Abstract
Background:
The hippocampus plays a central role in post-traumatic stress disorder (PTSD) pathogenesis, and the majority of neuroimaging research on PTSD has studied the hippocampus in its entirety. Although extensive literature demonstrates changes in hippocampal volume are associated with PTSD, fewer studies have probed the relationship between symptoms and the hippocampus’ functionally and structurally distinct subfields. We utilized data from a longitudinal study examining post-trauma outcomes to determine whether hippocampal subfield volumes change post-trauma and whether specific subfields are significantly associated with, or prospectively related to, PTSD symptom severity. As a secondary aim, we leveraged our unique study design sample to also investigate reliability of hippocampal subfield volumes using both cross-sectional and longitudinal pipelines available in FreeSurfer v6.0.
Methods:
Two-hundred and fifteen traumatically injured individuals were recruited from an urban Emergency Department. Two-weeks post-injury, participants underwent two consecutive days of neuroimaging (time 1: T1, and time 2: T2) with magnetic resonance imaging (MRI) and completed self-report assessments. Six-months later (time 3: T3), participants underwent an additional scan and were administered a structured interview assessing PTSD symptoms. First, we calculated reliability of hippocampal measurements at T1 and T2 (automatically segmented with FreeSurfer v6.0). We then examined the prospective (T1 subfields) and cross-sectional (T3 subfields) relationship between volumes and PTSD. Finally, we tested whether change in subfield volumes between T1 and T3 explained PTSD symptom variability.
Results:
After controlling for sex, age, and total brain volume, none of the subfield volumes (T1) were prospectively related to T3 PTSD symptoms nor were subfield volumes (T3) associated with current PTSD symptoms (T3). Tl – T2 reliability of all hippocampal subfields ranged from good to excellent (intraclass correlation coefficient (ICC) values > 0.83), with poorer reliability in the hippocampal fissure.
Conclusion:
Our study was a novel examination of the prospective relationship between hippocampal subfield volumes in relation to PTSD in a large trauma-exposed urban sample. There was no significant relationship between subfield volumes and PTSD symptoms, however, we confirmed FreeSurfer v6.0 hippocampal subfield segmentation is reliable when applied to a traumatically-injured sample, using both cross-sectional and longitudinal analysis pipelines. Although hippocampal subfield volumes may be an important marker of individual variability in PTSD, findings are likely conditional on the timing of the measurements (e.g. acute or chronic post-trauma periods) and analysis strategy (e.g. cross-sectional or prospective).
Keywords: FreeSurfer, Magnetic resonance imaging, PTSD, Hippocampal subfields, Test-retest reliability
1. Introduction
The hippocampus is a brain structure of the medial temporal lobe known primarily for its role in supporting learning and memory functions (Jin and Maren, 2015; Joshi et al., 2020; Knierim, 2015; Maren et al., 2013; Wixted and Squire, 2011). Work with rodents and human case studies with selective hippocampal damage (e.g., patient H.M.; Squire, 2009) has thoroughly documented the structure and function of the hippocampus (Bartsch and Wulff, 2015; Coburn, 2018; Knierim, 2015; Lupien and Lepage, 2001; Phillips and LeDoux, 2021; Preston-Ferrer and Burgalossi, 2018; Tatu and Vuillier, 2014; Witter et al., 2017). Comprised of several subfields with specialized cytoarchitecture, connectivity, and function including Cornu Ammonis (CA) 1–4, dentate gyrus (DG), presubiculum, subiculum, and parasubiculum, the hippocampus is integral for a myriad of mnemonic functions, such as the formation of fear memory traces (e.g., Coburn, 2018; El-Falougy and Benuska, 2006; Haukvik et al., 2018; Radonjic et al., 2014; Witter et al., 2017).
A substantial body of literature indicates the hippocampus is particularly vulnerable to stress from exposure to stress hormones produced by activity of the hypothalamic-pituitary-adrenal (HPA) axis (Bartsch and Wulff, 2015; Kim et al., 2015; Lupien and Lepage, 2001; McEwen et al., 2016; Miller and O’Callaghan, 2005; Ortiz and Conrad, 2018). Morphological, structural, and functional changes of the hippocampus have been reported in an array of psychological disorders, including posttraumatic stress disorder (PTSD; Besnard and Sahay, 2016; Coburn, 2018; Hayes et al., 2017; Lazarov et al., 2017; Logue et al., 2018; Malivoire et al., 2018; Rangaprakash et al., 2017; Tural et al., 2018; van Rooij et al., 2018). PTSD, which may develop as a consequence of experiencing a trauma, is a debilitating psychiatric disorder (Fenster et al., 2018; Mahan and Ressler, 2012). Symptoms include re-experiencing the traumatic event (e.g., flashbacks), avoidance of stimuli associated with the event, negative affect and cognition, and heightened arousal (American Psychiatric Association, 2013). Differences in hippocampus volume, as well as function, are theorized to underly memory issues frequently present in individuals with PTSD (Joshi et al., 2020; Liberzon and Sripada, 2007; McEwen et al., 2016; Shin, 2006).
A number of scholars have suggested hippocampal volume is a biomarker of risk for PTSD development (i.e., vulnerability factor; Gilbertson et al., 2002; Gurvits et al., 2006; Kremen et al., 2012; Wang et al., 2010; Xie et al., 2018) and/or asserted that changes in volume track with PTSD symptoms (i.e., are caused by the resulting psychological sequela; Apfel et al., 2011; Gurvits et al., 1996; Woon and Hedges, 2008). Although more sparse, additional work has demonstrated null findings, suggesting smaller hippocampus volume is neither a risk factor nor a consequence of PTSD (e.g., Bonne et al., 2001). Notably, the majority of this work has referenced the whole volume (Bonne et al., 2001; Chen et al., 2018).
Closer examination of hippocampal subfields may afford greater precision to the utility of the region as a biomarker of PTSD. In addition, each subfield may play a differential role in symptom development. Impaired extinction of fear memories and over-consolidation of fear, are two hallmarks of PTSD development which may result from specific subfield functional and/or structural abnormalities (Mahan and Ressler, 2012). Select studies, with both adolescents and adults, have segmented the hippocampus into its subfields and demonstrated that PTSD symptom severity is associated with smaller dentate gyrus (Hayes et al., 2017; Postel et al., 2019; Wang et al., 2010), CA1 (Chen et al., 2018), hippocampus-amygdala transition area (Ahmed-Leitao et al., 2016; Averill et al., 2017); , and parasubiculum (Ahmed-Leitao et al., 2016).
Specific neurocircuitry within the hippocampus, as described in animal models, would suggest particular behavioral effects (i.e., aberrant memory formation, consolidation, retrieval) emerge when different hippocampal subfields are perturbed (Phillips and LeDoux, 2021; Preston-Ferrer and Burgalossi, 2018). For example, decreased volume in the dentate gyrus, a region proposed to underlie pattern separation processes, may contribute to overgeneralization of fear, a common theoretical model of PTSD (Hayes et al., 2017). Together the dentate gyrus and CA3 also work together to encode and retrieve spatial information, while the CA1 is essential for a myriad of mnemonic tasks, including autobiographical memory (Bartsch et al., 2011). The parasubiculium is also linked to processing spatial information (Dalton and Maguire, 2017). Although future work is required, these findings collectively suggest PTSD is linked with decreased volume of hippocampal subfields responsible for holistic representations of scenes and offer a potential mechanism by which trauma impacts hippocampal activity and memory (Miller and Wiener, 2014).
The current evidence suggests that differences in hippocampal subfield volumes may reflect a predisposition to PTSD as well as correspond to post-trauma symptom trajectories. However, the current literature is lacking evidence as to whether hippocampal subfield volumes measured acutely post-trauma are prospectively related to PTSD symptoms. If subfield volumes are to be a useful biomarker for post-trauma individual risk and resilience, the measurement of the volumes must be valid – capture the individual differences associated with PTSD – and be reliably measured (Dhama et al., 2019; Lehrner and Yehuda, 2014; Mayeux, 2004). Therefore, reliable measurement of hippocampal structure and subfields is important for accurate monitoring of morphological and volumetric changes that accompany PTSD (Bartsch and Wulff, 2015; Burke and Barnes, 2010; Fröhner et al., 2019).
Although measurement of the whole hippocampus has proven reliable (Ahmed-Leitao et al., 2016; Brown et al., 2020; Hsu et al., 2002; Mulder et al., 2014; Schmidt et al., 2018), until recently, the small size of the subfields made assessing volumes challenging (Brown et al., 2020). Manual segmentation of the hippocampus and its subfields used to be the gold standard for segmentation despite the highly subjective process that depends heavily on the expertise of the evaluator (Dill et al., 2015; Yushkevich et al., 2015a,b). However, enhanced resolution of structural magnetic resonance imaging (MRI) technology and new segmentation programs have allowed for more quantitative approaches using atlases and probabilistic features of structural MRI data, making automated pipelines for hippocampal subfield segmentation a commonly used analytic tool (Dill et al., 2015). Although higher resolution images typically offer the most accurate segmentation (Wisse et al., 2016; Yushkevich et al., 2009), previous work has concluded automatic segmentation of hippocampal subfields in lower resolution images yields accurate measurements compared to manual edits (Yushkevich et al., 2015a,b).
FreeSurfer is perhaps the most widely used tool for automated tissue parcellation and cortical and subcortical segmentation (Fischl et al., 2002). Hippocampal subfield reliability processed through FreeSurfer has been evaluated across scanners (Marizzoni et al., 2015; Quattrini et al., 2020; Whelan et al., 2016) and across time on the scale of several months (Brown et al., 2020) to a year (Mulder et al., 2014). In the few studies that have assessed subfield reliability (Brown et al., 2020; Buser et al., 2020; Mulder et al., 2014), the majority appear to have moderate to good reliability, with the poorest reliability reported for the hippocampal fissure, which separates the dentate gyrus from the subiculum (Brown et al., 2020; Buser et al., 2020; Haładaj, 2020; Whelan et al., 2016). However, to our knowledge, day-to-day reliability, when a difference in hippocampal volume would be least expected, has not been evaluated. Moreover, reliability of hippocampal subfields has been predominately assessed in aging or healthy populations (Flores et al., 2015; Schmidt et al., 2018).
