Abstract
Background:
Balanoposthitis is defined as an inflammatory condition of glans penis and prepuce. There are wide variety of etiologies including both infectious and noninfectious conditions. This study attempts to throw light on information regarding clinical and microbiological aspects of balanoposthitis.
Objectives:
To study various clinical patterns, etiologies, and predisposing factors of balanoposthitis.
Methodology:
A descriptive study was undertaken on 106 cases who presented to sexually transmitted disease (STD) clinic with balanoposthitis between November 2017 and April 2019. A detailed history, physical examination, and investigations like KOH mount, leishman staining, gram staining, dark field microscopy, cultures, and other investigations were done wherever indicated. The data collected was tabulated and analyzed.
Results:
In our study, infectious etiology was the most common and was found in 77.36% cases. About 13.41% of cases with infectious balanoposthitis had multiple etiological agents. Noninfectious etiology was found in 22.64% cases. The most common infectious cause of balanoposthitis was candida, noted in 59.76% cases, followed by herpes simplex virus (19.51%), human papilloma virus (13.41%), and scabies (8.54%). Among noninfectious etiologies, adverse drug reaction (4.72% of total cases) was the most common, followed by lichen planus (3.77%) and psoriasis (3.77%). There was significantly higher incidence of phimosis in diabetic patients with candidal balanoposthitis.
Conclusion:
Identifying the etiology facilitates early treatment and hence reduces the infectivity and transmission of disease and also the disease complications like phimosis. In addition, multiple infectious etiologies should always be kept in mind while evaluating STDs.
Keywords: Balanoposthitis, candidal, herpes genitalis, genital warts
Introduction
Balanoposthitis is defined as an inflammatory condition of glans penis and prepuce.[1] It is commonly seen among male patients attending sexually transmitted disease (STD) clinics. Both infectious and noninfectious causes contribute to the etiology of balanoposthitis with former being the most common.[2] The most common infectious etiology of balanoposthitis is candida, followed by bacterial infections caused by Streptococcus species and Staphylococcus aureus, viral and parasitic infections.[2] The factors which predispose to candidal balanoposthitis include diabetes mellitus, immunosuppression, uncircumcised penis, poor hygiene of the genitals, and tight prepuce.[3]
Material and Methods
After obtaining institutional ethics committee approval, we conducted a prospective study on 106 cases with balanoposthitis over a period of 17 months (December 2017 - April 2019). All patients more than 12 years of age who presented with balanoposthitis during this period were included in our study. Informed and written consent was obtained from all subjects after explaining the details of the study. Demographic data like name, age, address, and telephone number were recorded. A detailed history including chief complaints related to skin, onset and duration of disease, sexual history, and associated medical or skin disorders were noted. A detailed examination was done and findings were noted. Investigations such as dark field microscopy, wetfilm, 10% KOH, Gram stain, Leishman stain, and culture were done wherever necessary. The collected data was tabulated and analyzed.
Results
This study included 106 subjects with the diagnosis of balanoposthitis. Age of patients in this study ranged between 13 and 68 years and the mean age was 37 years. Majority of patients (33 patients, 31.13%) were found in the age group of 21–30 years [Table 1].
Table 1.
Age distribution of cases
| Age categories | Frequency | Percent |
|---|---|---|
| <20 years | 12 | 11.32 |
| 21-30 years | 33 | 31.13 |
| 31-40 years | 20 | 18.87 |
| 41-50 years | 19 | 17.92 |
| 51-60 years | 17 | 16.04 |
| >60 years | 5 | 4.72 |
| Total | 106 | 100.00 |
Among 106 patients, 101 (95.28%) patients were uncircumcised, and 5 (4.72%) patients were circumcised. Among 106 patients, 73 (68.87%) patients were married and 33 (31.13%) patients were unmarried.
Categorization according to the site of involvement
Involvement of only glans penis (balanitis) was noted in 19 (17.92%) patients. Involvement of only prepuce (posthitis) was noted in 45 (42.45%) patients. Involvement of both glans penis and prepuce (balanoposthitis) was noted in 42 (39.62%) patients.
Clinical patterns observed
Erythema was the most common clinical pattern which was noted in 50 patients (47.17%), followed by fissuring over prepuce (28 patients, 26.42%), phimosis (25 patients, 23.58%), and ulcer (21 patients, 19.81%) [Table 2].
