Keywords: balance, gait, motor, neurodegeneration, Parkinson's disease, posture, rehabilitation, tremor
Abstract
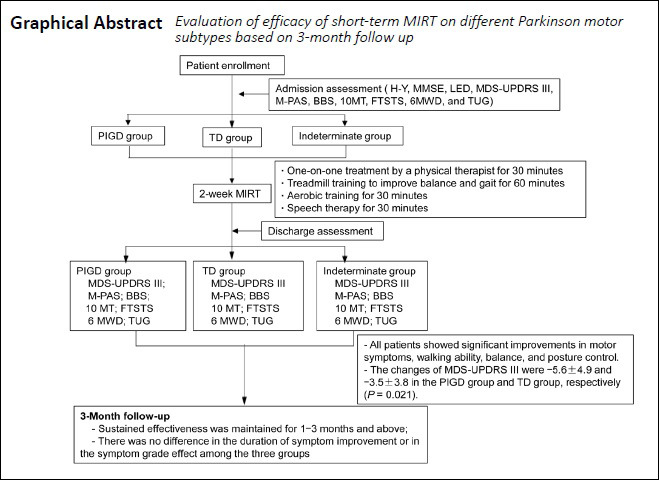
Parkinson’s disease (PD) can be classified into three motor-based subtypes: postural instability/gait difficulty (PIGD), tremor dominant (TD), and indeterminate. The neuropathophysiological mechanisms of the three motor subtypes are different, which may lead to different responses to therapy. Sixty-nine patients with idiopathic Parkinson’s disease (Hoehn–Yahr stage ≤ 3) were screened from 436 patients with Parkinsonism recruited through outpatient services and the internet. According to the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) TD/PIGD ratio, the patients were divided into PIGD (TD/PIGD ≤ 0.09; n = 36), TD (TD/PIGD ≥1.15; n = 19), and indeterminate (TD/PIGD = 0.90–1.15; n = 14) groups. All patients received 2 weeks of multidisciplinary intensive rehabilitation treatment (MIRT) during hospitalization, as well as a remote home rehabilitation health education class. Compared with the scores at admission, all patients showed significant improvements in their MDS-UPDRS III score, walking ability, balance, and posture control at discharge. Moreover, the MDS-UPDRS III score improvement was greater in the PIGD group than in the TD group. The follow-up data, collected for 3 months after discharge, showed that overall symptom improvement in each group was maintained for 1–3 months. Furthermore, there were no significant differences in the duration or grade effects of symptom improvement among the three groups. These findings suggest that 2 weeks of MIRT is effective for improving motor performance in all three motor subtypes. Patients in the PIGD group had a better response after hospitalization than those in the TD group. This study was approved by the Institutional Ethics Committee of Beijing Rehabilitation Hospital of Capital Medical University of China (approval No. 2018bkky022) on May 7, 2018 and registered with the Chinese Clinical Trial Registry (registration No. ChiCTR1900020771) on January 19, 2019.
