Abstract
Aim of the study
N-acetylcysteine (NAC) is the treatment of choice for acetaminophen-induced liver injury. However, recent years have witnessed growing interest in its role in the treatment of acute liver failure (ALF) due to other aetiologies. This study aims to determine both its safety and efficacy by pooling data from multiple studies.
Material and methods
A search was conducted for all controlled randomized/non-randomized studies that measured the efficacy and safety of NAC in adult patients with non-acetaminophen-induced acute liver failure (NAI-ALF). Transplant-free survival (TFS) was considered the primary endpoint, while secondary endpoints such as length of hospital stay, and incidence of adverse events during treatment, were included in our analysis. Data were pooled via a random-effects model, I2 was used as a measure of heterogeneity, and publication bias was assessed via a funnel plot.
Results
A total of 3 studies [2 randomized controlled trials (RCTs) and 1 non-randomized cohort] were pooled in this meta-analysis. TFS was significantly higher in patients given NAC, when compared to the placebo/control (PBO) group (RR = 1.54, CI = 1.19-1.98, p = 0.01, I2 = 0.0%). No secondary endpoint was observed to have improved significantly in patients prescribed NAC: length of hospital stay (SMD = –0.405, CI = –1.44-0.63, p = 0.445, I2 = 91.1%), renal failure (RR = 1.01, CI = 0.65-1.57, p = 0.967, I2 = 21.3%), infections (RR = 1.18, CI = 0.91-1.52, p = 0.208, I2 = 2.3%), pulmonary failure (RR = 1.19, CI = 0.57-2.49, p = 0.649, I2 = 84.6%). Minimal side effects were reported in around 10-14% of the patients prescribed NAC.
Conclusions
NAC was shown to significantly improve TFS in adult patients with NAI-ALF, while no significant benefit was observed concerning the secondary endpoints of length of hospital stay and incidence of adverse effects.
Keywords: meta-analysis, liver failure, acetylcysteine, non-acetaminophen, transplantfree survival
Introduction
Acute liver failure (ALF) is a relatively rare life-threatening condition with an annual incidence of around 80.2 people/million in East-Asian countries such as Taiwan [1]. The aetiology of ALF is strongly influenced by geographic and ethnic determinants, and comprises a host of factors ranging from viruses to alcohol and even toxins. While acetaminophen overdose remains the primary cause of ALF in developed countries such as the USA, viral hepatitis (particularly hepatitis E) is a far more common aetiology in South Asian countries such as Pakistan [2].
Given that ALF has no specific treatments, the management is primarily dependent on the case severity. Initial management comprises mainly treatment to address the cause of ALF in tandem with supportive care, while liver transplant may be considered down the line with progressing disease severity. In most cases, pharmacological therapy is usually ineffective as extensive liver damage reduces drug efficacy, thereby accounting for the treatment resistance observed in this disease. That being said, medication such as nucleoside analogues for hepatitis B and corticosteroids for autoimmune ALF, given before the onset of severe liver damage, have been proven effective in recovery [3, 4].
N-acetylcysteine (NAC), a derivative of the amino acid cysteine, is a well-known antidote for acetaminophen poisoning. It increases glutathione levels in the body, thereby helping neutralize the products of acetaminophen catabolism [3-5]. However, in recent years the role of NAC in treating non-acetaminophen-induced (NAI) ALF has become an area of interest, and several studies have been conducted to evaluate its safety and effectiveness [6].
A meta-analysis conducted in 2015 by Hu et al. examined both the safety and efficacy of acetylcysteine in patients with NAI-ALF. They included a total of 4 studies in their meta-analysis, of which 3 were randomized controlled trials (RCTs) and 1 was a prospective cohort study with historical controls. Of the aforementioned studies, 2 were conducted on a paediatric population while the others exclusively had adult participants. No significant differences between the NAC and placebo (PBO) groups were observed in the primary outcome of overall survival (RR = 1.16, CI = 0.81-1.67, p = 0.42). On the other hand, the secondary outcomes of transplant-free survival (RR = 1.61, CI = 1.11-2.34, p = 0.01) and post-transplant survival (RR = 2.44, CI = 1.11-5.37, p = 0.03) of the NAC group showed marked improvement when compared to the PBO group. However, as the study population included both adult and paediatric age groups, which differ considerably in their physiology, no definite conclusions regarding the use of NAC can be drawn from this study.