Table 2.
Clinical patterns observed
| Clinical pattern | Number | Percentage |
|---|---|---|
| Erosion | 7 | 6.60 |
| Fissuring | 28 | 26.42 |
| Erythema | 50 | 47.17 |
| Plaques | 20 | 18.87 |
| Verrucous growth | 11 | 10.38 |
| Ulcer | 21 | 19.81 |
| Phimosis | 25 | 23.58 |
| Ulceroproliferative growth | 2 | 1.89 |
| Subpreputial discharge | 9 | 8.49 |
| Urethral discharge | 2 | 1.89 |
Clinical patterns observed in candidal balanoposthitis
Among 49 patients with candidal balanoposthitis, fissuring was the common presentation (26 patients, 53.06%), followed by phimosis (25 patients, 51.02%), erythema (25 patients, 51.02%), and subpreputial discharge (8 patients, 16.33%) [Table 3].
Table 3.
Clinical patterns observed in candidal balanoposthitis
| Clinical pattern | Number | Percentage |
|---|---|---|
| Fissuring | 26 | 53.06 |
| Phimosis | 25 | 51.02 |
| Erythema | 25 | 51.02 |
| Subpreputial discharge | 8 | 16.33 |
| Swelling of prepuce | 7 | 14.29 |
| Plaques | 2 | 4.08 |
| Ulcer | 2 | 4.08 |
| Erythematous plaque | 2 | 4.08 |
| Erosion | 1 | 2.04 |
Among 82 patients with infectious balanoposthitis, 8 patients (9.76%) gave history of extramarital contact (EMC) and 8 patients (9.76%) gave history of premarital contact (PMC). Among 16 patients with EMC/PMC, 15 patients (93.75%) gave history of unprotected sex and 1 patient (6.25%) gave history of protected sex. Among 33 unmarried subjects, only 7 (21.21%) subjects gave history of sexual contact. The remaining 78.79% (26) of unmarried subjects denied history of any sexual contact.
Etiologies of balanoposthitis observed in the study
In our study, infectious etiology was the most common and was found in 82 (77.36%) patients. Noninfectious etiology was found in 24 (22.64%) patients.
Among infectious etiologies, fungal organisms (52 cases, 63.41%) were the most common, followed by viral (27 cases, 32.93%), bacterial (9 cases, 10.98%), and parasitic (7 cases, 8.54%).
Infectious etiologies
The most common cause of infectious balanoposthitis was candida, noted in 49 patients (59.76%), followed by herpes simplex virus (16 patients, 19.51%), human papilloma virus (11 patients, 13.41%), scabies (7 patients, 8.54%), syphilis (4.88%), and gonorrhea (3.66%) [Table 4] [Figures 1–5].
Table 4.
List of infectious etiologies of balanoposthitis
| Etiology | Frequency | Percentage of infectious cases (n=82) | Percentage of total cases (n=106) |
|---|---|---|---|
| Candidal | 49 | 59.76 | 46.23 |
| Dermatophytosis | 3 | 3.66 | 2.83 |
| Genital warts | 11 | 13.41 | 10.38 |
| Gonococcal | 3 | 3.66 | 2.83 |
| Herpes genitalis | 16 | 19.51 | 15.09 |
| Scabies | 7 | 8.54 | 6.60 |
| Scrub typhus | 1 | 1.22 | 0.94 |
| Syphilis | 4 | 4.88 | 3.77 |
| Streptococcal | 1 | 1.22 | 0.94 |
| Total | 95 |
Figure 1.
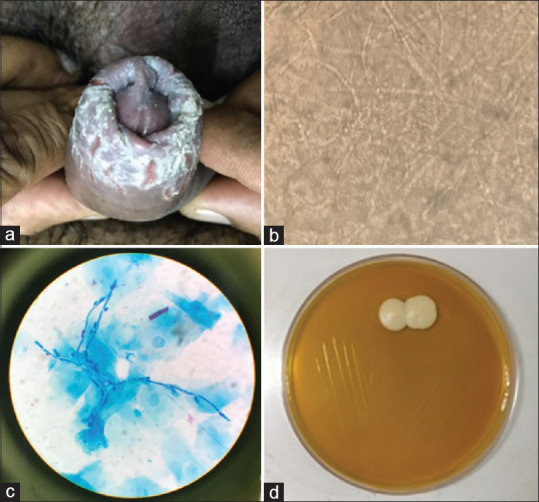
Candidal balanoposthitis. (a) Radial fissuring of prepuce. (b) KOH showing abundant pseudohyphae with spores. (c) Methylene blue staining showing psudohyphae with budding spores. (d) Culture showing growth of Candida albicans
Figure 5.
