Keywords: auditory, autonomic nerve system, disorder of consciousness, heart rate, misdiagnosis, music therapy, protection, repair, subjective score
Abstract
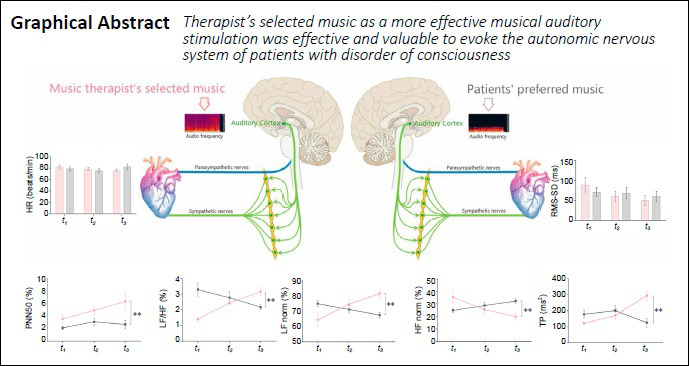
The current randomized controlled trial was performed at the China Rehabilitation Science Institute, China to test the hypothesis that musical auditory stimulation has positive effects on the autonomic nervous system of patients with disorder of consciousness. Although past studies have recommended that patients with disorder of consciousness listen to patient-preferred music, this practice is not universally accepted by researchers. Twenty patients with severe disorder of consciousness listened to either therapist-selected (n = 10, 6 males and 4 females; 43.33 ± 18.76 years old) or patient-preferred (n = 10, 5 males and 5 females, 48.83 ± 18.79 years old) musical therapy, 30 minutes/day, 5 times/week for 6 weeks. The results showed no obvious differences in heart rate variability-related parameters including heart rate, standard deviation of normal-to-normal R-R intervals, and the root-mean-square of successive heartbeat interval differences of successive heartbeat intervals between the two groups of patients. However, percentage of differences exceeding 50 ms between adjacent normal number of intervals, low-frequency power/high-frequency power, high-frequency power norm, low-frequency power norm, and total power were higher in patients receiving therapist-selected music than in patients receiving their own preferred music. In contrast, this relationship was reversed for the high-frequency power and very-low-frequency band. These results suggest that compared with preferred musical stimulation, therapist-selected musical stimulation resulted in higher interactive activity of the autonomic nervous system. Therefore, therapist-selected musical stimulation should be used to arouse the autonomic nervous system of patients with disorder of consciousness. This study was approved by the Institutional Ethics Committee of China Rehabilitation Research Center, China (approval No. 2018-022-1) on March 12, 2018 and registered with the Chinese Clinical Trial Registry (registration number ChiCTR1800017809) on August 15, 2018.
Chinese Library Classification No. R454.3; R741; B84
Introduction
Disorder of consciousness (DOC) is a disease that seriously affects a person’s ability to interact with the outside world, regardless of whether its origin is traumatic or non-traumatic (Erkkinen et al., 2018; Jang and Kwon, 2019; Owen, 2019; Jang and Lee, 2020; Liang et al., 2020; Mencarelli et al., 2020). According to the current ‘gold standard’ diagnosis criteria in the JFK Coma Recovery Scale – Revised (CRS-R) (Giacino et al., 2004), after the transition from coma, some patients who remain unresponsive to external stimulation will be diagnosed with unresponsive wakefulness syndrome (UWS). Moreover, patients who show fluctuating but definite behavioral evidence of self or environmental awareness can be diagnosed as in a minimally conscious state (MCS) (Giacino et al., 2002). Neurobehavioral and imaging studies have shown that the volatile nature of MCS awakening, including impaired visual, auditory, motor, and speech functions, might result in a lack of response by patients with MCS to clinical assessments (Arzi et al., 2020). Clinically, this can result in limited patient-examiner communication, which leads to a high rate of misdiagnosis (Binder et al., 2020). Although the clinical characteristics of DOC can be assessed using various behavioral observation scales (Riganello et al., 2018), behavior can still be misdiagnosed when scored manually. Although neuroimaging results have been increasingly used in the classification of DOC patients (Berlingeri et al., 2019; Binder et al., 2020; Kondziella et al., 2020), these technologies are often expensive, complex, and time-consuming. Given the severe neurological dysfunction in patients with DOC, assessing the autonomic nervous system (ANS) of patients with DOC can improve diagnosis accuracy.
Some researchers have proposed that the physiological signals of internal organs such as the heart can be used to determine ANS status of patients with DOC and to indicate the progress of clinical treatment (Riganello et al., 2012, 2019; Tobaldini et al., 2018). Heart rate (HR) variability (HRV) is the fluctuation in time between sequential heartbeats. These fluctuations represent the output of a complex brain-heart interaction system (Pan et al., 2018; Gao et al., 2019; Wu et al., 2020). The HRV test is non-invasive and allows clinicians to observe the immediate and flexible response to the environment, which reflects the sensitivity of the ANS (Arbit et al., 2015; Calabrò et al., 2017; Heinz et al., 2019; Dalise et al., 2020; Dorey et al., 2020; Ghiasi et al., 2020; Lin et al., 2020). The sympathetic (“fight or flight”) and parasympathetic (“rest and digest”) subdivisions of the ANS have antagonistic effects and are connected to the brain through the spinal cord (Riganello et al., 2015; Candemir et al., 2020). Low-frequency power (LF), high-frequency power (HF), total power (TP), and other variables in the HRV test reflect the body’s regulation of body temperature, feeling of pressure, renin-angiotensin-aldosterone balance, and atrial and ventricular pressure sensors (Hodges et al., 2019). Because of its low cost, ease of use, and the amount of measurement data that can be collected, many recent studies of ANS treatment for patients with DOC have used the HRV test.
