Keywords: endogenous stem cell, EphA4, ephrin-A2, ephrin-A3, ephrins, Müller cell, photoreceptor cell regeneration, retinal degeneration, retinal regeneration, retinal stem cell
Abstract
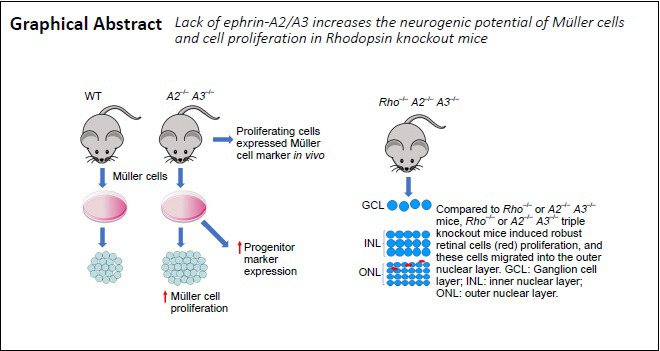
Müller cells (MC) are considered dormant retinal progenitor cells in mammals. Previous studies demonstrated ephrin-As act as negative regulators of neural progenitor cells in the retina and brain. It remains unclear whether the lack of ephrin-A2/A3 is sufficient to promote the neurogenic potential of MC. Here we investigated whether the MC is the primary retinal cell type expressing ephrin-A2/A3 and their role on the neurogenic potential of Müller cells. In this study, we showed that ephrin-A2/A3 and their receptor EphA4 were expressed in retina and especially enriched in MC. The level of ephrinAs/EphA4 expression increased as the retina matured that is correlated with the reduced proliferative and progenitor cell potential of MC. Next, we investigated the proliferation in primary MC cultures isolated from wild-type and A2–/–A3–/– mice by 5-ethynyl-2′-deoxyuridine (EdU) incorporation. We detected a significant increase of EdU+ cells in MC derived from A2–/–A3–/– mice. Next, we investigated the role of ephrin-A2/A3 in mice undergoing photoreceptor degeneration such as Rhodopsin knockout (Rho–/–) mice. To further evaluate the role of ephrin-A2/A3 in MC proliferation in vivo, EdU was injected intraperitoneally to adult wild-type, A2–/–A3–/– , Rho–/– and Rho–/–A2–/–A3–/– mice and the numbers of EdU+ cells distributed among different layers of the retina. EphrinAs/EphA4 expression was upregulated in the retina of Rho–/– mice compared to the wild-type mice. In addition, cultured MC derived from ephrin-A2–/–A3–/– mice also expressed higher levels of progenitor cell markers and exhibited higher proliferation potential than those from wild-type mice. Interestingly, we detected a significant increase of EdU+ cells in the retinas of adult ephrin-A2–/–A3–/– mice mainly in the inner nuclear layer; and these EdU+ cells were co-localized with MC marker, cellular retinaldehyde-binding protein, suggesting some proliferating cells are from MC. In Rhodopsin knockout mice (Rho–/–A2–/–A3–/– mice), a significantly greater amount of EdU+ cells were located in the ciliary body, retina and RPE than that of Rho–/– mice. Comparing between 6 and 12 weeks old Rho–/–A2–/–A3–/– mice, we recorded more EdU+ cells in the outer nuclear layer in the 12-week-old mice undergoing severe retinal degeneration. Taken together, Ephrin-A2/A3 are negative regulators of the proliferative and neurogenic potentials of MC. Absence of ephrin-A2/A3 promotes the migration of proliferating cells into the outer nuclear layer and may lead to retinal cell regeneration. All experimental procedures were approved by the Animal Care and Use Committee at Schepens Eye Research Institute, USA (approval No. S-353-0715) on October 24, 2012.
Chinese Library Classification No. R459.9; R364; R774
Introduction
Retinal degeneration is one of the leading causes of blindness and affects all ages (Pascolini and Mariotti, 2012). Retinal degeneration is a common pathological mechanism and an underlying cause of vision loss in various blinding conditions. This includes various forms of inherited retinal dystrophies, like retinitis pigmentosa, age-related macular degeneration, diabetic retinopathy, and retinal detachment (Chinskey et al., 2014).
The retina is a part of the central nervous system (CNS) located in the eye (Lamba et al., 2008). Like other CNS neurons in mammals, retinal neurons have little to no regeneration of lost neurons (Karl and Reh, 2010). Thus, loss of vision or retinal neurons in mammals is irreversible. However, in non-mammalian vertebrates, elegant endogenous repair processes in the retina could replenish the degenerated neurons very efficiently, even after a complete loss of retinal neurons (Lamba et al., 2008).
Müller cells are the major retinal glial cells. The processes of Müller cells span the entire thickness of the retina, allowing for interactions with adjacent neurons. Müller cells contribute to the maintenance of retinal homeostasis and visual function, including trophic support for retinal neurons that is important for neuronal survival. Recent studies showed that a subset of Müller cells represent neural progenitor cells (Karl and Reh, 2010; Goldman, 2014; Yu et al., 2014; Hamon et al., 2016) and express retinal progenitor cell markers such as Pax6, Ascl1, Chx10, Car2, and Dkk3, Sox2 and Pax6 (Reichenbach and Bringmann, 2013; Goldman, 2014; Enayati et al., 2020). Müller cells have been shown to maintain neural progenitor capacity and are able to regenerate the complex multi-layered retina following an injury of retinal tissue (Cameron, 2000; Fimbel et al., 2007). Studies have identified that Müller cells in the human retina possess a potential role as a neural precursor (Lawrence et al., 2007; Bhatia et al., 2009). However, Müller cells in mature mammals usually do not exhibit the neural progenitor cell capacity for regeneration of neurons. Awakening the neurogenic potential of Müller cells could be a potential strategy of repairing degenerating retina (Yu et al., 2014).
