Arthrospira maxima (Spirulina) is a cyanobacterium which is considered a nutraceutical because it has antioxidant, anti-inflammatory, and cytoprotective properties in different renal disease models (Rodriguez-Sánchez et al., 2012; Aziz et al., 2018; Memije-Lazaro et al., 2018). The therapeutic effects are due to the presence of metabolites with biological effects similar to those of essential fatty acids ω-3 and ω-6, vitamins A, C and E, and accessory pigments such as phycobiliproteins. One of the most abundant phycobiliproteins in A. maxima is C-phycocyanin (Mysliwa-Kurdziel and Solymosi, 2017). This molecule is responsible for nephroprotective action in a model of acute kidney injury (AKI) because it reduces oxidative stress and caspase activation (Rodriguez-Sánchez et al., 2012; Rojas-Franco et al., 2018). However, both A. maxima and its C-phycocyanin are related to the reduction of the redox environment. Thus, they probably help to maintain the adequate function of the intracellular organelles like the endoplasmic reticulum. However, this therapeutic action has not been evaluated previously.
In the laboratory, AKI is induced in animals by intoxication with inorganic mercury. The kidney is a target organ for inorganic mercury toxicity because the mercury binds to thiol-containing proteins in the tubular and glomerular nephron portion, disturbing the tubular transport mechanism and producing oxidative stress (Zalups, 2000; Orr et al., 2019). Besides these events, Hg2+ promotes the accumulation of misfolded and unfolded proteins in the endoplasmic reticulum, causing endoplasmic reticulum stress (ERS) (Stacchiotti et al., 2004; Rojas-Franco et al., 2019). The ERS activates three pathways: the protein kinase RNA-like ER-kinase (PERK) pathway, the activating transcription factor-6α (ATF-6α) pathway, and the inositol-requiring enzyme-1α (IRE-1α) pathway. When ERS cannot compensate the cell damage, these pathways promote cell death (Stacchiotti et al., 2004). Inorganic mercury intoxication in mice activates the PERK/eukaryotic initiation factor 2α (eIF2α) pathway in the kidney during the first 48 h without a protective response. Meanwhile, in the 72 h after intoxication, the PERK/ATF-4 activation branch causes the ATF-4, ATF-6α, and IRE-1α pathways to activate a cell death promoter response through growth arrest- and DNA damage-inducible gene 153 (GADD153) and subsequent activation of caspases 12 and 3 (Rojas-Franco et al., 2019).
Thus, in this work, we aimed to evaluate the effects of A. maxima and C-phycocyanin treatment on the three branches involved in ERS in mercury-caused AKI, to provide more information about the molecular mechanisms of nephroprotection of both nutraceuticals.
We used eighteen male NIH-Swiss albino mice weighing between 25 and 30 g. They were housed in groups of three in Plexiglas cages, and given food and water ad libitum in a room with constant temperature (21±2) ℃ and a 12-h light/dark cycle.
First, the A. maxima used in this experiment was cultured in our laboratory in Zarrouk medium, and C-phycocyanin was purified from it as described in our previous study (Rodriguez-Sánchez et al., 2012; Memije-Lazaro et al., 2018). Briefly, we used a Sephadex G-250 column equilibrated with 100 mmol/L phosphate buffer (PB; pH=7.4). The exclusion chromatography was eluted with PB (pH=7.4) with a linear gradient from 100.0 to 6.5 mmol/L. The protein was precipitated with (NH4)2SO4 at 4 ℃ and dialyzed. The resultant fraction was passed through ion-exchange chromatography using diethylaminoethyl (DEAE)-cellulose equilibrated with 50 mmol/L acetate buffer (pH=5.5). The column was eluted with a 50 mmol/L acetate buffer (pH=5.5). Finally, the protein was precipitated with (NH4)2SO4 at 4 ℃, dialyzed, and lyophilized.