Musical stimulation is an important therapeutic technique used to bring patients with severe DOC out of unconsciousness. Studies have compared the therapeutic effects of tactile stimulation and musical stimulation in patients with DOC and found that musical stimulation not only provides clues for how to awaken patients with DOC from unconsciousness, but also theoretical and clinical evidence for rehabilitation from severe brain trauma (Magee et al., 2016a). Riganello et al. (2015) stimulated patients with DOC using pleasant and uncomfortable music (typical examples from the four composers: Pyotr Ilyich Tchaikovsky, Modest Mussorgsky, Edvard Grieg, and Luigi Boccherini). Using a normalized unit of low frequency and HRV to observe the response of patients with DOC, they found that the neural network and structure of the patients’ brains changed depending on the musical composition. Perrin et al. (2015) reported that musical interventions, especially the use of live music, helped patients to strengthen their “real-time” localization capabilities. The beat (speed, rhythm) of music is a multi-sensory stimulus. According to the different stimuli, the beat can evoke attention and auditory perception, such as the name of the patient.
The use of specifically selected music for patients being treated with musical stimulation produces better results. One study compared the effects of four types of auditory stimulation (music liked by patients, entrained improvisation played by music therapists, music disliked by patients, and white noise) in 21 patients with MCS and found a clearer and more complete cognitive response in the patients who heard the music that they liked (O’Kelly et al., 2013). Some similar studies have shown that when patients with MCS listen to personalized music (preferred music), their brains show more active cortical activity (Magee et al., 2016a). However, according to the different responses shown by patients with DOC when listening to the live music played by the therapist, it is clear that in patients with MCS, targeted selection of music based on the patient’s response will also cause a clear cognitive response (Kotchoubey et al., 2015). Other studies (Proverbio et al., 2015; Riganello et al., 2015) have shown that using musical pieces selected by a professional clinical researcher (not the patient’s preferred music) has a more active effect on reticular activation of the auditory cortex in patients with DOC. In these studies, researchers have used classical European music for auditory stimulation interventions (Proverbio et al., 2015). Classical European music does not have the same cultural significance for Chinese listeners, and the listener needs to have a similar cultural background and a high level of appreciation (Fang et al., 2017). Therefore, based on the results of previous studies, there are two recommendations for the type of music that should be used in musical therapy for patients with DOC: 1) preferred music that is recommended by the patients’ families or related to the patients’ individualized experiences; 2) Classical musical pieces that are selected by a professional clinical music therapist. Therefore, the current study used HRV measurements to compare how well these two types of musical stimulation clinically aroused the ANS in patients with DOC.
Subjects and Methods
This study was approved by the Ethics Committee of China Rehabilitation Research Center (CRRC) (approval No. 2018-022-1) on March 12, 2018 (Additional file 1 (149.8KB, pdf) ), and informed consent (Additional file 2 (148.8KB, pdf) ) was obtained from the relatives or guardians of the participants before commencing the study. The study trial was registered with the Chinese Clinical Trial Registry (registration number ChiCTR1800017809) on August 15, 2018. The writing and editing of the research article were performed in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT) Statement (Additional file 3).
Additional file 3.
 CONSORT 2010 checklist of information to include when reporting a randomised trial*
CONSORT 2010 checklist of information to include when reporting a randomised trial*
| Section/Topic | Item No | Checklist item | Reported on page No |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | 1 | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | 1 | |
| Introduction | |||
| Background and | 2a | Scientific background and explanation of rationale | 1-2 |
| objectives | 2b | Specific objectives or hypotheses | 2 |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 2 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | 2 | |
| Participants | 4a | Eligibility criteria for participants | 2 |
| 4b | Settings and locations where the data were collected | 2 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 2-3 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 3 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | 3 | |
| Sample size | 7a | How sample size was determined | 3,4 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | 3 | |
| Randomisation: | |||
| Sequence | 8a | Method used to generate the random allocation sequence | 3 |
| generation | 8b | Type of randomisation; details of any restriction (such as blocking and block size) | 3 |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 3 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants tointerventions | 3 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | 3 |
| 11b | If relevant, description of the similarity of interventions | 3 | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 4 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 4 | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | 4 |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | 3,4 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 3,4 |
| 14b | Why the trial ended or was stopped | 3 | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 3 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 3,4 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 3,4 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | 4 | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | 3,4 |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | 5 |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 5 |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | 5 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 5 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | 1 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | 1 |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | 1,6 |
*We strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration for important clarifications on all the items. If relevant, we also recommend reading CONSORT extensions for cluster randomised trials, non-inferiority and equivalence trials, non-pharmacological treatments, herbal interventions, and pragmatic trials. Additional extensions are forthcoming: for those and for up to date references relevant to this checklist, see www.consort-statement. org.
Participants
Twenty-six participants were recruited from CRRC, Beijing. The inclusion criteria were as follows: 1) diagnosed as in a minimally conscious state on the Glasgow Coma Scale (GCS) (Tsai et al., 2020) and the Sensory Modality Assessment and Rehabilitation Techniques (SMART) scale (McAleese et al., 2018) for at least 1 month (inclusive) and hospitalized; 2) aged 18 to 70 years; 3) tolerance to therapy in a probe position for more than half an hour without postural hypotension; 4) receiving routine therapy (medicinal therapy: ATP, coenzyme A, citicoline, other brain-metabolism enhancers, and chlorhexidine awakening agents; physical therapy: occupational therapy and routine care); 5) no professional musical experience. The exclusion criteria were: 1) a history of any heart disease, heart surgery, or medication for heart disease; 2) severe auditory dysfunction; 3) having epilepsy, malignant arrhythmia, or other serious physical diseases. Criteria for withdrawal and termination: patients could be terminated if their condition changed, if they were discharged from the hospital, or if they voluntarily withdrew. Twenty participants completed the experiment. Six participants were withdrawn from the study because they did not meet the inclusion criteria. Participant characteristics are shown in Table 1.
Table 1.