Studies from our group and others demonstrated ephrinA/EphA system is one of the endogenous modulators of neurogenesis in the adult CNS. Ephrin-A2/A3 may act broadly by suppressing neurogenesis from dormant residential neural progenitors in diverse CNS regions (Jiao et al., 2008). Disrupting the interaction between ephrin-A2 and EphA7 in the adult brain of wild-type mice allows proliferation resulting in increased neurogenesis (Holmberg et al., 2005). Similar to Müller cells, the neurogenic potential of ciliary epithelium-derived cells is negatively regulated by ephrin-A3 and mediated through activation of an EphA4 receptor via suppression of Wnt3a/β-catenin signaling (Fang et al., 2013). This has led to our hypothesis that ephrin-A2/A3 may have a broad effect on inhibiting the regenerative potential of neural progenitors, including Müller cells in the retina of adult mice, and promote the photoreceptor regeneration in animal model of retinitis pigmentosa (RP). In the present study, we investigated whether the Müller cell is the key retinal cell type expressing ephrin-A2/A3 and the role of ephrin-A2/A3 on the neurogenic potential of Müller cells in vitro and in vivo.
Materials and Methods
Animals
Adult male C57BL/6J wild-type mice, aged 8–12 weeks, were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). The mice were maintained in standard cages in a light-controlled room (12-hour light/dark cycles) at 20–24°C and 30–75% relative humidity, with access to food and water ad libitum. Ephrin-A2/A3 double knockout mice (A2–/–A3–/– mice) were generated as previously described (Jiao et al., 2008; Fang et al., 2013). Rho–/– mice (a gift from Dr. Michael Young at Schepens Eye Research Institute, this line was originally developed by Dr. Peter Humphries, Trinity College, Dublin, Ireland) were crossed with A2–/–A3–/– mice to generate Rho–/–A2–/–A3–/– triple mutant mice. Experiments were conducted with 6–12 weeks old male A2–/–A3–/– mice and Rho–/–A2–/–A3–/– mice, and at least 6 mice in each group. All experimental procedures, uses, and care of animals followed a protocol S-353-0715 approved by the Animal Care and Use Committee at Schepens Eye Research Institute, USA on October 24, 2012 and adhered to the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research. We used 36 C57BL/6 and 12 A2–/–A3–/– pups for cell culture experiments, and 10 C57BL/6, 10 Rhodopsin–/– , 6 A2–/–A3–/– and 10 Rho–/–A2–/–A3–/– adult male and female mice for in vivo experiments.
Isolation of Müller cells
Mouse eyeballs were isolated from wild-type and postnatal day 5 to 15 A2–/–A3–/– mouse pups as previously described (Takeda et al., 2008; Zhu et al., 2014). The retinas were dissected out and dissociated into single cells using the Papain dissociation system (Worthington Biochemical Corporation, Lakewood, NJ, USA) according to the manufacturer’s instructions. The dissociated cells were cultured in Dulbecco’s modified Eagles’ medium and F12 (DMEM/F12, 1:1; Life Technologies, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Life Technologies, Grand Island, NY, USA) and 1% penicillin-streptomycin (Life Technologies). The medium was changed every 3 days. After 10–14 days of culture, over 95% of culture cells expressed Müller cell marker cellular retinaldehyde-binding protein (CRALBP) (Takeda et al., 2008).
Cell proliferation assay
To study Müller cell proliferation (Takeda et al., 2008), primary Müller cells were seeded at a density of 1 × 104 cells per well in 8-well culture chambers (Thermo Scientific, Waltham, MA, USA) that were pre-coated with poly-D-lysine (0.1 mg/mL, EMD Millipore, Billerica, MA, USA). The cells were cultured in a DMEM/F12 medium containing 10% FBS and cultured for 72 hours. 5-ethynyl-2′-deoxyuridine (EdU, 10 μM; Life Technologies) was added to the culture medium for incorporation into proliferating cells. The cells were fixed in 4% paraformaldehyde for 10 minutes on ice at 24 hours post-EdU treatment. Then the EdU incorporated proliferating cells were visualized using a Click-iT® EdU Alexa Fluor® 555 Imaging Kit (Life Technologies) according to the manufacturer’s instructions. Cell nuclei were stained with nuclear markers 4,6-diamidino-2-phenylindole dihydrochloride (DAPI; Vector Laboratories, Burlingame, CA, USA). The percentage of DAPI+ cells expressing EdU was recorded. The results presented were averaged from at least three independent experiments.
Reverse transcription polymerase chain reaction
Total RNA from primary Müller cell cultures or adult retinas were isolated as previously described (Zhu et al., 2014). Briefly, total RNA from primary Muller cells or adult mouse retinas were purified using RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA) in accordance with the manufacturer’s instructions. Then the purified mRNA was reverse-transcribed into cDNA using SuperScript® III First-Strand Synthesis SuperMix (Life Technologies). Quantification of specific gene expression such as retinal progenitor markers Pax6, Chx10, Ngn2, and Sox2 was performed by Sybr green based polymerase chain reaction (PCR) as previously described (Zhu et al., 2014). The primer sequences (Fang et al., 2013) are listed in Additional Table 1. Independent samples were analyzed at least in triplicate, and the relative amount of gene expression was determined by normalizing to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) level. GAPDH was chosen as an internal control gene because it has been shown that it is one of the constant expressing genes during retinal development (Adachi et al., 2015) and using GAPDH as a control gene has been widely performed in Müller cells studies (Ueno et al., 2017; Guimarães et al., 2018).
