Abstract
Aim of the study
This study was conducted to investigate the positive effect of silymarin on liver enzymes and antioxidant status in trauma patients with elevated liver enzymes due to trauma-induced liver injury, admitted to the intensive care unit.
Material and methods
This one-year, randomized, double-blinded, placebo-controlled clinical trial was conducted on 90 trauma patients. The participants were assigned to either receiving Livergol tablets containing 140 mg of silymarin or 140 mg of placebo three times daily for 14 days. Liver enzymes, including aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase (ALP), were measured at baseline and days 3, 7, 9 and 14 after intervention. Also, antioxidant markers were measured at baseline and day 14 after treatment.
Results
Receiving silymarin supplement significantly lowered the liver enzymes, compared to placebo (p < 0.05). The mean serum level of malondialdehyde (MDA) was significantly decreased and the mean serum levels of total antioxidant capacity (TAC) and thiol groups were significantly increased in the silymarin group from baseline to day 14. In the placebo group, mean serum levels of MDA and thiol groups were significantly increased, while serum level of TAC was not significantly changed at day 14, compared to baseline. Also, the mean serum level of MDA was significantly lower, while the serum levels of thiol groups and TAC were significantly higher in the silymarin group.
Conclusions
Silymarin supplementation significantly improved some antioxidant markers (TAC and thiol) and decreased liver enzymes in patients with trauma-induced liver injury.
Keywords: liver enzymes, antioxidant status, trauma, silymarin, liver injury
Introduction
Liver injury is the most common organ damage in patients with blunt multiple traumas, as well as mild abdominal trauma [1-3]. Thirty-one percent of patients with multiple trauma are at risk for abdominal injuries and 31% of these patients experience liver injury [4]. An elevation in liver enzymes is observed in trauma patients, which indicates damage to liver tissue [5].
In addition to imaging tests and physical examinations, biochemistry tests are also used in the evaluation of trauma patients [6]. Aminotransferases, including aspartate transaminase (AST) and alanine transaminase (ALT), are indicators of liver cell injury and are used in diagnosis of acute liver diseases such as acute hepatitis. AST is found in the liver, heart muscle, skeletal muscles, kidneys, brain, pancreas, lungs, leukocytes and red blood cells, in order from high to low concentrations, while ALT exists primarily in the liver and is considered as a specific indicator for liver injury. Low concentrations of aminotransferases are found in serum in normal conditions but following hepatic trauma and injury to hepatocyte membrane, these enzymes are released into the blood circulation and their blood concentration is significantly increased [7, 8]. The normal range of aminotransferases varies among different laboratories but generally is considered 10-40 IU/l [9, 10]. Thus, the changes in concentration of aminotransferases could be useful for diagnosis of various pathologic conditions [10, 11]. It has been observed that reactive oxygen species (ROS), such as hydrogen superoxide (H2O2), increase in liver ischemic injury [12]. The formation of ROS and oxidative stress are the main mechanisms of the disease in the majority of ischemic liver injuries [13].
Extracts of Silybum marianum leaves and fruits have been used for centuries to treat liver, spleen and gallbladder disorders. In the 1960s, the active ingredient in extracts of seeds and fruits was isolated and its chemical structure was clarified. This substance was called silymarin, which is a mixture of flavonolignans. Flavonoids are natural substances that have various medicinal and therapeutic properties. Some of them have an antioxidant effect due to their phenolic structures and inhibit free radical-induced processes [14, 15].
Silymarin possesses several properties, including antioxidant, anti-inflammatory, protein synthesis stimulator, hepatoprotective, anti-fibrotic, anti-cancer, anti-cell proliferation, immune response modulator and anti-viral. Silymarin is also capable of reduction or prevention of the toxic effects of drugs such as cisplatin, vincristine, acetaminophen and gentamicin on the kidneys [16-19]. The most important antioxidant mechanism of silymarin include prevention of free radical formation through inhibition of the enzymes involved in the production of ROS, direct destruction of free radicals, chelation of heavy metals in the intestine, stimulation of the synthesis of protective molecules against stressful stimuli, such as thioredoxin, sirtuins, activation of antioxidant enzymes such as superoxide dismutase, and stimulation of protein synthesis through hepatocyte survival [20-22].