Since then, the results of 2 new studies by Nabi et al. and Darweesh et al. have been published. Both trials were exclusively conducted on adult participants, and measured transplant-free survival as their primary outcome. Various secondary outcomes were also investigated such as length of hospital stay, overall survival, adverse effects during the course of the disease, and side effects due to treatment.
This new evidence coupled with the ambiguity regarding the role of NAC in managing adult patients with NAI-ALF necessitates a re-evaluation of its efficacy in the context of this patient demographic. The aim of this study is to bridge this gap by evaluating the mortality benefit of NAC in adult NAI-ALF patients who have not undergone transplant, and to assess its effect on disease course including system failure, length of hospital stay and overall survival.
Material and methods
A protocol for this study was designed and submitted for registration on PROSPERO (CRD42020163877) in January 2020, and the review was conducted in accordance with the PRISMA statement for meta-analysis and systematic reviews-2009 [7].
Search criteria
An exhaustive literature search of the following databases was conducted: PubMed, Cochrane Library, Google Scholar, Clinicaltrials.gov and TRIP database (Turning Research into Practice). All prospective studies including non-RCTs, RCTs and cohort studies published between 1st January 2000 and 1st October 2020 with full-text articles available in English were included. The search and screening were conducted independently by 2 reviewers, with a third one being consulted in case of any disagreements. A search string was constructed using the following key terms: “N-acetylcysteine”, “acetylcysteine”, “acetaminophen”, “paracetamol”, “acute liver failure”, “liver failure”, “acute”, “ALF”, “transplant free survival” (a detailed search strategy is available in the supplement).
Inclusion and exclusion criteria
The population of the studies selected for metaanalysis included patients over the age of 18, diagnosed with NAI-ALF as defined by the American Association for the Study of Liver Diseases based on the presence of clinical signs of coagulopathy [international normalized ratio (INR) ≥ 1.5] and encephalopathy that present within 26 weeks of the initial symptoms, in the absence of a pre-existing liver condition [8]. The intervention could be either oral or intravenous N-acetylcysteine, while the control was supportive treatment with or without a placebo.
Studies conducted on paediatric patients and those that did not measure transplant-free survival as an outcome were excluded from further analysis. Moreover, all uncontrolled trials, case reports and case series, abstracts, reviews, editorials, conference proceedings, open label trials and those with insufficient information regarding the study design and outcomes were also excluded.
Primary and secondary outcomes
The primary outcome of transplant-free survival was assessed as the proportion of the cohort that survived until the end of the specified study period without requiring a liver transplant. Multiple secondary endpoints such as the length of hospital stay and occurrence of adverse events (including renal failure, pulmonary failure, and infections) during treatment were also evaluated. Contrary to a previously conducted meta-analysis, overall survival was not included as a secondary endpoint as most of the included studies lacked information pertinent to transplants (such as transplantation rates and post-transplant survival).
Data extraction and risk of bias analysis
A standardized questionnaire was used to extract the relevant data required to conduct the systematic review and meta-analysis such as year of study, location, primary author, population size, demographic and clinical characteristics, details of intervention and control/supportive treatment, primary and secondary outcomes, and side-effects. The risk of bias of individual studies was evaluated using the Cochrane risk of bias tool (CRoBT) [9] for RCTs, and the modified Newcastle-Ottawa Scale (N-OS) [10] for non-randomized cohort studies.
Effect measures and statistical analysis
The Mantel-Haenszel random-effects model was chosen to pool data for meta-analysis. Risk ratios and Cohen’s d with their 95% confidence intervals were the respective effect measures used to compare categorical and continuous outcomes between studies. Forest plots were also made for all measured endpoints. Study heterogeneity was determined using the I2 method, and clinical heterogeneity expected between studies could be attributed to the variations in aetiologies of liver injury, degrees of encephalopathy, demographic factors such as age; and lifestyle factors based on cultural and geographical determinants. Moreover, publication bias was assessed using funnel plots and Harbord-Egger analysis.
Results
Our search identified a total of 2 RCTs [11, 12] and 2 prospective cohort studies [13, 14] that met our screening criteria for qualitative synthesis (Fig. 1). However, only 3 of these were included in the metaanalysis, with the study by Darweesh et al. being excluded as it included patients diagnosed with ALF in the absence of clinical signs of encephalopathy.
Fig. 1.