Gonorrhea. (a) clinical picture showing urethral discharge. (b) Gram stain showing intracellular diplococci
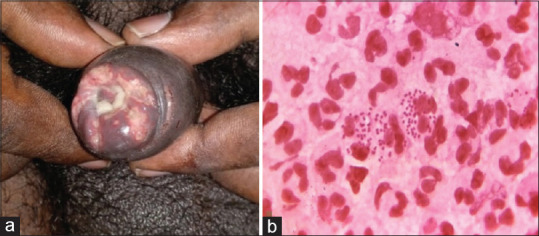
Figure 2.
Herpes genitalis. (a) Erosive ulcers with polycyclic margins. (b) Tzanck smear showing multinucleated giant cells
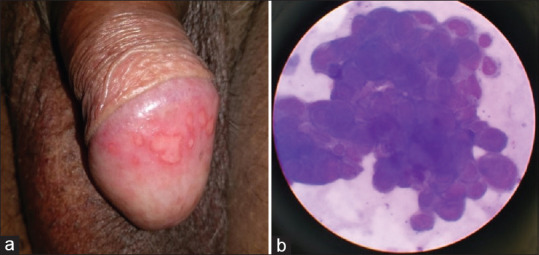
Figure 3.
Genital warts
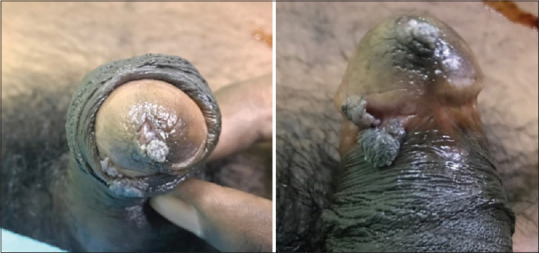
Figure 4.
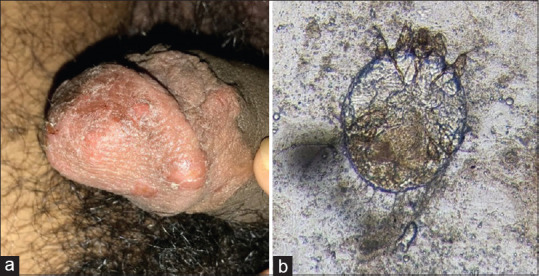
Scabies. (a) Excoriated papules over glans penis and prepuce. (b) Scabies mite visualized on scraping and microscopy
Association of candidal balanoposthitis with phimosis and diabetes mellitus
There were totally 35 (33.02%) diabetic patients in our study. Among 49 patients with candidal balanoposthitis, 25 (51.02%) patients had phimosis and 30 (61.22%) patients were diabetic. Among these 30 diabetic patients with candidal balanoposthitis, 18 (36.73%) patients were known diabetic and remaining 12 (24.49%) patients were newly diagnosed with diabetes mellitus after they presented to STD OPD with balanoposthitis. Diabetes mellitus was found to increase the risk of candidal balanoposthitis. This observation was found to be statistically significant (P < 0.05). In our study, mean HbA1c of patients with newly diagnosed diabetes was 7.0, while that of patients who were known case of diabetes was 5.7.
Comparison of occurrence of phimosis in candidal balanoposthitis patients with diabetes and in candidal balanoposthitis patients without diabetes
Phimosis was noted in 70% (21 cases) of candidal balanoposthitis patients with diabetes mellitus, whereas phimosis was noted only in 21.05% (4 cases) of candidal balanoposthitis patients without diabetes mellitus. It was found to be statistically significant that associated diabetes mellitus and candida balanoposthitis may increase the risk of phimosis (P < 0.05).