This study was conducted to investigate the positive effect of silymarin on liver enzymes and antioxidant status in trauma patients with elevated liver enzymes admitted to the intensive care unit (ICU).
Material and methods
Study design and setting
This randomized, double-blinded, placebo-controlled clinical trial was conducted in two ICUs of Shahid Rajaee Hospital, a teaching center affiliated to Shiraz University of Medical Sciences (SUMS), from August 2019 to August 2020.
Ethical approval
This study was approved by the Ethics Committee of the SUMS (IR.SUMS.REC.1398.768) and was registered in the Iranian Registry of Clinical Trials (IRCT20190911044744N1). Written informed consent was obtained from patients if conscious or their relatives. The trial was in accordance with the guidelines laid down in the 1975 Helsinki Declaration as revised in 2008.
Study population
All trauma patients with elevated liver enzymes (AST or ALT more than 2 times the upper limit normal) admitted in the ICU were included in the study if they met the following criteria: age of 16 or older, not having received a known hepatotoxic drug (ketoconazole, tetracyclines, isoniazid, rifampin, amoxicillin or clavulanate, macrolides, trimethoprim sulfamethoxazole, nitrofurantoin, clindamycin, phenytoin, valproic acid, carbamazepine, phenobarbital, acetaminophen, amiodarone, statins and propofol) [23-25], no history of oral silymarin administration over the past 7 days, not receiving concurrent medications with antioxidant properties, such as N-acetyl cysteine, vitamin C, E and A, willingness to participate in the study and no confirmed history of allergic reactions to silymarin. The patients were excluded from the study if they were pregnant or lactating or if they had a history of liver disease.
The patients were assigned to placebo or treatment groups using block randomization. Subjects in the treatment and placebo group received Livergol tablets containing 140 mg of silymarin three times daily (Goldaru Herbal Products Pharmaceutical Company, Isfahan, Iran) and 140 mg of placebo manufactured by the faculty of pharmacy of SUMS and similar to Livergol tablets in size, shape, weight, color, and taste three times daily, respectively.
Measurements and study outcomes
The patients’ information including demographic data, laboratory profiles, disease history and medications was recorded through reviewing the medical charts of the patients, as well as interviews with the patients or their relatives. Also, the Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II score on admission was recorded for each patient [26]. Blood samples (5 ml) were obtained at baseline; AST, ALT, alkaline phosphatase (ALP), direct bilirubin, albumin, prothrombin time (PT), serum electrolytes (potassium, sodium), blood urea nitrogen (BUN) and serum creatinine were all measured at baseline, as well as days 3, 7, 9 and 14 after Livergol or placebo administration. Also, prior to the intervention and at the end of the study (day 14), blood samples (5 ml) were collected from each patients and serum was separated by centrifugation at 2500 rpm for 10 min; following centrifugation, samples were stored at –80°C. At the end of the trial, the frozen blood samples were used for measuring total antioxidant capacity (TAC), malondialdehyde (MDA), and thiol groups. These measurements were performed using Zantox commercial kits (Kavosh Arian Azma Co, Iran).
For assessment of thiol groups, the Ellman’s reagent method was used [27]. Based on this method, free thiol groups react with 2-2/-dinitro-5,5/-dithiodibenzoic acid and a yellow complex is produced. The obtained complex was measured spectrophotometrically at 412 nm (EPOCH plate reader, Highlandpark, USA).
TAC was measured using the ferric reducing antioxidant power (FRAP) method [28], in which reduction of ferric (Fe3+) to ferrous (Fe2+) by electron donating antioxidants produces a blue complex under acidic pH and in the presence of 2,4,6-tripyridyl-s-triazine (TPTZ) reagent. The absorbance of the blue complex was measured using a spectrophotometer at 593 nm.