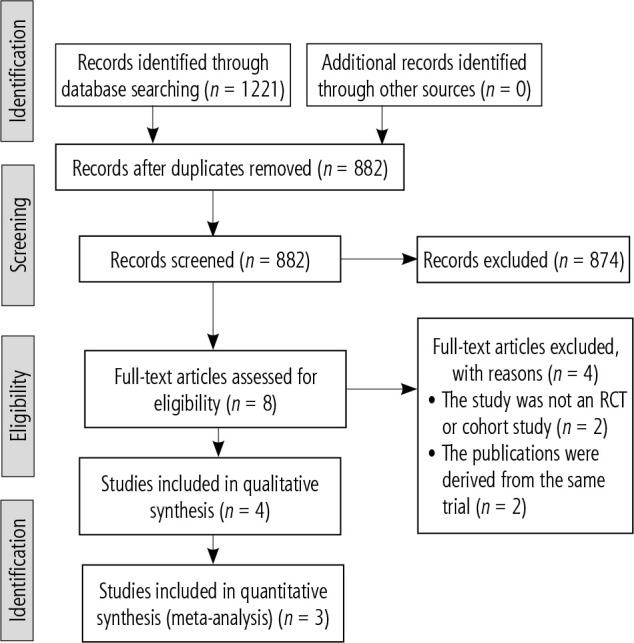
PRISMA diagram showing results of the search strategy employed
Table 1 shows a summary of basic study characteristics, while patient demographics along with relevant baseline clinical parameters and drug side-effects for each study are documented in Table 2. Demographic factors such as age and gender did not vary significantly between the NAC and PBO groups, enabling fair comparisons to be made. All studies excluded patients with prior history of liver disease. However, the use of biopsy to exclude acute-on-chronic liver disease was not documented in any study.
Table 1.
Baseline study characteristics
| Study | Study design | Duration of follow-up | Country | Population | ALF as defined by the study, and selection criteria | Intervention | Primary outcome | Outcomes | Prognostic system used for LT decision |
|---|---|---|---|---|---|---|---|---|---|
| Lee 2009 | Double blind RCT | 21 day | United States | NAC = 81 Placebo = 92 | ALF was defined as any degree of encephalopathy or coagulopathy (INR ≥ 1.5) due to an illness < 24 weeks Age > 18 years and < 70 years Patients excluded for known/suspected acetaminophen overdose or administration, hepatic ischemia, liver failure due to pregnancy or cancer, refractory hypotension, septic shock, or expected imminent transplantation (< 8 h) | NAC was administered with 5% dextrose solution. An initial loading dose of 150 mg/kg/h of NAC for one hour was followed by a dose of 12.5 mg/kg/h for 4 h, after which a continuous infusions of 6.25 mg/kg NAC was given for the remaining 67 h For the placebo group 5% dextrose solution was given | Overall survival at 3 weeks | 1. Transplant-free survival 2. Transplant rate 3. Length of hospital stay 4. No. of organ systems failing 5. Adverse events during the course of treatment |
MELD score |
| Nabi 2017 | Double blind RCT | Until discharge or death | Jammu and Kashmir | NAC = 40 Placebo = 40 | ALF was defined as biochemical evidence of ALF with INR of ≥ 1.5 and any degree of encephalopathy caused by illness of duration < 8 weeks (fulminant hepatic failure) Age > 18 years Patients excluded if history revealed acetaminophen induced ALF, pregnancy, prior acetaminophen exposure, acute on chronic liver failure, hepatic ischemia | IV NAC was administered with an initial loading dose of 150 mg/kg over 1 hour, followed by 12.5 mg/kg/h for 4 h after which a continuous infusion of 6.25 mg/kg/h was for remaining 67 h For the placebo group 5% IV dextrose solution was given for 72 h | Transplant-free survival | 1. Length of hospital stay 2. Adverse events during the course of treatment |
MELD score |
| Darweesh 2017 | Prospective cohort with historical control | 2-3 years | Kuwait | NAC = 85 Control = 70 | ALF was defined as jaundice (bilirubin [25 µmol/l) and coagulopathy (INR ≥ 1.5) with or without encephalopathy Patients excluded if clinical or historical evidence of acetaminophen overdose or prior liver disease | NAC was initially given as an infusion of 150 mg/kg in 100 ml dextrose 5% over 30 min, followed by 70 mg/kg in 500 ml dextrose 5% over 4 h, then 70 mg/kg in 500 ml dextrose 5% over 16 h. Subsequently, a continuous infusion of 150 mg/kg in 500 ml dextrose 5% over 24 h was continued until two consecutive INR results of less than 1.3 were noted with improving LFT at which point the patient was shifted to oral NAC in a dose of 600 mg/day. Patients were discharged 2-3 days after the change to oral NAC | Overall survival | 1. Transplant-free survival 2. Transplantation rate 3. Length of hospital stay 4. Adverse events during the course of treatment |
MELD score |
| Mumtaz 2009 | Prospective cohort with historical control | 60 days | Pakistan | NAC = 47 Control = 44 | Rapid development of acute liver injury with impaired synthetic function and encephalopathy in a person with a previously normal liver | Within 6 h of admission, oral NAC was administered at an initial dose of 140 mg/kg, followed by 17 doses of 70 mg/kg each 4 h apart | Transplant-free survival | 1. Length of hospital stay 2. Adverse events during the course of treatment |
King’s College Hospital prognostic criteria |
LT – liver transplantation, RCT – randomized controlled trial, NAC – N-acetylcysteine, ALF – acute liver failure, INR – international normalized ratio, MELD – Model of End-Stage Liver Disease, LFT – liver function test
Table 2.