Mice were randomly assigned into six groups (Fig. S1 shows a diagram of the groups). Three control groups received: (1) 100 mmol/L PB by oral gavage (og) per day as a vehicle for the nutraceuticals+0.9% saline solution (SS) intraperitoneally (ip); (2) 1 g/(kg·d) of A. maxima (og)+SS (ip); (3) 100 mg/(kg·d) of C-phycocyanin (og)+SS (ip). The other three groups received 5 mg/kg HgCl2 (ip) and each one received treatment with: (4) vehicle (100 mmol/L PB (og)); (5) 1 g/(kg·d) A. maxima (og); or (6) 100 mg/(kg·d) C-phycocyanin (og).
The administration of A. maxima and C-phycocyanin was done 30 min before saline and HgCl2 administration. Seventy-two hours after mercury intoxication, the mice were euthanized by cervical dislocation and their kidneys were dissected and frozen at -80 ℃ until we determined the ERS markers by western blotting (Rojas-Franco et al., 2019). The primary antibodies used for this study were caspase 12 (Millipore, Billerica, Massachusetts, USA; AB3613), ATF-6α, GADD34, X-box-binding protein 1 (XBP1), GADD153, and eIF2α (Santa Cruz Biotechnology, Dallas, Texas, USA; sc-22799, sc-8327, sc-575, sc-7160, and sc-517214, respectively), IRE-1α (Abcam, UK; ab37073), ATF-4, podocin, and nephrin (Biorbyt, Cambridge, UK; orb-129518, orb-337389, and orb-11107, respectively). Protein β-actin expression was used as a constitutive protein (Santa Cruz Biotechnology; sc-1615). According to ImageJ program specifications, we analyzed optical density (OD) from all bands obtained with the program.
All data in our results are presented as mean±standard error of the mean (SEM). We analyzed the data by two-way analysis of variance (ANOVA) and the Student's Newman-Keuls post-hoc test. The factors were treatment with nutraceutical and the presence of AKI. Values that presented at P<0.05 were considered statistically different.
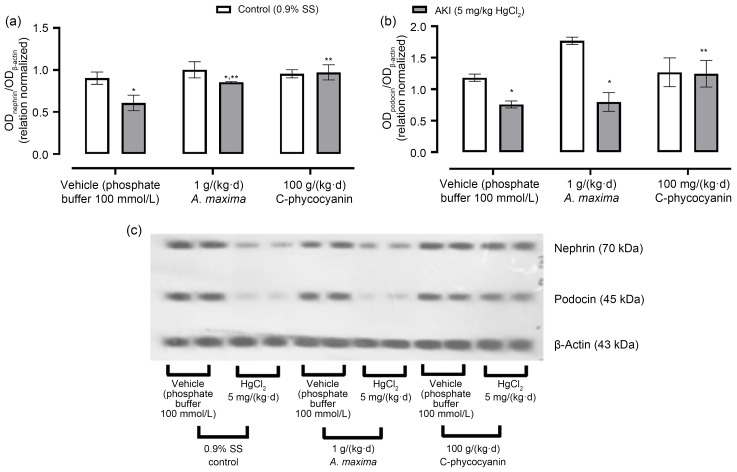
Fig. 1 shows the effects of nutraceutical treatment with A. maxima and its C-phycocyanin on nephrin and podocin expression in the kidneys of mice intoxicated with inorganic mercury. It also displays a representative blot for protein expression. It is evident that HgCl2-induced AKI reduced the renal expression of nephrin (29%) and podocin (45%). Meanwhile, A. maxima treatment prevented AKI-caused down-expression of both proteins. The reduction of the expression of nephrin was about 6%, and of podocin was about 33%. Also, C-phycocyanin prevented alteration in podocin and nephrin expression in the kidneys of mice with AKI.
Fig. 1. Effects of Arthrospira maxima and C-phycocyanin on renal expression of nephrin (a) and podocin (b) in the HgCl2-caused acute kidney injury (AKI) model. (c) Protein expression by western blotting. Data are represented as mean±standard error of the mean (SEM), n=3. * P<0.05 with respect to its control group; ** P<0.05 with respect to its vehicle group. SS: saline solution; OD: optical density.