MDA concentration was measured by the TBA reaction method [29]. Under acidic conditions and a temperature of 95°C, one molecule of MDA reacts with two molecules of TBA and this reaction produces a pink complex, which has maximum light absorption at a wavelength of 532 nm. The fluorescent intensity of the complex was measured at 553 nm with excitation at 515 nm using a spectrophotometer.
Statistical analysis
Statistical analyses were performed using the IBM SPSS Statistics Version 20 software (SPSS Inc./IBM Corp., Chicago, IL, USA). The analysis was conducted on data available to all subjects completing the study (per protocol analysis). The one-sample Kolmogorov-Smirnov test was performed to evaluate the normality of the distribution of the data. Categorical variables were presented as absolute and relative (percentage) frequencies. Continuous variables were expressed as mean ± standard deviation (SD).
The independent sample t-test was employed to compare parameters between silymarin and placebo groups. Also, the paired sample t-test was used for means comparison of variables in order to identify within-group differences. The repeated measure ANOVA test was applied to compare the changes in the investigated markers from baseline to 14 days follow-up between two groups. P values < 0.05 were considered statistically significant.
Results
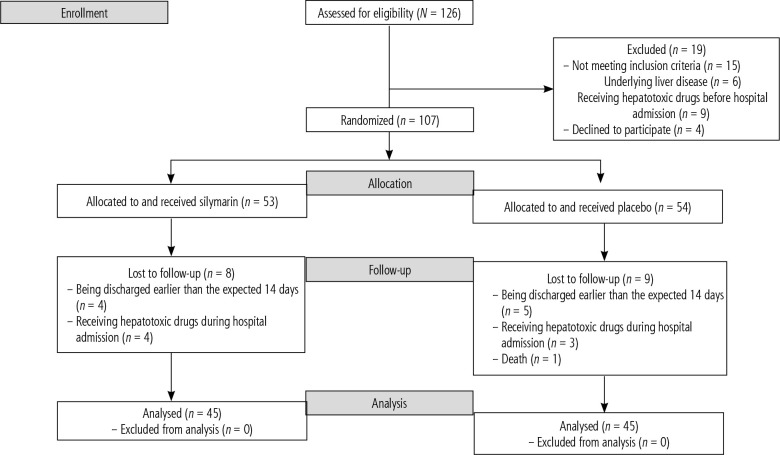
The consort diagram of the study is shown in Figure 1. During the study period, 126 patients were screened for recruitment, of whom 107 subjects were included in the trial, 53 in the silymarin group and 52 in the placebo group. Finally, 90 patients completed the study, including 45 cases in the silymarin group and 45 patients in the placebo group.
Fig. 1.
Flowchart of the study
The participants’ demographic and relevant clinical information is shown in Table 1. There was no significant difference between the two groups in terms of the baseline characteristics and clinical findings, including sex, age, weight, APACHE II score, serum electrolytes, serum creatinine, BUN, INR, direct bilirubin and serum albumin (p > 0.05).
Table 1.