Additional Table 1.
List of primer sequences used in real-time RT-PCR
| Gene names | Sequence (5’-3’) | Unigene # |
|---|---|---|
| Ephrin-A2 (147 bp) | F: GGC TAT ACC GTG GAG GTG R: CTG CCG GTG GTC ACA GGA | NM_007909.3 |
| Ephrin-A3 (134 bp) | F: CCG AGA AGT TCC AGC GTT ACA G R: GCA GCA GAC GAA CAC CTT CAT C | NM_010108.1 |
| EphA4 (300 bp) | F: ACC AGA TCG ACC AAA TGG AG R: AGA ATG ACC ACG AGG ACC AC | NM_007936.3 |
| Nestin (263 bp) | F: AGG ACC AGG TGC TTG AGA GA R: TTC GAG AGA TTC GAG GGA GA | NM_016701.3 |
| Sox2 (204 bp) | F: AGA ACC CCA AGA TGC ACA AC R: CTC CGG GAA GCG TGT ACT TA | NM_011443.3 |
| Ngn2 (67 bp) | F: TCA GAG CTG CTG GAG GAG AAC R: CCA GTT GCA TTC CCT CTG AGA | NM_009718.2 |
| Pax6 (206 bp) | F: AAC AAC CTG CCT ATG CAA CC R: ACT TGG ACG GGA ACT GAC AC | NM_013627.5 |
| Chx10 (228 bp) | F: TTC AAT GAA GCC CAC TAC CC R: ATC CTT GGC AGA CTT GAG GA | NM_007701.2 |
| Math5 (184 bp) | F: CAG GAC AAG AAG CTG TCC AA R: CAT AGG GCT CAG GGT CTA CCT | NM_016864.1 |
| Math1 (297 bp) | F: AGT TGC TGC AGA CTC CCA AT R: ACA GGT CCT TCT GTG CCA TC | NM_007500.4 |
| GAPDH (223 bp) | F: AAC TTT GGC ATT GTG GAA GG R: ACA CAT TGG GGG TAG GAA CA | NM_008084.3 |
F: Forward; R: reverse.
Labeling of proliferating Müller cells in vivo
Adult C57BL/6J wild-type, A2–/–A3–/– , Rho–/– and Rho–/–A2–/–A3–/– mice at the age of 6 and 12 weeks received daily intraperitoneal injections (5 mg/kg) of EdU for 7 consecutive days. The mice were sacrificed, and following the last dose of EdU injection, their eyeballs were collected immediately. EdU labeling was performed using a Click-iT® EdU Alexa Fluor® 555 Imaging Kit (Life Technologies) according to the manufacturer’s instructions. EdU+ cells on retinal sections were imaged by a Nikon epifluorescence microscope TE300 and then were counted manually with ImageJ (National Institutes of Health, Bethesda, MD, USA) on at least 12 non-consecutive retinal sections per mouse eye and 6 mouse eyeballs per group.
Immunofluorescence labeling
Immunofluorescence labeling was performed on retinal sections using a standard protocol as previously described (Fang et al., 2013). The eyeballs were collected and fixed in 4% paraformaldehyde at 4°C overnight. After washing with phosphate buffered saline (PBS), the eyeballs were cryoprotected in 30% sucrose solution, followed by embedding in optimal cutting temperature compound (Tissue-Tek). Ten μm thick frozen retinal sections were collected on glass slides. The sections were incubated in a blocking buffer (0.3% Triton-X 100, 1% bovine saline albumin (BSA) in PBS) for 1 hour at room temperature and then incubated with primary antibodies, including mouse anti-CRALBP antibody (1:500; Abcam, Cambridge, MA, USA) overnight at 4°C. Following washing in PBS, secondary antibody Cy2 conjugated anti-mouse IgG and anti-rabbit IgG (Jackson Immunoresearch Laboratories, West Grove, PA, USA) were used to visualize the signal of CRALBP and recoverin respectively. The nuclei on retina sections were counterstained with DAPI (Vector Laboratories) and mounted. Fluorescence images were captured by a Nikon epifluorescence microscope TE300.
Statistical analysis
Nonparametric unpaired t-test and one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test were used to compare the difference among groups using the software of Graphpad Prism 5 (GraphPad Software, La Jolla, CA, USA). Results are presented as the mean ± SD. and the significant level was set at P < 0.05.
Results
Enriched expression of ephrinA2/A3 and EphA4 receptor in Müller cells
We previously showed the expression of ephrin-A2, ephrin-A3 and their receptor EphA4 in adult mice retinas by immunolabeling, and EphA4 was co-localized with Müller cells (Zhu et al., 2014). We further demonstrated that the expression levels of ephrin-A3 and EphA4 are enriched in purified Müller cells by 3- and 7-fold higher, respectively, than that in the entire retina. The level of ephrin-A2 expression was similar in the retina and purified Müller cells (Figure 1A).
Figure 1.
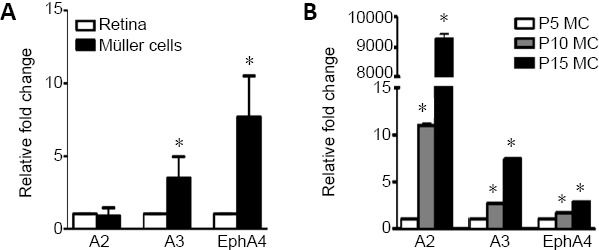
Expression of ephrinAs/EphA mRNA in the mouse retina and Müller cells.