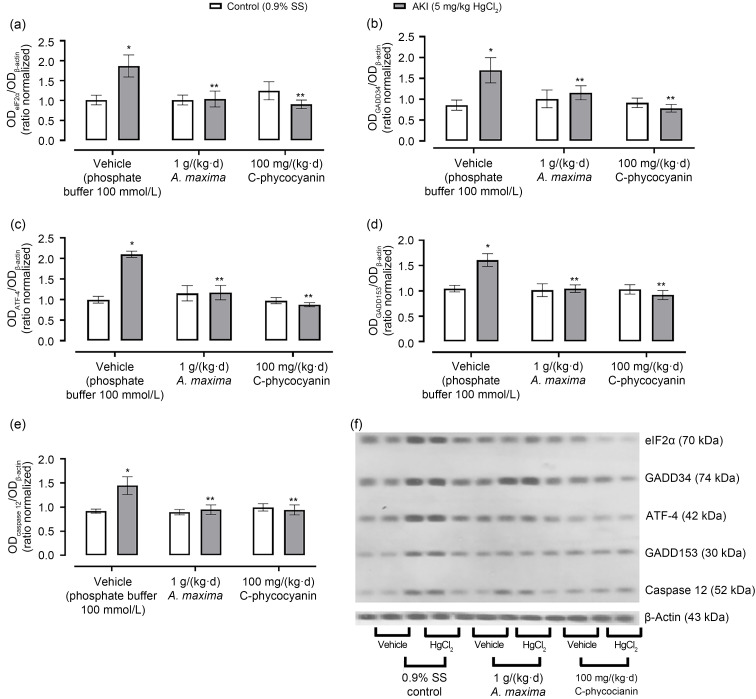
Fig. 2 shows the effects of A. maxima and C-phycocyanin on the PERK/eIF2α/ATF-4 signaling pathway. AKI induced by mercury intoxication caused over-expression of all proteins evaluated (eIEF2α=86.00%, GADD34=97.89%, ATF-4=101.14%, GADD153=60.48%, and caspase 12=45.79%). On the other hand, A. maxima treatment prevented increases in GADD34 (an increase of 35.68%) and caspase 12 (an increase of 4.11%). Also, it prevented changes in expression of GADD153 and ATF-4. With regard to C-phycocyanin treatment, it prevented altered expression of all proteins evaluated.
Fig. 2. Effects of Arthrospira maxima and C-phycocyanin on renal expression of PERK and ATF-4 signaling pathways in the HgCl2-caused acute kidney injury (AKI) model. The proteins that participate in these pathways are eIF2α (a), GADD34 (b), ATF-4 (c), GADD153 (d), and caspase 12 (e). (f) Protein expression by western blotting. Data are represented as mean±standard error of the mean (SEM), n=3. * P<0.05 with respect to its control group; ** P<0.05 with respect to its vehicle group. OD: optical density; SS: saline solution; eIF2α: eukaryotic initiation factor 2α; GADD34/153: growth arrest- and DNA damage-inducible gene 34/153; ATF-4: activating transcription factor-4; PERK: protein kinase RNA-like ER-kinase.
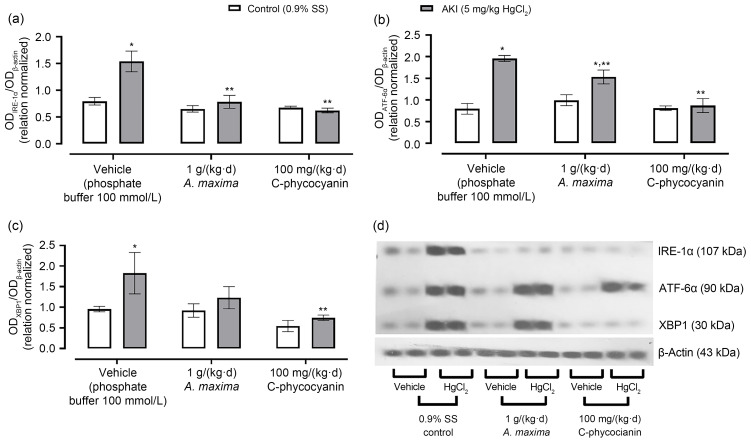
Fig. 3 shows the effects of A. maxima or C-phycocyanin on the IRE-1α and ATF-6α branches in the kidneys of mice intoxicated with inorganic mercury, including the changes in expression of IRE-1α; it is evident that mercury enhanced the effects by about 100%, and treatment with A. maxima only increased them by about 20%. Meanwhile, C-phycocyanin treatment prevented the altered expression of IRE-1α in mercury-caused AKI. Mercury also caused an over-expression of ATF-6α (145%) and XBP1 (50%). The A. maxima and C-phycocyanin treatments only enhanced the expression of ATF-6α (by about 54% and 7%, respectively) while XBP1 only expressed about 33% with Spirulina treatment and 37% with C-phycocyanin treatment.