Herein, we examined the relationship between hippocampal subfield volumes and PTSD in a longitudinal study of psychological outcomes following a traumatic injury, using the probabilistic atlas-based procedure within FreeSurfer (version 6.0). As a secondary aim, we assessed the reliability of hippocampal subfield measurement. The participants in the study were scanned at three times, on two consecutive days approximately 2-weeks after their traumatic injuries (time 1: T1, and time 2: T2), and 6 months (time 3: T3) after their injury. This design allowed us to address four critical aims: 1) assess the reliability of hippocampal subfields on two consecutive days of scanning (T1 – T2), 2) determine whether hippocampal subfield measurements acutely post-trauma (T1) prospectively relate to future PTSD (T3), 3) examine the more routinely investigated cross-sectional association between subfield measurements at follow-up (T3) and current PTSD symptoms (T3), and 4) evaluate whether change in hippocampal volume (T1 – T3) relates to future PTSD symptoms (T3).
Based on the aforementioned research, we hypothesized that smaller global hippocampal volume (T1) would prospectively relate to T3 PTSD symptoms (Gilbertson et al., 2002; Gurvits et al., 2006; Kremen et al., 2012; Wang et al., 2010; Xie et al., 2018). We also hypothesized smaller global hippocampal volume (Apfel et al., 2011; Gurvits et al., 1996; Woon and Hedges, 2008), as well as smaller dentate gyrus/CA4 and CA1 (measured at T3) would be significantly related to T3 PTSD symptoms (Hayes et al., 2017). Finally, we anticipated there would be a significant change between T1 and T3 volumes, such that decreases in dentate gyrus and CA1 volume would track with PTSD symptoms (Chen et al., 2018; Hayes et al., 2017).
2. Method
2.1. Participants
Nine-hundred sixty-nine trauma survivors treated for their injuries at the Emergency Department (ED) at Froedtert Hospital (Milwaukee, Wisconsin, USA) were recruited for the Imaging Study on Trauma & Resilience (iSTAR study). Participants were recruited and screened for eligibility directly from the ED or by phone following discharge. After expressing interest in study participation, the participant received a complete verbal overview of the study and provided written informed consent. All procedures were approved by the Medical College of Wisconsin Institutional Review Board.
Of the 969 recruited for the study, 215 met eligibility criteria and were enrolled. Individuals were eligible if their trauma exposure met criterion A of PTSD diagnosis as defined by the Diagnostic and Statistical Manual - 5th edition (DSM-5; American Psychiatric Association, 2013), scored a minimum of three on the Predicting PTSD Questionnaire (Rothbaum et al., 2014; represents a greater risk of PTSD development), if they were English speaking, between the ages of 18–60 years, and able to schedule their first research visit within 30 days of their trauma. Exclusion criteria included contraindications for MRI scanning including metal objects or fragments in the body, claustrophobia, and pregnancy or planned pregnancy within the next 6 months, head injury more severe than a mild traumatic brain injury (score of less than 13 on the Glasgow Coma Scale; Sternbach, 2000), spinal cord injury with neurological deficit, self-inflicted injury, severe vision or hearing impairments, history of psychotic or manic symptoms, currently on antipsychotic medications, substance abuse noted in medical record, or on police hold following their injury. Sample characteristics are reported in Table 1.
Table 1.
Sample Characteristics.
| Variable | Percent (%) | Mean | SD | Range |
|---|---|---|---|---|
| Age (years) | 33.1 | 10.8 | ||
| Sex | ||||
| Female | 55% | |||
| Race and Ethnicity | ||||
| African American/Black | 60% | |||
| White | 26% | |||
| More than one race/Other | 8% | |||
| Unknown/Not reported | 6% | |||
| Education | ||||
| Less than high school/GED | 9% | |||
| High school/GED | 31% | |||
| Some post-secondary education/college | 25% | |||
| Associate degree | 14% | |||
| Bachelor’s degree or beyond | 16% | |||
| Not reported | 5% | |||
| Mechanism of Injury | ||||
| Motor Vehicle Crash | 68% | |||
| Assault/Altercation | 13% | |||
| Other (Fall, Pedestrian Struck, Crush Injury) | 19% | |||
| Days Since Injury | ||||
| T1 | 16.2 | 5.1 | ||
| T3 | 183.6 | 12.6 | ||
| CAPS-5 Total Symptom Severity (N = 139) | 11.69 | 10.73 | 0–63 | |
| CAPS-5 PTSD Dx (N = 139) | 18% (N = 26) |
Note: Demographic data presented for all participants with T1 Scans (N = 197); PTSD symptom severity is presented for subjects who completed T3 scans and the structured interview (N = 139). CAPS-5, Clinician Administered PTSD Scale for DSM-5.
2.2. Procedure
Participants attended research visits at three time points; within 2–3 weeks on two consecutive days (T1, T2) and 6 months (T3) following the trauma that resulted in their ED admission. At all visits, a large battery of behavioral and cognitive tasks, demographics, self-report questionnaires, physiologic, biologic, and neuroimaging data were collected. Here we report on select study measures and the structural MRI data from all time points. Of the 215 initially enrolled in the study, 208 were scanned at T1 (96.7% retention), 185 at T2 (86.0% retention), and 160 at T3 (74.0% retention). Reductions in sample sizes at each time point were the result of expected losses to follow-up due to scheduling conflicts or discontinued interest in study participation. However, final sample sizes in the reliability analyses were further reduced due to qualitative assessment of motion artifacts (i.e. large-scale ghosting, zippering, blurring, signal-dropout, etc.) within anatomical scans (usable scans: T1 = 197, T2 = 178, T3 = 153) or due to missing scans at relevant time points. Therefore, our final sample size for the T1 – T2 reliability analysis consisted of 175 with usable (motion artifact free) anatomical scans at both T1 and T2 (81.4% retention). Similarly, the final sample size for the analysis on T1 – T3 change over time and PTSD symptoms, as well as the T1 – T3 reliability analysis (included in Supplemental Material), included 141 participants with usable scans at both T1 and T3 (65.5% retention).
At T3, the Clinician Administered PTSD Scale for DSM-5 (CAPS-5) was administered by a trained staff member to evaluate PTSD symptoms with respect to the index trauma (Weathers et al., 2018). CAPS-5 is considered the gold-standard of PTSD psychodiagnostic assessments and has good validity with other measures of PTSD and high internal consistency (Weathers et al., 2018). The interview consists of 30 items, with the first 20 corresponding to symptoms of PTSD included in the DSM-5 (American Psychiatric Association, 2013). The interviewer rated each symptom on severity and frequency, with individual item scores ranging from 0 to 4. A total PTSD symptom severity score was created by summing the first 20 items. In the current study, 20% of the CAPS were subject to reliability checks and the total symptom severity scores had excellent reliability (interclass correlation coefficient = 0.96, 95% Confidence Interval [0.93–0.98]).
2.3. MRI acquisition
Structural MRI scans were collected on one scanner: a 3.0T short bore GE Signa Excite system with a 32-channel head-coil. High resolution spoiled gradient recalled (SPGR) T1-weighted images were acquired in sagittal slices (voxel size = 1 × 0.9375 × 0.9375 mm, TR = 8.2 ms; TE = 3.2 ms; FOV = 240 mm; flip angle = 12°, slice thickness = 1 mm, # slices = 150, matrix = 150 × 256 × 256).
2.4. FreeSurfer processing pipeline
Anatomical T1-weighted scans from T1, T2, and T3 were all processed cross-sectionally in the FreeSurfer v6.0 recon-all pipeline for automated cortical and subcortical parcellations and tissue segmentation (https://surfer.nmr.mgh.harvard.edu/). The technical details of the pipeline have been described extensively in previous publications (Dale et al., 1999; Dale and Sereno, 1993; Fischl, 2004; Fischl et al., 1999a,b, 2001, 2002, 2004; Fischl and Dale, 2000; Han et al., 2006; Jovicich et al., 2006; Reuter et al., 2010, 2012; Ségonne et al., 2004). Resultant reconstructions were visually inspected for quality control ensuring appropriate parcellations and segmentations were completed; however, no manual edits were made to limit experimenter bias (McCarthy et al., 2015). One subject was excluded from all analyses due to limited contrast resulting in poor reconstruction through the FreeSurfer pipeline (N = 175 for T1 – T2, N = 141 for T1 – T3).
As part of a supplemental analysis to compare reliability and performance of FreeSurfer processing pipelines, T1 and T3 (N = 141) scans were also processed through FreeSurfer’s longitudinal processing stream (Reuter et al., 2010, 2012). Thus, hippocampal subfield volume reliability was compared between outputs from the cross-sectional and longitudinal processing streams (see Supplemental Material).
2.4.1. Hippocampal subfields
An automated pipeline for hippocampal subfield segmentation is included in FreeSurfer v6.0. This pipeline can be implemented on cross-sectional data and on the within-subject template from the longitudinal processing stream in FreeSurfer. The specific details of the steps within this pipeline are described in the original methods paper (Iglesias et al., 2015). Outputs from the analysis include volume estimates for each hemisphere of the following hippocampal subfields: hippocampal tail, subiculum, CA1, hippocampal fissure, presubiculum, parasubiculum, molecular layer, granule cell layer of the dentate gyrus (GC-DG), CA3, CA4, fimbria, hippocampal-amygdaloid transition area (HATA), and the whole hippocampus. See Fig. 1 for hippocampal subfield segmentation from a representative participant.
Fig. 1.