(A) Relative expression of ephrin-A2 (A2), ephrin-A3 (A3) and their receptor EphA4 mRNA in adult retina (white bar) and purified mouse Müller cells (black bar). Note ephrin-A2/A3 and EphA4 are enriched in Müller cells. Statistical analysis was performed by unpaired t-test. *P < 0.05, vs. the corresponding mRNA expression in retina. (B) Relative expression of ephrin-A2/A3 and EphA4 mRNA in Müller cells derived from postnatal retinas and their expressions were normalized to that from postnatal day 5 (P5) Müller cells (MC). *P < 0.05, vs. the corresponding mRNA expression in P5 Müller cells. Statistical analysis was performed by one-way analysis of variance followed by Tukey’s post hoc test. Note ephrin-A2/A3 and EphA4 are highly expressed with maturation of Müller cells. n = 6 animals. Data are expressed as the mean ± SD.
We reasoned that if ephrin-A2/A3 are negative regulators of the proliferative potential of Müller cells, the levels of ephrinAs expression would increase following Müller cell maturation and cessation of cell division after birth. Retinal cell division peaks around the day of birth and ceases after 5–6 days in the center of the retina and after 11 days in the periphery (Young, 1985; Rapaport et al., 1996). Müller cells are among the last cell types to commit post-mitotic proliferation and differentiation. Once cell division ceases, there is little evidence for renewed proliferation of either progenitors nor Müller cells in mammalian retina (Close et al., 2005). To test whether expression of ephrin-A2/A3 from Müller cells correlates to the termination of cell division in retina, we isolated Müller cells from pups at postnatal days 5, 10, and 15. We detected a dramatic increase of ephrin-A2, ephrin-A3, and EphA4 expression in Müller cells from postnatal day 5 to 15 (Figure 1B). The data suggest that ephrinAs/EphA4 signals participate as negative regulators in the proliferative and neurogenic potential of Müller cells.
Absence of ephrin-As/EphA4 signaling enhances neurogenic potential and proliferation ability of Müller cells
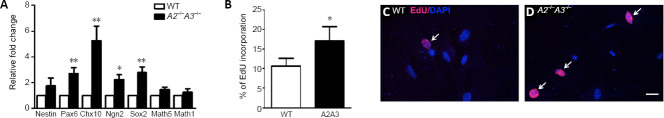
To test if ephrin-A2/A3 are essentially involved in the regulation of regenerative potential of Müller cells, we investigated the progenitor cell markers expressions and proliferation ability of Müller cells derived from ephrin-A2/A3 deficient (A2–/–A3–/–) mice. Our result showed A2–/–A3–/– deficient Müller cells significantly increased in the expression of retinal progenitor markers such as Pax6, Chx10, Ngn2, and Sox2 compared to age-matched wild-type (WT) mice (Figure 2A). Moreover, Müller cells derived from A2–/–A3–/– mice showed a significant increase in EdU incorporation, indicating increased cell proliferation (Figure 2B–D). This data support that ephrin-A2/A3 negatively regulate the neural progenitor cell fate and proliferative potential of Müller cells.
Figure 2.
Lack of ephrin-A2/A3 enhances progenitor cell potency of Müller cells in vitro.
(A) Relative expression of progenitor cell markers in Müller cells derived from wild-type (WT) and A2–/–A3–/– mice. Note significantly increased levels of Pax6, Chx10, Ngn2, and Sox2 mRNAs in Müller cells of A2–/–A3–/– mice. (B) Quantitation of 5-ethynyl-2′-deoxyuridine (EdU) incorporated Müller cell cultures derived from WT and A2–/–A3–/– mice. n = 6 animals. Statistical analysis was performed by unpaired t-test. Data are expressed as the mean ± SD. *P < 0.05, **P < 0.01, vs. the corresponding genes in wild type mice. (C, D) Representative epifluorescence photomicrographs showing the expression of EdU (pink; arrows) in the cultures of Müller cell derived from wild-type (C) and A2–/–A3–/– (D) mice. The nuclei of cultured cells were counterstained by 4,6-diamidino-2-phenylindole dihydrochloride(blue). Scale bar: 20 µm.
Absence of ephrin-A2/A3 increases Müller cell proliferation in vivo
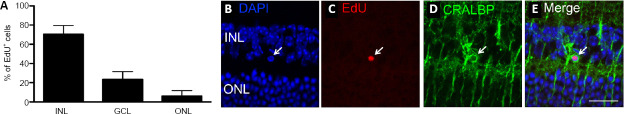
We previously showed lack of ephrin-A2/A3 increased cell proliferation in ciliary body, retina, and retinal pigment epithelium (Zhu et al., 2014). Here, by counting EdU+ cells across different retinal layers, we detected approximately 70% of EdU+ cells were located in the inner nuclear layer of A2–/–A3–/– mice (Figure 3A), where Müller cell bodies normally reside. This suggests that most proliferating cells originated from Müller cells. Only 23% of EdU+ cells were found in the ganglion cell layer, and 6% in the outer nuclear layer (Figure 3A). To investigate whether the EdU+ cells in the retina were derived from Müller cells, a single dose of EdU was injected intraperitoneally to adult A2–/–A3–/– mice. We detected co-localization of EdU and Müller cell marker CRALBP at 7 days post-EdU injection (Figure 3B–E). Taken together, our data suggest that ephrin-A2/A3 play as key inhibitory signals suppressing the proliferative potential of Müller cells in vivo.
Figure 3.
Lack of ephrin-A2/A3 promotes retinal cell proliferation in vivo.