Participant characteristics
| Intervention group | Control group | t | P | |
|---|---|---|---|---|
| Total number | 10 | 10 | ||
| Gender | ||||
| Male (n) | 6 | 5 | ||
| Female (n) | 4 | 5 | ||
| Age (yr) | 43.33 ± 18.76 | 48.83 ± 18.79 | 0.9254 | > 0.05 |
| Years since injury | 1.03 ± 0.62 | 1.26 ± 1.06 | 0.03926 | > 0.05 |
| GCS classification | ||||
| MCS | 10 | 10 | ||
| SMART classification | ||||
| MCS | 10 | 10 |
Data are expressed as a number in total number, gender, and GCS classification. Other data are expressed as the mean ± SD, and analyzed by paired t-test. Intervention group: therapist-selected music; control group: patient-preferred music. GCS: Glasgow Coma Scale classification; MCS: minimally consciousness state; SAMRT: Sensory Modality Assessment and Rehabilitation Technique.
Study design
The study was a randomized controlled trial with a pretest-midtest-posttest design. It included two groups: the intervention group (n = 10) and the control group (n = 10). After screening by the neurological specialists, computer-generated sequences (by Excel 2013; Microsoft office software, Seattle, WA, USA) were used to randomly assign the patients into the two groups. This study adopted a double-blind design — neither the participants nor the data analyst knew which group of data was being tested and analyzed. The intervention group received therapist-selected music, while the control group received their own preferred music. The study was conducted from June 2019 until May 2020 at CRRC. The cost of music therapy for both groups was supported by the fund for this study.
Procedure
After obtaining approval from the Scientific Research Foundation of CRRC, participants were screened by the neurorehabilitation specialists. Patients who were diagnosed as MCS by the GCS and SAMRT scale were referred to the Music Therapy Department at CRRC. Participants were reviewed by the researchers to identify potential interventional objectives based on the inclusion and exclusion criteria of the study. Once potential participants were identified, an invitation to participate in the study was sent to their family members. The invitation included the purpose, procedures, risks, benefits, confidentiality, and participants’ rights. Once we acquired the consent forms, the participants were divided into two groups based on the GCS classification. Music therapy researchers screened patients based on previous outcomes for similar GCS scores to assess whether they had an abnormal auditory function, and took the SMART assessment as a reference (Magee et al., 2016b). After the screening, computer-generated sequences (by Excel 2013, Microsoft office software, Seattle, WA, USA) were used to randomly assign the patients into two groups. The participants in the intervention group were treated by music therapists for 6 weeks with therapist-selected musical stimulation, while participants in the control group were treated with their preferred music for 6 weeks. The enrollment and allocation of participants are shown in Figure 1.
Figure 1.
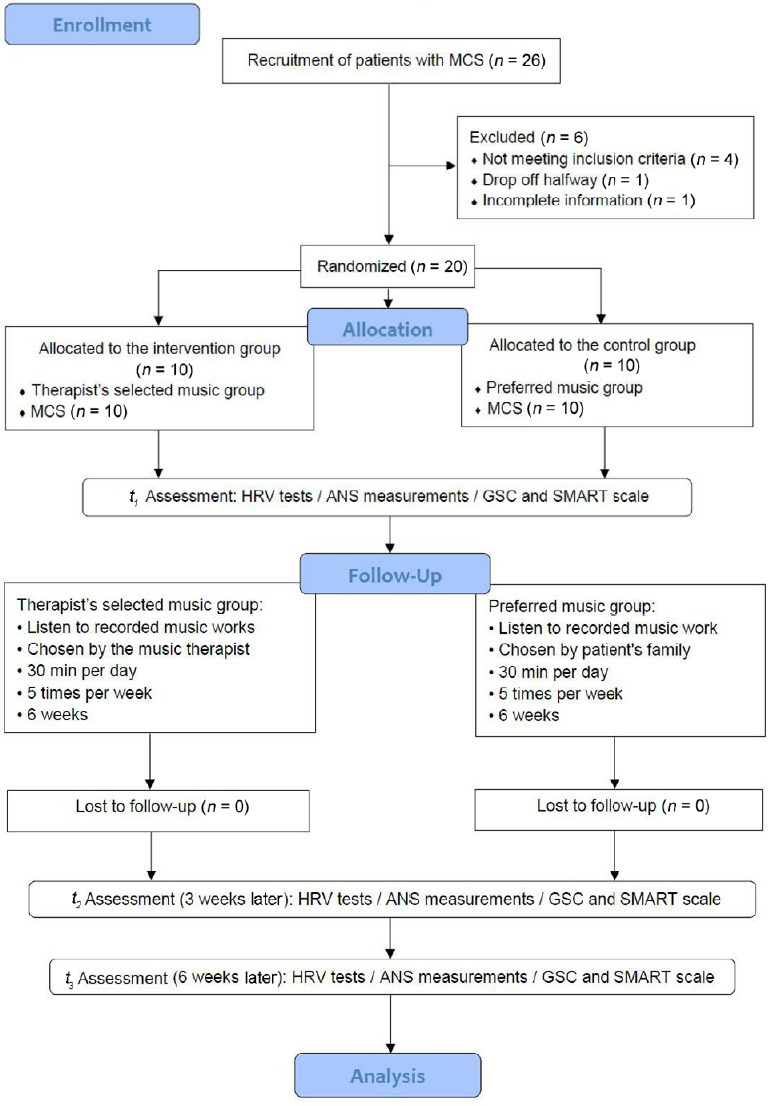
Flow diagram for participant recruitment and allocation.
ANS: Autonomic nervous system; GSC: Glasgow Coma Scale; HRV: heart rate variability; MCS: minimum conscious state; SMART: Sensory Modality Assessment and Rehabilitation Techniques.
Figure 1 shows that 26 participants were enrolled in the study, 6 were withdrawn because they did not meet the inclusion criteria (n = 4), did not complete the study (n = 1), or their information was incomplete (n = 1). Evaluations were conducted three times during the experiment: t1 (baseline), t2 (3 weeks after), and t3 (6 weeks after). The data analysis included a sample of 20 patients with MCS.