Demographic information and baseline laboratory findings in silymarin and placebo groups
| Variables | Mean ± SD or n (%) | P-value | |
|---|---|---|---|
| Silymarin group | Placebo group | ||
| Sex | |||
| Male | 25 (55.6) | 21 (46.7) | 0.52 |
| Female | 20 (44.4) | 24 (53.3) | |
| Age (years) | 52.02 ±14.92 | 54.15 ±17.38 | 0.53 |
| Weight (kg) | 65.66 ±12.67 | 67.22 ±10.59 | 0.53 |
| APACHE II score | 14.48 ±4.92 | 14.60 ±4.64 | 0.91 |
| Mg (mg/dl) | 1.59 ±0.42 | 1.61 ±0.49 | 0.85 |
| Na (mEq/l) | 138.37 ±2.41 | 138.84 ±2.90 | 0.41 |
| K (mEq/l) | 4.30 ±0.67 | 4.27 ±0.50 | 0.80 |
| Ca (mg/l) | 8.63 ±0.48 | 8.58 ±0.57 | 0.60 |
| Albumin (g/dl) | 3.34 ±0.49 | 3.26 ±0.65 | 0.50 |
| Direct bilirubin (mg/dl) | 0.4 ±0.21 | 0.43 ±0.19 | 0.54 |
| INR | 1.21 ±0.21 | 1.22 ±0.21 | 0.72 |
| Serum creatinine | 1.47 ±81 | 1.42 ±67 | 0.76 |
| BUN | 21.02 ±7.47 | 21.08 ±5.90 | 0.96 |
SD – standard deviation, APACHE II score – Acute Physiology and Chronic Health Evaluation II Score, INR – international normalized ratio, BUN – blood urea nitrogen
The level of liver enzymes before and on different days of the intervention are shown in Table 2. At baseline, there were no significant differences between the silymarin and placebo group in terms of ALT, AST and ALP. AST and ALP levels were not significantly different between the two groups on day 3 (p = 0.43 and p = 0.75, respectively) but they were significantly lower on days 7, 9 and 14 in silymarin treated patients (p = 0.04, p < 0.001 and p < 0.001 for AST and p = 0.041, p = 0.027 and p < 0.001 for ALP, respectively). ALT level was also significantly lower in the silymarin group on days 9 and 14, compared to the placebo group (p < 0.001 for both days), while no significant difference was found on days 3 and 7.
Table 2.
Comparison between mean aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase on different days in silymarin and placebo groups
| Variables | Day | Silymarin | Placebo | P-value |
|---|---|---|---|---|
| AST (U/l) | Baseline | 215.14 ±91.61 | 201.88 ±65.78 | 0.43 |
| Day 3 | 180.42 ±85.22 | 197.48 ±58.31 | 0.27 | |
| Day 7 | 144.24 ±73.37 | 184.93 ±57.02 | 0.04 | |
| Day 9 | 111.33 ±61.43 | 161.37 ±55.01 | < 0.001 | |
| Day 14 | 79.26 ±49.95 | 133.97 ±52.94 | < 0.001 | |
| ALT (U/l) | Baseline | 181.84 ±81.59 | 178.22 ±65.78 | 0.81 |
| Day 3 | 149.15 ±74.19 | 176.04 ±55.50 | 0.055 | |
| Day 7 | 116.55 ±63.39 | 164.82 ±55.19 | 0.062 | |
| Day 9 | 84.86 ±52.28 | 138.73 ±53.63 | < 0.001 | |
| Day 14 | 58.75 ±42.10 | 112.75 ±51.39 | < 0.001 | |
| ALP (U/l) | Baseline | 272.15 ±44.06 | 268.05 ±54.11 | 0.79 |
| Day 3 | 254.15 ±42.09 | 258.70 ±50.77 | 0.75 | |
| Day 7 | 222.40 ±39.19 | 252.30 ±49.68 | 0.041 | |
| Day 9 | 197.60 ±39.42 | 229.07 ±50.80 | 0.027 | |
| Day 14 | 154.80 ±40.11 | 209.45 ±49.69 | < 0.001 |
AST – aspartate aminotransferase, ALT – alanine transaminase, ALP – alkaline phosphatase
Moreover, the results from repeated measure analysis showed that AST, ALT and ALP levels significantly decreased (p < 0.001) in both silymarin and placebo groups. This decrease in liver enzymes was significantly higher in silymarin treated patients than the placebo group (p < 0.001). Also, no significant differences were observed in the mean change in AST, ALT, and ALP values at baseline, and days 3, 7, 9, and 14 in placebo and silymarin treated patients (p > 0.05).
Regarding international normalized ratio (INR) and albumin values, no significant differences were observed between the study groups on days 3, 7, 9 and 14 (all p > 0.05).