Description of baseline demographic and clinical characteristics
| Study | Age (mean years) | Male (%) | Bilirubin(mg/dl) | ALT (mg/dl) | Time from jaundice to hepatic encephalopathy (days) | Encephalopathy | Reported side-effects | ||
|---|---|---|---|---|---|---|---|---|---|
| 0 | I-II | III-IV | |||||||
| Darweesh 2017 | 33.5/34.8 | 60/60 | 5.34/5.93 | 3144/2993** | – | 68.4 | 25.2 | 6.5 | Prolonged cholestasis present in 82 (96.4%) patients, fever and allergic reaction were reported in 3 (10%) patients, and dyspepsia in 11 (13.3%) |
| Nabi 2017 | 30.60/38.48 | 42.5/60 | 21.12/20.67 | 1051/1056 | 22 ±11.4/28 ±18.2 | 0.0 | 65.0 | 35.0 | No adverse effects attributable to NAC noted |
| Mumtaz 2009 | 27.74/37.52 | 55.3/54.5 | 20.63/14.36 | 1926/1457 | 8.87 ±9.47/13 ±18.24 | 0.0 | 42.9 | 57.1 | Noted in 6 (12.7%) patients within 4 h after NAC administration. These were nonspecific maculopapular rash in 2 patients, transient bronchospasm in 1 patient, and vomiting in 4 patients |
| Lee 2009a | 42/40.5 | 53/32 | 22.3/20.3 | 999/756** | 7 (0-153)/12 (0-165)*** | 0.0 | 65.9 | 34.1 | Nausea and vomiting present in 14% (95% CI = 6%, 22%, n = 81) of NAC treated patients and 4% (95% CI = 0%, 9%, n = 92) of placebo patients (p = 0.031) |
ALT – alanine transaminase, NAC – N-acetylcysteine
In cells with „/”, values represent NAC/control
DILI – drug induced liver injury, HILI – hepatitis-induced liver injury, other: fatty liver due to pregnancy, Wilson’s-induced liver injury, SLE
a Values for ALT and bilirubin reported as medians
Values are in IU/l
A significant difference was observed between NAC and placebo concerning median time in days from jaundice to HE (range)
Table 3 shows an aetiology wise breakdown of each study population. With the exception of Mumtaz et al. (concerning patients with hepatitis D superinfection-induced ALF), no significant differences between the frequencies of NAC and PBO populations were reported, with reference to any given aetiology. Data concerning aetiology-wise posttreatment transplant-free survival (TFS) outcomes were only reported by Nabi et al. and Lee et al. Nabi et al. reported significantly higher TFS in NAC patients with non-acetaminophen drug-induced liver failure (DILF) compared to their placebo counterparts. Moreover, Lee et al. reported that compared to their PBO counterparts, NAC patients with drug-induced or hepatitis B-induced acute liver failure had better overall survival and TFS than those with auto-immune or indeterminant ALF.
Table 3.