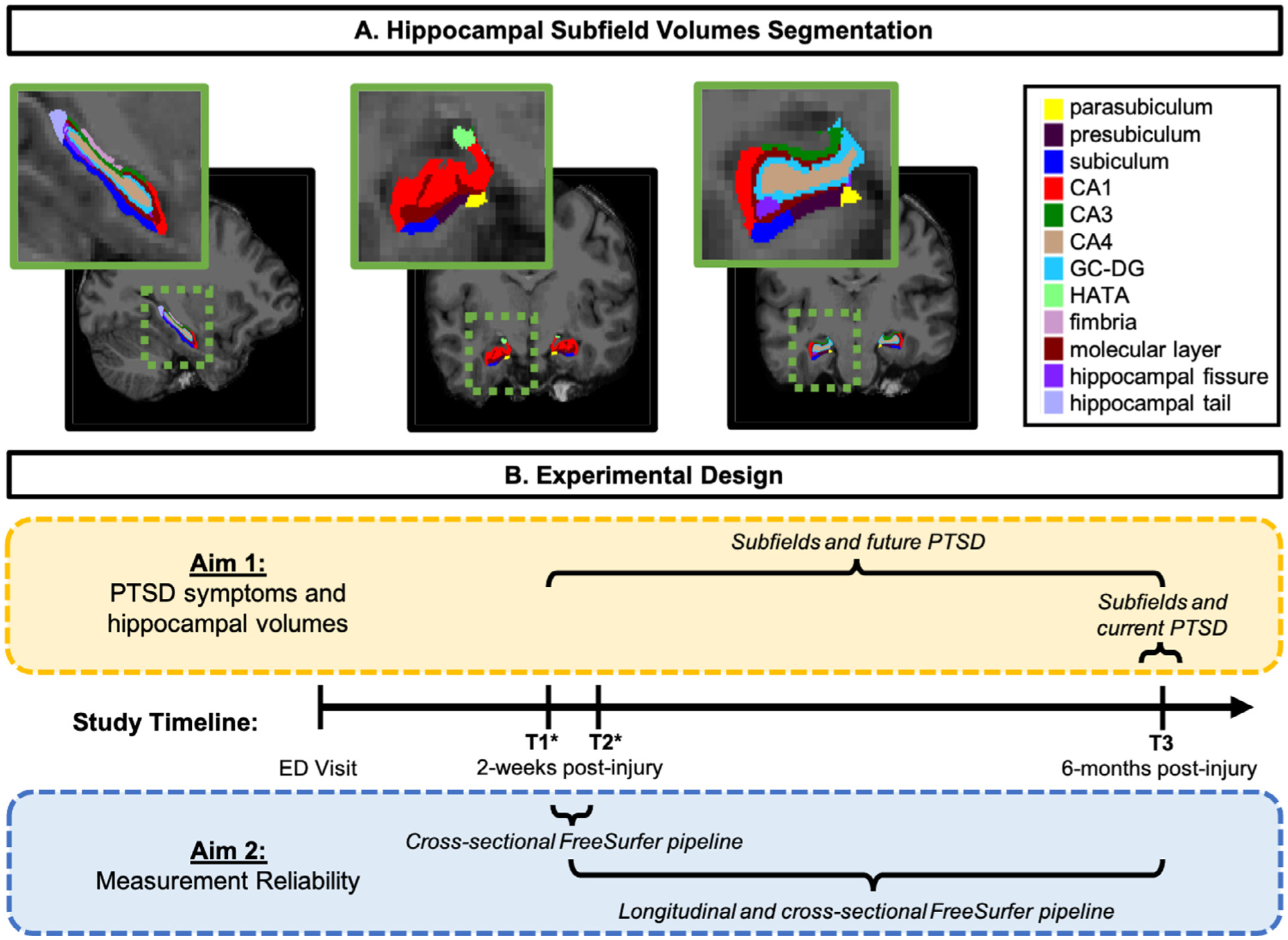
A) Hippocampal subfield segmentations from a representative participant. CA, cornu ammonis; GC-DG, granule cell layer of the dentate gyrus; HATA, hippocampal-amygdaloid transitional area. B) Schematic of experimental design including the analytic strategy for Aim 1 (yellow box) and Aim 2 (blue box) as well as the study timeline. Following the participant’s Emergency Department (ED) visit and recruitment into the study, MRI structural scans occurred at all study appointments: timepoint one (T1; two-weeks post-trauma), timepoint two (T2; two-weeks post-trauma), and timepoint three (T3; six-months post-trauma). Note: * T1 and T2 study appointments occurred on two consecutive days. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.5. Statistical analysis
2.5.1. T1 – T2 hippocampal subfield measurement reliability: percent volume difference (PVD) and intraclass correlation coefficients (ICC)
Average percent volume difference (PVD, Eq. 1) was calculated as in Brown et al. (2020) and (Morey et al., 2009, 2010) for each hemisphere and each subfield to determine volumetric correspondence between T1 – T2 (N = 175).
| (1) |
In a similar manner, intra-class correlation coefficients (ICC) were calculated to assess within-subject variability of hippocampal subfield measurement across time. Using the statistical package “irr” in R (Gamer et al., 2012), ICC(3,1) was used to estimate the agreement of hippocampal subfield measurements for T1 – T2 scans (N = 175). The ICC was modeled by a two-way mixed-effects model with random subject and fixed session effects. For both PVD and ICC, calculations for T1 – T2 were done using outputs from FreeSurfer’s cross-sectional processing stream.
In addition, we explored reliability (PVD and ICC) of hippocampal subfield measurement between T1 – T3, without considering PTSD symptoms, using both the cross-sectional and longitudinal processing streams in FreeSurfer. The results of this analysis can be found in the Supplemental Material.
2.5.2. Hippocampal subfield volumes and PTSD symptoms
Of the 197 subjects with scans at T1, 30 did not complete the CAPS-5 at T3 and were therefore excluded from the analyses investigating PTSD symptoms. Thus, 167 individuals were included in the analysis examining T1 volumes and T3 PTSD symptoms and 139 subjects were analyzed in the tests assessing T3 volumes and T3 symptoms (two individuals who underwent T3 scanning did not complete the interview).
Bivariate relationships between PTSD symptom severity, age, and hippocampal subfields were first assessed using Pearson’s correlations whereas the relationship between numeric variables and sex (coded “0” for males and “1” for females) were evaluated using point bi-serial correlation (see Supplemental Material). Considering we had no a-priori hypotheses regarding hemispheric differences, left and right hemispheres for each subfield, as well as whole hippocampus, were summed to yield a bilateral volume. In the primary analyses, general linear models were conducted to determine whether subfield volumes were prospectively related to T3 PTSD symptoms, or whether T3 subfield volumes were associated with T3 PTSD symptoms, after adjustment for sex, age, and total brain volume (total gray matter + total white matter). For all statistical tests, a Holm-Bonferroni correction was applied to correct for multiple comparisons (alpha = 0.05; Holm, 1979).
2.5.3. Change in hippocampal subfield volumes and PTSD symptoms
Finally, we examined the relationship of PVD (Eq. 1) in hippocampal subfields across time (T1 – T3) in relation to future PTSD symptoms (T3). Of the 141 participants with scans at T1 and T3, 4 did not complete the CAPS-5 at T3, therefore 137 participants were included in this analysis. Left and right hemispheres for each subfield were summed to yield a bilateral PVD measure. Thirteen (12 subfields + whole hippocampus) general linear models (GLMs) were run with CAPS-5 (T3) as the dependent variable, and bilateral PVD of a given hippocampal subfield (T1 – T3) as the independent variable while controlling for sex, age, and total brain volume. For all statistical tests, a Holm-Bonferroni correction was applied to correct for multiple comparisons (alpha = 0.05; Holm, 1979).
For this analysis, we used volume measurements from FreeSurfer’s longitudinal processing stream; however, for completeness, we repeated the above analysis with volume measurements from the cross-sectional processing stream. Complete results for both versions of the analysis can be found in the supplement (Supplemental Table 4 and 5).
3. Results
3.1. PVD (T1 – T2)
Fig. 2 depicts average PVD for hippocampal subfield measurements acquired across two consecutive days (T1 – T2; N = 175). The subfields demonstrating highest consistency (PVD < 3%) included the molecular layer and whole hippocampal volume. The left fissure, bilateral parasubiculum, and HATA show the least consistency when processed showing approximately a 10% difference in volume across the two scans.
Fig. 2.
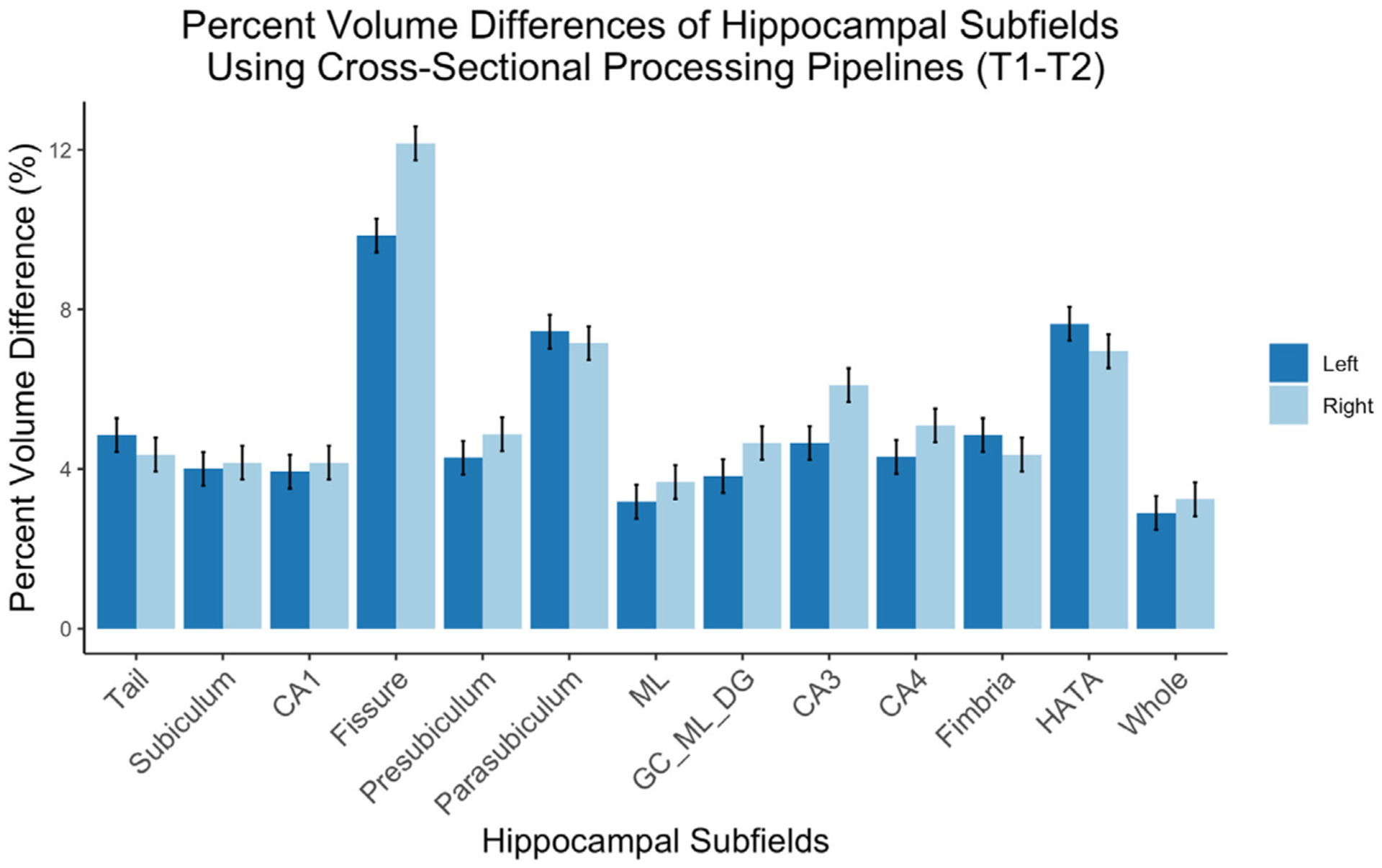
Percent Volume Differences for all hippocampal subfields across two consecutive scan days (T1 – T2). Error bars represent standard error. Left, left hemisphere; Right, right hemisphere; CA, cornu ammonis; ML, molecular layer; GC_ML_DG, granule cell layer of the dentate gyrus; HATA, hippocampal-amygdaloid transition area; Whole, whole hippocampal volume. N = 175. ICC (T1 – T2).