With respect to antioxidant indices, including MDA, TAC and thiol groups, there were no significant differences between groups at baseline (Table 3). MDA level on day 14 was marginally significantly different between the groups (p = 0.051). Also, TAC and thiol groups were significantly higher in silymarin treated patients (both p < 0.001). In the silymarin group, MDA decreased significantly at the end of the treatment period compared to baseline (p < 0.001), while both TAC and thiol groups significantly increased (both p < 0.001). In the placebo group, MDA and thiol groups significantly changed on day 14, compared to the baseline. TAC decreased over time in the placebo group; however, this change was not statistically significant (p = 0.9).
Table 3.
Comparison between mean malondialdehyde, thiol groups and total antioxidant capacity at baseline and at the end of the study in silymarin and placebo groups
| Variables | Day | Silymarin | Placebo | P-value |
|---|---|---|---|---|
| MDA | Baseline | 0.76 ±0.72 | 0.76 ±0.95 | 0.80 |
| Day 14 | 0.54 ±0.10 | 0.58 ±0.008 | 0.051 | |
| P-value | < 0.001 | < 0.001 | ||
| Thiol | Baseline | 385.91 ±157.37 | 379.80 ±17.44 | 0.08 |
| Day 14 | 416.01 ±17.82 | 365.15 ±17.52 | < 0.001 | |
| P-value | < 0.001 | < 0.001 | ||
| TAC | Baseline | 798.92 ±17.13 | 802.96 ±13.61 | 0.21 |
| Day 14 | 878.78 ±27.84 | 802.83 ±14.58 | < 0.001 | |
| P-value | < 0.001 | 0.9 |
MDA – malondialdehyde, TAC – total antioxidant capacity
The mean length of hospital stay was not significantly different between silymarin and placebo groups (23.8 ±5.11 vs. 24.2 ±3.81, p = 0.64). No adverse effects associated with silymarin were observed and there were no reports of gastrointestinal symptoms. All subjects tolerated silymarin well. No mortality was found in the silymarin group, while one patient died in the placebo group.
Discussion
This study was conducted to evaluate the efficacy of silymarin supplementation on liver injury induced by trauma and its related markers. To the best of our knowledge, this clinical trial is the first human study testing the effects of silymarin on serum levels of antioxidant markers and liver enzymes in trauma-induced liver injury.
The primary findings of this study suggested that receiving 140 mg of silymarin supplement three times daily for 14 days could significantly lower the liver enzymes, including ALT, AST and ALP, compared to placebo.
Several beneficial effects of silymarin have been identified, including antioxidant, anti-inflammatory, hepatoprotective and anti-fibrotic properties, as well as modulation of insulin resistance [30, 31]. In line with our results, the findings of two animal studies indicated the protective effects of silymarin against liver injury. Kim et al. reported that oral administration of silymarin in mice can prevent stress-induced liver damage through its antioxidant and anti-inflammatory activities. Significantly higher serum levels of ALT and AST were observed in the control group in this study. Also, Yamisen et al. evaluated the positive effects of silymarin in burn-induced liver injury in burned rats and observed that both topical and systemic treatments with silymarin were effective in reversing this type of liver injury [32].
Several clinical trials have been conducted on the hepatoprotective effects of silymarin in different pathologic situations. Luangchosiri et al. reported that patients receiving 140 mg of silymarin three times daily were at lower risk for antituberculosis drug-induced liver injury. However, the median changes of serum transaminase, glutathione and MDA were not significantly different between the silymarin and control group [33].
In contrast to our results, another randomized trial evaluating the effect of silymarin in patients with acute clinical hepatitis with any etiology reported no detectable effect on biomarkers of the hepatocellular inflammatory process, including ALT and AST. However, silymarin significantly improved subjective symptoms and signs related to biliary retention [34].