Interventions
Once the participants for each group were identified, an intervention delivery schedule was developed. The intervention group was stimulated by the therapist-selected music. Each patient received auditory stimulation for 30 minutes, five times per week for 6 weeks. The music was selected by the music therapist according to the following principles: (1) New Age music pieces composed by Chinese and Japanese composers; (2) a clear and simple melodic line (homophonic music promotes attention and concentration); (3) speed between 80–120 beats/minute (consistent with HR norm); (4) the original recording volume (sound pressure level) was between 40–60 dB, 20–20 kHz (within hearing range for the human ear, moderate volume, appropriate frequency); (5) positive emotional orientation. Based on these principles, the represented musical pieces and composers selected by the music therapist were as follows: (1) “Summer in Satoyama” by Japanese composer Masaaki Kishibe; (2) “The Sun Rises as Usual” by Japanese composer Joe Hisaishi; (3) “Summer” by Japanese composer Joe Hisaishi; (4) “Four Seasons in Beijing - Colorful Butterflies Dance Summer” by Chinese composer Chen-Chen Ho; (5) “New Ambush on All Sides” by Chinese composer Zhao Cong. The control group received musical pieces chosen by the participants’ families, most were pop songs or old folk music that contain lyrics. All patients underwent routine treatment during the study period, including taking medication and other care and support.
Measurements
The following were conducted at each measurement time point: (1) HRV tests; (2) ANS measurements; (3) GCS Scale, and SMART Scale (McAleese et al., 2018; Tsai et al., 2020). No participant wore chest binders, earrings, rings, or other aids likely to affect heart function or autonomic nervous function during assessment or training.
HRV and ANS tests
We recorded the HR with the HR Bodyguard device (Ver. 3.1; Meiyang Ltd., Beijing, China). The HR was calculated as the R to R wave measures at a 1000-Hz sampling frequency. HRV was analyzed with a standard program (Ver. 3.1, Meiyang Ltd.) for the following measurement time intervals. The HRV data (Nolan et al., 1998) include: (1) HR; (2) standard deviation of normal-to-normal R-R intervals (SDNN), where R is the peak of a QRS complex heartbeat; (3) root-mean-square of successive heartbeat interval differences (RMS-SD). All the data were recorded during the music monitoring process, which corresponds to the transmural dispersion of repolarization. HRV includes time-domain measures (HR, SDNN, RMS-SD, and PNN50—the percentage of differences exceeding 50 ms between successive normal number of intervals) calculated within 30-minute periods. The evaluation of sympathetic and parasympathetic nervous systems within the ANS was accomplished using an ANS guard device (Ver. 3.1; Meiyang Ltd.) and the following indicators: (1) PNN50; (2) LF; (3) HF; (4) LF norm = LF / (LF + HF) × 100; (5) HF norm = HF / (LF + HF) × 100; (6) TP; and (7) very-low-frequency band (VLF). LF and HF components were obtained by integrating the power spectra over their respective ranges of 0.04–0.15 Hz and 0.15–0.40 Hz. The magnitude of the HF and the ratio of LF to HF (LF/HF) correspond to the strength of the vagal activity and the sympathovagal balance, respectively. The magnitude of the LF involves both vagal and sympathetic nerve activity (Furlan et al., 2019). The magnitude of each spectral component was evaluated using the natural logarithms of the power (lnLF and lnHF). The ratio of the LF component to the HF component (LF/HF ratio) was evaluated by dividing lnLF by lnHF (lnLF/lnHF).
GCS
The GCS includes three aspects: eye-opening response, verbal response, and body movement. The sum of these three scores is the coma index, with lower scores indicating a more severe disorder. The highest score is 15, indicating clear consciousness, 12–14 is classified as MCD, 9–11 is classified as moderate DOC, and a score below 8 is a coma (Tsai et al., 2020).
SMART
The SMART was developed as both an assessment and treatment tool. This diagnostic neurobehavioral tool determines vegetative state. In addition to structured behavioral observations, the SMART Profile describes both the highest SMART level and frequency of responses for the sensory, motor function, and functional communication modalities. The SMART levels range from 1 (no response) to 5 (differentiating responses) (McAleese et al., 2018). This combination can also be used to define an individual’s diagnosis and point on the diagnostic spectrum. The lowest response is SMART Level 1 and the highest is SMART Level 5 Consistent (5C). Responses in any modality between 1 and 3 are indicative of a vegetative state, SMART levels 4 and 5 are MCS/MCS+ , depending on the type and frequency of responses observed.
For all the assessments, HRV and ANS tests were administered by the same experienced operator. GCS and SMART questionnaires were evaluated by professionals. All the evaluators were registered research assistants who worked as health care professionals and who had 5 years of clinical experience.
Statistical analysis
According to the formula n = Z2 ·σ2 /d2 (Antonisamy et al., 2010), n is the minimum sample size, Z is the confidence interval, which is generally 95%, σ is the standard deviation, and d is the sampling error range, normally 0.5. The minimum sample size n is therefore calculated as ≈ 19.88, and the sample size is thus approximately equal to 20 (n = 20).
The measurement data from the two groups were collected at three time points: before intervention (t1), 3 weeks later (t2), and 6 weeks later (t3). We obtained the mean and the standard deviation (SD) for each group at each time point. We conducted two-way analyses of variance and paired t-tests to find the effects of Group, Time Point, and Group × Time Point interactions. SPSS statistical software, version 22.0 (IBM, Armonk, NY, USA) was used for statistical analysis. Before analysis, basic frequencies were run on the data to screen for missing values and outliers, and to establish data entry accuracy. Data were analyzed using two-way ANOVAs and paired t-tests to determine the specific effects of the interventions.
Results
Therapist-selected music and patient-preferred music have equal effects on HRV
There were no significant differences between groups at t2 for HR (F = 0.41, P = 0.67), SDNN (F = 2.07, P = 0.14), or RMS-SD (F = 1.65, P = 0.20). Similarly, no significant differences were found at t3 (HR: F = 0.03, P = 0.86; SDNN: F = 0.13, P = 0.72; RMS-SD: F = 4.53, P = 0.99) (Figure 2).
Figure 2.

Comparison of heart rate variability in patients with DOC who received musical stimulation.
The intervention group received the therapist-selected music; the control group received preferred music. t1: Baseline; t2: after 3 weeks of treatment; t3: after 6 weeks of treatment. (A–C) HR, SDNN, and RMS-SD. Data are expressed as mean ± SD (n = 10), and analyzed by two-way analysis of variance. DOC: Disorder of consciousness; HR: heart rate; RMS-SD: root mean square of successive heartbeat interval differences; SDNN: standard deviation of normal to normal R-R intervals.