Breakdown of population by aetiology
| Study | Aetiology of ALF | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-acetaminophen drug- induced liver injury | Hepatitis B virus | Hepatitis A virus | Hepatitis E virus | Undetermined aetiology | Other aetiologies* | ||||
| Darweesh 2017 | 31/28 (36.5,40.0) | 11/14 (12.9, 20.0) | 12/10 (14.1, 14.3) | 6/5 (7.1, 7.1) | – | 25/13 (29.4, 18.6) | |||
| Nabi 2017 | 10/5 (25.0, 12.5)*** | 4/4 (10.0, 10.0) | 5/3 (12.5, 7.5) | 7/7 (17.5, 17.5) | 13/17 (32.5, 42.5) | 1/4 (2.5, 10.0) | |||
| Mumtaz 2009 | 3/8 (6.4, 18.2) | 11/14 (23.4, 31.8) | 2/0 (4.3, 0.0) | 24/16 (51.1, 37.2) | 1/4 (2.1, 9.1) | 6/2 (12.7, 4.5)** | |||
| Lee 2009 | 19/26 (27.1, 32.9) | 25/12 (35.7, 15.2) | – | – | 15/26 (21.4, 32.9) | 11/15 (15.7, 19.0) | |||
ALF – acute liver failure
In cells with „/”, values represent NAC/control (NAC %, control %)
Indicates ALF due to autoimmune conditions, other viral infections, Wilson’s-induced ALF, etc.
Statistically significant difference between NAC and placebo frequencies concerning hepatitis D superinfection
TFS of NAC patients was significantly higher than the PBO group
The results of the quality assessment of the studies, conducted using the CRoBT and N-OS, are shown in Supplementary Tables 1-2. The RCT by Lee et al. was of good quality with low risk of bias (RoB) in almost all categories, while the RoB in the study by Nabi et al. was generally unclear. On the other hand, the Newcastle-Ottawa Scale showed that both cohort studies had a low RoB.
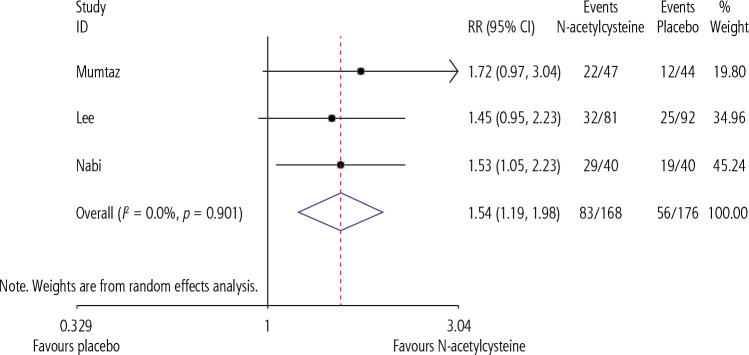
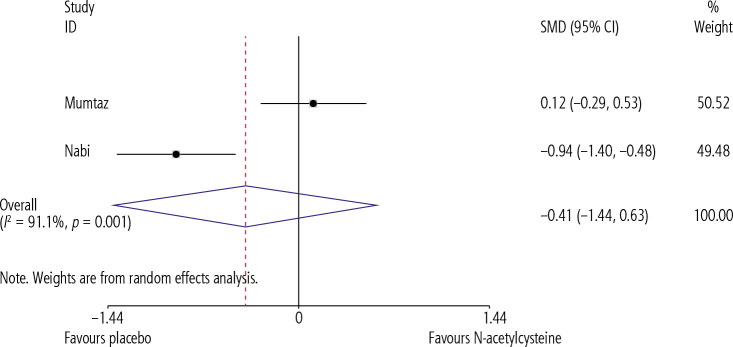
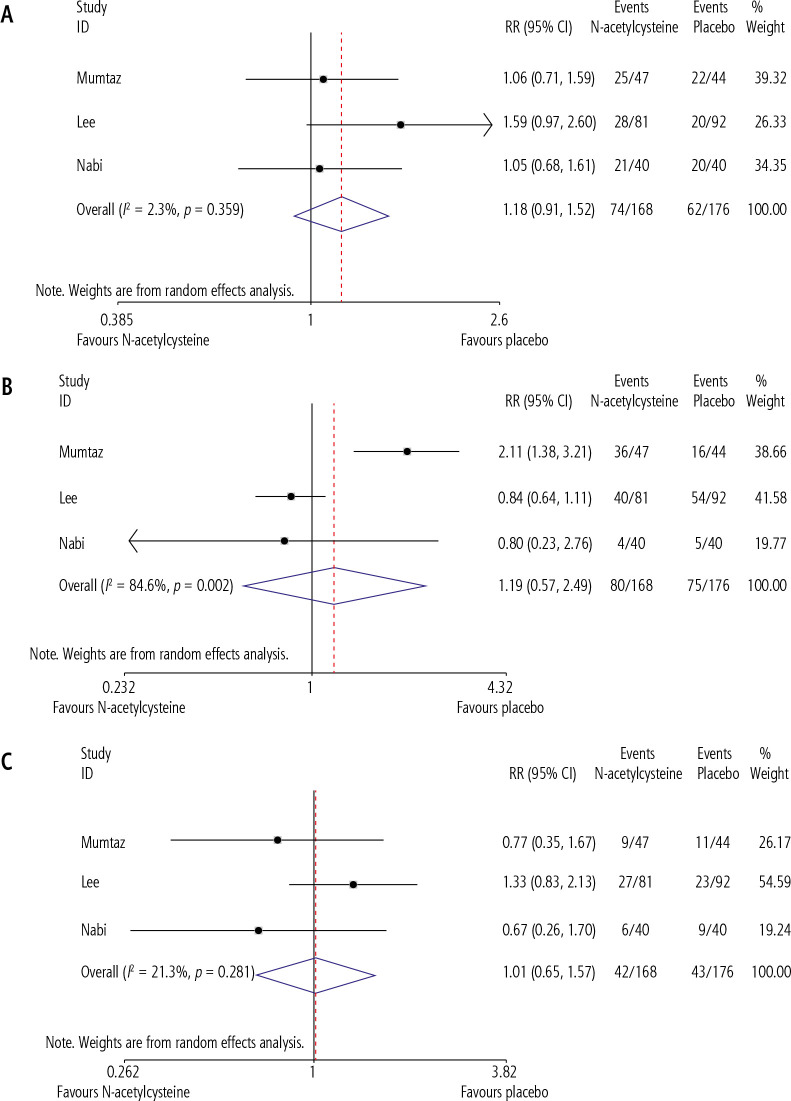
Our pooled analysis showed that N-acetylcysteine significantly improved our primary endpoint of TFS (RR = 1.54, CI = 1.19-1.98, p = 0.01, I2 = 0.0%) when compared to placebo (Fig. 2). However, N-acetylcysteine was not shown to significantly influence any of our secondary endpoints, which included length of hospital stay (SMD = –0.405, CI = –1.44-0.63, p = 0.445, I2 = 91.1%) (Fig. 3), and incidence of adverse events during the course of treatment (Fig. 4).
