.
Forest Plot showing the adjusted odds ratios and 95% confidence intervals from the multivariable logistic regression model used to assess decrease in in-person visits during the pandemic period compared to the baseline period, increase in telehealth visits during the pandemic period compared to the baseline period, pandemic-related treatment and imaging delays, and COVID-19 diagnoses among patients with lung cancer compared to patients with other solid tumors and hematological malignancies.
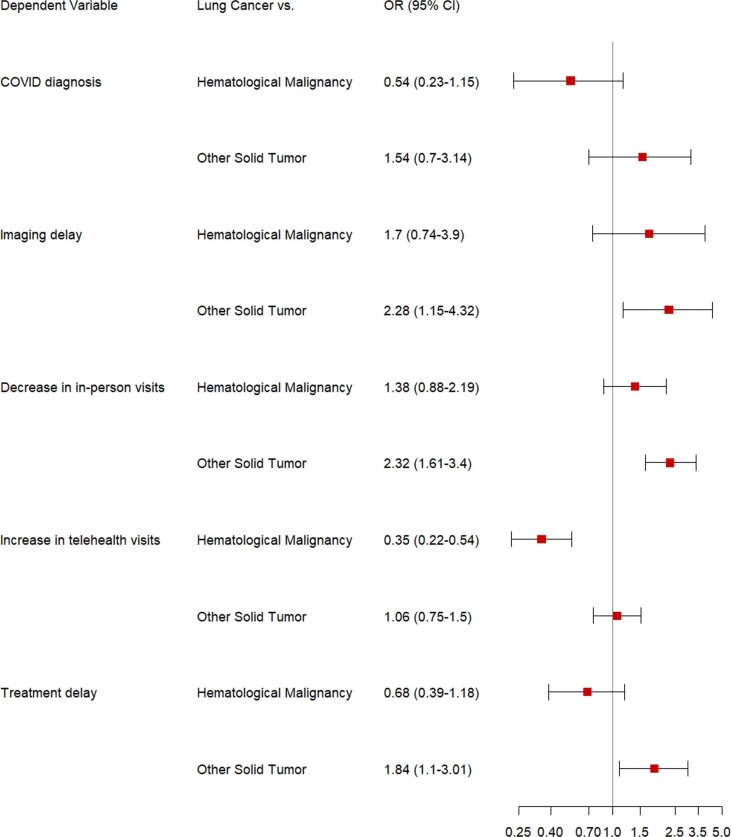
When comparing data from the pandemic period to the baseline period, 209 patients with LC had a decrease in the number of in-person outpatient visits (LC: 209 [66.8%] vs. other solid tumors: 749 [47.5%] and hematological malignancies: 260 [54.9%]). On multivariable analysis, patients with LC were significantly more likely to have a decrease in in-person visits compared to patients with other solid tumors (aOR 1.94; 95% CI, 1.46–2.58), but not compared to those with hematological malignancies (aOR 1.20; 95% CI, 0.85–1.71). Given patients requiring active therapy may face increased negative consequences from cancer care changes, we also compared differences in in-person visits among patients on systemic therapy during the index week. Similar to the overall cohort, patients with LC on systemic therapy were more likely have a decrease in in-person visits compared to patients with other solid tumors (aOR 2.32; 95% CI, 1.61–3.40), but not compared to those with hematological malignancies (aOR 1.38; 95% CI, 0.88–2.19) (Fig. 2 ).
Fig. 2.
Forest Plot showing the adjusted odds ratios and 95% confidence intervals from the multivariable logistic regression model used to assess decrease in in-person visits during the pandemic period compared to the baseline period, increase in telehealth visits during the pandemic period compared to the baseline period, pandemic-related treatment and imaging delays, and COVID-19 diagnoses among patients with lung cancer on systemic therapy during the index week compared to patients with other solid tumors and hematological malignancies on systemic therapy during the index week.
When comparing data from the pandemic period to the baseline period, 126 (40.3%) patients with LC had an increase in the number of telehealth visits (vs. other solid tumors: 465 [29.5%] and hematological malignancies: 168 [35.4%]). On multivariable analysis, patients with LC were less likely to have an increase in telehealth visits compared to patients with hematological malignancies (aOR 0.47; 95% CI, 0.32–0.67). Notably, patients with LC were not more likely to have an increase in telehealth visits compared to patients with other solid tumors (aOR 1.13; 95% CI 0.85–1.50), despite the higher likelihood of decreased in-person visits noted earlier. A similar trend was seen for patients on systemic therapy during the index week; patients with LC were less likely to have an increase in telehealth visits compared to patients with hematological malignancies (aOR 0.35; 95% CI, 0.22–0.54).