As mentioned above, considering the potential anti-inflammatory and antioxidant effects of silymarin, as well as its free radical scavenging activities, it has been proposed that it can be beneficial in management of pathological conditions associated with oxidative stress and free radicals, such as diabetes, a wide range of cancers, alcoholic liver diseases, liver cirrhosis, Amanita mushroom poisoning, viral hepatitis, toxic and drug-induced liver diseases, drug-induced kidney injury and end stage renal disease [35-39]. However, no studies have been conducted on the effect of silymarin on trauma-induced liver injury. Trauma can interrupt the blood supply to the liver and consequently cause hepatic ischemia/reperfusion injury. During the reperfusion phase, the release of ROS activates Kupffer cells, and stimulates inflammatory cytokine production, as well as infiltration of leucocytes, which in turn induces damage to liver cells [40]. Thus, this evidence supports the use of silymarin in trauma-induced liver injury.
In this manner, our results showed that the mean serum level of MDA was significantly decreased and the mean serum levels of TAC and thiol groups were significantly increased in the silymarin group from baseline to day 14. In the placebo group, mean serum levels of MDA and thiol groups were significantly increased, while serum level of TAC was not significantly changed on day 14, compared to baseline. Comparing placebo and treatment groups revealed that the mean serum level of MDA was significantly lower, while the serum levels of thiol groups and TAC were significantly higher in the silymarin group. Ebrahimpour et al. in a clinical trial evaluating the effect of silymarin supplementation on antioxidant status in patients with type 2 diabetes mellitus reported that TAC, superoxide dismutase, and glutathione peroxidase activity levels were significantly higher in the treatment group compared to the placebo group. Also, MDA was significantly decreased in the silymarin group compared to the baseline value [39]. Our results were in agreement with these results.
The most important side effects of silymarin are gastrointestinal symptoms including diarrhea, dyspepsia, irregular stools and nausea [41]. All the patients treated with silymarin tolerated it well and no adverse effects were observed among the participants. Our results are in line with previous studies, which confirmed the safety of silymarin [19, 42].
Our study had some limitations which should be addressed. First, the sample size in this study was relatively small and the intervention period was short, limited to two weeks (α = 5% and β = 10%, with a statistical power of 80%). Second, due to limitations, the antioxidant status markers were measured only at two time points and the daily change trend was determined. Another limitation was the possibility of gradual degradation of antioxidant markers in serum samples during storage in the freezer at –80°C for a relatively long period of time (up to one year for the first collected samples). A further limitation is using the conventional form of silymarin, which is associated with lower bioavailability, more extensive metabolism and lower permeability through the intestine, compared to modified formulations.
Conclusions
In conclusion, our results showed that supplementation with 140 mg of silymarin three times daily could significantly lower the serum levels of AST, ALT and ALP in trauma patients with increased levels of liver enzymes. Also, a significant reduction in MDA level and an increase in TAC and thiol groups were observed in the silymarin treated group. Silymarin could be a helpful complementary treatment in trauma-induced liver injury. Further studies with larger sample sizes and longer follow-up durations are required to better determine the efficacy of this treatment modality.
Acknowledgements
The authors would like would like to express their gratitude to Shiraz University of Medical Sciences, Shiraz, Iran and also the staff of Shahid Shahid Rajaee Hospital for their assistance. The authors want to dedicate this article to the memory of Maryam Rahimi, one of the ICU nurses who sadly passed away in the fight against COVID-19.
This article was extracted from the PhD thesis written by Dr. Ehsan Mirzaei for the Degree of Subspecialty in Clinical Pharmacy (#1398-06-02-17788) and supported by the Vice Chancellor for Research of Shiraz University of Medical Sciences.
Disclosure
The authors declare no conflict of interest.