Chinese Library Classification No. R455; R493; R742
Introduction
Parkinson’s disease (PD) is a neurodegenerative, chronic, and progressive disease (Dorsey and Bloem, 2018; Gao et al., 2019; Dani et al., 2020; Goulding et al., 2020). It is also the fastest growing neurological disorder. A previous study reported that the number of people with PD is expected to increase from 6 million in 2015 to 12 million in 2040 (Dorsey et al., 2018). PD is characterized by resting tremor, rigidity, bradykinesia, and postural instability (Bari et al., 2020). Based on these symptoms, PD is commonly divided into the postural instability/gait difficulty (PIGD), tremor dominant (TD), and indeterminate subtypes, according to the DATATOP study (Jankovic et al., 1990) and the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) TD/PIGD ratio (Stebbins et al., 2013). From a treatment perspective, the neuropathophysiological mechanisms are different in the three motor subtypes, which may result in different responses to therapy (Thenganatt and Jankovic, 2014; Vervoort et al., 2015, 2016). Previous studies have reported that, compared with the TD subtype, the PIGD subtype is related to greater risk of cognitive impairment, more rapid disease progression, and poorer response to dopaminergic treatment and deep brain stimulation (DBS) (Alves et al., 2006; Rajput et al., 2009; Katz et al., 2015; Arie et al., 2017). This suggests that an effective treatment for the PIGD subtype is urgently needed. There is growing evidence that rehabilitation, as an effective and complementary treatment of PD, plays an important role in PD treatment (Bloem et al., 2015; Mak et al., 2017; Fox et al., 2018) by improving both motor symptoms (MS) and non-motor symptoms (NMS) in people with PD. However, because of the diversity of rehabilitation programs and the methodological shortcomings of previous studies, there are currently no optimal rehabilitation programs for PD. Multidisciplinary intensive rehabilitation treatment (MIRT) is a multidisciplinary, aerobic, intensive, and goal-oriented rehabilitation program specifically designed for people with PD (Frazzitta et al., 2012, 2015). Its short- and long-term effects on MS and NMS in people with PD have been demonstrated in numerous studies (Trend et al., 2002; Frazzitta et al., 2012, 2015, 2018). Although MIRT has beneficial effects on PD symptoms, both the type of patients that respond best to MIRT and the optimal initial intervention time remain unknown (Radder et al., 2018). It has been noted that PD is characterized by large phenotypic heterogeneity, but it remains unclear whether specific clinical subtypes differ in their responses to rehabilitation interventions (Abbruzzese et al., 2016). To treat PD more effectively, further studies into the responses of different PD motor subtypes to treatment should be conducted (Chase, 2015). In clinical practice, we have observed that patients with the PIGD subtype seem to make better progress after MIRT. However, only one study has investigated whether there are differences in the effects of MIRT between different PD motor subtypes. After a 4-week MIRT program, Frazzitta et al. (2013a) reported that MIRT was effective in improving the motor performance of patients in the TD and PIGD groups; the improvement was the same in the two groups. However, few studies have addressed the long-term effects of MIRT in these two subtypes. Based on previous studies, we included patients with early- and medium-stage PD in this study, and used an allocation method that was more detailed than in previous studies. In addition, we performed a 3-month follow-up to investigate whether there were any differences among the three groups in the long-term effects of MIRT on symptoms.
The aim of this study was to investigate whether the PIGD subtype benefited more from the 2-week MIRT program than the other two motor subtypes, in both the short- and long-term, to provide a more effective and personalized rehabilitation program for PD.
Subjects and Methods
Patients
This prospective cohort study is part of an ongoing project (Active and passive biofeedback and neuromodulation collaborative therapy system evaluation and clinical validation, ChiCTR1900020771, registered on January 19, 2019). This project investigated the effectiveness of traditional rehabilitation methods on different subtypes of PD, and was written as per the requirements of the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement. Briefly, we screened 436 parkinsonism patients between February 2019 and April 2019 from outpatient services and the internet. Patients who met the inclusion criteria and were willing to participate in the project were included. Standardized examinations were conducted by trained members of the study group, and 69 eligible patients were finally included in this study. According to the subtyping methods proposed by Stebbins et al. (2013), 69 patients were divided into the PIGD group (TD/PIGD ratio ≤ 0.09), the TD group (TD/PIGD ratio ≥ 1.15) and the indeterminate group(0.9 < TD/PIGD ratio < 1.15).
Inclusion criteria
(1) Idiopathic PD diagnosed by a neurologist according to the Movement Disorder Society criteria (Postuma et al., 2015), with Hoehn–Yahr (H-Y) stage ≤ 3; (2) stable vital signs, with no serious cardiopulmonary disease or osteoarthropathy; (3) stable medication, with no drug adjustment within 3 months; (4) if patients had other diseases, they needed no special treatment during hospitalization; (5) no DBS or in vivo implantation treatment; and (6) were able to understand each item of the informed consent, were willing to sign the informed consent, and promised to complete the assessments and treatments.