KOH and culture in candidal balanoposthitis
Among 49 patients with candidal balanoposthitis, 36 (73.47%) patients were found to be KOH positive. In remaining 13 patients with candidal balanoposthitis with negative KOH, fungal culture was done and was positive for candidal growth in 12 patients (92.31%). All positive cultures showed growth of Candida albicans. The remaining one patient with candidal balanoposthitis who was negative for both KOH and culture had typical clinical feature of candidal balanoposthitis.
In the present study, about 3.66% (three) patients had dermatophytosis over prepuce. Two of the three patients had associated lesions over groin also. And one patient had isolated lesion over genitals (shaft of penis and prepuce).
A total of 3 (3.66% of infectious balanoposthitis) cases were diagnosed with gonococcal etiology. Among those 3 cases, only one had only gonococcal etiology, whereas the other two cases had mixed infections.
In our study, all the patients with herpes genitalis except one, presented with erosive ulcers with either circular or polycyclic margins, whereas the remaining one patient presented with radial fissuring of prepuce.
Presence of multiple infectious etiologies was noted in 13.41% of study population [Table 5].
Table 5.
List of multiple etiologies causing balanoposthitis
| Etiologies | Number of cases | Percentage of infectious cases (n=82) | Percentage of total cases (n=106) |
|---|---|---|---|
| Candidal + genital warts | 3 | 3.66 | 2.83 |
| Candidal + herpes genitalis | 2 | 2.44 | 1.89 |
| Syphilis + herpes genitalis | 2 | 2.44 | 1.89 |
| Candidal + scabies | 1 | 1.22 | 0.94 |
| Herpes genitalis + genital warts | 1 | 1.22 | 0.94 |
| Gonococcal + genital warts | 1 | 1.22 | 0.94 |
| Syphilis + herpes genitalis + gonococcal + candidal | 1 | 1.22 | 0.94 |
| Total | 11 | 13.41 | 10.38 |
Only 27 subjects gave consent for HIV testing and they were all negative.
Partner screening was possible only in 5 subjects (3 cases of candidal balanoposthitis and 2 cases of herpes genitalis). Rest of the patients failed to bring their partner for screening, despite the counseling for same. Partners of all 3 cases of candida balanoposthitis had vulvovaginitis, while partners of cases of genital herpes were asymptomatic.
Noninfectious etiologies
Among noninfectious etiologies, adverse drug reaction (5 cases, 4.72% of total cases) was the most common, followed by lichen planus (4 cases, 3.77%) and psoriasis (4 cases, 3.77%) [Table 6] [Figure 6].
Table 6.
Noninfectious etiologies of balanoposthitis
| Etiology | Number of cases | % of total cases |
|---|---|---|
| Adverse drug reaction | 5 | 4.72 |
| Lichen planus | 4 | 3.77 |
| Psoriasis | 4 | 3.77 |
| Insect bite allergy | 2 | 1.89 |
| Carcinoma | 2 | 1.89 |
| Granuloma annulare | 1 | 0.94 |
| Irritant contact | 1 | 0.94 |
| Paederus dermatits | 1 | 0.94 |
| Circinate balanitis | 1 | 0.94 |
| Sebaceous cyst | 1 | 0.94 |
| Vitiligo | 1 | 0.94 |
| Zoon’s balanitis | 1 | 0.94 |
| Total | 24 | 22.64 |
Figure 6.
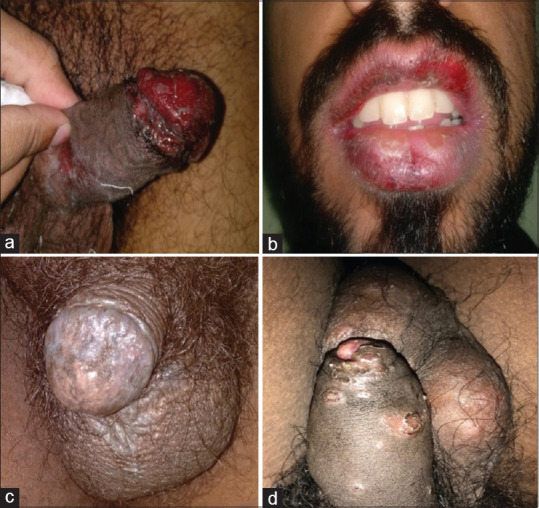
Noninfectious balanoposthitis. (a) Adverse drug reaction showing erosions over glans penis and prepuce. (b) Adverse drug reaction in the same patient showing erosions over lips. (c) Genital lichen planus. (d) Genital psoriasis
A total of 5 (4.72%) patients had balanoposthitis due to adverse drug reaction. All the patients had fixed drug eruption. All the 5 patients had erosions over glans penis and prepuce. Two of the five patients had associated oral lesions also and the remaining three patients had only genital lesions.