References
- 1.Matthes G, Stengel D, Seifert J, et al. Blunt liver injuries in polytrauma: results from a cohort study with the regular use of whole-body helical computed tomography. World J Surg 2003; 27: 1124-1130. [DOI] [PubMed] [Google Scholar]
- 2.Meredith JW, Young JS, Bowling J, et al. Nonoperative management of blunt hepatic trauma: the exception or the rule? J Trauma 1994; 36: 529-534; discussion 534-535. [DOI] [PubMed] [Google Scholar]
- 3.Feliciano DV, Pachter HL. Hepatic trauma revisited. Curr Probl Surg 1989; 26: 453-524. [DOI] [PubMed] [Google Scholar]
- 4.Leenen LP. Abdominal trauma: from operative to nonoperative management. Injury 2009; 40 Suppl 4: S62-68. [DOI] [PubMed] [Google Scholar]
- 5.Arslan G, Gemici AA, Yirgin IK, et al. Liver trauma grading and biochemistry tests. Emerg Radiol 2013; 20: 379-384. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie AH, Williscroft DM. Elevated liver enzymes as a predictor of liver injury in stable blunt abdominal trauma patients: case report and systematic review of the literature. Can J Rural Med 2006; 11: 283-287. [PubMed] [Google Scholar]
- 7.Cogbill TH, Moore EE, Feliciano DV, et al. Hepatic enzyme response and hyperpyrexia after severe liver injury. Am Surg 1992; 58: 395-399. [PubMed] [Google Scholar]
- 8.Strawn T, Williams HC, Flint LM, Jr. Prognostic significance of serum biochemical changes following liver trauma. Am Surg 1980; 46: 111-115. [PubMed] [Google Scholar]
- 9.Mohankumar N, Ranjan P, Kumari A. Drug-induced liver injury: Diagnosing (and treating) it early. J Fam Pract 2015; 64: 634-644. [PubMed] [Google Scholar]
- 10.Kaplan MM. Alanine aminotransferase levels: what’s normal? Ann Intern Med 2002; 137: 49-51. [DOI] [PubMed] [Google Scholar]
- 11.Lala V, Goyal A, Bansal P, et al. Liver function tests. StatPearls Publishing, Treasure Island (FL) 2020.
- 12.Zhang M, Yang D, Gong X, et al. Protective benefits of AMP-activated protein kinase in hepatic ischemia-reperfusion injury. Am J Transl Res 2017; 9: 823-829. [PMC free article] [PubMed] [Google Scholar]
- 13.Elias-Miró M, Jiménez-Castro MB, Rodés J, et al. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic Res 2013; 47: 555-568. [DOI] [PubMed] [Google Scholar]
- 14.Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs 2001; 15: 465-489. [DOI] [PubMed] [Google Scholar]
- 15.Kidd P, Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos). Altern Med Rev 2005; 10: 193-203. [PubMed] [Google Scholar]
- 16.Roozbeh J, Shahriyari B, Akmali M, et al. Comparative effects of silymarin and vitamin E supplementation on oxidative stress markers, and hemoglobin levels among patients on hemodialysis. Ren Fail 2011; 33: 118-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain SA, Jassim NA, Numan IT, et al. Anti-inflammatory activity of silymarin in patients with knee osteoarthritis. A comparative study with piroxicam and meloxicam. Saudi Med J 2009; 30: 98-103. [PubMed] [Google Scholar]
- 18.Song JH, Choi HJ. Silymarin efficacy against influenza A virus replication. Phytomedicine 2011; 18: 832-835. [DOI] [PubMed] [Google Scholar]
- 19.Mahi-birjand M, Karimzadeh I, Zarban A, et al. Protective effects of silymarin on gentamicin-induced nephrotoxicity in infectious patients: a randomized double blinded placebo-controlled clinical trial. Pharm Sci 2020; 26: 287-295. [Google Scholar]
- 20.Abenavoli L, Izzo AA, Milić N, et al. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother Res 2018; 32: 2202-2213. [DOI] [PubMed] [Google Scholar]
- 21.Boigk G, Stroedter L, Herbst H, et al. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology 1997; 26: 643-649. [DOI] [PubMed] [Google Scholar]