Fig. 3. Effects of Arthrospira maxima and C-phycocyanin on expression of IRE-1α (a), ATF-6α (b), and XBP1 (c) in the HgCl2-caused acute kidney injury (AKI) model. (d) Protein expression by western blotting. Data are represented as mean±standard error of the mean (SEM), n=3. * P<0.05 with respect to its control group; ** P<0.05 with respect to its vehicle group.OD: optical density; SS: saline solution; IRE-1α: inositol-requiring enzyme-1α; ATF-6α: activating transcription factor-6α; XBP1: X-box-binding protein 1.
Nutraceutical demand stimulates the food industry to develop new products that claim beneficial health effects. However, it is crucial to demonstrate the mechanism of these therapeutic effects. In the case of A. maxima and its C-phycocyanin, research showed that they had a nephroprotective effect in animal models of AKI caused by inorganic mercury intoxication. The nephroprotective effect is related to the antioxidant and anti-apoptotic effects in the kidney (Rodriguez-Sánchez et al., 2012; Rojas-Franco et al., 2018). Likewise, some researchers demonstrated that A. maxima and C-phycocyanin prevented cell death in RINm5F cell cultures through activation of the reactive oxygen species (ROS)/Akt/nuclear factor-κB (NF-κB) pathway to reduce ERS, but they only evaluated spliced XBP1 (spXBP1) and GADD153 messenger RNA (mRNA) synthesis (Lee et al., 2017). However, this was the first study to evaluate the effect of A. maxima and its C-phycocyanin treatments on the three branches of ERS (PERK, ATF-4, and ATF-6α) in the model of mercury-caused AKI.
HgCl2-caused AKI is characterized by renal dysfunction due to tubular necrosis and glomerular damage (Rojas-Franco et al., 2018). This glomerular damage model is associated with slit membrane damage of the podocyte. Two essential proteins of the slit membrane evaluated in this study were podocin and nephrin. Inorganic mercury intoxication reduced their expression, and proteinuria occurred because of the organizational change in the slit diaphragm. Another point is that low expression of nephrin is associated with a reduction in pro-survival signaling into podocytes because nephrin activates the phosphoinositide 3-kinase (PI3K)/Akt pathway with an anti-apoptotic effect (Shankland, 2006). In our study, A. maxima and its C-phycocyanin treatments prevented low expression of nephrin and podocin, and the treatments were associated with the anti-apoptotic effects previously reported (Rojas-Franco et al., 2018).