Four (3.77%) patients in our study had lichen planus. All the 4 patients had violaceous plaques over prepuce and glans. Two patients with genital lichen planus had both associated oral and skin lesions, one patient had associated oral lesions alone, and the remaining one patient had isolated genital lesion.
Four (3.77%) patients in our study had psoriatic plaques over glans penis and prepuce. While 3 patients had associated psoriatic plaques over skin also, 1 patient had isolated genital psoriasis. In all the 4 patients, lesions were well defined, scaly, erythematous plaques, while lesions over the skin of prepuce were more scaly when compared to lesions over glans penis.
In our study, one (0.94%) patient had irritant contact dermatitis to Dettol application over glans penis.
Unusual cases of noninfectious balanoposthitis found in our study included
Carcinoma penis,
Granuloma annulare,
Sebaceous cyst,
Insect bite allergy,
Zoon's balanitis,
Paederus dermatitis.
Association with genital hygiene
For analytical purposes, genital hygiene of patients was classified into three categories as follows:
Good (washing the genitals >5 times per week)
Moderate (washing the genitals 3 to 5 times per week)
Poor (washing the genitals <3 times per week).
Among 106 patients with balanoposthitis, 44 patients (41.51%) had poor genital hygiene, 22 patients (20.75%) had moderate genital hygiene, and 40 patients (37.74%) had good genital hygiene.
Complications noted
Phimosis was the most common complication noted in 25 (23.58%) subjects, followed by paraphimosis (1 subject, 0.94%) and meatitis (1 subject, 0.94%).
Discussion
In our study of 106 patients with balanoposthitis, the age of patients ranged from 13–68 years with the most common age group from 21–30 years (31.13%). The mean age of the study population was 37 years with the standard deviation of 14.02 years.
In the present study, 101 patients (95.28%) were uncircumcised, and only 5 patients (4.72%) were circumcised. This is similar to the study conducted by Raju and Prakash,[4] where 69 patients (92%) were uncircumcised and only 6 cases (8%) were circumcised.
In the present study, 73 (68.87%) subjects were married. Among 33 unmarried subjects, only 7 (21.21%) subjects gave history of sexual contact.
In the present study, erythema was the most common clinical feature which was noted in 47.17% cases, followed by fissuring over prepuce (26.42% cases), phimosis (23.58% cases), ulcer (19.81% cases), and subpreputial discharge (8.49% cases). According to Lisboa et al.,[5] erythema of the glans and prepuce and subpreputial discharge were the most common presenting feature. Erythema, ulceration, and exudates were the common clinical features noted by Rajiah, et al.[6]
Factors like poor hygiene and irritation by smegma favor the development of balanoposthitis. In our study, about 41.5% had poor genital hygiene. Local hygienic measures have been suggested for the treatment of nonspecific balanoposthitis.[7]
With regards to site of involvement, involvement of only glans penis was noted in 17.92% patients, only prepuce in 42.45% patients, and both glans penis and prepuce in 39.62% patients.
In the present study, infectious causes were found to be the etiology for 77.36% cases. Infectious causes outnumbered noninfectious causes. This is in comparison with the study by Krishnamurthy et al.,[8] who found incidence of infectious causes (65.74%) to be more than noninfectious causes.
Among 82 patients with infectious balanoposthitis, 8 patients (9.76%) gave history of extramarital contact (EMC) and 8 patients (9.76%) gave history of premarital contact (PMC). Among 16 patients with EMC/PMC, 15 patients (93.75%) gave history of unprotected sex and 1 patient (6.25%) gave history of protected sex. Hence, patients must be educated about the importance of safe sex practices in prevention of spread of sexually transmitted infections.
