Abstract
Aim of the study
Vitamin D deficiency is known to be associated with disease severity, unresponsiveness to treatment, and morbidity among patients with chronic viral hepatitis B and C, autoimmune hepatitis, and alcoholic hepatitis. This study aims to research vitamin D levels in patients suffering from cirrhotic and non-cirrhotic phases of hepatitis D.
Material and methods
170 individuals in total were included in the study in the form of two groups: the first group of 100 patients with chronic hepatitis D (CHD), 30 of whom had cirrhosis, and the second control group of 70 individuals with similar characteristics to those of the first group in terms of age, type, and seasonal sampling. Levels of 25-hydroxy vitamin D [25(OH)D] were measured in the serum collected from patients and the control group.
Results
The lowest 25(OH)D levels were identified in patients with cirrhotic CHD. When these levels were compared with those of the control group, they were found to be significant (15.30 ±6.92 and 18.90 ±8.30 ng/ml, respectively, p = 0.04). 25(OH)D deficiency (< 10 ng/ml) was detected at significantly higher rates in patients with both cirrhotic and non-cirrhotic CHD compared to the healthy controls (30%, 25%, and 8.5%, respectively, p = 0.01). A significant correlation was established between 25(OH)D levels and bilirubin in patients with CHD (r = 0.252, p = 0.012). Multivariate analysis showed that chronic hepatitis D (odds ratio [OR] = 3.608, 95% confidence interval [CI]: 1.31-9.89, p = 0.013) and age (OR = 1.04, 95% CI: 1.00-1.08, p = 0.033) were associated with vitamin D deficiency.
Conclusions
Frequency of 25(OH)D vitamin deficiency is higher in patients with CHD. The identification of vitamin D levels and the replacement of any deficiency may create a positive effect on disease progression, morbidity, and mortality levels.
Keywords: hepatitis delta virus, liver cirrhosis, 25-hydroxyvitamin D2
Introduction
Hepatitis D is the most serious form of viral hepatitis in humans. Whereas concurrent infection evolves into chronicity in only 2% of cases, superinfection leads to chronic infection in over 90% of cases [1, 2]. Chronic hepatitis D (CHD) poses a higher risk for cirrhosis, decomposition, and hepatocellular carcinoma when compared to chronic hepatitis B mono-infection because of the faster progression of fibrosis in this disease. It is estimated that 15 to 20 million people worldwide are infected with hepatitis D virus (HDV) [3]. Despite a range of drug trials, the only therapeutic option for this disease is observed to be pegylated interferon. Nevertheless, the virological response rate achieved through this form of therapy does not exceed approximately 30% [4].
In adults, deficiency of vitamin D can be a preliminary factor aggravating osteopenia and osteoporosis and may lead to osteomalacia, as well as muscle weakness, and increase fracture risk [5, 6]. Several findings indicate that it is associated with a wide spectrum of diseases ranging from autoimmune diseases to cancer in addition to its impact on the musculoskeletal system [7]. Besides calcium metabolism, vitamin D shows effects on significant cellular functions such as proliferation and differentiation [8]. The liver plays an important role in the metabolism of vitamin D. In the liver, vitamin D derived from the skin and diet is hydroxylated into 25-hydroxy vitamin D [25(OH)D], which represents the major circulating form of vitamin D and which is used to determine a patient’s vitamin D status [9].
The literature has reported many studies concerning vitamin D levels in chronic liver diseases of varying etiologies and their association with disease severity [10-15]. Some of these studies established an association between low vitamin D levels and disease severity [10-14], while others failed to support this finding [15]. Chronic hepatitis D is an important public health problem in the South-Eastern region of Turkey where the study was conducted. Our aim in the present study is to investigate vitamin D levels among patients with chronic hepatitis D and the association between these levels and disease phases.
Material and methods
Patients
The study was undertaken in compliance with the principles of the Helsinki Declaration and was approved by the Local Ethics Committees of the Dicle University School of Medicine with serial number 2014/375.
The patient population of the present study comprises 100 patients with CHD, 30 of them cirrhotic, who had subsequently applied as outpatients to the Gastroenterology Clinic of the Faculty of Medicine under Dicle University between January 2014 and March 2015. The disease was diagnosed according to the following criteria for CHD infection: positive finding for hepatitis B surface antigen (HBsAg) and anti-HDV antibodies for at least 6 months, serum alanine or aspartate aminotransferase activity higher than the normal threshold, and positive finding for HDV RNA. Histopathological evaluations were performed to determine the Ishak scores [16]. Liver cirrhosis was diagnosed histopathologically [16] or by comprehensively reviewing laboratory parameters and physical, endoscopic, and/or ultrasonographic findings.