Therapist-selected music outperformed patient-preferred music at stimulating aspects of the ANS in patients with DOC
Compared with the control group, PNN50 (F = 14.49, P = 0.0004), LF/HF (F = 12.84, P = 0.0001), LF norm (F = 7.44, P = 0.0001), and TP (F = 12.22, P = 0.0001) were higher in the intervention group at t3. In contrast, HF norm (F = 7.99, P = 0.0009) and VLF (F = 12.22, P = 0.0001) were lower in the intervention group at t3 (Figure 3).
Figure 3.
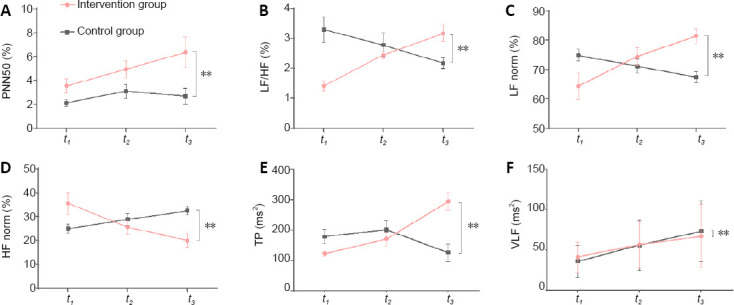
Comparison of the autonomic nervous system metrics in patients with DOC who received musical stimulation.
The intervention group received therapist-selected music; the control group received preferred music group. t1: baseline; t2: at 3 weeks of treatment; t3: at 6 weeks of treatment. (A–F) PNN50 (A), LF/HF (B), LF norm (C), HF norm (D), TP (E), and VLF (F). Data are expressed as the mean ± SD (n = 10), and analyzed by repeated-measures analysis of variance. **P < 0.01. DOC: Disorder of consciousness; HF: high-frequency power; LF: low-frequency power; PNN50: percentage of differences exceeding 50 ms between adjacent normal number of intervals; TP: total power; VLF: low-frequency power. HF norm (%) = HF / (LF + HF) × 100; LF norm (%) = LF / (LF + HF) × 100.
Discussion
In this study, preferred music and therapist-selected music were used as auditory stimulation for arousing patients with DOC from unconsciousness. Compared with previously reported methods that only used “name-calling” (Schnakers et al., 2016) or “preferred music” as the auditory stimulation, we found that although “preferred music” is recommended in the literature, it did not have a longitudinal positive effect in the clinic. Instead, we found that when professional music therapists selected representative famous music pieces as the auditory stimulation, it performed better than preferred music in stimulating the ANS.
HRV
HRV is the fluctuation of the time interval between adjacent heartbeats. HRV contains a variety of indicators, among which are the HR, SDNN, and RMS-SD. Analyzing all these variables gives information about the ANS. The RMSSD is used to estimate vagal activity and is strongly correlated to the PNN50. The SDNN represents the contribution of both branches of the ANS. The brainstem (medulla and pons) functions in the tonic control of blood pressure, respiratory rhythm, and autonomic reflexes. The brain controls homeostasis, emotion, and stress responses, which are relatively intuitive indicators for judging the activity of the autonomous nervous system (Riganello et al., 2012, 2018).
In this study, we compared the effects patient-preferred music and therapist-selected music on HRV-related physiological indicators in patients with DOC. We implemented a randomized control design for grouping and ensured that the general characteristics of the two groups were not significantly different. The patients in the intervention group were given the therapist-selected music, while the control group received patient-preferred music. After each patient was given musical stimulation, the variability of the HR, SDNN, and RMS-SD was recorded. We found that regardless of the group, the HR, SDNN, and RMS-SD did not differ between groups or across time points. Because HR, SDNN, and RMS-SD are regulated by the brainstem and the lower part of the medulla oblongata, not the cerebral cortex, we can reasonably infer that for patients with DOC, whether they listen to preferred music or therapist-selected music, any difference in how HR-related indicators are regulated is not obvious. These results also show that at a certain level, musical auditory stimulation has no obvious effect on the heart rate or its root mean square in patients with DOC.
ANS
We found a significant difference in ANS between groups. When listening to musical works recommended by clinical music therapists, which are faster than the average HR and have high melody recognition (without lyrics), patients exhibited higher sympathetic nerve excitability, increased myocardial consumption of oxygen volume, and a higher degree of active arousal. Additionally, the HF norm was lower in the intervention group, which suggests a reduction of parasympathetic activity. Because the effects of sympathetic and parasympathetic nerves are opposite, we can conclude that the nervous systems in patients with DOC exhibited higher levels of awakening when listening to the therapist-selected music than did the patients who heard preferred music. Looking at TP, results suggest that while listening to professionally selected music, patients with DOC showed higher overall activity and regulatory ability of the ANS. Compared with the control group, the intervention group showed an improved VLF index, which hints at higher body stability in terms of thermoregulation, renin–angiotensin balance, and endothelial influences on the heart. Thus, the intervention group showed a tendency for stabilized temperature regulation, with certain advantages compared with the control group. The two groups of GCS scores were not significantly different, so they were not qualitatively comparable (Additional Figure 1A (1.2MB, tif) ).