Infectious balanoposthitis
In our study, fungal organisms were the most common infectious agents causing balanoposthitis (63.41%), followed by viral (32.93%), bacterial (10.98%), and parasitic (8.54%). In the study by Deepa et al.,[9] candidal balanoposthitis was the most common infectious cause (about 56%). But in contrast, according to Raju and Prakash,[4] the common infective causes following fungal infection (47.91%) were bacterial infections (37.05%), viral infections (Condylomata acuminate-6.72% and Herpes genitalis-4.16%), and parasitic infection (Scabies 4.16%). In a study by Devi et al.,[10] Candida spp. remained the commonest percentage (29%) followed by Staphylococcus aureus (13%) and herpes simplex (12%). In a recent study by Goel et al.,[11] fungal STIs (candidal) and viral STIs (herpes genitalis and condylomata acuminata) are on the rise and bacterial STIs (syphilis and chancroid) are declining.
Among 49 patients with candidal balanoposthitis, 10% KOH was positive in 73.47% patients. KOH negative patients were sent for candida culture. Culture was positive in 92.31% patients and all cultures showed growth of Candida albicans. In the study by Lisboa et al.[5], C. albicans was the most common candidal species found in 76–89%, followed by Candida glabrata and others. But C. glabrata was the more frequent species isolated according to Franscia, et al.[12]
In our study, among patients with candidal balanoposthitis, fissuring was the common clinical pattern (53.06%), followed by phimosis (51.02%), erythema (51.02%), and subpreputial discharge (16.33%).
The other common infective causes, next to candidal balanoposthitis include herpes simplex virus (19.51%), human papilloma virus (13.41%), and scabies (8.54%).
In our study, all the patients with herpes genitalis except one, presented with erosive ulcers with either circular or polycyclic margins, which is the most common presentation in herpes genitalis,[13] whereas one patient presented with radial fissuring of prepuce, which was clinically similar to candidal posthitis. KOH and fungal culture were done for the patient and were negative. But tzanck smear showed multinucleated giant cells and the patient was diagnosed as herpes genitalis and subsequently treated with acyclovir.
About 13.41% of our patients with infectious balanoposthitis had multiple etiological agents in same individual. A study conducted by Prabhakar et al.[14] showed 9% of mixed infections in genital ulcer disease. In addition, the study by Sumathi et al.[15] showed multiple etiological agents in 11.11% of patients with genital ulcer disease. Hence, one should always consider the possibility of multiple etiologies, while evaluating STDs. This will lead to faster resolution of genital lesions, and hence reduce the risk of disease transmission to uninfected partner.
Poorly controlled levels of blood sugar can favour proliferation of Candida over glans and prepuce, causing balanoposthitis.[16] In our study, diabetes mellitus was present in 33% of total patients (both infectious and noninfectious) and in 61.22% of patients with candidal balanoposthitis. Thus, diabetes mellitus is a risk factor for candidal balanoposthitis (significant association noted in our study). Among the diabetic patients with candidal balanoposthitis, 60% patients were known diabetic, while 40% patients were newly diagnosed with diabetes after they presented to STD OPD with balanoposthitis. A study by Drivsholm et al.[17] had reported that balanitis could be associated with 12% of newly diagnosed diabetics. According to the study conducted by Swamiappan et al.,[18] 47.22% of patients with candida balanoposthitis were known diabetic and 43.52% were newly diagnosed with diabetes mellitus.
In our study, phimosis was seen in 70% of patients who had both diabetes and candida balanoposthitis, but in only 21.05% of candidal balanoposthitis patients without diabetes mellitus. Hence, diabetes mellitus and candidal balanoposthitis together may increase the risk of phimosis and this was found to be statistically significant in our study.
Unusual cases of infectious balanoposthitis
1) In the present study, about 3.66% cases had dermatophytosis over prepuce. Dermatophytosis of genital region is relatively rare when compared to tinea cruris, as per literature. Only few such cases have been reported so far.[19] In this era of resistant dermatophytoses, the trend of genital dermatoses is changing and dermatophytic infection is becoming common nowadays. A study by Singhal and Nair[20] reported it as the second most common nonvenereal male genital dermatoses.