Patients who had concomitant hepatitis C virus (HCV), human immunodeficiency virus (HIV) infection, acute exacerbation of chronic hepatitis B, autoimmune hepatitis, alcoholic hepatitis, hepatic and extra-hepatic malignancies, inflammatory bowel disorders, and drugs known to affect vitamin D metabolism and vitamin/mineral supplements were excluded from the study.
Demographic and clinical characteristics and laboratory results, including age, sex, presence or absence of diabetes, body mass index, biochemical, and hemogram tests were recorded.
Controls
We included age- and sex-matched healthy subjects who applied to our outpatient clinic for health screening in the control group. Hepatitis B virus (HBV), HCV, HDV, HIV serology results of these patients were negative. Liver function test results were normal. Patients were not taking vitamin D or any other vitamin supplements. Seasonal variability is frequently observed for 25(OH)D levels in human serum samples. Therefore, serum samples were taken from the patient and control groups at similar time intervals.
Blood collection
Blood samples were obtained following overnight fasting from healthy volunteers and patients with CHD after the collection of informed consent. The serum was then separated from the cells by centrifugation at 3000 rpm for 10 minutes. The serum was isolated and kept at –80°C until subsequent analysis.
Quantification of 25(OH)D serum levels
The analysis of serum 25(OH)D was performed through the use of high-performance liquid chromatography (ImmuChrom GmbH, Germany). Cases with vitamin D concentrations of < 20 and < 10 ng/ml were defined as insufficiency and deficiency, respectively, whereas concentrations ≥ of 20 ng/ml were considered adequate [17].
Virological assessment
Levels of HBsAg (qualitative), anti-HBs, hepatitis B e antigen (HBeAg), anti-HBe, anti-HCV, and anti-HIV were determined in the microbiology laboratory of our hospital by the macro-EIA method using Cobas e 601 by Roche with its original kits. The total anti-HDV was determined on the Triturus model of Grifols employing the micro-ELISA method. HDV RNA was measured on LightCycler 2.0 by Roche (Roche Diagnostics GmbH, Manheim Germany) using real-time RT-PCR.
Statistical analysis
The analysis of the data was performed using the statistical software SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Inter-group comparisons were performed using Student’s test where numeric data demonstrated a normal distribution and using the Mann-Whitney U test where numeric data did not demonstrate a normal distribution. Groups with categorical variables were compared using the Pearson χ2 test. The binary logistic regression analysis was performed to detect the independent predictors of deficiency of 25(OH)D. Any p-value smaller than 0.05 was considered statistically significant.
Results
The mean age of the patient population of 44 females and 56 males was 41.6 ±12.1 years (range: 16-71 years) and 30 of these patients were cirrhotic. The control group was comparable for age, sex, body mass index (BMI), and sampling season with the CHD group (Table 1). 30 of the remaining 51 patients did not undergo liver biopsy due to cirrhosis, and 29 patients started pegylated interferon (PEG-IFN) therapy without a biopsy. A total of 58 patients have received PEG-IFN therapy. Forty-nine of the patients had undergone liver biopsy. Clinical and laboratory baseline characteristics of the CHD patients with and without cirrhosis are shown in Table 2.
Table 1.