On the other hand, inorganic mercury enhanced the expressed proteins associated with unfolding protein response (UPR), such as GADD34, heat shock protein 72 (HSP72), HSP60, and glucose-regulated protein 75 (GRP75) (Stacchiotti et al., 2004). In a previous report, HgCl2 caused ERS in the kidney because the PERK/eIF2α/ATF-4/GADD153 pathway activated during the first 48 h after intoxication. However, it had a protective response that activated IRE-1α/XBP1, promoting cell death by activating caspases 12 and 3, 72 h after inorganic mercury intoxication (Rojas-Franco et al., 2019). Concerning A. maxima and C-phycocyanin, a few studies have demonstrated a reduction of the UPR in cell culture by treating with phycocyanin only (Lee et al., 2017). Our study is the first that reveals that A. maxima and its C-phycocyanin prevent HgCl2-caused ERS in the kidney in mammals. First, A. maxima and C-phycocyanin administrations limit ROS production and disturbance in the antioxidant system of renal cells, preventing disruption of the ERS. In particular, the treatments with A. maxima and C-phycocyanin prevented activation of the three branches of ERS. A. maxima contains several natural polyphenols that modulate phosphorylation of the ER transmembrane sensor and prevent GRP78 from dissociating the sensory proteins in the membrane; they also inhibit ERS (Liu et al., 2016). On the other hand, A. maxima contains C-phycocyanin, which by itself exerts nutraceutical effects on ERS. C-phycocyanin prevents activation of the PERK/eIF2α/ATF-4/GADD153 pathway that promotes cell death because the molecule treatment reduces ATF-4 and GADD153 expression (Bhardwaj et al., 2020). Also, C-phycocyanin reduces the activity of the IRE-1α/XBP1 signaling pathway in the kidneys of animals intoxicated with mercury. This branch is a pro-survival pathway that operates through the over-expression of several chaperones. However, under a sustained engaged stimulus such as mercury intoxication, IRE-1α stimulates c-Jun N-terminal kinase (JNK)/mitogen-activated protein kinase 8 (MAPK8)/stress/abscisic acid (ABA)-activated protein kinase 1 (SAPK1) pathway (Iurlaro and Muñoz-Pinedo, 2016). This idea is supported by the fact that in cell culture, C-phycocyanin decreases the expression of phosphorylated ERK (p-ERK), p-JNK, p-p38, Bcl-2-associated X protein (Bax), caspase 9, and caspase 3, but increases expression of Bcl-2 (Lim et al., 2012). Finally, we propose that C-phycocyanin treatment prevents ERS by reducing caspase 12-mediated cell death in the kidneys of animals intoxicated with inorganic mercury. Also, C-phycocyanin reduces the activity of caspases 3 and 9 in kidneys exhibiting HgCl2-caused AKI (Rojas-Franco et al., 2018).
In this paper, we propose that the nephroprotective action of A. maxima and C-phycocyanin against mercury-caused AKI is related to preventing reduction of nephrin and podocin expression, as well as prevention of ERS. In conclusion, treatment with A. maxima reduced over-expression of GADD34, IRE-1α, ATF-6α, and caspase 12, which are related to HgCl2-caused ERS. Meanwhile, C-phycocyanin treatment prevented the ERS caused by inorganic mercury because it normalized all protein expression except that of ATF-6α. Finally, we suggest A. maxima and C-phycocyanin as an alternative therapy to prevent HgCl2-caused AKI.
Supplementary information
Acknowledgments
This study was supported by the Secretaría de Investigación y Posgrado, Instituto Politécnico Nacional (SIP-IPN; Nos. 20200521, 20201091, 20200493, 20200402, and 20200327). We should like to thank Dr. En Tao WANG-HU (Departamento de Microbiología, Escuela Nacional de Ciencias Biológicas (ENCB)-IPN) for help us with the abstract in Chinese.
Author contributions
Edgar CANO-EUROPA and Margarita FRANCO-COLÍN designed the research. Placido ROJAS-FRANCO and Vanessa BLAS-VALDIVIA conducted the experiments. María Estela MELENDEZ-CAMARGO performed the data analysis. All authors participated in writing the article and have read and approved the final manuscript.
Compliance with ethics guidelines
Placido ROJAS-FRANCO, Margarita FRANCO-COLÍN, Vanessa BLAS-VALDIVIA, María Estela MELENDEZ-CAMARGO, and Edgar CANO-EUROPA declare that they have no conflict of interest.
All experimental procedures described in this research study followed the Mexican Laws and Codes (NOM-062-ZOO-1999). Also, the protocol was approved by the Internal Bioethics Committee of the Escuela Nacional de Ciencias Biológicas (ENCB, IPN), México (CEI-ENCB 019/2014).