Differences between therapist-selected music and preferred music
There are different opinions in the literature as to whether music therapy for awakening patients with DOC should use therapist-selected music or patient-preferred music. We found that the sympathetic excitability of the patients of DOC tended to be greater, while parasympathetic activity tended to be reduced after listening to the therapist-selected music than after listening to patient-preferred music. Additionally, temperature regulation (stability) was better in the intervention group than in the control group. However, we must note that the “preferred music” was usually chosen by the patients’ families. Due to the severe cognitive dysfunction of patients with DOC, they cannot express their musical preferences by themselves. Therefore, the “preferred music” that clinical researchers was based on information provided by families or guardians. As such, it was inherently biased. In fact, music selected by anyone other than a patient themselves cannot guarantee that it would be liked by the patient. At this time, auditory stimulation using musical pieces selected by a therapist with music experience is the more professional clinical choice. After comparing the audio frequency of therapist-selected music (Additional Figure 1A (1.2MB, tif) ) and the preferred music (Additional Figure 1B (1.2MB, tif) ), we found that the frequency of the therapist-selected music was between 20 and 20,000 Hz, which was a much wider range than that of the preferred music (0 to 5000 Hz). Additionally, the musical structure was more regular. Therefore, the advantages of professionally recommended therapist-selected music are: (1) the wider audio frequency band can give the primary auditory cortex a wider range of stimulation, activate more neural networks, and thus act on the regulation of the ANS; (2) the regular trilogy form provides stable structural predictability for auditory stimuli. The trilogy form is form of music that generally consists of three sections: section A, section B, and section A again. The first section and the third section have the same phrase melody and musical structure, giving people a sense of repetition and expectation.
Music pieces can have a powerful impact on emotions and mood (Magee et al., 2016a, 2017). Faster paced music could also act to speed up HR and breathing more than slower music can, which could have a significant effect on arousal and the sympathetic system. In this study, although professionally selected music had a more significant effect on sympathetic and parasympathetic activation in the process of awakening patients with DOC, we found no significant differences in GSC or SMART between groups. Thus, we can conclude that regulation and activation of the ANS in patients with DOC is more effective using professional music therapist-recommended suitable music as auditory stimulation based on the individual differences of patients with DOC than using music not selected at the professional level.
Limitations
This study has several limitations. First is the small sample size, as detailed in the methods. Six participants were excluded from analysis, which may have caused the variance in group allocation. If another control group (noise stimulation, no stimulation) was added, the comparison might have been more accurate. If larger sized studies are conducted in the future, the therapeutic outcomes could be more precisely observed.
Implications for clinical practice
In previous reports (O’Kelly et al., 2013; Magee et al., 2016a), clinicians usually used preferred music to awaken patients with DOC, rather than using music selected by professionals. There is only one study reporting the application of music recommended by professional composers for treating patients with DOC in a vegetative state, suggesting the feasibility of professional music selection (Riganello et al., 2015). The current study confirmed the positive effect of therapist-selected music in 20 patients. All participants in the experimental group showed more active ANS arousal than the control group. Owing to the individual differences in preferred music and the difficulty in musical quality control, the musical pieces making up the patient-preferred music have common characteristics that are difficult to unify. Creating standards for patients with DOC is therefore difficult with such a non-rigorous selection process. However, therapist-selected music with professional standards that follow strict rules and the consistency of professional control during the treatment process have produced long-term positive effects in activating the ANS of patients with DOC. Therefore, the selection of music in our study provides practical significance for the standardization of clinical treatment for awakening patients with DOC.
Conclusions
Therapist-selected music improved ANS activity in patients with DOC. Therefore, we propose that clinicians and professional music therapists work together to make clinical treatment for DOC more effective.
Additional files:
Additional file 1 (149.8KB, pdf) : Hospital Ethics Approval (Chinese).
Additional file 2 (148.8KB, pdf) : Informed Consent Form (Chinese).
Additional file 3: CONSORT checklist.
Additional file 4: Open peer review reports 1 (106.8KB, pdf) and 2 (90.6KB, pdf) .
Additional Figure 1 (1.2MB, tif) : Audio frequency of therapist’s selected music (A) and preferred music (B).
Audio frequency of therapist's selected music (A) and preferred music (B).
The spectrogram plot shows the intensity of the audio frequency for the selected music signal as it changes over the time. The vertical axis indicates frequency, the horizontal axis indicates time, and the colored scales indicate the intensity. The color bar goes from left to right, indicating from light to deep. Magenta is the color of the highest intensity. Purple is the color of the secondary intensity. Yellow is the color of the lowest intensity. The higher the vertical axis of magenta and purple distribution, the wider the frequency range is.
Acknowledgments
This research was supported by China Rehabilitation Research Center. We thank our colleagues from Music Therapy Center, Department of Psychology, China Rehabilitation Research Center; Chinese Institute of Rehabilitation Science; Center of Neural Injury and Repair; Department of Spinal and Neural Functional Reconstruction and Beijing Key Laboratory of Neural Injury and Rehabilitation, China Rehabilitation Research Center; who provided technical support, modification advice, and statistical recommendations. It is greatly assisted to the research. We thanked Meng-Yang Lu for music therapy in the control group, and Yi-Zheng Wang for project application and the patients’ allocation.
Footnotes
P-Reviewers: Riganello F, Gao T; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
Conflicts of interest: The authors declare that there are no conflicts of interest in this work.
Financial support: This study was supported by the Beijing Science and Technology Project Foundation of China, No. Z181112661718066 (to HTL). The funding source had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: This study was approved by the Ethics Committee of China Rehabilitation Research Center (approval No. 2018-022-1) on March 12, 2018, and was registered with the Chinese Clinical Trial Registry (registration No. ChiCTR1800017809) on August 15, 2018.
Declaration of patient consent: The authors certify that they have obtained the consent forms from patients’ legal guardians. In the form, patients’ legal guardians have given their consent for patients’ images and other clinical information to be reported in the journal. The patients’ legal guardians understand that patients’ names and initials will not be published.
Reporting statement: The writing and editing of the article were performed in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT) Statement.
Biostatistics statement: The statistical methods of this study were reviewed by the epidemiologist of Capital Medical University, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: The individual deidentified participant data (including data dictionaries) will be shared; the data in particular will be shared; the additional, related documents will be available (study protocol, statistical analysis plan, etc.). The data will become available in 5 years after publication. Research colleagues in the same field can access the data through the China Clinical Trials Registry.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Francesco Riganello, S. Anna Institute and Research in Advanced Neurorehabilitation, Italy; Tian Gao, The University of Texas at San Antonio, USA.
Funding: This study was supported by the Beijing Science and Technology Project Foundation of China, No. Z181112661718066 (to HTL).