Demographic characteristics of patients with chronic hepatitis D and the healthy group
| HDV patients and healthy controls | Age | Sex | Season | BMI | DM | Creatinine | Albumin | Child score | MELD score | Fibrosis stage | INR | Total bilirubin | Total protein | BUN | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HDV patients and healthy controls | r | 1 | –0.075 | 0.045 | 0.030 | 0.127 | –0.130 | . c | . c | . c | . c | . c | . c | . c | . c | . c |
| p | 0.331 | 0.559 | 0.702 | 0.100 | 0.091 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| n | 170 | 170 | 170 | 170 | 170 | 170 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| Age | r | –0.075 | 1 | –0.240** | 0.075 | –0.027 | 0.084 | –0.051 | –0.331** | 0.228* | 0.157 | 0.090 | 0.145 | 0.065 | –0.186 | –0.005 |
| p | 0.331 | 0.002 | 0.333 | 0.730 | 0.275 | 0.613 | 0.001 | 0.022 | 0.120 | 0.545 | 0.151 | 0.521 | 0.064 | 0.958 | ||
| n | 170 | 170 | 170 | 170 | 170 | 170 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| Sex | r | 0.045 | –0.240** | 1 | 0.033 | 0.043 | –0.065 | –0.113 | –0.041 | –0.048 | –0.002 | –0.125 | –0.015 | –0.247* | –0.136 | –0.040 |
| p | 0.559 | 0.002 | 0.674 | 0.580 | 0.399 | 0.262 | 0.687 | 0.636 | 0.981 | 0.399 | 0.885 | 0.013 | 0.177 | 0.695 | ||
| n | 170 | 170 | 170 | 170 | 170 | 170 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| Season | r | 0.030 | 0.075 | 0.033 | 1 | 0.102 | 0.005 | –0.053 | –0.002 | –0.117 | –0.109 | –0.251 | –0.177 | –0.184 | –0.092 | –0.044 |
| p | 0.702 | 0.333 | 0.674 | 0.187 | 0.943 | 0.599 | 0.981 | 0.245 | 0.280 | 0.085 | 0.079 | 0.067 | 0.362 | 0.664 | ||
| n | 170 | 170 | 170 | 170 | 170 | 170 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| BMI | r | 0.127 | –0.027 | 0.043 | 0.102 | 1 | –0.055 | 0.109 | 0.013 | 0.097 | 0.161 | –0.103 | –0.055 | –0.091 | 0.144 | –0.161 |
| p | 0.100 | 0.730 | 0.580 | 0.187 | 0.477 | 0.279 | 0.899 | 0.338 | 0.110 | 0.484 | 0.587 | 0.369 | 0.152 | 0.109 | ||
| n | 170 | 170 | 170 | 170 | 170 | 170 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| DM | r | –.130 | 0.084 | –.065 | .005 | –.055 | 1 | –0.002 | 0.068 | –0.121 | –0.123 | -0.160 | –0.080 | 0.024 | 0.043 | –0.081 |
| p | 0.091 | 0.275 | .399 | .943 | .477 | 0.987 | 0.500 | 0.230 | 0.222 | 0.278 | 0.429 | 0.810 | 0.669 | 0.424 | ||
| n | 170 | 170 | 170 | 170 | 170 | 170 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| Crea | r | . c | –0.051 | –.113 | –.053 | .109 | –.002 | 1 | 0.124 | –0.174 | –0.188 | –0.203 | –0.107 | –0.100 | 0.077 | – 0.060 |
| p | 0.000 | 0.613 | .262 | .599 | .279 | .987 | 0.221 | 0.083 | 0.060 | 0.166 | 0.289 | 0.322 | 0.448 | 0.552 | ||
| n | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| Albumin | r | . c | –0.331** | –.041 | –.002 | .013 | .068 | 0.124 | 1 | –0.261** | –0.218* | –0.010 | –0.182 | –0.196 | 0.414** | 0.083 |
| p | 0.000 | 0.001 | .687 | .981 | .899 | .500 | 0.221 | 0.009 | 0.029 | 0.944 | 0.070 | 0.051 | 0.000 | 0.410 | ||
| n | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| Child score | r | . c | 0.228* | –.048 | –.117 | .097 | –.121 | –0.174 | –0.261** | 1 | 0.924** | 0.414** | 0.632** | 0.422** | –0.021 | 0.158 |
| p | 0.000 | 0.022 | .636 | .245 | .338 | .230 | 0.083 | 0.009 | 0.000 | 0.003 | 0.000 | 0.000 | 0.832 | 0.116 | ||
| n | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| MELD score | r | . c | 0.157 | –.002 | –.109 | .161 | –.123 | –0.188 | –0.218* | 0.924** | 1 | 0.427** | 0.584** | 0.367** | –0.033 | 0.109 |
| p | 0.000 | 0.120 | .981 | .280 | .110 | .222 | 0.060 | 0.029 | 0.000 | 0.002 | 0.000 | 0.000 | 0.742 | 0.280 | ||