Exclusion criteria
(1) Patients with fractures or psychotic symptoms; and (2) patients with Clinical Dementia Rating (CDR) (Morris, 1993) scores > 0.5, or with vision or hearing impairments, who were unable to complete the rehabilitation protocol.
This study was approved by the Institutional Ethics Committee of Beijing Rehabilitation Hospital of Capital Medical University of China (approval No. 2018bkky022) on May 7, 2018. It was performed in accordance with the Declaration of Helsinki. All patients signed their informed consent before inclusion.
Flow chart of the study is shown in Figure 1.
Figure 1.
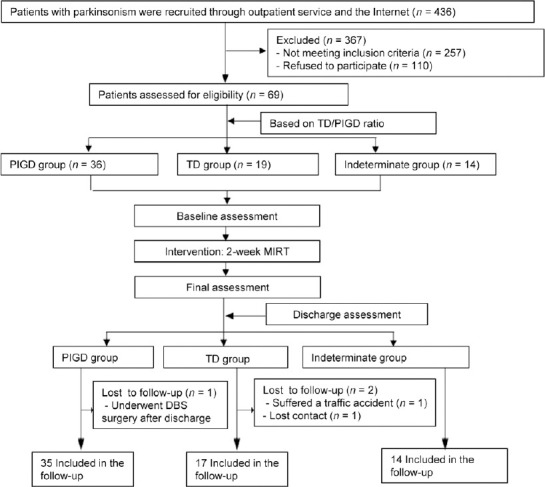
Flow chart of the study.
MIRT: Multidisciplinary intensive rehabilitation treatment; PIGD: postural instability/gait difficulty; TD: tremor dominant.
MIRT procedure
All patients underwent a 2-week MIRT program, which aimed to combine motor relearning with external and internal cue strategies (Frazzitta et al., 2012, 2015). The 2-week program was conducted in a hospital setting, 5 days per week, and was composed of four daily rehabilitation sessions. The duration of each session was 30–60 minutes.
The first session included one-on-one treatment with a physical therapist for 30 minutes. It included warm-up activities, followed by active and passive exercises to improve stretching of the abdominal muscles, enhancement of the paraspinal muscles, adjustment of posture, and control of balance and posture.
The second session used C-MiLL (Motek, Amsterdam/Culemborg, Netherlands) and Balance Tutor (Meditouch, Netanya, Israel) to improve balance and gait: the treadmill training had auditory cues, visual cues, and an anti-interference platform with feedback. Patients were trained for 30 minutes, twice per day: once in the morning and once in the afternoon.
The third session was aerobic training. Patients performed a 30-minute aerobic training on an upper and lower limb trainer (T5XR; Nustep, Ann Arbor, MI, USA).
The fourth session included speech therapy for half an hour. In this session, we proposed three possible kinds of interventions:
-
(1)
counselling for the management of swallowing and language problems for patients and pertinent caregivers;
-
(2)
individual swallowing training to correctly ingest foods and liquids, and meal monitoring;
-
(3)
speech therapy to treat hypokinetic dysarthria (facial exercises to improve mouth motion and facial expressions; breathing exercises to alleviate the pressure of speech; and exercises to improve vocalization, articulation, and speech prosody).
During all training sessions, each patient’s heart rate was kept at 70–80% of the maximum rate.
When discharged, a specialized rehabilitation therapist introduced the PD management platform developed by our team to the patients, and showed them how to use it to ensure that patients could independently use the platform for home-based rehabilitation training after discharge. To motivate and supervise patients for home-based rehabilitation training, patients were required to sign in after completing their daily exercises.
Data acquisition and assessment
The primary outcome of this study was the MDS-UPDRS III score. The secondary outcomes were the Modified Parkinson Activity Scale (M-PAS), 10-Meter Walk (10MT), 6-Minute Walk Distance (6MWD), Berg Balance Scale (BBS), Timed Get Up and Go (TUG), and Five Times Sit to Stand (FTSTS) results. All patients were evaluated by the same physiotherapist and neurologist at admission and discharge. The pre-treatment assessment was completed within 1–2 days after each patient was included in the study, and the post-treatment assessment was carried out within 1–2 days after the MIRT. All assessments were made in the ON state (1–2 hours after medication).