- 22.Pietrangelo A, Borella F, Casalgrandi G, et al. Antioxidant activity of silybin in vivo during long-term iron overload in rats. Gastroenterology 1995; 109: 1941-1949. [DOI] [PubMed] [Google Scholar]
- 23.Lat I, Foster DR, Erstad B. Drug-induced acute liver failure and gastrointestinal complications. Crit Care Med 2010; 38 (6 Suppl): S175-187. [DOI] [PubMed] [Google Scholar]
- 24.Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med 2006; 354: 731-739. [DOI] [PubMed] [Google Scholar]
- 25.Lescot T, Karvellas C, Beaussier M, et al. Acquired liver injury in the intensive care unit. Anesthesiology 2012; 117: 898-904. [DOI] [PubMed] [Google Scholar]
- 26.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818-829. [PubMed] [Google Scholar]
- 27.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963; 61: 882-888. [PubMed] [Google Scholar]
- 28.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996; 239: 70-76. [DOI] [PubMed] [Google Scholar]
- 29.Richard MJ, Portal B, Meo J, et al. Malondialdehyde kit evaluated for determining plasma and lipoprotein fractions that react with thiobarbituric acid. Clin Chem 1992; 38: 704-709. [PubMed] [Google Scholar]
- 30.Gillessen A, Schmidt HHJ. Silymarin as supportive treatment in liver diseases: a narrative review. Adv Ther 2020; 37: 1279-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saller R, Melzer J, Reichling J, et al. An updated systematic review of the pharmacology of silymarin. Complement Med Res 2007; 14: 70-80. [DOI] [PubMed] [Google Scholar]
- 32.Yemişen E, Yarat A, Akbay TT, et al. The effect of silymarin on the liver in thermal burn injury. Marmara Pharm J 2014; 18: 56-61. [Google Scholar]
- 33.Luangchosiri C, Thakkinstian A, Chitphuk S, et al. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. MC Complement Altern Med 2015; 15: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Kamary SS, Shardell MD, Abdel-Hamid M, et al. A randomized controlled trial to assess the safety and efficacy of silymarin on symptoms, signs and biomarkers of acute hepatitis. Phytomedicine 2009; 16: 391-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roozbeh J, Shahriyari B, Akmali M, et al. Comparative effects of silymarin and vitamin E supplementation on oxidative stress markers, and hemoglobin levels among patients on hemodialysis. Ren Fail 2011; 33: 118-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahbazi F, Dashti-Khavidaki S, Khalili H, et al. Potential renoprotective effects of silymarin against nephrotoxic drugs: a review of literature. J Pharm Pharm Sci 2012; 15: 112-123. [DOI] [PubMed] [Google Scholar]
- 37.Karimi G, Vahabzadeh M, Lari P, et al. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran J Basic Med Sci 2011; 14: 308-317. [PMC free article] [PubMed] [Google Scholar]
- 38.Pradhan S, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res 2006; 124: 491-504. [PubMed] [Google Scholar]
- 39.Gargari BP, Mobasseri M, Valizadeh H, et al. Effects of Silybum marianum (L.) Gaertn. (silymarin) extract supplementation on antioxidant status and hs-CRP in patients with type 2 diabetes mellitus: a randomized, triple-blind, placebo-controlled clinical trial.Phytomedicine 2015; 22: 290-296. [DOI] [PubMed] [Google Scholar]
- 40.Mizar SM, Omar HA, El Sherbiny GA, et al. Nebivolol and chrysin protect the liver against ischemia/reperfusion-induced injury in rats. Beni-Suef Univ J Basic Appl Sci 2015; 4: 86-92. [Google Scholar]
- 41.Saller R, Brignoli R, Melzer J, et al. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed 2008; 15: 9-20. [DOI] [PubMed] [Google Scholar]
- 42.Soleimani V, Delghandi PS, Moallem SA, et al. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytother Res 2019; 33: 1627-1638. [DOI] [PubMed] [Google Scholar]