Results of the ICC analysis indicated good (between 0.75–0.9) to excellent (greater than 0.9; Koo and Li, 2016) scan-rescan reliability (ranged from 0.83 to 0.94) across the two consecutive scanning days using the cross-sectional processing stream (T1 – T2; Table 2).
Table 2.
Intraclass correlations coefficients for hippocampal subfields (T1 – T2) processed through cross-sectional pipelines with 95% confidence intervals.
| Subfield | Hemi | Cross-sectional | ||
|---|---|---|---|---|
| ICC | Lower bound | Upper bound | ||
| Hippocampal tail | L | 0.91 | 0.88 | 0.94 |
| R | 0.94 | 0.91 | 0.95 | |
| Subiculum | L | 0.94 | 0.92 | 0.96 |
| R | 0.92 | 0.89 | 0.94 | |
| CA1 | L | 0.93 | 0.90 | 0.95 |
| R | 0.89 | 0.85 | 0.92 | |
| Hippocampal fissure | L | 0.85 | 0.80 | 0.89 |
| R | 0.83 | 0.77 | 0.87 | |
| Presubiculum | L | 0.94 | 0.92 | 0.95 |
| R | 0.91 | 0.87 | 0.93 | |
| Parasubiculum | L | 0.88 | 0.84 | 0.91 |
| R | 0.92 | 0.90 | 0.94 | |
| Molecular Layer | L | 0.94 | 0.92 | 0.95 |
| R | 0.90 | 0.86 | 0.92 | |
| GC-DG | L | 0.93 | 0.90 | 0.95 |
| R | 0.90 | 0.86 | 0.92 | |
| CA3 | L | 0.94 | 0.92 | 0.95 |
| R | 0.91 | 0.88 | 0.93 | |
| CA4 | L | 0.91 | 0.89 | 0.94 |
| R | 0.89 | 0.82 | 0.92 | |
| Fimbria | L | 0.91 | 0.88 | 0.94 |
| R | 0.94 | 0.91 | 0.95 | |
| HATA | L | 0.89 | 0.85 | 0.91 |
| R | 0.88 | 0.84 | 0.91 | |
| Whole hippocampus | L | 0.94 | 0.91 | 0.95 |
| R | 0.90 | 0.86 | 0.92 | |
Hemi, hemisphere; ICC, intraclass correlation; L, left; R, right; CA, cornu ammonis; GC-DG, granule cell layer of dentate gyrus; HATA, hippocampal-amygdaloid transitional area, N = 175.
3.2. Hippocampal subfield volumes (T1) and future PTSD symptoms (T3)
Bivariate relationships between hippocampal subfields (T1), sex, age, and T3 CAPS-5 total scores are presented in Supplemental Table 1. Even before adjustment for multiple comparisons, none of the 12 subfield volumes were associated with T3 PTSD symptoms over and above total brain volume, age, and sex (Table 3; all full model uncorrected p’s > 0.05; Hippocampal tail: R2 = 0.02, F(4, 162) = 0.87, p = .482; Subiculum: R2 = 0.01, F(4, 162) = 0.52, p = .719; CA1: R2 = 0.01, F(4, 162) = 0.68, p = .601; Fissure: R2 = 0.01, F(4, 162) = 0.53, p = .707; Presubiculum: R2 = 0.01, F(4, 162) = 0.683, p = .601; Parasubiculum: R2 = 0.02, F(4, 162) = 0.86, p = .483; Molecular Layer: R2 = 0.01, F(4, 162) = 0.67, p = .609; GC-ML-DG: R2 = 0.02, F(4, 162) = 1.17, p = .323; CA3: R2 = 0.02, F(4, 162) = 1.00, p = .405; CA4: R2 = 0.03, F(4, 162) = 1.31, p = .267; Fimbria: R2 = 0.01, F(4, 162) = 0.54, p = .701; HATA: R2 = 0.01, F(4, 162) = 0.72, p = .573). The whole hippocampus volume was not prospectively related to T3 PTSD symptoms, R2 = 0.01, F(4, 162) = 0.61, p = .654.
Table 3.
Hippocampal volumes from cross-sectional processing stream (T1) and future PTSD Symptoms (T3).
| Bilateral Subfield Volume (T1) | B | ß | T | p | |
|---|---|---|---|---|---|
| Hippocampal Tail | (Intercept) | 7.04 | 0.00 | 0.48 | 0.634 |
| Hippocampal Tail | 0.01 | 0.10 | 1.22 | 0.224 | |
| Sex | 2.07 | 0.09 | 0.86 | 0.389 | |
| Age | −0.07 | −0.06 | −0.75 | 0.455 | |
| Total brain volume | −0.00 | −0.02 | −0.19 | 0.851 | |
| Subiculum | (Intercept) | 14.00 | −0.00 | 0.97 | 0.334 |
| Subiculum | −0.00 | −0.03 | −0.33 | 0.743 | |
| Sex | 2.31 | 0.10 | 0.96 | 0.337 | |
| Age | −0.07 | −0.06 | −0.74 | 0.461 | |
| Total brain volume | 0.00 | 0.03 | 0.24 | 0.808 | |
| CA1 | (Intercept) | 15.25 | −0.00 | 1.07 | 0.287 |
| CA1 | −0.01 | −0.08 | −0.87 | 0.384 | |
| Sex | 2.24 | 0.09 | 0.94 | 0.350 | |
| Age | −0.06 | −0.05 | −0.65 | 0.519 | |
| Total brain volume | 0.00 | 0.06 | 0.51 | 0.612 | |
| Hippocampal Fissure | (Intercept) | 11.30 | −0.00 | 0.78 | 0.438 |
| Hippocampal Fissure | 0.01 | 0.03 | 0.42 | 0.679 | |
| Sex | 2.42 | 0.10 | 1.01 | 0.316 | |
| Age | −0.07 | −0.07 | −0.83 | 0.409 | |
| Total brain volume | 0.00 | 0.01 | 0.06 | 0.953 | |
| Presubiculum | (Intercept) | 16.30 | −0.00 | 1.12 | 0.264 |
| Presubiculum | −0.01 | −0.08 | −0.87 | 0.383 | |
| Sex | 2.10 | 0.09 | 0.87 | 0.384 | |
| Age | −0.07 | −0.06 | −0.76 | 0.446 | |
| Total brain volume | 0.00 | 0.05 | 0.47 | 0.642 | |
| Parasubiculum | (Intercept) | 15.37 | −0.00 | 1.09 | 0.278 |
| Parasubiculum | −0.07 | −0.11 | −1.22 | 0.225 | |
| Sex | 2.09 | 0.09 | 0.87 | 0.384 | |
| Age | −0.06 | −0.06 | −0.70 | 0.484 | |
| Total brain volume | 0.00 | 0.05 | 0.51 | 0.611 | |
| Molecular Layer | (Intercept) | 16.03 | −0.00 | 1.11 | 0.270 |
| Molecular Layer | −0.01 | −0.08 | −0.85 | 0.399 | |
| Sex | 2.29 | 0.10 | 0.96 | 0.339 | |
| Age | −0.06 | −0.05 | −0.67 | 0.503 | |
| Total brain volume | 0.00 | 0.06 | 0.52 | 0.604 | |
| GC-DG | (Intercept) | 17.91 | −0.00 | 1.26 | 0.211 |
| GC-DG | −0.03 | −0.16 | −1.64 | 0.103 | |
| Sex | 2.10 | 0.09 | 0.88 | 0.378 | |
| Age | −0.04 | −0.03 | −0.40 | 0.690 | |
| Total brain volume | 0.00 | 0.11 | 0.93 | 0.356 | |
| CA3 | (Intercept) | 15.50 | −0.00 | 1.10 | 0.272 |
| CA3 | −0.03 | −0.13 | −1.42 | 0.157 | |
| Sex | 2.25 | 0.09 | 0.95 | 0.346 | |
| Age | −0.04 | −0.03 | −0.42 | 0.675 | |
| Total brain volume | 0.00 | 0.07 | 0.68 | 0.500 | |
| CA4 | (Intercept) | 18.27 | −0.00 | 1.28 | 0.201 |
| CA4 | −0.04 | −0.17 | −1.80 | 0.074 | |
| Sex | 2.17 | 0.09 | 0.91 | 0.363 | |
| Age | −0.03 | −0.03 | −0.37 | 0.710 | |
| Total brain volume | 0.00 | 0.11 | 0.97 | 0.335 | |
| Fimbria | (Intercept) | 13.10 | −0.00 | 0.93 | 0.352 |
| Fimbria | −0.02 | −0.04 | −0.45 | 0.650 | |
| Sex | 2.17 | 0.09 | 0.89 | 0.373 | |
| Age | −0.07 | −0.06 | −0.80 | 0.424 | |
| Total brain volume | 0.00 | 0.03 | 0.25 | 0.799 | |
| HATA | (Intercept) | 13.94 | −0.00 | 0.99 | 0.322 |
| HATA | −0.07 | −0.09 | −0.96 | 0.337 | |
| Sex | 2.34 | 0.10 | 0.98 | 0.329 | |
| Age | −0.03 | −0.03 | −0.36 | 0.718 | |
| Total brain volume | 0.00 | 0.06 | 0.53 | 0.594 | |
| Whole hippocampus | (Intercept) | 15.82 | −0.00 | 1.08 | 0.282 |
| Whole hippocampus | −0.00 | −0.07 | −0.68 | 0.496 | |
| Sex | 2.29 | 0.10 | 0.96 | 0.341 | |
| Age | −0.06 | −0.06 | −0.68 | 0.497 | |
| Total brain volume | 0.00 | 0.05 | 0.44 | 0.659 |
Note.