Motor function assessment
MDS-UPDRS III
The third part of the MDS-UPDRS assesses motor function. It contains 18 items; each item is rated from 0 to 4 scores, as follows: 0: normal; 1: slight (symptoms with sufficiently low frequency or intensity as to cause no impact on function); 2: mild (symptoms of sufficient frequency or intensity to cause a modest impact on function); 3: moderate (symptoms sufficiently frequent or intense as to have a considerable impact, but not prevent function); and 4: severe (symptoms that prevent function) (Goetz et al., 2008). Higher scores indicate more severe MS. According to the MS that are observed in PD, MDS-UPDRS III scores can be subdivided into the rigidity, tremor, axial, and bradykinesia subscales (Li et al., 2018).
M-PAS
The M-PAS is designed to assess PD patients’ activities of daily living. It consists of 14 items and can be divided into three domains: gait akinesia (6 items), chair transfer (2 items), and bed mobility (6 items) (Keus et al., 2009). M-PAS scores range from 0 to 56 (higher values indicate better performance).
Walking ability
The 10MT is used to assess walking speed, including comfortable and fast gait speeds (Lang et al., 2016). The 6MWD gauges the distance a patient can walk quickly on a hard, flat surface in 6 minutes (Laboratories, 2002).
Balance and posture control
The BBS is used to assess balance control (Berg et al., 1995). BBS scores range from 0 to 56 (higher values indicate better balance), as follows: 0–20: poor balance, needs wheelchair to move around; 21–40: fair balance, can walk with assistance; 41–56: good balance, can walk independently. The TUG is a measure of functional mobility, and indirectly reflects dynamic balance (Podsiadlo and Richardson, 1991; Morris et al., 2001; Opara et al., 2017). The FTSTS is mostly related to balance and bradykinesia in PD (Duncan et al., 2011).
Assessment of long-term effects at the 3-month follow-up
Three months after MIRT, a professional staff member sent questionnaires to the patients. This staff member also provided instructions on how to fill out and submit the questionnaire, to ensure the quality of questionnaire answers, reduce the difficulty of the task, and increase patient compliance. We contacted any patients who failed to submit the questionnaire within 2 days, and we conducted a phone interview if there was still no response. The long-term effects of MIRT were evaluated using patient-reported outcome measures, which consisted of two parts: the duration of the rehabilitation effect and the overall effect grading questionnaire.
The options when reporting the duration of the rehabilitation effects were as follows: A. The duration of the effects lasted less than 1 month; B. The duration of the effects lasted 1 month; C. The duration of the effects lasted 2 months; D. The duration of the effects lasted 3 months or more. When filling out the questionnaire, patients could only choose one answer.
The contents of the questionnaire were graded according to the overall effects of the treatment, as follows: 0: the improvement was less than 25%, or there was no improvement or worsening; 1: the improvement was between 26% and 50%; 2: the improvement was between 51% and 75%; 3: the improvement was between 76% and 95%; and 4: the improvement was more than 95% (Czarnecki et al., 2012).