| n | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| Fibrosis stage | r | . c | 0.090 | –.125 | –.251 | –.103 | –.160 | –0.203 | –0.010 | 0.414** | 0.427** | 1 | 0.312* | 0.189 | 0.192 | 0.135 |
| p | 0.000 | 0.545 | .399 | .085 | .484 | .278 | 0.166 | 0.944 | 0.003 | 0.002 | 0.031 | 0.198 | 0.192 | 0.362 | ||
| n | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | |
| INR | r | . c | 0.145 | –.015 | –.177 | –.055 | –.080 | –0.107 | –0.182 | 0.632** | 0.584** | 0.312* | 1 | 0.415** | –0.006 | 0.047 |
| p | 0.000 | 0.151 | .885 | .079 | .587 | .429 | 0.289 | 0.070 | 0.000 | 0.000 | 0.031 | 0.000 | 0.955 | 0.643 | ||
| n | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| Total bilirubin | r | . c | 0.065 | –.247* | –.184 | –.091 | .024 | –0.100 | –0.196 | 0.422** | 0.367** | 0.189 | 0.415** | 1 | 0.050 | 0.136 |
| p | 0.000 | 0.521 | .013 | .067 | .369 | .810 | 0.322 | 0.051 | 0.000 | 0.000 | 0.198 | 0.000 | 0.624 | 0.176 | ||
| n | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| Total protein | r | . c | –0.186 | –.136 | –.092 | .144 | .043 | 0.077 | 0.414** | –0.021 | –0.033 | 0.192 | –0.006 | 0.050 | 1 | 0.102 |
| p | 0.000 | 0.064 | .177 | .362 | .152 | .669 | 0.448 | 0.000 | 0.832 | 0.742 | 0.192 | 0.955 | 0.624 | 0.313 | ||
| n | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
| BUN | r | . c | –0.005 | –.040 | –.044 | –.161 | –.081 | –0.060 | 0.083 | 0.158 | 0.109 | 0.135 | 0.047 | 0.136 | 0.102 | 1 |
| p | 0.000 | 0.958 | .695 | .664 | .109 | .424 | 0.552 | 0.410 | 0.116 | 0.280 | 0.362 | 0.643 | 0.176 | 0.313 | ||
| n | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 48 | 100 | 100 | 100 | 100 | |
BMI – body mass index, DM – diabetes mellitus, Crea – creatinine, INR – international normalized ratio, BUN – blood urea nitrogen
Correlation is significant at the 0.01 level (2-tailed)
Correlation is significant at the 0.05 level (2-tailed)
Cannot be computed because at least one of the variables is constant
Table 2.
Clinical and laboratory baseline characteristics of the chronic hepatitis D patients with and without cirrhosis
| Risk factor | B | SE | p | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Sex | 0.443 | 0.424 | 0.295 | 1.558 | 0.679 | 3.576 |
| Age | 0.036 | 0.019 | 0.055 | 1.037 | 0.999 | 1.076 |
| Presence of hepatitis D viral infection | 1.395 | 0.489 | 0.004 | 4.037 | 1.548 | 10.526 |
| Season | 0.534 | 0.418 | 0.201 | 1.705 | 0.752 | 3.865 |
| Constant | –0.401 | 0.776 | 0.605 | 0.670 | ||
Risk factors for vitamin D deficiency in patients with chronic hepatitis D viral infection as identified by multivariate logistic regression. B – Beta coefficient, SE – standard error, OR – odds ratio, CI – confidence interval
Serum 25(OH)D levels
Serum 25(OH)D levels were 18.28 ±12.07 ng/ml and 18.90 ±8.30 ng/ml in the patient and control groups, respectively (p = 0.061). Mean serum 25(OH)D levels were lower, 15.30 ±6.92 ng/ml, in cirrhotic patients with CHD than in non-cirrhotic ones, 19.56 ±13.55 ng/ml, p = 0.393. The lowest 25(OH)D levels were identified in patients with cirrhotic CHD. When these levels, 15.30 ±6.92 ng/ml, were compared with those of the control group, 18.90 ±8.30 ng/ml, p = 0.025, they were found to be significant. Insufficient levels of serum 25(OH)D were found in 76.6%, 65.7%, and 57.1% of cirrhotic patients with CHD, non-cirrhotic patients with CHD, and healthy controls, respectively (p = 0.164). No correlation was established between vitamin D levels and HDV RNA, HBV DNA, and histopathological findings.