p < .05 uncorrected, CA, cornu ammonis; GC-DG, granule cell layer of dentate gyrus; HATA, hippocampal-amygdaloid transitional area. N = 167.
T1 hippocampal subfield volumes separated by hemisphere were also examined. After correction for multiple comparisons, still no subfields were related to future symptoms.
3.3. Hippocampal subfield volumes (T3) associated with current PTSD symptoms (T3)
Bivariate relationships between hippocampal subfields (T3; obtained via cross-sectional pipeline), sex, age, and current PTSD symptoms are presented in Supplemental Table 2.
None of the subfields were associated with PTSD symptoms even before correction for multiple comparisons (all full model uncorrected p’s > 0.05; Tail: R2 = 0.03, F(4, 134) = 1.22, p = .301; Subiculum: R2 = 0.003, F(4, 134) = 0.11, p = .976; CA1: R2 = 0.005, F(4, 134) = 0.18, p = .946; Fissure: R2 = 0.003, F(4, 134) = 0.11, p = .977; Presubiculum: R2 = 0.005, F(4, 134) = 0.19, p = .941; Parasubiculum: R2 = 0.01, F(4, 134) = 0.41, p = .801; Molecular Layer: R2 = 0.006, F(4, 134) = 0.23, p = .917; GC-ML-DG: R2 = 0.01, F(4, 134) = 0.547, p = .700; CA3: R2 = 0.001, F(4, 134) = 0.59, p = .667; CA4: R2 = 0.01, F(4, 134) = 0.563, p = .689; Fimbria: R2 = 0.004, F(4, 134) = 0.13, p = .968; HATA: R2 = 0.007, F(4, 134) = 0.24, p = .914), furthermore, whole hippocampus volume was not significantly associated with CAPS-5 scores, R2 = 0.002, F(4, 134) = 0.09, p = .983.
Again, we examined the same set of relationships separately for each hemisphere, still no subfield volumes at T3 were related to T3 PTSD symptoms after correction for multiple comparisons.
3.4. Change in subfield volume and PTSD symptoms
Full model results of the GLM analysis of subfield PVD (T1 – T3) associated with CAPS symptom severity (T3) using the longitudinal stream can be found in Supplemental Table 4. Results using the longitudinal stream outputs indicated there were differences in subfield significance (namely, bilateral fissure and subiculum); however, no results of this analysis survived correction for multiple comparisons using the Holm-Bonferroni method (all adjusted p > .80; Holm, 1979).
Though the primary evaluation in this analysis utilized the longitudinal stream outputs, examination of results using the cross-sectional stream outputs were also examined (Supplemental Table 5). No results of this analysis survived correction for multiple comparisons. Thus, in either analysis stream, change in hippocampal subfield volume over time (PVD T1 – T3) was not related to future PTSD symptoms (T3). When hippocampal subfield volumes were examined separately by hemisphere, for either cross-sectional or longitudinal stream, no changes in volumes were related to PTSD symptoms. Table 4
Table 4.
Hippocampal volumes from cross-sectional processing stream (T3) associated with current PTSD Symptoms (T3).
| Bilateral Subfield Volume (T3) | B | ß | T | p | |
|---|---|---|---|---|---|
| Hippocampal Tail | (Intercept) | 10.66 | −0.00 | 0.81 | 0.420 |
| Hippocampal Tail | 0.01 | 0.20 | 2.15 | 0.034 | |
| Sex | −0.55 | −0.03 | −0.24 | 0.811 | |
| Age | −0.05 | −0.05 | −0.55 | 0.587 | |
| Total brain volume | −0.00 | −0.12 | −1.05 | 0.294 | |
| Subiculum | (Intercept) | 17.58 | 0.00 | 1.32 | 0.188 |
| Subiculum | −0.00 | −0.04 | −0.41 | 0.685 | |
| Sex | −0.04 | −0.00 | −0.02 | 0.986 | |
| Age | −0.04 | −0.04 | −0.41 | 0.684 | |
| Total brain volume | −0.00 | −0.01 | −0.05 | 0.959 | |
| CA1 | (Intercept) | 17.92 | 0.00 | 1.36 | 0.177 |
| CA1 | −0.01 | −0.07 | −0.65 | 0.514 | |
| Sex | −0.08 | −0.00 | −0.04 | 0.971 | |
| Age | −0.03 | −0.03 | −0.32 | 0.749 | |
| Total brain volume | 0.00 | 0.01 | 0.09 | 0.925 | |
| Hippocampal Fissure | (Intercept) | 17.75 | 0.00 | 1.33 | 0.187 |
| Hippocampal Fissure | −0.01 | −0.04 | −0.39 | 0.699 | |
| Sex | −0.13 | −0.01 | −0.06 | 0.956 | |
| Age | −0.04 | −0.04 | −0.39 | 0.694 | |
| Total brain volume | −0.00 | −0.02 | −0.21 | 0.831 | |
| Presubiculum | (Intercept) | 18.47 | 0.00 | 1.39 | 0.168 |
| Presubiculum | −0.01 | −0.08 | −0.68 | 0.495 | |
| Sex | −0.15 | −0.01 | −0.06 | 0.949 | |
| Age | −0.04 | −0.04 | −0.41 | 0.682 | |
| Total brain volume | 0.00 | 0.02 | 0.12 | 0.907 | |
| Parasubiculum | (Intercept) | 17.93 | −0.00 | 1.37 | 0.173 |
| Parasubiculum | −0.07 | −0.12 | −1.15 | 0.250 | |
| Sex | −0.17 | −0.01 | −0.07 | 0.941 | |
| Age | −0.03 | −0.03 | −0.35 | 0.725 | |
| Total brain volume | 0.00 | 0.03 | 0.23 | 0.819 | |
| Molecular Layer | (Intercept) | 18.32 | 0.00 | 1.38 | 0.168 |
| Molecular Layer | −0.01 | −0.09 | −0.80 | 0.427 | |
| Sex | −0.00 | −0.00 | −0.00 | 0.998 | |
| Age | −0.03 | −0.03 | −0.32 | 0.746 | |
| Total brain volume | 0.00 | 0.03 | 0.23 | 0.820 | |
| GC-DG | (Intercept) | 18.65 | −0.00 | 1.42 | 0.157 |
| GC-DG | −0.03 | −0.17 | −1.37 | 0.172 | |
| Sex | −0.19 | −0.01 | −0.08 | 0.934 | |
| Age | −0.01 | −0.01 | −0.12 | 0.903 | |
| Total brain volume | 0.00 | 0.08 | 0.59 | 0.556 | |
| CA3 | (Intercept) | 17.81 | −0.00 | 1.37 | 0.174 |
| CA3 | −0.03 | −0.15 | −1.44 | 0.153 | |
| Sex | −0.06 | −0.00 | −0.03 | 0.980 | |
| Age | −0.01 | −0.01 | −0.11 | 0.913 | |
| Total brain volume | 0.00 | 0.05 | 0.43 | 0.666 | |
| CA4 | (Intercept) | 18.41 | −0.00 | 1.41 | 0.161 |
| CA4 | −0.03 | −0.16 | −1.40 | 0.165 | |
| Sex | −0.09 | −0.00 | −0.04 | 0.969 | |
| Age | −0.01 | −0.01 | −0.14 | 0.892 | |
| Total brain volume | 0.00 | 0.08 | 0.57 | 0.571 | |
| Fimbria | (Intercept) | 17.43 | 0.00 | 1.32 | 0.188 |
| Fimbria | −0.02 | −0.05 | −0.49 | 0.626 | |
| Sex | −0.18 | −0.01 | −0.08 | 0.938 | |
| Age | −0.04 | −0.04 | −0.50 | 0.616 | |
| Total brain volume | −0.00 | −0.02 | −0.14 | 0.885 | |
| HATA | (Intercept) | 16.81 | −0.00 | 1.29 | 0.200 |
| HATA | −0.06 | −0.09 | −0.81 | 0.418 | |
| Sex | 0.03 | 0.00 | 0.01 | 0.989 | |
| Age | −0.01 | −0.01 | −0.12 | 0.902 | |
| Total brain volume | 0.00 | 0.02 | 0.16 | 0.871 | |
| Whole hippocampus | (Intercept) | 17.38 | 0.00 | 1.30 | 0.195 |
| Whole hippocampus | −0.00 | −0.03 | −0.28 | 0.783 | |
| Sex | −0.01 | −0.00 | −0.01 | 0.995 | |
| Age | −0.04 | −0.04 | −0.41 | 0.680 | |
| Total brain volume | −0.00 | −0.01 | −0.08 | 0.936 |
Note.
p < .01 uncorrected, CA, cornu ammonis; GC-DG, granule cell layer of dentate gyrus; HATA, hippocampal-amygdaloid transitional area. N = 139.