References
- 1.Antonisamy B, Premkumar P, Christopher S. New Delhi: McCrow Hill; 2010. Principles and practice of biostatistics. [Google Scholar]
- 2.Arbit B, Azarbal B, Hayes SW, Gransar H, Germano G, Friedman JD, Thomson L, Berman DS. Prognostic contribution of exercise capacity, heart rate recovery, chronotropic incompetence, and myocardial perfusion single-photon emission computerized tomography in the prediction of cardiac death and all-cause mortality. Am J Cardiol. 2015;116:1678–1684. doi: 10.1016/j.amjcard.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 3.Arzi A, Rozenkrantz L, Gorodisky L, Rozenkrantz D, Holtzman Y, Ravia A, Bekinschtein TA, Galperin T, Krimchansky BZ, Cohen G, Oksamitni A, Aidinoff E, Sacher Y, Sobel N. Olfactory sniffing signals consciousness in unresponsive patients with brain injuries. Nature. 2020;581:428–433. doi: 10.1038/s41586-020-2245-5. [DOI] [PubMed] [Google Scholar]
- 4.Berlingeri M, Magnani FG, Salvato G, Rosanova M, Bottini G. Neuroimaging studies on disorders of consciousness: a meta-analytic evaluation. J Clin Med. 2019;8:516. doi: 10.3390/jcm8040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder M, Górska U, Pipinis E, Voicikas A, Griskova-Bulanova I. Auditory steady-state response to chirp-modulated tones: A pilot study in patients with disorders of consciousness. Neuroimage Clin. 2020;27:102261. doi: 10.1016/j.nicl.2020.102261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calabrò RS, Naro A, Manuli A, Leo A, De Luca R, Lo Buono V, Russo M, Bramanti A, Bramanti P. Pain perception in patients with chronic disorders of consciousness: What can limbic system tell us. Clin Neurophysiol. 2017;128:454–462. doi: 10.1016/j.clinph.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Candemir M, Candemir B, Ertürk A. Evaluation of cardiac autonomic nervous system in patients with ankylosing spondylitis using 12-lead electrocardiography and Holter monitoring. Clin Rheumatol. 2020;39:2631–2639. doi: 10.1007/s10067-020-05046-y. [DOI] [PubMed] [Google Scholar]
- 8.Dalise AM, Prestano R, Fasano R, Gambardella A, Barbieri M, Rizzo MR. Autonomic nervous system and cognitive impairment in older patients: evidence from long-term heart rate variability in real-life setting. Front Aging Neurosci. 2020;12:40. doi: 10.3389/fnagi.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorey TW, Moghtadaei M, Rose RA. Altered heart rate variability in angiotensin II-mediated hypertension is associated with impaired autonomic nervous system signaling and intrinsic sinoatrial node dysfunction. Heart Rhythm. 2020;17:1360–1370. doi: 10.1016/j.hrthm.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10:a033118. doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang L, Shang J, Chen N. Perception of western musical modes: a chinese study. Front Psychol. 2017;8:1905. doi: 10.3389/fpsyg.2017.01905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furlan R, Heusser K, Minonzio M, Shiffer D, Cairo B, Tank J, Jordan J, Diedrich A, Gauger P, Zamuner AR, Dipaola F, Porta A, Barbic F. Cardiac and vascular sympathetic baroreflex control during orthostatic pre-syncope. J Clin Med. 2019;8 doi: 10.3390/jcm8091434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J, Wu M, Wu Y, Liu P. Emotional consciousness preserved in patients with disorders of consciousness. Neurol Sci. 2019;40:1409–1418. doi: 10.1007/s10072-019-03848-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiasi S, Greco A, Barbieri R, Scilingo EP, Valenza G. Assessing autonomic function from electrodermal activity and heart rate variability during cold-pressor test and emotional challenge. Sci Rep. 2020;10:5406. doi: 10.1038/s41598-020-62225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 16.Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, Kelly JP, Rosenberg JH, Whyte J, Zafonte RD, Zasler ND. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 17.Heinz G, De Angelis K, Dal Corso S, Sousa MHG, Viana A, Dos Santos F, Corrêa JCF, Corrêa FI. Effects of transcranial direct current stimulation (tDCS) and exercises treadmill on autonomic modulation of hemiparetic patients due to stroke-clinic test, controlled, randomized, double-blind. Front Neurol. 2019;10:1402. doi: 10.3389/fneur.2019.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodges GJ, Ferguson SAH, Cheung SS. Cardiac autonomic function during hypothermia and its measurement repeatability. Appl Physiol Nutr Metab. 2019;44:31–36. doi: 10.1139/apnm-2018-0248. [DOI] [PubMed] [Google Scholar]
- 19.Jang SH, Lee HD. Recovery of an injured ascending reticular activating system with recovery from a minimally conscious state to normal consciousness in a stroke patient: a diffusion tensor tractography study. Neural Regen Res. 2020;15:1767–1768. doi: 10.4103/1673-5374.276362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang SH, Kwon YH. Neuroimaging characterization of recovery of impaired consciousness in patients with disorders of consciousness. Neural Regen Res. 2019;14:1202–1207. doi: 10.4103/1673-5374.251299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondziella D, Bender A, Diserens K, van Erp W, Estraneo A, Formisano R, Laureys S, Naccache L, Ozturk S, Rohaut B, Sitt JD, Stender J, Tiainen M, Rossetti AO, Gosseries O, Chatelle C EAN Panel on Coma, Disorders of Consciousness. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020;27:741–756. doi: 10.1111/ene.14151. [DOI] [PubMed] [Google Scholar]
- 22.Kotchoubey B, Pavlov YG, Kleber B. Music in research and rehabilitation of disorders of consciousness: psychological and neurophysiological foundations. Front Psychol. 2015;6:1763. doi: 10.3389/fpsyg.2015.01763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang Z, Shao S, Lv Z, Li D, Sleigh JW, Li X, Zhang C, He J. Constructing a consciousness meter based on the combination of non-linear measurements and genetic algorithm-based support vector machine. IEEE Trans Neural Syst Rehabil Eng. 2020;28:399–408. doi: 10.1109/TNSRE.2020.2964819. [DOI] [PubMed] [Google Scholar]