We also assessed seasonal changes in 25(OH)D levels in CHD patients and the control group. Serum 25(OH)D levels were significantly lower in both CHD patients and the control group where serum samples had been taken in Winter and Fall seasons, 13.79 ±6.18 ng/ml and 23.34 ±14.87 ng/ml, p = 0.001, compared to those taken in Summer and Spring seasons, 16.88 ±6.96 ng/ml and 20.92 ±9.1 ng/ml, p = 0.032 (Fig. 1). There was a significant difference between CHD and control groups in terms of 25(OH)D levels in the same season only in the Winter and Fall period, 13.79 ±6.18 ng/ml and 16.88 ±6.96 ng/ml, p = 0.023 (Fig. 1).
Fig. 1.
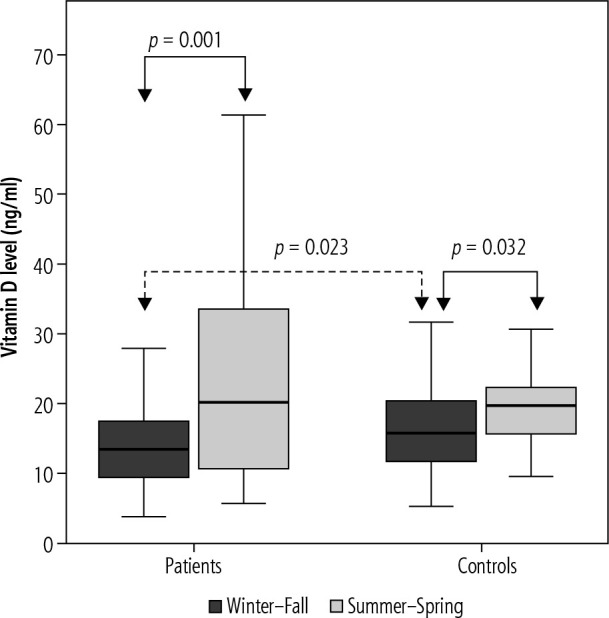
Vitamin D levels are characterized by seasonal variation in patient with chronic hepatitis D and healthy controls
Vitamin D deficiency (< 10 ng/ml) in patients with chronic hepatitis D
Deficiency of serum 25(OH)D was found in 30%, 25%, and 8.5% of CHD patients with cirrhosis, CHD patients without cirrhosis, and healthy controls, respectively (p = 0.01). Among the studied variables, total bilirubin concentration showed a significant correlation with 25(OH)D levels (r = –0.252, p = 0.012). However, there was no relationship between aspartate transaminase (AST), alanine transaminase (ALT), platelet count, albumin, international normalized ratio, and vitamin D levels. Multivariate analysis showed that chronic hepatitis D (odds ratio [OR] = 3.608, 95% confidence interval [CI]: 1.31-9.89, p = 0.013) and age (OR = 1.04, 95% CI: 1.00-1.08, p = 0.033) were associated with vitamin D deficiency, whereas CHD with cirrhosis (OR = 0.65, 95% CI: 0.24-1.76, p = 0.42) was not. However, the other variables in the model including sex, BMI, and seasonal status did not predict the vitamin D concentrations.
Discussion
The present study concluded that 25(OH)D deficiency was more pronounced among cirrhotic patients and at a higher incidence among patients with CHD than healthy controls.
Chronic hepatitis D infection progresses more rapidly than other types of hepatitis. Cirrhosis and complications due to cirrhosis may develop in the early phase of patients with CHD. Vitamin D levels have been studied in patients with CHD in our study, which is so important; our study will make important contributions to the literature.
Vitamin D not only acts as a significant regulator in calcium metabolism but also influences several different cellular functions, from proliferation to immunomodulation [18]. Vitamin D exerts its biological effects through its attachment to nuclear vitamin D receptors situated in a large number of tissues ranging from liver cells to immune system cells [19].
Vitamin D produced in the skin or ingested through the diet is biologically inert and requires two successive hydroxylations [20]. Once cytochrome is hydroxylated through the P450 enzyme system in the liver it is converted thereby into 25(OH)D. Vitamin D enters the circulation again being mostly attached to the binding protein [21]. It is also significant that the synthesis of vitamin D binding proteins occurs in liver cells. As there is no feedback regulation of hydroxylation of vitamin D in the liver, when liver parenchyma disease occurs, the impaired hydroxylation of vitamin D can also lead to vitamin D deficiency or shortage [22, 23]. Although this metabolite is the biologically inactive form, 25(OH)D has the highest quantity and the longest half-life in the blood. For these reasons, 25(OH)D is a reflection of the total quantity of vitamin D taken in through exposure to sunlight and dietary means and released in liver deposits [24]. Therefore, serum concentrations provide the most reliable marker that reflects the situation concerning vitamin D [24]. In the present study, liquid chromatography-mass spectrometry (LC-MS) was used as a method that has been primarily preferred in the determination of vitamin D and metabolites in recent years [22].