4. Discussion
We assessed the relationship between subfields and the development of PTSD symptoms and the stability of hippocampal subfield volumes after trauma in a traumatically injured sample. Our longitudinal design, which consisted of two consecutive scans acutely post-trauma (T1 and T2) and one scan 6-months post-injury (T3), provided a unique opportunity to evaluate measurement reliability and utilize both the cross-sectional and longitudinal processing streams within FreeSurfer. We found the associations (although nonsignificant after correcting for multiple comparisons) between subfields and PTSD symptoms varied depending on whether the measurement was acquired acutely post-trauma (T1) or at follow-up (T3) and whether the analysis used the cross-sectional or longitudinal pipeline.
Reliability between Tl and T2 scans of hippocampal subfields ranged from good to excellent, with all ICC values over 0.83 (Koo and Li, 2016). Change in volume did not significantly relate to future PTSD symptoms, therefore, we were also interested in measurement differences between T1 – T3. Reliability between T1 and T3 (Supplemental Material) also ranged from good to excellent with ICC values over 0.86 for both FreeSurfer processing streams (Koo and Li, 2016). In both sets of reliability analyses (T1 – T2 and T1 – T3), we replicated previous work showing excellent reliability in the whole hippocampus and the molecular layer (Brown et al., 2020) with poorer reliability in the hippocampal fissure (Quattrini et al., 2020). Percent volume difference metrics revealed similar outcomes; the lowest percent difference between T1 and T2 was in the whole hippocampus and molecular layer whereas the hippocampal fissure, HATA, and parasubiculum had the largest differences. Using the longitudinal preprocessing pipeline (T1 – T3) revealed the smallest percent differences; subfields demonstrating highest consistency (PVD < 3%) included the bilateral hippocampal tail, subiculum, CA1, molecular layer, and whole hippocampal volume. For both processing streams, the bilateral fissure, parasubiculum, and HATA showed the least consistency (PVD > 5%).
These results replicate and further support the reliability of FreeSurfer hippocampal subfield segmentation as demonstrated in other studies comparing varying sample sizes, scanners, and time intervals between scans (Brown et al., 2020; Whelan et al., 2016). Moreover, our traumatically injured sample yields a unique measurement of hippocampal volumes post-trauma that would not otherwise be reported in a healthy sample. Thus, reliable measurement across both sets of time-points is important in disentangling volumetric differences in subfields attributed to trauma-related outcomes rather than measurement biases over time.
Decreased bilateral dentate gyrus/CA4 volume (T1) did not relate to greater PTSD symptom severity (T3). Though the dentate gyrus has been demonstrated to be associated with current PTSD symptoms (Hayes et al., 2017), our results suggest that, in this sample, the dentate gyrus is not prospectively related to, or associated with PTSD symptoms. The size of the dentate gyrus may not be predisposing of PTSD, rather it may be sensitive to the stress associated with PTSD in specific samples, particularly those that are comprised of highly symptomatic participants or individuals who experienced sustained trauma exposure (e.g., combat veterans; Zimmerman et al., 2016).
Chronic stress in the environment that individuals return to after trauma may impact hippocampal volumes (Haddad et al., 2015). The majority of neuroimaging work has been conducted with predominately White participants. Our sample is distinctly comprised of participants from diverse racial, ethnic, and socioeconomic backgrounds. As more data emerges on the neural impact of socioeconomic position (e.g., Johnson et al., 2016; Noble et al., 2012), racism and race-based stressors (Carter, 2007), and chronic exposure to environmental/societal stress (e.g., community violence, environmental toxins, etc.), we encourage future neuroscience research to consider how other forms of traumatic and stressful exposures (e.g., racism, sexism, poverty) may be impacting brain regions highly vulnerable to stress such as the hippocampus.
Previous work has demonstrated smaller whole hippocampus volume is associated with PTSD (e.g., Logue et al., 2018; Salminen et al., 2019; Xie et al., 2018). Surprisingly, we did not find a bivariate association between hippocampal volume and PTSD symptoms, nor was global hippocampal volume a significant term in the regression analysis. It is important to note that a number of studies have not demonstrated a relationship between whole hippocampal volume and PTSD (e.g., Bonne et al., 2001; Chen et al., 2018; see meta-analysis by Logue et al., 2018); perhaps indicating the association is not as robust as widely assumed and that trauma type and timing of measurement are important factors.
Our results, regardless of processing pipeline, do not clearly align with the framework describing differences in hippocampal subfields as either a vulnerability factor of PTSD development or as part of the subsequent post-trauma neurobiological changes. Rather, they suggest the two hypotheses may not be mutually exclusive. Our unique experimental design also stressed the importance of considering timing of structural measurements. The lack of consensus between our results and the majority of previously published findings (c.f. Bonne et al., 2001) is less surprising given a large recent study found that major depressive disorder, a common co-morbid diagnoses with PTSD, was a better predictor of hippocampal subfields than PTSD (Salminen et al., 2019). Future research should attempt to disentangle the effects of PTSD and depression on hippocampal structure and should extend research efforts across various post-trauma timepoints.
4.1. Limitations
Despite being a relatively large sample, the current results represent data from the same participants collected on the same scanner. To further validate the reliability of FreeSurfer’s hippocampal subfield segmentation, larger samples should be collected on several scanners and with varying scan acquisition parameters. Greater resolution of anatomical scans would also likely enhance performance of the reconstruction pipeline. In addition, FreeSurfer’s hippocampal subfield segmentation pipeline permits the inclusion of additional T2 weighted hippocampal scans to enhance segmentation reliability. Such scans were not collected in the current study and results still demonstrated reliable subfield estimation. However, future reliability examinations of this pipeline in FreeSurfer should include the additional T2 hippocampal scans.
The current sample was underpowered to investigate group differences (PTSD +/−) in hippocampal subfield volumes. Although participants in the current study were traumatically injured, the rates of PTSD (18% PTSD+) and PTSD symptoms in the sample are rather low (Mean CAPS-5 Total Severity = 11.77, N = 140). Similarly, the majority of the sample was injured in a motor vehicle crash yielding a sample less generalizable to samples with greater variability in trauma exposures (i.e., assault, combat, falls, etc.). Finally, we did not acquire a pre-trauma scan and therefore we were unable to explore whether differences in structure that predate the trauma can predict future trauma outcomes. The combination of these factors may explain the lack of replication of the well described smaller hippocampus and PTSD relationship (Hayes et al., 2017; Logue et al., 2018; Salminen et al., 2019). Though our reliability results closely resemble those reported from samples of healthy adults (Brown et al., 2020; Quattrini et al., 2020), and we excluded participants with head injury greater than mild TBI, using acute trauma survivors may confound hippocampal subfield reliability estimates as effects of physical trauma on volumes cannot be ruled out.
The hippocampus volume differences between individuals are relatively small. The average hippocampal reduction associated with a PTSD diagnosis is typically subtle, especially when trauma types are collapsed (mixed-trauma sample; Salminen et al., 2019). Coupled with the variability in measurement reliability, caution should be taken when interpreting only change in hippocampal subfields over time.
Conclusions
The current study demonstrated excellent reliability of FreeSurfer 6.0 hippocampal subfield segmentation, on scans acquired on two consecutive days and six months apart, within a large trauma-exposed sample. Findings replicate and extend previous work examining FreeSurfer reliability by using a larger sample and time points not previously examined. Reliability of automated hippocampal subfield segmentations is crucial to research examining diseases and disorders affecting the hippocampus. Though ongoing validation is necessary, the current results contribute to the promise of robust methodology within FreeSurfer in examining brain-related changes associated with trauma exposure.
Although in our sample the hippocampal subfields volumes did not prospectively relate to or track with PTSD symptoms, future work should still consider how the function and structure of the distinct subfields may underlie pathogenesis of PTSD symptoms. Elucidating the role of hippocampal subfields in PTSD may lead to more effective treatments of specific symptoms (e.g., impaired extinction and over-consolidation of fear).
Supplementary Material
Acknowledgments
We would like to extend a special thanks to the iSTAR research team, especially Kate Isely and Sarah Stevens. The authors are also grateful to Milwaukee Trauma Outcomes Project research team, who have provided valuable feedback.
Financial disclosures
Support for the research, authorship, and/or publication of this article include the following: a NIH grant R01-M1H106574 (PI: Larson). E.K.W is supported by the National Center for Advancing Translational Sciences, National Institutes of Health (TL1TR001437). The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the NIH.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2021.118076.