(A) Distribution of EdU+ proliferating cells in retinal laminations of A2–/–A3–/– mice. Data are expressed as the mean ± SD. (B–E) Co-localization of EdU and Müller cell marker cellular retinaldehyde-binding protein (CRALBP, arrow). n = 6 animals. Scale bar: 20 µm. EdU: 5-Ethynyl-2′-deoxyuridine; GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer.
Absence of ephrin-A2/A3 increases proliferation of retinal cell in Rho–/– mice
Lower vertebrates, such as fish, are able to regenerate retina upon retinal injury. However, mammalian retina lacks such regenerative ability. We hypothesize that retinal injury in mice would further suppress the neurogenic and proliferation potential of Müller cells by upregulation of negative regulators, such as ephrin-As.
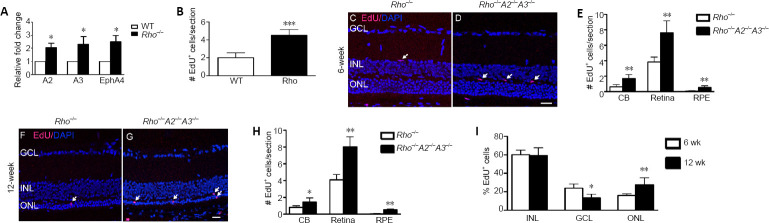
To test this hypothesis, Rhodopsin knockout (Rho–/–) mice were used as a model of retinal degeneration. Rho–/– is a well-known model of retinitis pigmentosa in which rod photoreceptors are dysfunctional and display a progressive loss of rod. Subsequently, it compromises the cone survival (Humphries et al., 1997; Hobson et al., 2000; Jaissle et al., 2001). We compared the ephrinAs/EphA expression with RT-PCR in 3 months old Rho–/– mice and WT mice. In agreement with our hypothesis, the expression of ephrin-A2/A3 and EphA4 were significantly higher in the retinas of Rho–/– mice (Figure 4A). The data suggested increased ephrin-As expression in Rho–/– mice further inhibits the neurogenic potential of Müller cells.
Figure 4.
Retinal cell proliferation in Rho–/– A2–/– A3–/– mice.
(A) Expression of ephrin-A2/A3 and EphA4 mRNA in the retina of 6 weeks old wild-type (WT) mice and Rho–/– mice. *P < 0.05, vs. wild type mice. (B) Quantification of EdU+ cells in retinas derived from 6-week-old WT and Rho–/– mice. Representative photomicrographs showing EdU+ cells (red; white arrows) on retinal sections counter stained by DAPI (blue) and quantification of EdU+ cells in ciliary body (CB), retina and retinal pigment epithelium (RPE) in 6-week-old (C–E) and 12-week-old (F–H) Rho–/– and Rho–/–A2–/–A3–/– mice. Scale bars in C: 20 µm. ***P < 0.001, vs. wild type mice in panel B; *P < 0.05, **P < 0.01, vs. Rho–/– mice in panel E and panel H. (I) Quantification of EdU+ cells in INL, GCL and ONL of 6- and 12-week-old Rho–/–A2–/–A3–/– mice. *P < 0.05, **P < 0.01, vs. 6-week-old Rho–/–A2–/–A3–/– mice. n = 6 animals. Error bar = mean ± SD. Statistical analysis was performed by unpaired t-test. DAPI: 4,6-Diamidino-2-phenylindole dihydrochloride; EdU: 5-ethynyl-2′-deoxyuridine; GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer.
To study whether the lack of ephrin-A2/A3 further promotes retinal cell proliferation in mice undergoing retinal degeneration, we injected EdU intraperitoneally into 6 week old WT, Rho–/– and Rho–/–A2–/–A3–/– mice and 12-week-old Rho–/– and Rho–/–A2–/–A3–/– mice for 7 consecutive days to label the proliferating cells in vivo. In comparing the number of proliferating cells in retinas of 6-week-old WT and Rho–/– mice, we detected more EdU+ cells in Rho–/– mice (Figure 4B). As expected, we found more EdU+ cells in ciliary body, retina, and RPE in both 6 and 12 weeks old Rho–/–A2–/–A3–/– mice than in the age-matched Rho–/– mice (Figure 4C–H). This data suggest that an increase of cell proliferation occurred in retina undergoing retinal degeneration once the negative regulators of neurogenic potency molecules, such as ephrin-A2/A3, were knocked out.
Next we asked whether these proliferating cells in Rho–/–A2–/–A3–/– mice have a tendency to migrate to the retinal layer undergoing neuronal degeneration. To compare the distribution of EdU+ cells in the retinas derived from 6 and 12 weeks old Rho–/–A2–/–A3–/– mice, the 12-week-old Rho–/–A2–/–A3–/– mice (only 1 to 2 layers of photoreceptors survived) had almost 2-fold increase of proliferating cells in the outer nuclear layer (ONL). On the contrary, approximately 50% less EdU+ cells were detected in the ganglion cell layer (GCL) in the 12-week-old mice than that of the 6-week-old Rho–/–A2–/–A3–/– mice (Figure 4I). Taken together, the data suggest that more proliferating cells were migrating into the ONL in the 12-week-old Rho–/–A2–/–A3–/– mice with severe photoreceptor degeneration.