During the pandemic period, 33 (10.5%) patients with LC experienced treatment delays due to the pandemic (vs. other solid tumors: 127 [8.0%] and hematological malignancies: 79 [16.7%]) and 26 (8.3%) patients with LC experienced delays in imaging or diagnostic procedures due to the pandemic. On multivariable analysis, patients with LC were more likely to experience treatment delays than patients with other solid tumors (aOR 1.80; 95% CI 1.13–2.80) and were more likely to experience imaging/diagnostic procedure delays than patients with other solid tumors (aOR 2.59; 95% CI, 1.46–4.47) and hematological malignancies (aOR 2.01; 95% CI, 1.02–3.93). Patients with LC on systemic therapy were more likely to have treatment and imaging delays than patients with other solid tumors on systemic therapy (aOR 1.84; 95% CI 1.10–3.01 and aOR 2.28; 95% CI 1.15–4.32, respectively).
4. Discussion
Patients with LC have experienced significant cancer care disruption due to the COVID-19 pandemic [6], [7]. In this prospective cohort study, patients with LC were at greater risk of COVID-19-related care disruption compared to patients with other malignancies; they experienced decreased in-person visits and increased treatment delays compared to patients with other solid tumors, as well as increased imaging/diagnostic delays compared to patients with both other solid and hematological malignancies. Among patients on systemic therapy during the index week, patients with LC were also at greater risk for decreased in-person visits and increased treatment delays compared to patients with other solid tumors. These findings may reflect the perception that patients were at increased risk of severe complications if they developed COVID-19 secondary to compromised pulmonary function or comorbidities prevalent in this population, leading to care alterations aimed at reducing infective risk. This perception aligns with the results of real-world studies which demonstrate that mortality from COVID-19 among patients with lung cancer early in the pandemic ranged from 25% to 33% [1], [8], [9].
This study examines the initial phase of the pandemic, when COVID-19 testing, treatment, and availability of telehealth was limited compared to today. Additionally, the non-lung cancer cohorts do not reflect the prevalence of these cancers in the general population and were based on available data from two US academic cancer centers. Even in view of these limitations, this study highlights that patients with LC were at increased risk for care disruption. These care alterations included increasing the duration between chemotherapy and immunotherapy cycles, as well as utilizing telehealth visits instead of in-person visits for patients on targeted oral therapy or active surveillance.
The consequences of delaying treatment, routine surveillance, and foregoing evidence-based cancer care among this high-risk population must be balanced against the competing risk of infection. Long-term effects of care change in patients undergoing lung cancer treatment or surveillance remain unknown, although alterations are unlikely to improve outcomes as most lead to fewer interactions with healthcare providers or decreased cancer-directed therapy. Literature detailing the impact of COVID-19 on new cancer diagnoses and prognosis is emerging, with fewer LC cases diagnosed during the pandemic period compared to the prior year and an increase in symptomatic and severe cases of LC diagnosed during the pandemic period [10], [11].
To date, no standard-of-care approach exists for treating patients with LC during the pandemic. Several organizations and groups of experts shared general recommendations for providers early in the pandemic [12], [13], [14]. The European Society of Medical Oncology (ESMO), for example, recommended prioritizing outpatient visits for individuals with new diagnosis of LC with disease-related symptoms or suspicion of clinical stage II-IV NSCLC and SCLC and those on current treatment [13]. Nonetheless, decisions must be individualized to each patient’s specific circumstance and preferences and discussed within a multidisciplinary setting [15].