- 24.Lin FV, Tao Y, Chen Q, Anthony M, Zhang Z, Tadin D, Heffner KL. Processing speed and attention training modifies autonomic flexibility: A mechanistic intervention study. Neuroimage. 2020;213:116730. doi: 10.1016/j.neuroimage.2020.116730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magee WL, Tillmann B, Perrin F, Schnakers C. Editorial: Music and disorders of consciousness: emerging research, practice and theory. Front Psychol. 2016a;7:1273. doi: 10.3389/fpsyg.2016.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magee WL, Clark I, Tamplin J, Bradt J. Music interventions for acquired brain injury. Cochrane Database Syst Rev. 2017;1:CD006787. doi: 10.1002/14651858.CD006787.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magee WL, Siegert RJ, Taylor SM, Daveson BA, Lenton-Smith G. Music Therapy Assessment Tool for Awareness in Disorders of Consciousness (MATADOC): reliability and validity of a measure to assess awareness in patients with disorders of consciousness. J Music Ther. 2016b;53:1–26. doi: 10.1093/jmt/thv017. [DOI] [PubMed] [Google Scholar]
- 28.McAleese A, Wilson CF, McEvoy M, Caldwell S. Comparison of SMART and WHIM as measurement tools in routine assessment of PDOC patients. Neuropsychol Rehabil. 2018;28:1266–1274. doi: 10.1080/09602011.2016.1264977. [DOI] [PubMed] [Google Scholar]
- 29.Mencarelli L, Biagi MC, Salvador R, Romanella S, Ruffini G, Rossi S, Santarnecchi E. Network mapping of connectivity alterations in disorder of consciousness: towards targeted neuromodulation. J Clin Med. 2020;9:828. doi: 10.3390/jcm9030828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, Baig W, Flapan AD, Cowley A, Prescott RJ, Neilson JM, Fox KA. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998;98:1510–1516. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- 31.O’Kelly J, James L, Palaniappan R, Taborin J, Fachner J, Magee WL. Neurophysiological and behavioral responses to music therapy in vegetative and minimally conscious states. Front Hum Neurosci. 2013;7:884. doi: 10.3389/fnhum.2013.00884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owen AM. The Search for Consciousness. Neuron. 2019;102:526–528. doi: 10.1016/j.neuron.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Pan J, Xie Q, Huang H, He Y, Sun Y, Yu R, Li Y. Emotion-related consciousness detection in patients with disorders of consciousness through an EEG-based BCI system. Front Hum Neurosci. 2018;12:198. doi: 10.3389/fnhum.2018.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrin F, Castro M, Tillmann B, Luauté J. Promoting the use of personally relevant stimuli for investigating patients with disorders of consciousness. Front Psychol. 2015;6:1102. doi: 10.3389/fpsyg.2015.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proverbio AM, Manfrin L, Arcari LA, De Benedetto F, Gazzola M, Guardamagna M, Lozano Nasi V, Zani A. Non-expert listeners show decreased heart rate and increased blood pressure (fear bradycardia) in response to atonal music. Front Psychol. 2015;6:1646. doi: 10.3389/fpsyg.2015.01646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riganello F, Garbarino S, Sannita WG. Heart rate variability, homeostasis, and brain function: A tutorial and review of application. J Psychophysiol. 2012;26:178–203. [Google Scholar]
- 37.Riganello F, Cortese MD, Arcuri F, Quintieri M, Dolce G. How can music influence the autonomic nervous system response in patients with severe disorder of consciousness. Front Neurosci. 2015;9:461. doi: 10.3389/fnins.2015.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riganello F, Larroque SK, Di Perri C, Prada V, Sannita WG, Laureys S. Measures of CNS-autonomic interaction and responsiveness in disorder of consciousness. Front Neurosci. 2019;13:530. doi: 10.3389/fnins.2019.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riganello F, Larroque SK, Bahri MA, Heine L, Martial C, Carrière M, Charland-Verville V, Aubinet C, Vanhaudenhuyse A, Chatelle C, Laureys S, Di Perri C. A heartbeat away from consciousness: heart rate variability entropy can discriminate disorders of consciousness and is correlated with resting-state fMRI brain connectivity of the central autonomic network. Front Neurol. 2018;9:769. doi: 10.3389/fneur.2018.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnakers C, Magee WL, Harris B. Sensory stimulation and music therapy programs for treating disorders of consciousness. Front Psychol. 2016;7:297. doi: 10.3389/fpsyg.2016.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tobaldini E, Toschi-Dias E, Trimarchi PD, Brena N, Comanducci A, Casarotto S, Montano N, Devalle G. Cardiac autonomic responses to nociceptive stimuli in patients with chronic disorders of consciousness. Clin Neurophysiol. 2018;129:1083–1089. doi: 10.1016/j.clinph.2018.01.068. [DOI] [PubMed] [Google Scholar]
- 42.Tsai SHL, Goyal A, Alvi MA, Kerezoudis P, Yolcu YU, Wahood W, Habermann EB, Burns TC, Bydon M. Hospital volume-outcome relationship in severe traumatic brain injury: stratified analysis by level of trauma center. J Neurosurg. 2020 doi: 10.3171/2020.1.JNS192115. doi:103171/20201JNS192115. [DOI] [PubMed] [Google Scholar]
- 43.Wu M, Li F, Wu Y, Zhang T, Gao J, Xu P, Luo B. Impaired frontoparietal connectivity in traumatic individuals with disorders of consciousness: a dynamic brain network analysis. Aging Dis. 2020;11:301–314. doi: 10.14336/AD.2019.0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Audio frequency of therapist's selected music (A) and preferred music (B).
The spectrogram plot shows the intensity of the audio frequency for the selected music signal as it changes over the time. The vertical axis indicates frequency, the horizontal axis indicates time, and the colored scales indicate the intensity. The color bar goes from left to right, indicating from light to deep. Magenta is the color of the highest intensity. Purple is the color of the secondary intensity. Yellow is the color of the lowest intensity. The higher the vertical axis of magenta and purple distribution, the wider the frequency range is.