2) In the present study, one of the patients had four etiologies: herpes genitalis with candidal balanoposthitis, syphilis, and gonorrhea. Though multiple etiologies can occur in same individual, it is uncommon to find four etiologies in the same patient.
3) One patient with fever of unknown origin was found to have eschar over prepuce. Serology for scrub typhus was done and was found to be positive. He was subsequently treated with doxycycline.
Noninfectious balanoposthitis
Among noninfectious etiologies, adverse drug reaction (4.72% of total patients) was the most common, followed by lichen planus (3.77%) and psoriasis (3.77%). Similarly, in the study conducted by Raju and Prakash,[4] FDE constituted the most common cause (8%), followed by irritant dermatitis (2.67%) and erythema multiforme (1.33%).
A total of 5 (4.72%) patients had balanoposthitis due to adverse drug reaction. All the patients had fixed drug eruption. Co-trimoxazole, NSAIDs, and tetracyclines are the most common drugs implicated in fixed drug eruption.[21] In our study, one patient developed FDE with ciprofloxacin + tinidazole, one with norfloxacin, one with NSAIDs, and one with ofloxacin. And in one more patient, patient was not aware of the drug details.
Complications noted
Phimosis was the most common complication noted in 23.58% cases, followed by paraphimosis (0.94%) and meatitis (0.94%). Phimosis and meatal stenosis were the complications noted by Rajiah, et al.[6]
Summary and Conclusion
In our study, infectious etiologies of balanoposthitis were more common than noninfectious etiologies. Common causes of infectious balanoposthitis included candidal balanoposthitis, followed by herpes genitalis, genital warts, scabies, syphilis, and gonorrhea. Unusual causes of infectious balanoposthitis like dermatophytosis and scrub typhus were also found in our study. About 13.41% of patients with infectious balanoposthitis had multiple etiological agents. Hence, the possibility of multiple etiologies should always be kept in mind while diagnosing STDs. This facilitates early treatment and hence reduces the risk of transmission of disease. Among the diabetic patients with candidal balanoposthitis, 60% patients were known diabetic, while 40% patients were newly diagnosed as diabetic after they presented to STD OPD with balanoposthitis. Hence, blood sugar and HbA1c levels must be checked as a routine in patients presenting with balanoposthitis. Phimosis was seen in 70% of patients who had both diabetes and candidal balanoposthitis, but in only 21.05% of candidal balanoposthitis patients without diabetes mellitus. This suggests that candidal infection and diabetes mellitus, when present together, increase the incidence of phimosis. Among noninfectious etiologies, adverse drug reaction was the most common, followed by lichen planus and psoriasis. Unusual causes of balanoposthitis like insect bite allergy, carcinoma penis, granuloma annulare, irritant contact dermatitis, paederus dermatitis, circinate balanitis, sebaceous cyst, vitiligo, and Zoon's balanitis were also found in our study. All lesions which occur on genitalia are not necessarily sexually transmitted. Nonvenereal conditions can also occur over the genitalia. All clinicians should examine these conditions with an open mind and treat them with appropriate drugs. Identifying the common nonvenereal genital conditions and reassuring the patients will help to remove venereophobia and avoids unnecessary mental distress. Patients, who have fear and a lack of knowledge about safe sex practices and genital hygiene, need more attention and adequate education.
Limitations
-
1)
Some patients denied consent for HIV testing. Hence, the incidence of HIV might be more if serology were done in all patients.