References
- Aziz MM, Eid NI, Nada AS, et al. , 2018. Possible protective effect of the algae spirulina against nephrotoxicity induced by cyclosporine A and/or gamma radiation in rats. Environ Sci Pollut Res, 25(9): 9060-9070. 10.1007/s11356-017-1146-0 [DOI] [PubMed] [Google Scholar]
- Bhardwaj M, Leli NM, Koumenis C, et al. , 2020. Regulation of autophagy by canonical and non-canonical ER stress responses. Semin Cancer Biol, 66: 116-128. 10.1016/j.semcancer.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurlaro R, Muñoz-Pinedo C, 2016. Cell death induced by endoplasmic reticulum stress. FEBS J, 283(14): 2640-2652. 10.1111/febs.13598 [DOI] [PubMed] [Google Scholar]
- Lee J, Park A, Kim MJ, et al. , 2017. Spirulina extract enhanced a protective effect in type 1 diabetes by anti-apoptosis and anti-ROS production. Nutrients, 9(12): 1363. 10.3390/nu9121363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BJ, Jeong JY, Chang YK, et al. , 2012. C-phycocyanin attenuates cisplatin-induced nephrotoxicity in mice. Ren Fail, 34(7): 892-900. 10.3109/0886022X.2012.690925 [DOI] [PubMed] [Google Scholar]
- Liu H, Yang JQ, Li LF, et al. , 2016. The natural occurring compounds targeting endoplasmic reticulum stress. Evid Based Complement Alternat Med, 2016: 7831282. 10.1155/2016/7831282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memije-Lazaro IN, Blas-Valdivia V, Franco-Colín M, et al. , 2018. Arthrospira maxima (Spirulina) and C-phycocyanin prevent the progression of chronic kidney disease and its cardiovascular complications. J Funct Foods, 43: 37-43. 10.1016/j.jff.2018.01.013 [DOI] [Google Scholar]
- Mysliwa-Kurdziel B, Solymosi K, 2017. Phycobilins and phycobiliproteins used in food industry and medicine. Mini Rev Med Chem, 17(3): 1173-1193. 10.2174/1389557516666160912180155 [DOI] [PubMed] [Google Scholar]
- Orr SE, Barnes MC, Joshee L, et al. , 2019. Potential mechanisms of cellular injury following exposure to a physiologically relevant species of inorganic mercury. Toxicol Lett, 304: 13-20. 10.1016/j.toxlet.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sánchez R, Ortiz-Butrón R, Blas-Valdivia V, et al. , 2012. Phycobiliproteins or C-phycocyanin of Arthrospira (Spirulina) maxima protect against HgCl2-caused oxidative stress and renal damage. Food Chem, 135(4): 2359-2365. 10.1016/j.foodchem.2012.07.063 [DOI] [PubMed] [Google Scholar]
- Rojas-Franco P, Franco-Colín M, Camargo MEM, et al. , 2018. Phycobiliproteins and phycocyanin of Arthrospira maxima (Spirulina) reduce apoptosis promoters and glomerular dysfunction in mercury-related acute kidney injury. Toxicol Res Appl, 2: 1-10. 10.1177/2397847318805070 [DOI] [Google Scholar]
- Rojas-Franco P, Franco-Colín M, Torres-Manzo AP, et al. , 2019. Endoplasmic reticulum stress participates in the pathophysiology of mercury-caused acute kidney injury. Ren Fail, 41(1): 1001-1010. 10.1080/0886022X.2019.1686019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankland SJ, 2006. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int, 69(12): 2131-2147. 10.1038/sj.ki.5000410 [DOI] [PubMed] [Google Scholar]
- Stacchiotti A, Lavazza A, Rezzani R, et al. , 2004. Mercuric chloride-induced alterations in stress protein distribution in rat kidney. Histol Histopathol, 19(4): 1209-1218. 10.14670/HH-19.1209 [DOI] [PubMed] [Google Scholar]
- Zalups RK, 2000. Molecular interactions with mercury in the kidney. Pharmacol Rev, 52(1): 113-143. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.