Fig. 2.
Comparison of the primary outcome (transplant-free survival) between NAC and PBO groups
Fig. 3.
Comparison of length of hospital stay between NAC and PBO groups
Fig. 4.
Comparison of adverse events between NAC and PBO groups during treatment. A) Incidence of renal failure, B) incidence of infections, C) incidence odrenal failure
While Nabi et al. found an inverse positive correlation between length of hospital stay and use of NAC (NAC = 8.2 ±2.1 days, PBO = 10.7 ±3.1 days, p = 0.002), such findings were reported by neither Mumtaz et al. (NAC = 9.4 ±7.26 days, PBO = 8.39 ±9.55, p = 0.56) nor Lee et al. (NAC = 9 days, PBO = 13 days, p = 0.056).
With reference to these adverse events, none were significantly affected by the administration of NAC (renal failure – RR = 1.01, CI = 0.65-1.57, p = 0.967, I2 = 21.3%, incidence of infections – RR = 1.18, CI = 0.91-1.52, p = 0.208, I2 = 2.3%, pulmonary failure – RR = 1.19, CI = 0.57-2.49, p = 0.649, I2 = 84.6%). Regarding the safety of NAC, most studies reported side-effects in only a minority of the cohort (Table 2). Moreover, depending on the study there was considerable variability in the incidence of side effects between control and intervention groups (Table 2). The reported side-effects, however, were generally not a major cause of concern, being limited to nausea, vomiting, fever, allergic reactions, dyspepsia, and rash. The trial conducted by Darweesh et al. was a notable exception in this regard as 96.4% of the enrolled patients suffered from prolonged cholestasis, which the authors attributed to NAC administration.
A funnel plot was made to evaluate the risk of bias across studies (Fig. 5) and Harbord’s modified test for small study effects was performed, with no significant bias, across studies, being found (bias = 1.08, CI = –13.6-14.7, p = 0.521).
Fig. 5.