Statistical analysis
Normally distributed data are expressed as the mean ± SD, while non-normal data are reported as the median (interquartile range). Data normality was verified using the Shapiro–Wilk test. The Wilcoxon signed-rank test was used to compare axial subscores, bradykinesia subscores, tremor subscores, rigidity subscores, M-PAS, BBS, FTSTS, 6MWD, and TUG, before and after the intervention. Paired samples t-tests were used to analyze MDS-UPDRS III scores, 10MT-comfortable gait speed, and 10MT-fast gait speed, before and after the intervention. Between-group comparisons for H-Y stage, disease duration, age, Mini-Mental State Examination (MMSE) score, axial subscores, bradykinesia subscores, tremor subscores, rigidity subscores, M-PAS, BBS, FTSTS, 6MWD, and TUG were assessed using the Kruskal–Wallis test, while mean L-dopa equivalent dose (LED), MDS-UPDRS III, 10MT-comfortable gait speed, and 10MT-fast gait speed were assessed using the one-way analysis of variance at baseline. The sex ratio was compared using the chi-squared test, while the proportion of fluctuating patients was analyzed using Fisher’s exact test. To correct the baseline imbalance factor (for H-Y stage, axial subscores, and tremor subscores), multiple linear regression was used. The model met the six conditions of multiple linear regression. The formula was as follows: ΔY = a + X1 + X2 + X3 + X4 + Y baseline data, in which a is a constant term, X1 is H-Y stage, X2 is axial subscores, X3 is tremor subscores, and X4 is the group (the dummy variable was set with the PIGD group as the reference). Ordinal logistic regression was used to analyze the grade effect of efficacy between the three groups. Missing data were imputed with the median of the indicator in each group. There was statistical significance if P < 0.05. All analyses were performed using SPSS Statistics version 25.0 software (IBM, Armonk, NY, USA).
Results
Characteristics of the PD patients in each group
After 2 weeks of MIRT, all patients (n = 69; 36 in the PIGD group, 19 in the TD group, and 14 in the indeterminate group) took part in the short-term assessment, within 1–2 days after the MIRT. Three patients were lost to follow-up: one in the PIGD group (the patient underwent DBS surgery after discharge) and two in the TD group (one suffered a traffic accident, and the other lost contact). Sixty-six patients (35 in the PIGD group, 17 in the TD group, and 14 in the indeterminate group) participated in the 3-month follow-up.
Of the 69 total subjects, there were 34 males and 35 females. The mean age was 60.6 ± 7.0 years (range 37–71 years), and the duration of PD was 6.0 ± 3.2 years (range 2–19 years). The MMSE scores ranged from 17 to 30, with a mean of 27.0 ± 2.7. The H-Y stage ranged from 1.5 to 3, with a mean of 2.5 ± 0.5. The patients were divided into the PIGD, TD, and indeterminate groups according to their TD/PIGD ratio. The results are shown in Table 1. There were no differences in age, sex ratio, disease duration, MMSE, mean LED, proportion of fluctuating patients, MDS-UPDRS III, bradykinesia subscores, rigidity subscores, M-PAS, BBS, 10MT-comfortable gait speed, 10MT-fast gait speed, FTSTS, 6MWD, or TUG among the three groups at baseline (P > 0.05). However, there were significant differences in H-Y stage, axial subscores, and tremor subscores between the three groups at baseline (P < 0.05). The results of pairwise comparisons revealed significant differences in H-Y stage (P = 0.001), axial subscores (P < 0.01), and tremor subscores (P < 0.01) between the PIGD and TD groups. Furthermore, tremor subscores were significantly different between the PIGD and indeterminate groups (P < 0.01), and axial subscores were significantly different between the TD and indeterminate groups (P = 0.02). There were no significant differences in H-Y stage (P = 0.05) or tremor subscores (P = 0.098) between the TD and indeterminate groups. There were also no significant differences in H-Y stage (P = 0.334) or axial subscores (P = 0.271) between the PIGD and indeterminate groups.
Table 1.