Vitamin D deficiency is reported at rates reaching 60% among patients with chronic hepatitis B [25, 26]. Farnik et al. found vitamin D insufficiency (> 10 ng/ml – < 20 ng/ml) and deficiency (< 10 ng/ml) among patients with chronic hepatitis B at rates of 47% and 34%, respectively, and below those of healthy controls. The present study identified an inverse relationship between vitamin D levels and HBV DNA [25]. Another study on CHB established, once more, lower vitamin D levels at a higher extent and a negative correlation between vitamin D and HBV DNA. Similarly, vitamin D levels exhibited a significant increase after successful long-term antiviral treatment. However, the present study did not establish such a correlation in HBV DNA-positive patients. This may be attributed to the differences in the natural course of both diseases depending on the suppression of HBV by HDV [1, 2].
Several studies report higher ratios of vitamin D deficiency among patients with chronic hepatitis C compared to those in patients with CHB (92%) [27]. Interestingly, a poor vitamin D status has been linked to fibrosis and low responsiveness to therapy in patients with chronic hepatitis C [28, 29].
Vitamin D deficiency was associated with relevant studies with many mediators and pathways affecting fibrosis [30-34]. Changes in the composition of the extracellular matrix in chronic liver diseases play an important role in the development of fibrosis. These changes are induced by fibroblasts and vascular smooth muscle cells. Vitamin D regulates important functions of these cells including differentiation, proliferation, and migration. Liver cells are the main source of matrix metalloproteinase inhibitors. There is an association between increases in matrix metalloproteinases 2 and 9, a function of fibronectin and collagen IV degradation, and deficiency in vitamin D [30, 31]. Also, vitamin D plays an important role in the activation of T-lymphocytes. Deficiency of vitamin D leads to reductions in the capability of T-lymphocytes to inactivate the virus responsible for hepatitis C, and the consequent increase in necroinflammation exacerbates fibrosis [32]. Because of the anti-apoptotic effect of vitamin D on liver cells, its deficiency emerges as another factor of importance that affects fibrosis [33, 34]. As emphasized above, due to its effects on a range of cellular functions, vitamin D deficiency may lead to advanced fibrosis in patients with chronic hepatitis. In addition to these clinical and experimental observations, relevant studies reported that there was an increase in permanent virological responses through the supplementation of treatment regimens with interferon and vitamin D [35, 36]. There is currently no alternative to interferon treatment even though its success rates in the treatment of chronic hepatitis D are not high. In this context, the supplementation of interferon treatment with vitamin D can contribute to the success rates in treatment for patients with CHD who suffer from vitamin D deficiency, as is the case in chronic hepatitis C treatment.
Studies on cirrhosis indicated vitamin D deficiency in approximately 80% of patients [22, 37]. Also, studies indicated an inverse correlation between this deficiency and scores that indicate disease severity as Child-Pugh [37, 38]. Similarly, a significant correlation was identified between serum 25(OH)D levels and the laboratory parameters of albumin levels, bilirubin concentrations, and international normalized ratios [37-40]. Among patients with cirrhosis, considering the role of the relationship between disease severity and laboratory parameters and vitamin D in the metabolism of this vitamin in the liver, any decrease in the production of carrier protein is emphasized to be one of the most important factors [22]. Lower serum concentrations of vitamin D carrier proteins from reduced production in the liver affect serum 25(OH)D levels. The present study found a correlation only between bilirubin and vitamin D levels, and could not identify any correlation with other parameters. In the present study we found the lower rates of vitamin D deficiency and less correlations of the parameters among cirrhotic patients compared to the previous literature. This difference may be due to the fact that most of our patients are diagnosed with compensated cirrhosis.
Studies undertaken with general populations indicated that deficiency in vitamin D led to significant increases in the risk of death from all causes [41, 42]. In patients suffering from chronic liver disease, an association between vitamin D deficiency and an increase in the risk of death was reported [37, 43]. Stoke et al. established vitamin D levels below 6 ng/ml as the single independent factor determining mortality [43].