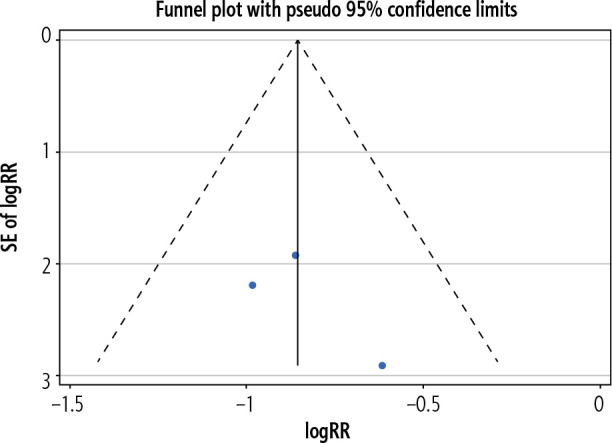
Funnel Plot for assessment of publication bias in the primary outcome
Discussion
Results of the meta-analysis revealed that administration of NAC significantly improved the TFS in NAI-ALF patients. This was found to be consistent with both a previously conducted meta-analysis by Hu et al. and the individual findings of the included studies. However, it should be noted that Lee et al. reported a survival benefit of NAC in only patients with encephalopathy grades 1 and 2 (NAC = 52%, PBO = 30%, p = 0.010). Furthermore, no significant correlation was found between our secondary end point of duration of hospital stay, with administration of NAC. The results of this meta-analysis should, however, be interpreted with caution in light of the high heterogeneity observed (I2 = 91.1%). This heterogeneity may be attributed to two main factors: exclusion of Lee et al. from the pooled analysis as it reported outcomes as medians, and the study design limitations of Mumtaz et al. (cohort with retrospective controls). Use of retrospective controls may have introduced a degree of bias in the comparison, as treatment standards and clinical protocols evolve over time, resulting in potentially shorter durations of hospital stay.
While certain aetiologies of ALF have a better prognosis than others [8], the question whether patients of specific aetiologies respond more favourably to NAC remains unclear. Both Nabi et al. and Lee et al. reported that NAC patients in the DILF subpopulations had better TFS than PBO. Moreover, according to Lee et al. the same was also true concerning patients with hepatitis B-induced ALF. However, no firm conclusions can be drawn upon these findings as the sample population in either case is too small.
With regards to the adverse events included in the analysis (renal failure, infections and pulmonary failure), none were shown to differ significantly between the NAC and PBO groups. These results are in accordance with previous studies that report no significant effect of NAC on improving renal function [15]. This is because kidney injury in NAI-ALF is primarily attributed to hypoperfusion, which is not reported to be ameliorated by NAC [15]. Similarly, individual studies reported conflicting findings regarding the relationship between NAC and pulmonary failure, which may be potentially explained by the difference in diagnostic criteria for pulmonary failure used by the studies, as this information was not reported.
While not included in the meta-analysis, a multicentre, prospective cohort study with historical controls was published by Darweesh et al. in 2017. As patients without signs of encephalopathy at the time of presentation were also included in the study, it was removed from the meta-analysis to allow for a fair pooling of results. This study reported a significantly higher TFS in NAC patients (96.4%) compared to the control (23.3%), in accordance with the meta-analysis. Moreover, overall survival (NAC = 96.7%, control = 76.7%, p < 0.01) and length of hospital stay (NAC = 10.1 days, control = 28 days, p < 0.01) were also reported to be significantly better in the NAC groups compared to the control.
Although the exact mechanism of action for NAC in patients with NAI-ALF still remains unclear, a recent study by Stravitz et al. [16] provided some new insights into the subject. In accordance with previous research, this study reported that ALF patients had increased levels of proinflammatory cytokines such as interleukin (IL)-1β, IL-2, IL-6, IL-10, IL-13, IL-17, interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α). However, the novel finding of this recent study was that only IL-17 levels significantly influenced the transplant-free survival and transplantation rate (OR = 3.46, p = 0.016) in such patients. Studies have shown that IL-17 promotes a pro-inflammatory response of neutrophils and potentiates the secretion of cytokines via positive feedback loops in the liver [17, 18]. It is also reported to enhance the permeability of the blood-brain barrier to both cytokines and T-lymphocytes, thereby providing a possible link to encephalopathy [19]. Stravitz et al. also reported that in patients given NAC, IL-17 decreased to undetectable levels over the course of treatment (p = 0.042), and was significantly lower than that observed in the PBO group (p = 0.045). While no other study to our knowledge has reported a mechanism of action for the decrease in IL-17 due to NAC, similar findings were reported by a study based on mice [20].