Discussion
In the present study, we showed that ephrinAs/EphA4 was significantly enriched in Müller cells, which are thought to be a source of dormant endogenous stem-like cells in the retina (Ahmad et al., 2011; Lenkowski and Raymond, 2014). Expression of ephrin-A2/A3 and EphA4 significantly increased in Müller cell culture derived from P5, P10 and P15 pups; especially ephrin-A2. Note the expression level of ephrin-A2 is similar in the retina and Müller cells. The data suggest eprhin-A2/A3 expressions are significantly increased after maturation of the retina. It further supports the inhibitory role of ephrin-As on the neurogenic potential of Müller cells. Here, we showed that Müller cells derived from A2–/–A3–/– mice exhibit higher proliferative and neurogenic potentials than those from wild-type mice in vitro. In Rho–/–A2–/–A3–/– mice, absence of ephrin-A2/A3 promoted retinal cell proliferation. Other than Müller cells, our previous study demonstrated that lack of ephrin-A3 promoted the neurogenic potential of ciliary epithelium in mice, which is also known as a source of retinal progenitor cells (Fang et al., 2013). These results support a broad inhibitory role of ephrin-A2/A3 in the regulation of the proliferative and regenerative potentials of retinal progenitors or Müller cells that are not limited to inhibiting the neurogenic potential of neural progenitors in the CNS (Jiao et al., 2008).
Müller cells are the major glial component in retinas. Following retinal insult, Müller cells would exhibit a gliotic response (Graca et al., 2018). Interestingly, these Müller cells in zebrafish will be undergoing reprogramming and manifest neurogenic property; however, the majority of Müller cells in mammals will still be quiescent (Lahne et al., 2020). It remains unclear the determinant pathway responsible for the Müller cell fate in such scenario. Evidence shows Müller cells possess progenitor characteristics, and studies suggest it could be a novel endogenous source to regenerate retinal neurons for cell replacement therapy (Ahmad et al., 2011; Goldman, 2014). Accumulating studies using pharmacological and genetic engineering approaches indicate that Wnt/β-catenin (Osakada et al., 2007; Fang et al., 2013), sonic hedgehog (Wan et al., 2007), epidermal growth factor (EGF)/EGF receptor (EGFR) (Wan et al., 2012), glutamate (Takeda et al., 2008), and Ascl1-dependent signaling events (Fausett et al., 2008) could stimulate a small proportion of Müller cells to proliferate and express specific markers of retinal neurons in the injured mammalian retina (Goldman, 2014). Recently, the Reh group reported that a combination of Ascl1 and histone deacetylation inhibitor could induce Müller cells to proliferate and differentiate into neurons in adult mouse retina (Jorstad et al., 2017). This data suggests that mobilizing the neurogenic potential of Müller cells for cell replacement study could be possible. Nevertheless, it remains unclear whether these proliferating cells have a preference migrating into the niche of retinal layers undergoing neuronal degeneration or if the degenerating neurons would attract the cells. Here, we provided evidence that more proliferating cells are present in the retinal layer undergoing severe inherited photoreceptor degeneration. Our data showed more proliferating cells were detected in the ONL of 12 weeks old than in 6-week-old Rho–/– ephrin A2–/–A3–/– mice. It would be interesting to investigate if combinational treatment of multiple factors is efficient to mobilize more Müller cells and ultimately rescue vision.
Our previous study demonstrated that glutamate analogue α-aminoadipate stimulated Müller cells to proliferate and trans-differentiate into photoreceptors and other retinal neurons in vivo by expressing specific markers (Takeda et al., 2008). Studies of ours and others have reported that EphrinAs/EphA system are inhibitors of neurogenesis in the brain and ciliary epithelium (Gao et al., 2000; Jiao et al., 2008; Fang et al., 2013). Our results demonstrate that ephrin-A2/A3 and their receptor EphA4 are highly expressed in the adult retina and enriched in Müller cells. In agreement with the inhibitory role of ephrinAs/EphA4 in the retinal progenitors and Müller cells, expression of ephrin-A2/A3 and EphA4 parallels the cessation of both Müller cell proliferation and retinal neurogenesis during development (Close et al., 2005). In our previous study, we have demonstrated that the absence of ephrin-A2/A3 promotes the proliferation of retinal cells compared to wild-type mice (Zhu et al., 2014). Most of proliferating cells (70%) were detected in the inner nuclear layer, where Müller cell bodies reside. After 1 day EdU injection, the EdU+ cells were double labeled with Müller cell marker CRALBP, which suggests that they arise from Müller cells.
Rho–/– mouse is a commonly used mouse model of retinitis pigmentosa in which photoreceptor degeneration begins around 2 weeks after birth, and at 12 weeks old, only one to two layers of photoreceptors can be detected in the retina (Jaissle et al., 2001). RP is a heterogenous set of inherited photoreceptor degenerative diseases involving at least 60 mutated genes. We adopted Rho–/– mice in this study because mutated Rhodopsin genes accounts for 20–25% patients with dominant RP (Dryja et al., 2000). In addition, the period of photoreceptor degeneration in Rho–/– mice is longer than in other RP mice, such as rd1 and rd10 mice (Pennesi et al., 2012). Thus, it provides a longer duration to investigate the role of ephrin-A2/A3 to the neurogenic potential of Müller cells during photoreceptor degeneration.