The present study has a few limiting aspects. The first one is the fact that this single-center study was performed on a low number of patients. Secondly, its nature as a case-control study does not allow us to provide a causal interpretation between vitamin D deficiency and CHD. Finally, the study was not conducted on a complete set of data concerning diet, exposure to sunlight, and parathormone levels, all of which have an impact on vitamin D metabolism. However, this issue will not constitute a significant bias, as these data were lacking for both the control group and the patient group, and those included in the study were from a climatically uniform geographical region.
Conclusions
The present study shows that vitamin D deficiency is more pronounced among patients with chronic hepatitis D and especially among those with cirrhosis. Nevertheless, it is necessary to conduct studies concerning the effects of vitamin D replacement on the rates of response to interferon treatment among patients with CHD suffering from vitamin D deficiency and on the morbidity and mortality rates of patients with end-stage liver diseases.
Acknowledgments
We thank Ismail Sari for providing statistical assistance in the preparation of this manuscript.
Disclosure
The authors declare no conflict of interest.
References
- 1.Farci P, Niro GA. Clinical features of hepatitis D. Semin Liver Dis 2012; 32: 228-236. [DOI] [PubMed] [Google Scholar]
- 2.Smedile A, Farci P, Verme G, et al. Influence of delta infection on severity of hepatitis B. Lancet 1982; 2: 945-947. [DOI] [PubMed] [Google Scholar]
- 3.Ryu WS, Bayer M, Taylor J. Assembly of hepatitis delta virus particles. J Virol 1992; 66: 2310-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alves C, Branco C, Cunha C. Hepatitis delta virus: a peculiar virus. Adv Virol 2013; 2013: 560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357: 266-281. [DOI] [PubMed] [Google Scholar]
- 6.Looker AC, Mussolino ME. Serum 25-hydroxyvitamin D and hip fracture risk in older US white adults. J Bone Miner Res 2008; 23: 143-150. [DOI] [PubMed] [Google Scholar]
- 7.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest 2005; 35: 290-304. [DOI] [PubMed] [Google Scholar]
- 8.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 2005; 26: 662-687. [DOI] [PubMed] [Google Scholar]
- 9.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004; 80 (Suppl): 1689S-1696S. [DOI] [PubMed] [Google Scholar]
- 10.Tsuneoka K, Tameda Y, Takase K, Nakano T. Osteodystrophy in patients with chronic hepatitis and liver cirrhosis. J Gastroenterol 1996; 31: 669-678. [DOI] [PubMed] [Google Scholar]
- 11.Masuda S, Okano T, Osawa K, et al. Concentrations of vitamin D-binding protein and vitamin D metabolites in serum of patients with liver cirrhosis. J Nutr Sci Vitaminol (Tokyo) 1989; 35: 225-234. [DOI] [PubMed] [Google Scholar]
- 12.Bouillon R, Auwerx J, Dekeyser L, et al. Serum vitamin D metabolites and their binding protein in patients with liver cirrhosis. J Clin Endocrinol Metab 1984; 59: 86-89. [DOI] [PubMed] [Google Scholar]
- 13.Chen CC, Wang SS, Jeng FS, Lee SD. Metabolic bone disease of liver cirrhosis: is it parallel to the clinical severity of cirrhosis? J Gastroenterol Hepatol 1996; 11: 417-421. [DOI] [PubMed] [Google Scholar]
- 14.Hepner GW, Roginsky M, Moo HF. Abnormal vitamin D metabolism in patients with cirrhosis. Am J Dig Dis 1976; 21: 527-532. [DOI] [PubMed] [Google Scholar]
- 15.Duarte MP, Farias ML, Coelho HS, et al. Calcium-parathyroid hormone-vitamin D axis and metabolic bone disease in chronic viral liver disease. J Gastroenterol Hepatol 2001; 16: 1022-1027. [DOI] [PubMed] [Google Scholar]
- 16.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22: 696-699. [DOI] [PubMed] [Google Scholar]
- 17.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med 2011; 364: 248-254. [DOI] [PubMed] [Google Scholar]
- 18.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 2005; 26: 662-687. [DOI] [PubMed] [Google Scholar]
- 19.Bookout AL, Jeong Y, Downes M, et al. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 2006; 126: 789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets 2011; 12: 4-18. [DOI] [PubMed] [Google Scholar]