Regarding the adverse effects of NAC, studies reported it to be relatively safe with minimal side-effects (Table 2). Most studies reported that these side effects either resolved spontaneously with little to no treatment or were attributed to progressing ALF rather than NAC administration. A notable exception to this was the study by Darweesh et al., who reported prolonged cholestasis in a considerable number of patients (96.4%). However, this could easily be a consequence of the disease itself or the use of historical controls, leaving the exact role of NAC uncertain.
It should be noted that certain limitations need to be considered before drawing any concrete conclusions from this meta-analysis. Firstly, there were discrepancies in the study design, follow-up durations, population demographics, and baseline clinical characteristics of the studies from which data were pooled. Secondly, the influence of variables such as the grade of encephalopathy and aetiology of ALF should be considered when evaluating the role of NAC in increasing TFS. Thirdly, there is an inadequate amount of high-quality published evidence examining both overall survival (including post-transplant survival) and transplant-free survival, thereby hindering a meta-analysis of the overall survival outcome. Therefore, further research needs to be conducted on this subject for a definitive conclusion to be reached.
Conclusions
The findings of this study suggest NAC has a proven positive impact on TFS in adult patients with NAI-ALF. Its use is relatively safe, with minimal side-effects, and no significant increase in the incidence of major adverse events (renal failure, infections, and pulmonary failure). However, more randomized trials need to be conducted to conclusively ascertain its role in improving overall survival and duration of hospital stay.
Disclosure
The authors declare no conflict of interest.
The supplementary tables are available on journal website.
References
- 1.Ho C, Lee C, Wang J, et al. Nationwide longitudinal analysis of acute liver failure in Taiwan. Medicine 2014; 93: e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khokhar N, Niazi TK. Acute liver failure: an update. Pak J Med Res 2012; 51: 107-110. [Google Scholar]
- 3.Bernal W, Lee WM, Wendon J, et al. Acute liver failure: A curable disease by 2024? J Hepatol 2015; 62 (Suppl 1): S112-120. [DOI] [PubMed] [Google Scholar]
- 4.Karkhanis J, Verna EC, Chang MS, et al. Steroid use in acute liver failure. Hepatology 2014; 59: 612-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acetylcysteine monograph for professionals . Drugs.com, https://www.drugs.com/monograph/acetylcysteine.html (accessed: 14th March, 2020).
- 6.Hu J, Zhang Q, Ren X, et al. Efficacy and safety of acetylcysteine in “non-acetaminophen” acute liver failure: A meta-analysis of prospective clinical trials. Clin Res Hepatol Gastroenterol 2015; 39: 594-599. [DOI] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee W, Stravitz R, Larson A. Introduction to the revised American Association for the Study of Liver Diseases position paper on acute liver failure 2011. Hepatology 2012; 55: 965-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottawa Hospital Research Institute, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed: 26th June, 2020).
- 11.Nabi T, Nabi S, Rafiq N, Shah A. Role of N-acetylcysteine treatment in non-acetaminophen-induced acute liver failure: a prospective study. Saudi J Gastroenterol 2017; 23: 169-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee W, Hynan L, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009; 137: 856-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mumtaz K, Azam Z, Hamid S, et al. Role of N-acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation. Hepatol Int 2009; 3: 563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darweesh SK, Ibrahim MF, El-Tahawy MA. Effect of N-Acetylcysteine on mortality and liver transplantation rate in non-acetaminophen-induced acute liver failure: a multicenter study. Clin Drug Investig 2017; 37: 473-482. [DOI] [PubMed] [Google Scholar]
- 15.Tujios SR, Hynan LS, Vazquez MA, et al. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin Gastroenterol Hepatol 2015; 13: 352-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stravitz R, Sanyal A, Reisch J, et al. Effects of N-acetylcysteine on cytokines in non-acetaminophen acute liver failure: potential mechanism of improvement in transplant-free survival. Liver Int 2013; 33: 1324-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasumi Y, Takikawa Y, Endo R, Suzuki K. Interleukin-17 as a new marker of severity of acute hepatic injury. Hepatol Res 2007; 37: 248-254. [DOI] [PubMed] [Google Scholar]
- 18.Lafdil F, Miller AM, Ki SH, Gao B. Th17 cells and their associated cytokines in liver diseases. Cell Mol Immunol 2010; 7: 250-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 2007; 13: 1173-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bemeur C, Vaquero J, Desjardins P, Butterworth RF. N-acetylcysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure in mice: antioxidant and anti-inflammatory mechanisms. Metab Brain Dis 2010; 25: 241-249. [DOI] [PubMed] [Google Scholar]