-
2)
Many patients did not bring partner for screening, despite counseling regarding the same. Hence, partner assessment could not be done.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cuckow PM. FORESKIN [Internet]. Pediatric Urology. 2nd ed. W. B. Saunders; 2009. [Google Scholar]
- 2.Lisboa C, Ferreira A, Resende C, Rodrigues AG. Infectious balanoposthitis: Management, clinical and laboratory features. Int J Dermatol. 2009;48:121–4. doi: 10.1111/j.1365-4632.2009.03966.x. [DOI] [PubMed] [Google Scholar]
- 3.Cree GE, Willis AT, Phillips KD, Brazier JS. Anaerobic balanoposthitis. Br Med J (Clin Res Ed) 1982;284:859–60. doi: 10.1136/bmj.284.6319.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raju J, Prakash BG. Balanoposthitis: A clinical study. J Evid Based Med Healthcare. 2015;2:249–60. [Google Scholar]
- 5.Lisboa c, Santos A, Dias C, Azevedo F, Pina-Vaz C, Rodrigues A. Candida balanitis: Risk factors. J Eur Acad Dermatol Venereol. 2010;24:820–6. doi: 10.1111/j.1468-3083.2009.03533.x. [DOI] [PubMed] [Google Scholar]
- 6.Rajiah K, Veettil SK, Kumar S, Mathew EM. Study on various types of infections related to balanitis in circumcised or uncircumcised male and its causes, symptoms and management. Afr J Pharm Pharmacol. 2012;6:74–83. [Google Scholar]
- 7.Fahmy MAB. Normal and Abnormal Prepuce. 2020 doi: 10.1007/978-3-030-37621-5. [Google Scholar]
- 8.Krishnamurthy VR, et al. “An aetiological study of balanoposthitis in Thanjavur. Ind Jr STD. 1982;3:70–1. [Google Scholar]
- 9.Deepa K, Chitra CL, Manipriya R. A clinico etiological study of balanoposthitisin male patients attending the sexual transmitted diseases outpatient department. Int J Res Dermatol. 2019;5:123. [Google Scholar]
- 10.Devi B, Lego K, Goswami A. A study of etiological profile of balanoposthitis in patients attending. RIMS hospital Imphal. 2009 [Google Scholar]
- 11.Goel S, Chopra D, Choudhary V, Riyat A, Chopra S. Changing trends of sexually transmitted infections and estimation of partner notification at a tertiary care center in North India. Indian J Sex Transm Dis. 2020;41:176–80. doi: 10.4103/ijstd.IJSTD_10_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okungbowa FI, Isikhuemen OS, Dede AP. The distribution frequency of Candida species in the genitourinary tract among symptomatic individuals in Nigerian cities. Rev Iberoam Micol. 2003;20:60–3. [PubMed] [Google Scholar]
- 13.Sharma VK. A Textbook of the Indian Association for the Study of Sexually Transmitted Diseases and AIDS. 2nd ed. India: Viva Books Pvt Ltd; 2009. Sexually transmitted diseases and HIV/AIDS. [Google Scholar]
- 14.Prabhakar P, Narayanan P, Deshpande GR, Das A, Neilsen G, Mehendale S, et al. Genital ulcer disease in India: Etiologies and performance of current syndrome guidelines. Sex Transm Dis. 2012;39:906–10. doi: 10.1097/OLQ.0b013e3182663e22. [DOI] [PubMed] [Google Scholar]
- 15.Muralidhar S, Talwar R, Anil Kumar D, Kumar J, Bala M, Khan N, et al. Genital ulcer disease: How worrisome is it today A Status Report from New Delhi. India J Sex Transm Dis. 2013;3 doi: 10.1155/2013/203636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cold CJ, Taylor JR. The prepuce. BJU Int. 1999;83(Suppl 1):34–44. doi: 10.1046/j.1464-410x.1999.0830s1034.x. [DOI] [PubMed] [Google Scholar]
- 17.Drivsholm T, de Fine Olivarius N, Nielsen AB, Siersma V. Symptoms, signs and complications in newly diagnosed type 2 diabetic patients, and their relationship to glycaemia, blood pressure and weight. Diabetologia. 2005;48:210–4. doi: 10.1007/s00125-004-1625-y. [DOI] [PubMed] [Google Scholar]
- 18.Swamiappan M, Chandran V, Ramasamy S, Sridhar V, Vanathi T. Candidal balanoposthitis--A retrospective study in a tertiary care centre of South India. J Evol Med Dent Sci. 2016;5:7042–6. [Google Scholar]
- 19.Das JK, Sengupta S, Gangopadhyay A. Dermatophyte infection of the male genitalia. Indian J Dermatol. 2009;54:21. [Google Scholar]
- 20.Singhal RR, Nair PA. A study on clinical patterns of nonvenereal male genital dermatoses at a rural-based tertiary care center. Clin Dermatol Rev. 2021;5:78–84. [Google Scholar]
- 21.Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D. West Sussex: John Wiley & Sons Inc; 2016. Rooks Textbook of Dermatology Chichester. [Google Scholar]