- 21.Ho AS, Cheng CC, Lee SC, et al. Novel biomarkers predict liver fibrosis in hepatitis C patients: alpha 2 macroglobulin, vitamin D binding protein and apolipoprotein AI. J Biomed Sci 2010; 17: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stokes CS, Volmer DA, Grünhage F, Lammert F. Vitamin D in chronic liver disease. Liver Int 2013; 33: 338-352. [DOI] [PubMed] [Google Scholar]
- 23.Nair S. Vitamin D deficiency and liver disease. Gastroenterol Hepatol (N Y) 2010; 6: 491-493. [PMC free article] [PubMed] [Google Scholar]
- 24.Heaney RP. The Vitamin D requirement in health and disease. J Steroid Biochem Mol Biol 2005; 97: 13-19. [DOI] [PubMed] [Google Scholar]
- 25.Farnik H, Bojunga J, Berger A, et al. Low vitamin D serum concentration is associated with high levels of hepatitis B virus replication in chronically infected patients. Hepatology 2013; 58: 1270-1276. [DOI] [PubMed] [Google Scholar]
- 26.Chen EQ, Bai L, Zhou TY, et al. Sustained suppression of viral replication in improving vitamin D serum concentrations in patients with chronic hepatitis B. Sci Rep 2015; 5: 15441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci 2010; 55: 2624-2628. [DOI] [PubMed] [Google Scholar]
- 28.Petta S, Camma C, Scazzone C, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology 2010; 51: 1158-1167. [DOI] [PubMed] [Google Scholar]
- 29.Bitetto D, Fattovich G, Fabris C, et al. Complementary role of vitamin D deficiency and the interleukin-28B rs12979860 C/T polymorphism in predicting antiviral response in chronic hepatitis C. Hepatology 2011; 53: 118-126. [DOI] [PubMed] [Google Scholar]
- 30.Timms PM, Mannan N, Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM 2002; 95: 787-796. [DOI] [PubMed] [Google Scholar]
- 31.Han YP. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J Gastroenterol Hepatol 2006; 21: S88-S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen L. Vitamin D controls T cell activation: implication for causal association between vitamin D deficiency and fibrosis in chronic hepatitis C. Hepatology 2010; 52: 1864. [DOI] [PubMed] [Google Scholar]
- 33.Nakano T, Cheng YF, Lai CY, et al. Impact of artificial sunlight therapy on the progress of non-alcoholic fatty liver disease in rats. J Hepatol 2011; 55: 415-425. [DOI] [PubMed] [Google Scholar]
- 34.Garciade León M del C, Montfort I, Tello Montes E, et al. Hepatocyte production of modulators of extracellular liver matrix in normal and cirrhotic rat liver. Exp Mol Pathol 2006; 80: 97-108. [DOI] [PubMed] [Google Scholar]
- 35.Bitetto D, Fabris C, Fornasiere E, et al. Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transpl Int 2011; 24: 43-50. [DOI] [PubMed] [Google Scholar]
- 36.Abu-Mouch S, Fireman Z, Jarchovsky J, et al. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol 2011; 17: 5184-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Putz-Bankuti C, Pilz S, Stojakovic T, et al. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int 2012; 32: 845-851. [DOI] [PubMed] [Google Scholar]
- 38.Fisher L, Fisher A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol 2007; 5: 513-520. [DOI] [PubMed] [Google Scholar]
- 39.Chen CC, Wang SS, Jeng FS, Lee SD. Metabolic bone disease of liver cirrhosis: Is it parallel to the clinical severity of cirrhosis? J Gastroenterol Hepatol 1996; 11: 417-421. [DOI] [PubMed] [Google Scholar]
- 40.Rode A, Fourlanos S, Nicoll A. Oral vitamin D replacement is effective in chronic liver disease. Gastroenterol Clin Biol 2010; 34: 618-620. [DOI] [PubMed] [Google Scholar]
- 41.Zittermann A, Iodice S, Pilz S, et al. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr 2012; 95: 91-100. [DOI] [PubMed] [Google Scholar]
- 42.Pilz S, Tomaschitz A, März W, et al. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf) 2011; 75: 575-584. [DOI] [PubMed] [Google Scholar]
- 43.Stokes CS, Krawczyk M, Reichel C, et al. Vitamin D deficiency is associated with mortality in patients with advanced liver cirrhosis. Eur J Clin Invest 2014; 44: 176-183. [DOI] [PubMed] [Google Scholar]