We observed higher expression of ephrin-A2, ephrin-A3 and EphA4 in Rho–/– mice compared to that in WT mice, which is consistent with other reports, showing an increased ephrin/Eph expression after CNS injury (Willson et al., 2002; Goldshmit et al., 2006). It seems that the mammalian CNS seems to suppress the regenerative mechanisms. The underlying mechanisms or purpose remain unclear. Our data also showed that a lack of ephrin-A2/A3 allows more proliferating cells in the retinas of Rho–/–A2–/–A3–/– mice. By comparing the distribution of proliferation cells in mice undergoing various stages of retinal degeneration (6 weeks vs. 12 weeks Rho–/–A2–/–A3–/– mice), we detected proliferating cells across the retina including INL, GCL and ONL. Interestingly, we detected significantly more proliferating cells in the ONL than that in the GCL of 12 weeks old Rho–/–A2–/–A3–/– mice, which are in an advanced stage of RP. The data suggest migration of retinal progenitor toward the ONL in the mice with severe photoreceptor degeneration. As there are very few proliferating cells in the WT retina and most of which were found at the very end of the peripheral retina, we were not able to quantify their retinal layer distribution. We recently showed that delivery of non-invasive electrical stimulation (Yu et al., 2020) on the eye lid induced proliferation of Müller cells, migrating into the outer nuclear layer and expressing recovery following retinal detachment. It suggests that the EdU+ proliferating cells detected in the ONL of Rho–/–A2–/–A3–/– mice may have potential to be differentiated and express photoreceptor receptor markers. Nevertheless, the number of proliferating cells in Rho–/–A2–/–A3–/– mice were relatively small, and the present study did not compare the changes of spatial vision in Rho–/– and Rho–/–A2–/–A3–/– mice. Future study is needed to further investigate the impact of ephrin-A2/A3 on endogenous retinal cell regeneration and visual function.
In summary, a lack of ephrin-A2/A3 could increase cell proliferation in retina, likely from Müller cells, and showed increased migration into the outer nuclear layer of Rho–/–A2–/–A3–/– mice. This suggests mobilizing Müller cells could be a potential strategy for replacing degenerating neurons in retinal diseases and may even improve visual function.
Additional file:
Additional Table 1: List of primer sequences used in real-time RT-PCR.
Acknowledgments
Rho–/– mice was a gift from Dr. Michael Young at Schepens Eye Research Institute, this mouse line was originally developed by Dr. Peter Humphries, Trinity College, Dublin.
Footnotes
C-Editors: Zhao M, Li CH; T-Editor: Jia Y
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This study was supported by the grants from Lion’s Foundation Grant and BrightFocus Foundation (to KSC) and the National Natural Science Foundation of China, No. 81600727 (to RLZ).
Institutional review board statement: All experimental procedures were approved by the Animal Care and Use Committee at Schepens Eye Research Institute (approval No. S-353-0715) on October 24, 2012.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the grants from Lion’s Foundation Grant and BrightFocus Foundation (to KSC) and the National Natural Science Foundation of China, No. 81600727 (to RLZ).
References
- 1.Adachi H, Tominaga H, Maruyama Y, Yoneda K, Maruyama K, Yoshii K, Kinoshita S, Nakano M, Tashiro K. Stage-specific reference genes significant for quantitative PCR during mouse retinal development. Genes Cells. 2015;20:625–635. doi: 10.1111/gtc.12254. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad I, Del Debbio CB, Das AV, Parameswaran S. Muller glia: a promising target for therapeutic regeneration. Invest Ophthalmol Vis Sci. 2011;52:5758–5764. doi: 10.1167/iovs.11-7308. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia B, Singhal S, Lawrence JM, Khaw PT, Limb GA. Distribution of Muller stem cells within the neural retina: evidence for the existence of a ciliary margin-like zone in the adult human eye. Exp Eye Res. 2009;89:373–382. doi: 10.1016/j.exer.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Cameron DA. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis Neurosci. 2000;17:789–797. doi: 10.1017/s0952523800175121. [DOI] [PubMed] [Google Scholar]
- 5.Chinskey ND, Besirli CG, Zacks DN. Retinal cell death and current strategies in retinal neuroprotection. Curr Opin Ophthalmol. 2014;25:228–233. doi: 10.1097/ICU.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 6.Close JL, Gumuscu B, Reh TA. Retinal neurons regulate proliferation of postnatal progenitors and Muller glia in the rat retina via TGF beta signaling. Development. 2005;132:3015–3026. doi: 10.1242/dev.01882. [DOI] [PubMed] [Google Scholar]
- 7.Dryja TP, McEvoy JA, McGee TL, Berson EL. Novel rhodopsin mutations Gly114Val and Gln184Pro in dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2000;41:3124–3127. [PubMed] [Google Scholar]
- 8.Enayati S, Chang K, Achour H, Cho KS, Xu F, Guo S, K ZE, Xie J, Zhao E, Turunen T, Sehic A, Lu L, Utheim TP, Chen DF. Electrical stimulation induces retinal müller cell proliferation and their progenitor cell potential. Cells. 2020;9:781. doi: 10.3390/cells9030781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang Y, Cho KS, Tchedre K, Lee SW, Guo C, Kinouchi H, Fried S, Sun X, Chen DF. Ephrin-A3 suppresses Wnt signaling to control retinal stem cell potency. Stem Cells. 2013;31:349–359. doi: 10.1002/stem.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28:1109–1117. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao PP, Sun CH, Zhou XF, DiCicco-Bloom E, Zhou R. Ephrins stimulate or inhibit neurite outgrowth and survival as a function of neuronal cell type. J Neurosci Res. 2000;60:427–436. doi: 10.1002/(SICI)1097-4547(20000515)60:4<427::AID-JNR1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 13.Goldman D. Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431–442. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldshmit Y, McLenachan S, Turnley A. Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res Rev. 2006;52:327–345. doi: 10.1016/j.brainresrev.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Graca AB, Hippert C, Pearson RA. Müller glia reactivity and development of gliosis in response to pathological conditions. Adv Exp Med Biol. 2018;1074:303–308. doi: 10.1007/978-3-319-75402-4_37. [DOI] [PubMed] [Google Scholar]