As the pandemic continues worldwide, providers must continually balance the risks of COVID-19 infection versus the risks of delaying cancer treatment and surveillance. It is essential to minimize delays in these evidence-based practices that are known improve patient outcomes. Prioritizing vaccination among patients is one essential step to resume continuity of care in this vulnerable population.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Sheena Bhalla: Conceptualization, Methodology, Investigation, Writing – original draft, Writing - review & editing. Ziad Bakouny: Conceptualization, Methodology, Formal analysis, Investigation, Writing - review & editing. Andrew L. Schmidt: Conceptualization, Methodology, Investigation, Writing - review & editing. Chris Labaki: Investigation, Writing - review & editing. John A. Steinharter: Investigation, Writing - review & editing. Douglas A. Tremblay: Investigation, Writing - review & editing. Mark M. Awad: Resources, Writing - review & editing. Alaina J. Kessler: Investigation, Writing - review & editing. Robert I. Haddad: Resources, Writing - review & editing. Michelle Evans: Investigation, Writing - review & editing. Fiona Busser: Investigation, Writing - review & editing. Michael Wotman: Investigation, Writing - review & editing. Catherine R. Curran: Investigation, Writing - review & editing. Brittney S. Zimmerman: Investigation, Writing - review & editing. Gabrielle Bouchard: Investigation, Writing - review & editing. Tomi Jun: Investigation, Writing - review & editing. Pier V. Nuzzo: Investigation, Writing - review & editing. Qian Qin: Investigation, Writing - review & editing. Laure Hirsch: Investigation, Writing - review & editing. Jonathan Feld: Investigation, Writing - review & editing. Kaitlin M. Kelleher: Investigation, Writing - review & editing. Danielle Seidman: Investigation, Writing - review & editing. Hsin-Hui Huang: Formal analysis, Writing - review & editing. Heather M. Anderson-Keightly: Investigation, Writing - review & editing. Talal El Zarif: Investigation, Writing - review & editing. Sarah Abou Alaiwi: Investigation, Writing - review & editing. Talia D. Rosenbloom: Investigation, Writing - review & editing. Penina S. Stewart: Investigation, Writing - review & editing. Matthew D. Galsky: Conceptualization, Methodology, Writing - review & editing. Toni K. Choueiri: Conceptualization, Methodology, Writing - review & editing. Deborah B. Doroshow: Conceptualization, Methodology, Investigation, Writing – original draft, Writing - review & editing. : .
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Z. Bakouny: Non-financial support, Bristol Myers Squibb; research support, Genentech/ImCore; Honoraria from UpToDate. A. Schmidt: Educational travel support, Pfizer and Astellas. M.M. Awad: Research support, Bristol-Myers Squibb Lilly, AstraZeneca, and Genentech; consulting/advisory role, Bristol-Myers Squibb, Lilly, AstraZeneca, Genentech, Merck, Achilles, and Abbvie. R. Haddad: Consulting/Advisory role, BMS, Merck, Pfizer, Genetech, Astra Zeneca, and GSK; Research grant/funding, Merck, BMS, Pfizer, Genentech, GSK, and Astra Zeneca. S Tolaney: institutional research funding from AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Exelixis, Bristol-Myers Squibb, Eisai, Nanostring, Cyclacel, Odonate, and Seattle Genetics; has served as an advisor/consultant to AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Bristol-Myers Squibb, Eisai, Nanostring, Puma, Sanofi, Celldex, Paxman, Puma, Silverback Therapeutics, G1 Therapeutics, AbbVie, Anthenex, OncoPep, Outcomes4Me, Kyowa Kirin Pharmaceuticals, Daiichi-Sankyo, and Samsung Bioepsis Inc. M.D. Galsky reports: Stock, Rappta Therapeutics; consulting/advisory role, BioMotiv, Janssen, Dendreon, Merck, GlaxoSmithKline, Lilly, Astellas, Genentech, Bristol-Myers Squibb, Novartis, Pfizer, EMD Serono, AstraZeneca, Seattle Genetics, Incyte, Alleron Therapeutics, Dracen, Inovio Pharmaceuticals, NuMab, Dragonfly Therapeutics; institutional research funding, Janssen, Dendreon, Novartis, Bristol-Myers Squibb, Merck, AstraZeneca, Genentech/Roche. T. Choueiri: Research support, AstraZeneca, Alexion, Bayer, Bristol MyersSquibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Ipsen, 16 Tracon, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Lilly, Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Corvus, Calithera, Analysis Group, Sanofi/Aventis, Takeda; honoraria, AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol Myers-Squibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Roche, Roche Products Limited, F. Hoffmann-La Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, NCCN, Michael J. Hennessy (MJH) Associates, Inc (Healthcare Communications Company with several brands such as OnClive, PeerView and PER), Research to Practice, L-path, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, NEJM, Lancet Oncology, Heron Therapeutics, Lilly Oncology; consulting or advisory role, AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol Myers-Squibb/ER Squibb and sons LLC, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Heron Therapeutics, Lilly, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, Pionyr, Tempest, Lilly Ventures; owns stocks of Pionyr and Tempest; leadership role, Director of Genitourinary Oncology Division at Dana-Farber and past President of Medical Staff at Dana-Farber), member of NCCN Kidney panel and the GU Steering Committee, past chairman of the Kidney Cancer Association Medical and Scientific Steering Committee), KidneyCan Advisory board, Kidney cancer Research Summit co-chair (2019-); patents, royalties or other intellectual properties: International Patent Application No. PCT/US2018/12209, entitled “PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response,” filed January 3, 2018, claiming priority to U.S. Provisional Patent Application 17 No. 62/445,094, filed January 11, 2017; International Patent Application No. PCT/US2018/058430, entitled “Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy,” filed October 31, 2018, claiming priority to U.S. Provisional Patent Application No. 62/581,175, filed November 3, 2017; travel, accommodations, expenses, in relation to consulting, advisory roles, or honoraria; medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies (ClinicalThinking, Envision Pharma Group, Fishawack Group of Companies, Health Interactions, Parexel, Oxford PharmaGenesis, and others). Dr. Choueiri’s institution (Dana-Farber Cancer Institute) may have received additional independent funding of drug companies or/and royalties potentially involved in research around the subject matter. Dr. Choueiri has mentored several non-US citizens on research projects with potential funding (in part) from non-US sources/Foreign Components. D. Doroshow: Consulting/Advisory role, Ipsen, Boeringer Ingelheim, Atheneum Partners, Boston Healthcare Associates, Dedham Group, Guidepoint Global Advisors; travel expenses, Ipsen. All other authors have declared no conflicts of interest.]
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.lungcan.2021.07.002.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Garassino M.C., Whisenant J.G., Huang L.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L., Zhu F., Xie L., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q., Berger N.A., Xu R. Analyses of Risk, Racial Disparity, and Outcomes Among US Patients With Cancer and COVID-19 Infection. JAMA Oncol. 2021;7(2):220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lièvre A., Turpin A., Ray-Coquard I., et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: A French nationwide cohort study (GCO-002 CACOVID-19) Eur. J. Cancer. 2020;141:62–81. doi: 10.1016/j.ejca.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt A.L., Bakouny Z., Bhalla S., et al. Cancer Care Disparities during the COVID-19 Pandemic: COVID-19 and Cancer Outcomes Study. Cancer Cell. 2020;38(6):769–770. doi: 10.1016/j.ccell.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkrief A., Kazandjian S., Bouganim N. Changes in Lung Cancer Treatment as a Result of the Coronavirus Disease 2019 Pandemic. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calles A., Alva M., Aparicio I., et al. Impact of COVID-19 in continuity of cancer treatment for lung cancer patients. Clin. Cancer Res. 2020;26(18 Supplement) PO-021. [Google Scholar]
- 8.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo J., Rizvi H., Preeshagul I.R., et al. COVID-19 in patients with lung cancer. Ann. Oncol. 2020;31(10):1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.R. Reyes, R. López-Castro, E. Auclin, et al., Impact of COVID-19 Pandemic in the Diagnosis and Prognosis of Lung Cancer. 2020 World Conference on Lung Cancer Singapore; January 28-31; Virtual. Abstract 3700.
- 11.Bakouny Z., Paciotti M., Schmidt A.L., Lipsitz S.R., Choueiri T.K., Trinh Q.D. Cancer Screening Tests and Cancer Diagnoses During the COVID-19 Pandemic. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2020.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dingemans A.C., Soo R.A., Jazieh A.R., et al. Treatment Guidance for Patients With Lung Cancer During the Coronavirus 2019 Pandemic. J. Thorac. Oncol. 2020;15(7):1119–1136. doi: 10.1016/j.jtho.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ESMO. ESMO Management and Treatment Adapted Recommendations in the COVID-19 Era: Lung Cancer. 2020. Accessed March 11, 2021. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/lung-cancer-in-the-covid-19-era. [DOI] [PMC free article] [PubMed]
- 14.Singh A.P., Berman A.T., Marmarelis M.E., et al. Management of Lung Cancer During the COVID-19 Pandemic. JCO Oncol. Pract. 2020;16(9):579–586. doi: 10.1200/OP.20.00286. [DOI] [PubMed] [Google Scholar]
- 15.Hartman H.E., Sun Y., Devasia T.P., et al. Integrated Survival Estimates for Cancer Treatment Delay Among Adults With Cancer During the COVID-19 Pandemic. JAMA Oncol. 2020;6(12):1881–1889. doi: 10.1001/jamaoncol.2020.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.