References
- Ahmed-Leitao F, Spies G, van den Heuvel L, Seedat S, 2016. Hippocampal and amygdala volumes in adults with posttraumatic stress disorder secondary to childhood abuse or maltreatment: A systematic review. Psychiatry Research: Neuroimaging 256, 33–43. doi: 10.1016/j.pscychresns.2016.09.008. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5, 5 edition American Psychiatric Publishing. [Google Scholar]
- Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, Marmar CR, Weiner MW, Schuff N, Neylan TC, 2011. Hippocampal volume differences in gulf war veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol. Psychiatry 69 (6), 541–548. doi: 10.1016/j.biopsych.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill CL, Satodiya RM, Scott JC, Wrocklage KM, Schweinsburg B, Averill LA, Akiki TJ, Amoroso T, Southwick SM, Krystal JH, Abdallah CG, 2017. Post-traumatic stress disorder and depression symptom severities are differentially associated with hippocampal subfield volume loss in combat veterans. Chron. Stress 1. doi: 10.1177/2470547017744538, 2470547017744538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch T, Wulff P, 2015. The hippocampus in aging and disease: from plasticity to vulnerability. Neuroscience 309, 1–16. doi: 10.1016/j.neuroscience.2015.07.084. [DOI] [PubMed] [Google Scholar]
- Bartsch Thorsten, Döhring J, Rohr A, Jansen O, Deuschl G, 2011. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc. Natl. Acad. Sci 108 (42), 17562–17567. doi: 10.1073/pnas.1110266108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard A, Sahay A, 2016. Adult hippocampal neurogenesis, fear generalization, and stress. Neuropsychopharmacology 41 (1), 24–44. doi: 10.1038/npp.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY, 2001. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am. J. Psychiatry 158 (8), 1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, Pierce ME, Clark DC, Fischl BR, Iglesias JE, Milberg WP, McGlinchey RE, Salat DH, 2020. Test-retest reliability of FreeSurfer automated hippocampal subfield segmentation within and across scanners. Neuroimage 210, 116563. doi: 10.1016/j.neuroimage.2020.116563. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA, 2010. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 33 (3), 153–161. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser NJ, Madan CR, Hanson JL, 2020. Quantifying numerical and spatial reliability of amygdala and hippocampal subdivisions in FreeSurfer [Preprint]. Neuroscience doi: 10.1101/2020.06.12.149203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RT, 2007. Racism and psychological and emotional injury: recognizing and assessing race-based traumatic stress. Couns. Psychol 35 (1), 13–105. doi: 10.1177/0011000006292033. [DOI] [Google Scholar]
- Chen LW, Sun D, Davis SL, Haswell CC, Dennis EL, Swanson CA, Whelan CD, Gutman B, Jahanshad N, Iglesias JE, Thompson P, Wagner HR, Saemann P, LaBar KS,Morey RA, 2018. Smaller hippocampal CA1 subfield volume in posttraumatic stress disorder. Depress. Anxiety 35 (11), 1018–1029. doi: 10.1002/da.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn D, 2018. Using MR to view PTSD’s effect on the amygdala and hippocampus. Radiol. Technol 89 (5), 5. [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. Neuroimage 9 (2), 179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI, 1993. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J. Cogn. Neurosci 5 (2), 162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dalton MA, Maguire EA, 2017. The pre/parasubiculum: a hippocampal hub for scene-based cognition? Curr. Opin. Behav. Sci 17, 34–40. doi: 10.1016/j.cobeha.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K, Latheef SK, Dadar M, Samad HA, Munjal A, Khandia R, Karthik K, Tiwari R, Yatoo Mohd.I., Bhatt P, Chakraborty S, Singh KP, Iqbal HMN, Chaicumpa W, Joshi SK, 2019. Biomarkers in stress related diseases/disorders: diagnostic, prognostic, and therapeutic values. Front. Mol. Biosci 6. doi: 10.3389/fmolb.2019.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill V, Franco AR, Pinho MS, 2015. Automated methods for hippocampus segmentation: the evolution and a review of the state of the art. Neuroinformatics 13 (2), 133–150. doi: 10.1007/s12021-014-9243-4. [DOI] [PubMed] [Google Scholar]
- El-Falougy H, Benuska J, 2006. History, anatomical nomenclature, comparative anatomy and functions of the hippocampal formation. Bratisl. Lek. Listy 107 (4), 103–106. [PubMed] [Google Scholar]
- Fenster RJ, Lebois LAM, Ressler KJ, Suh J, 2018. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat. Rev. Neurosci 19 (9), 535–551. doi: 10.1038/s41583-018-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, 2004. Automatically parcellating the human cerebral cortex. Cereb. Cortex 14 (1), 11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM, 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. In: Proceedings of the National Academy of Sciences, 97, pp. 11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM, 2001. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging 20 (1), 70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM, 1999a. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp 8 (4), 272–284. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl Bruce, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM, 2002. Whole brain segmentation. Neuron 33 (3), 341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Fischl Bruce, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM, 2004. Sequence-independent segmentation of magnetic resonance images. Neuroimage 23, S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl Bruce, Sereno MI, Dale AM, 1999b. Cortical surface-based analysis. Neuroimage 9 (2), 195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Flores R.de, Joie RL, Landeau B, Perrotin A, Mézenge F, de L. Sayette V, Eustache F, Desgranges B, Chételat G, 2015. Effects of age and Alzheimer’s disease on hippocampal subfields. Hum. Brain Mapp 36 (2), 463–474. doi: 10.1002/hbm.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhner JH, Teckentrup V, Smolka MN, Kroemer NB, 2019. Addressing the reliability fallacy in fMRI: similar group effects may arise from unreliable individual effects. Neuroimage 195, 174–189. doi: 10.1016/j.neuroimage.2019.03.053. [DOI] [PubMed] [Google Scholar]
- Gamer M, Lemon J, Singh I, 2012. Irr: Various coefficients of Interrater Reliability and Agreement (Version 0.84.1) https://CRAN.R-project.org/package=irr.
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK, 2002. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci 5 (11), 1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvits TV, Metzger LJ, Lasko NB, Cannistraro PA, Tarhan AS, Gilbertson MW, Orr SP, Charbonneau AM, Wedig MM, Pitman RK, 2006. Subtle neurologic compromise as a vulnerability factor for combat-related posttraumatic stress disorder: results of a twin study. Arch. Gen. Psychiatry 63 (5), 571–576. doi: 10.1001/arch-psyc.63.5.571. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK, 1996. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol. Psychiatry 40 (11), 1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad L, Schäfer A, Streit F, Lederbogen F, Grimm O, Wüst S, Deuschle M, Kirsch P, Tost H, Meyer-Lindenberg A, 2015. Brain structure correlates of urban upbringing, an environmental risk factor for schizophrenia. Schizophr. Bull 41 (1), 115–122. doi: 10.1093/schbul/sbu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haładaj R, 2020. Anatomical variations of the dentate gyrus in normal adult brain. Surg. Radiol. Anat 42 (2), 193–199. doi: 10.1007/s00276-019-02298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B, 2006. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 32 (1), 180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Haukvik UK, Tamnes CK, Söderman E, Agartz I, 2018. Neuroimaging hippocampal subfields in schizophrenia and bipolar disorder: a systematic review and meta-analysis. J. Psychiatr. Res 104, 217–226. doi: 10.1016/j.jpsychires.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Hayes JP, Hayes S, Miller DR, Lafleche G, Logue MW, Verfaellie M, 2017. Automated measurement of hippocampal subfields in PTSD: evidence for smaller dentate gyrus volume. J. Psychiatr. Res 95, 247–252. doi: 10.1016/j.jpsychires.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S, 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat 6 (2), 65–70. [Google Scholar]
- Hsu Y-Y, Schuff N, Du A-T, Mark K, Zhu X, Hardin D, Weiner MW, 2002. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J. Magn. Reson. Imaging : JMRI 16 (3), 305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, Roy N, Frosch MP, McKee AC, Wald LL, Fischl B, Van Leemput K, 2015. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115, 117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Maren S, 2015. Prefrontal-hippocampal interactions in memory and emotion. Front. Syst. Neurosci 9. doi: 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Riis JL, Noble KG, 2016. State of the art review: poverty and the developing brain. Pediatrics (4) 137. doi: 10.1542/peds.2015-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SA, Duval ER, Kubat B, Liberzon I, 2020. A review of hippocampal activation in post-traumatic stress disorder. Psychophysiology 57 (1), e13357. doi: 10.1111/psyp.13357. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, MacFall J, Fischl B, Dale A, 2006. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 30 (2), 436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Pellman B, Kim JJ, 2015. Stress effects on the hippocampus: a critical review. Learn. Mem 22 (9), 411–416. doi: 10.1101/lm.037291.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, 2015. The hippocampus. Curr. Biol 25 (23), R1116–R1121. doi: 10.1016/j.cub.2015.10.049. [DOI] [PubMed] [Google Scholar]
- Koo TK, Li MY, 2016. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med 15 (2), 155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Koenen KC, Afari N, Lyons MJ, 2012. Twin studies of posttraumatic stress disorder: differentiating vulnerability factors from sequelae. Neuropharmacology 62 (2), 647–653. doi: 10.1016/j.neuropharm.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov A, Zhu X, Suarez-Jimenez B, Rutherford BR, Neria Y, 2017. Resting-state functional connectivity of anterior and posterior hippocampus in posttraumatic stress disorder. J. Psychiatr. Res 94, 15–22. doi: 10.1016/j.jpsychires.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrner A, Yehuda R, 2014. Biomarkers of PTSD: military applications and considerations. Eur. J. Psychotraumatol 5. doi: 10.3402/ejpt.v5.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, & Sripada CS (2007). The functional neuroanatomy of PTSD: a critical review. In De Kloet ER, Oitzl MS, & Vermetten E (Eds.), Progress in Brain Research (Vol. 167, pp. 151–169). Elsevier. 10.1016/S0079-6123(07)67011-3 [DOI] [PubMed] [Google Scholar]
- Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, Densmore M, Haswell CC, Ipser J, Koch SBJ, Korgaonkar M, Lebois LAM, Peverill M, Baker JT, Boedhoe PSW, Frijling JL, Gruber SA, Harpaz-Rotem I, Jahanshad N, Morey RA, 2018. Smaller hippocampal volume in post-traumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol. Psychiatry 83 (3), 244–253. doi: 10.1016/j.biopsych.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M, 2001. Stress, memory, and the hippocampus: can’t live with it, can’t live without it. Behav. Brain Res 127 (1–2), 137–158. doi: 10.1016/S0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ, 2012. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 35 (1), 24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malivoire BL, Girard TA, Patel R, Monson CM, 2018. Functional connectivity of hippocampal subregions in PTSD: relations with symptoms. BMC Psychiatry 18 (1), 129. doi: 10.1186/s12888-018-1716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I, 2013. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci 14 (6), 417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marizzoni M, Antelmi L, Bosch B, Bartrés-Faz D, Müller BW, Wiltfang J, Fiedler U, Roccatagliata L, Picco A, Nobili F, Blin O, Bombois S, Lopes R, Sein J, Ranjeva J-P, Didic M, Gros-Dagnac H, Payoux P, Zoccatelli G, Jovicich J, 2015. Longitudinal reproducibility of automatically segmented hippocampal subfields: a multisite European 3T study on healthy elderly. Hum. Brain Mapp 36 (9), 3516–3527. doi: 10.1002/hbm.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, 2004. Biomarkers: potential uses and limitations. NeuroRx 1 (2), 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy CS, Ramprashad A, Thompson C, Botti J-A, Coman IL, Kates WR, 2015. A comparison of FreeSurfer-generated data with and without manual intervention. Front Neurosci 9, 379. doi: 10.3389/fnins.2015.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD, 2016. Stress EFFECTS ON NEURONAL STRUCTURE: HIPPOCAMPUS, AMYGDALA, AND PREFRONTAL COrtex. Neuropsychopharmacology 41 (1), 3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP, 2005. Aging, stress and the hippocampus. Ageing Res. Rev 4 (2), 123–140. doi: 10.1016/j.arr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Miller JK, Wiener JM, 2014. PTSD recovery, spatial processing, and the val66met polymorphism. Front. Hum. Neurosci 8. doi: 10.3389/fnhum.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R, Petty C, Xu Y, Hayes J, Wagner H, Lewis D, LaBar K, Styner M, McCarthy G, 2009. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage 45 (3), 855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R, Selgrade E, Wagner H, Huettel S, Wang L, McCarthy G, 2010. Scan–rescan reliability of subcortical brain volumes derived from automated segmentation. Human Brain Mapping 31 (11), 1751–1762. doi: 10.1002/hbm.20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder ER, de Jong RA, Knol DL, van Schijndel RA, Cover KS, Visser PJ, Barkhof F, Vrenken H, 2014. Hippocampal volume change measurement: quantitative assessment of the reproducibility of expert manual outlining and the automated methods FreeSurfer and FIRST. Neuroimage 92, 169–181. doi: 10.1016/j.neuroimage.2014.01.058. [DOI] [PubMed] [Google Scholar]
- Noble KG, Grieve SM, Korgaonkar MS, Engelhardt LE, Griffith EY, Williams LM, Brickman AM, 2012. Hippocampal volume varies with educational attainment across the life-span. Front. Hum. Neurosci 6. doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz JB, Conrad CD, 2018. The impact from the aftermath of chronic stress on hippocampal structure and function: is there a recovery? Front. Neuroendocrinol 49, 114–123. doi: 10.1016/j.yfrne.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE, 2021. Differential Contribution of Amygdala and Hippocampus to Cued and Contextual Fear Conditioning, p. 12. [DOI] [PubMed]
- Postel C, Viard A, André C, Guénolé F, Flores R.de, Baleyte J-M, Gerardin P, Eustache F, Dayan J, Guillery-Girard B, 2019. Hippocampal subfields alterations in adolescents with post-traumatic stress disorder. Hum. Brain Mapp 40 (4), 1244–1252. doi: 10.1002/hbm.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston-Ferrer P, Burgalossi A, 2018. Linking neuronal structure to function in rodent hippocampus: a methodological prospective. Cell Tissue Res. 373 (3), 605–618. doi: 10.1007/s00441-017-2732-7. [DOI] [PubMed] [Google Scholar]
- Quattrini G, Pievani M, Jovicich J, Aiello M, Bargalló N, Barkhof F, Bartres-Faz D, Beltramello A, Pizzini FB, Blin O, Bordet R, Caulo M, Constantinides M, Didic M, Drevelegas A, Ferretti A, Fiedler U, Floridi P, Gros-Dagnac H, Marizzoni M, 2020. Amygdalar nuclei and hippocampal subfields on MRI: test-retest reliability of automated volumetry across different MRI sites and vendors. Neuroimage 218, 116932. doi: 10.1016/j.neuroimage.2020.116932. [DOI] [PubMed] [Google Scholar]
- Radonjic V, Malobabic S, Radonjic V, Puskas L, Stijak L, Aksic M, Filipovic B, 2014. Hippocampus: why is it studied so frequently? Vojnosanit. Pregl 71 (2), 195–201. doi: 10.2298/VSP130222043R. [DOI] [PubMed] [Google Scholar]
- Rangaprakash D, Deshpande G, Daniel TA, Goodman AM, Robinson JL, Salibi N, Katz JS, Denney TS, Dretsch MN, 2017. Compromised hippocampus-striatum pathway as a potential imaging biomarker of mild-traumatic brain injury and post-traumatic stress disorder: hippocampus-Striatum Pathway as a Biomarker of mTBI and PTSD. Hum. Brain Mapp 38 (6), 2843–2864. doi: 10.1002/hbm.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, Fischl B, 2010b. Highly accurate inverse consistent registration: a robust approach. Neuroimage 53 (4), 1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas DH, Fischl B, 2010. Highly accurate inverse consistent registration: A robust approach. Neuroimage 53 (4), 1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B, 2012. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61 (4), 1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Kearns MC, Reiser E, Davis JS, Kerley KA, Rothbaum AO, Mercer KB, Price M, Houry D, Ressler KJ, 2014. Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. J. Clin. Psychiatry 75 (12), 1380–1387. doi: 10.4088/JCP.13m08715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen L, Sämann P, Zheng Y, Dennis E, Clarke E, Jahanshad N, Iglesias J, Whelan C, Bruce S, Hayes J, Seedat S, Averill C, Baugh L, Bomyea J, Bright J, Buckle C, Choi K, Davenport N, Davidson R, 2019. Hippocampal Subfield Volumes are Uniquely Affected in PTSD and Depression: International analysis of 31 Cohorts from the PGC-ENIGMA PTSD Working Group doi: 10.1101/739094. [DOI]
- Schmidt MF, Storrs JM, Freeman KB, Jack CR, Turner ST, Griswold ME, Mosley TH, 2018. A comparison of manual tracing and FreeSurfer for estimating hippocampal volume over the adult lifespan. Hum. Brain Mapp 39 (6), 2500–2513. doi: 10.1002/hbm.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B, 2004. A hybrid approach to the skull stripping problem in MRI. Neuroimage 22 (3), 1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shin LM, 2006. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. N. Y. Acad. Sci 1071 (1), 67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Squire LR, 2009. The legacy of patient H.M. for neuroscience. Neuron 61 (1), 6–9. doi: 10.1016/j.neuron.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternbach GL, 2000. The Glasgow coma scale. J. Emerg. Med 19 (1), 67–71. doi: 10.1016/s0736-4679(00)00182-7. [DOI] [PubMed] [Google Scholar]
- Tatu L, Vuillier F, 2014. Structure and vascularization of the human hippocampus. In: Frontiers of Neurology and Neuroscience, 34, pp. 18–25. doi: 10.1159/000356440 S. KARGER AG. [DOI] [PubMed] [Google Scholar]
- Tural Ü, Aker AT, Önder E, Sodan HT, Ünver H, Akansel G, 2018. Neurotrophic factors and hippocampal activity in PTSD. PLoS ONE 13 (5), e0197889. doi: 10.1371/journal.pone.0197889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Stevens JS, Ely TD, Hinrichs R, Michopoulos V, Winters SJ, Ogbonmwan YE, Shin J, Nugent NR, Hudak LA, Rothbaum BO, Ressler KJ, Jovanovic T, 2018. The role of the hippocampus in predicting future posttraumatic stress disorder symptoms in recently traumatized civilians. Biol. Psychiatry 84 (2), 106–115. doi: 10.1016/j.biopsych.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, Weiner MW, Schuff N, 2010. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch. Gen. Psychiatry 67 (3), 296. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, Keane TM, Marx BP, 2018. The clinician-administered PTSD Scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol. Assess 30 (3), 383–395. doi: 10.1037/pas0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan CD, Hibar DP, van Velzen LS, Zannas AS, Carrillo-Roa T, McMahon K, Prasad G, Kelly S, Faskowitz J, deZubiracay G, Iglesias JE, van Erp TGM, Frodl T, Martin NG, Wright MJ, Jahanshad N, Schmaal L, Sämann PG, Thompson PM, 2016. Heritability and reliability of automatically segmented human hippocampal formation subregions. Neuroimage 128, 125–137. doi: 10.1016/j.neuroimage.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse LEM, Kuijf HJ, Honingh AM, Wang H, Pluta JB, Das SR, Wolk DA, Zwanenburg JJM, Yushkevich PA, Geerlings MI, 2016. Automated hippocampal subfield segmentation at 7T MRI. Am. J. Neuroradiol 37 (6), 1050–1057. doi: 10.3174/ajnr.A4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Kleven H, Kobro Flatmoen A, 2017. Comparative contemplations on the hippocampus. Brain Behav. Evol 90 (1), 15–24. doi: 10.1159/000475703. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR, 2011. The medial temporal lobe and the attributes of memory. Trends Cogn. Sci 15 (5), 210–217. doi: 10.1016/j.tics.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon FL, Hedges DW, 2008. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus 18 (8), 729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Xie H, Claycomb Erwin M, Elhai JD, Wall JT, Tamburrino MB, Brickman KR, Kaminski B, McLean SA, Liberzon I, Wang X, 2018. Relationship of hippocampal volumes and posttraumatic stress disorder symptoms over early post-trauma periods. Biol. Psychiatry: Cognit. Neurosci. Neuroimaging 3 (11), 968–975. doi: 10.1016/j.bpsc.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Amaral RSC, Augustinack JC, Bender AR, Bernstein JD, Boccardi M, Bocchetta M, Burggren AC, Carr VA, Chakravarty MM, Chetelat G, Daugherty AM, Davachi L, Ding S-L, Ekstrom A, Geerlings MI, Hassan A, Huang Y, Iglesias E, Zeineh MM, 2015a. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. Neuroimage 111, 526–541. doi: 10.1016/j.neuroimage.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Avants BB, Pluta J, Das S, Minkoff D, Mechanic-Hamilton D, Glynn S, Pickup S, Liu W, Gee JC, Grossman M, Detre JA, 2009. A high-resolution computational atlas of the human hippocampus from post-mortem magnetic resonance imaging at 9.4 T. Neuroimage 44 (2), 385–398. doi: 10.1016/j.neuroimage.2008.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Pluta JB, Wang H, Xie L, Ding S-L, Gertje EC, Mancuso L, Kliot D, Das SR, Wolk DA, 2015b. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum. Brain Mapp 36 (1), 258–287. doi: 10.1002/hbm.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Ezzati A, Katz MJ, Lipton ML, Brickman AM, Sliwinski MJ, Lipton RB, 2016. Perceived stress is differentially related to hippocampal subfield volumes among older adults. PLoS ONE 11 (5), e0154530. doi: 10.1371/journal.pone.0154530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