- 16.Guimarães RPM, Landeira BS, Coelho DM, Golbert DCF, Silveira MS, Linden R, de Melo Reis RA, Costa MR. Evidence of Müller glia conversion into retina ganglion cells using neurogenin2. Front Cell Neurosci. 2018;12:410. doi: 10.3389/fncel.2018.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamon A, Roger JE, Yang XJ, Perron M. Müller glial cell-dependent regeneration of the neural retina: An overview across vertebrate model systems. Dev Dyn. 2016;245:727–738. doi: 10.1002/dvdy.24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobson AH, Donovan M, Humphries MM, Tuohy G, McNally N, Carmody R, Cotter T, Farrar GJ, Kenna PF, Humphries P. Apoptotic photoreceptor death in the rhodopsin knockout mouse in the presence and absence of c-fos. Exp Eye Res. 2000;71:247–254. doi: 10.1006/exer.2000.0878. [DOI] [PubMed] [Google Scholar]
- 19.Holmberg J, Armulik A, Senti KA, Edoff K, Spalding K, Momma S, Cassidy R, Flanagan JG, Frisen J. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19:462–471. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- 21.Jaissle GB, May CA, Reinhard J, Kohler K, Fauser S, Lutjen-Drecoll E, Zrenner E, Seeliger MW. Evaluation of the rhodopsin knockout mouse as a model of pure cone function. Invest Ophthalmol Vis Sci. 2001;42:506–513. [PubMed] [Google Scholar]
- 22.Jiao JW, Feldheim DA, Chen DF. Ephrins as negative regulators of adult neurogenesis in diverse regions of the central nervous system. Proc Natl Acad Sci U S A. 2008;105:8778–8783. doi: 10.1073/pnas.0708861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorstad NL, Wilken MS, Grimes WN, Wohl SG, VandenBosch LS, Yoshimatsu T, Wong RO, Rieke F, Reh TA. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature. 2017;548:103–107. doi: 10.1038/nature23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karl MO, Reh TA. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med. 2010;16:193–202. doi: 10.1016/j.molmed.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahne M, Nagashima M, Hyde DR, Hitchcock PF. Reprogramming Müller glia to regenerate retinal neurons. Annu Rev Vis Sci. 2020;6:171–193. doi: 10.1146/annurev-vision-121219-081808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamba D, Karl M, Reh T. Neural regeneration and cell replacement: a view from the eye. Cell Stem Cell. 2008;2:538–549. doi: 10.1016/j.stem.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence JM, Singhal S, Bhatia B, Keegan DJ, Reh TA, Luthert PJ, Khaw PT, Limb GA. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem cells. 2007;25:2033–2043. doi: 10.1634/stemcells.2006-0724. [DOI] [PubMed] [Google Scholar]
- 28.Lenkowski JR, Raymond PA. Muller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res. 2014;40:94–123. doi: 10.1016/j.preteyeres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 31.Pennesi ME, Michaels KV, Magee SS, Maricle A, Davin SP, Garg AK, Gale MJ, Tu DC, Wen Y, Erker LR, Francis PJ. Long-term characterization of retinal degeneration in rd1 and rd10 mice using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:4644–4656. doi: 10.1167/iovs.12-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rapaport DH, Rakic P, LaVail MM. Spatiotemporal gradients of cell genesis in the primate retina. Perspect Dev Neurobiol. 1996;3:147–159. [PubMed] [Google Scholar]
- 33.Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61:651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- 34.Takeda M, Takamiya A, Jiao JW, Cho KS, Trevino SG, Matsuda T, Chen DF. alpha-Aminoadipate induces progenitor cell properties of Müller glia in adult mice. Invest Ophthalmol Vis Sci. 2008;49:1142–1150. doi: 10.1167/iovs.07-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueno K, Iwagawa T, Ochiai G, Koso H, Nakauchi H, Nagasaki M, Suzuki Y, Watanabe S. Analysis of Müller glia specific genes and their histone modification using Hes1-promoter driven EGFP expressing mouse. Sci Rep. 2017;7:3578. doi: 10.1038/s41598-017-03874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22:334–347. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan J, Zheng H, Xiao HL, She ZJ, Zhou GM. Sonic hedgehog promotes stem-cell potential of Muller glia in the mammalian retina. Biochem Biophys Res Commun. 2007;363:347–354. doi: 10.1016/j.bbrc.2007.08.178. [DOI] [PubMed] [Google Scholar]
- 38.Willson CA, Irizarry-Ramirez M, Gaskins HE, Cruz-Orengo L, Figueroa JD, Whittemore SR, Miranda JD. Upregulation of EphA receptor expression in the injured adult rat spinal cord. Cell Transplant. 2002;11:229–239. [PubMed] [Google Scholar]
- 39.Wong IY, Poon MW, Pang RT, Lian Q, Wong D. Promises of stem cell therapy for retinal degenerative diseases. Graefes Arch Clin Exp Ophthalmol. 2011;249:1439–1448. doi: 10.1007/s00417-011-1764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 41.Yu H, Vu TH, Cho KS, Guo C, Chen DF. Mobilizing endogenous stem cells for retinal repair. Transl Res. 2014;163:387–398. doi: 10.1016/j.trsl.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu H, Enayati S, Chang K, Cho KS, Lee SW, Talib M, Zihlavnikova K, Xie J, Achour H, Fried SI, Utheim TP, Chen DF. Noninvasive electrical stimulation improves retinal function and induces neural regeneration in mice with inherited photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2020 doi: 10.1167/iovs.61.4.5. doi:101167/iovs6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu R, Cho KS, Chen DF, Yang L. Ephrin-A2 and -A3 are negative regulators of the regenerative potential of Müller cells. Chin Med J (Engl) 2014;127:3438–3442. [PubMed] [Google Scholar]