Demographic information and characteristics of the three groups
| Items | PIGD group (n = 36) | TH group (n = 19) | Indeterminate group (n = 14) | P-value |
|---|---|---|---|---|
| Gendera [male, n(%)] | 18(50.0) | 8(42.1) | 8(57.1) | 0.689* |
| H-Y stageb | 3.0(1.0) | 2.0(1.0) | 2.5 (1.0) | 0.003‡ |
| Disease durationb (yr) | 6.5(5.0) | 5.0(4.0) | 4.5 (4.0) | 0.443‡ |
| Ageb (yr) | 62.5(11.0) | 60.0(10.0) | 63.0 (6.0) | 0.120‡ |
| MMSEb | 28(2) | 27(6) | 28(3) | 0.857‡ |
| Mean L-dopa equivalent dose (LED)c (mg) | 613.9±279.7 | 445.4±199.3 | 560.8±221.9 | 0.077† |
| Motor fluctuationa | 7(19.4) | 1(5.3) | 1(7.1) | 0.317§ |
| MDS-UPDRS IIIc (scores) | 29.1±11.2 | 28.1±10.7 | 30.1±10.0 | 0.861† |
| Axial subscoresb | 5(3) | 3(2) | 5(1) | 0.002‡ |
| Bradykinesia subscoresb | 13.5(9) | 12(9) | 11.5(6) | 0.358‡ |
| Tremor subscoresb | 0(3) | 7(6) | 5(5) | < 0.001‡ |
| Rigidity subscoresb | 7(6) | 6(3) | 5(5) | 0.248‡ |
| M-PASb | 50(9) | 54(5) | 52(10) | 0.104‡ |
| BBSb | 53(4) | 55(3) | 55(2) | 0.061‡ |
| 10MT-Comfortable gait speedc (m/s) | 1.09±0.17 | 1.19±0.17 | 1.13±0.14 | 0.122† |
| 10MT-Fast gait speedc (m/s) | 1.44±0.23 | 1.48±0.22 | 1.52±0.22 | 0.550† |
| FTSTSb (s) | 11.2(3.4) | 10.6(3.2) | 9.0(2.2) | 0.059‡ |
| 6MWDb (m) | 463(111) | 466(137) | 473.5(139) | 0.739‡ |
| TUGb (s) | 9.9(2.3) | 9.3(2.6) | 9.2(3.2) | 0.372‡ |
aCount data are expressed as n(%), bnon-normal data are reported as the median (interquartile range), and cnormally distributed data are expressed as the mean ± SD. *P: Chi-squared test; ‡P: Kruskal-Wallis test; §P: Fisher’s exact test; †P: one-way analysis of variance, df = 2. 10MT: 10-Meter Walk; 6MWD: 6-Minute Walk Distance; BBS: Berg Balance Scale; FTSTS: Five Times Sit to Stand; H-Y: Hoehn–Yahr stage; LED: L-dopa equivalent dose; MDS-UPDRS III: Movement Disorder Society-Unified Parkinson’s Disease Rating Scales III; MMSE: Mini-Mental State Examination; M-PAS: Modified Parkinson Activity Scale; n: number of patients; PIGD: postural instability/gait difficulty; SD: standard deviation; TD: tremor dominant; TUG: Timed Get Up and Go.
Motor function outcomes after MIRT
After 2 weeks of MIRT, patients with PD had significant improvements in MDS-UPDRS III, M-PAS, BBS, 10MT-comfortable gait speed, 10MT-fast gait speed, FTSTS, 6MWD, and TUG (P < 0.01; Table 2). When comparing the MDS-UPDRS III subgroup scores, all MS, including tremor, rigidity, bradykinesia, and axial symptoms, improved significantly in the patients with PD (all P < 0.01; Figure 2).
Table 2.
Motor function assessments of all patients pre- and post-MIRT
| Items | Pre-MIRT (n = 69) | Post-MIRT (n = 69) | P-value |
|---|---|---|---|
| MDS-UPDRS IIIa (scores) | 29.0±10.7 | 24.3±10.4 | < 0.001 |
| M-PASb | 52(9) | 54(6) | < 0.001 |
| BBSb | 54(4) | 55(3) | < 0.001 |
| 10MT-Comfortable gait speeda (m/s) | 1.13±0.17 | 1.24±1.89 | < 0.001 |
| 10MT-Fast gait speeda (m/s) | 1.47±0.22 | 1.59±0.23 | < 0.001 |
| FTSTSb (s) | 10.6(3.5) | 9.1(3.1) | < 0.001 |
| 6MWDb (m) | 465(117) | 510(98) | < 0.001 |
| TUGb (s) | 9.6(2.5) | 8.5(1.8) | < 0.001 |
aNormally distributed data are expressed as the mean ± SD, while bnon-normal data are reported as the median (interquartile range) (paired samples t-test or Wilcoxon signed-rank test). 10MT: 10-Meter Walk; 6MWD: 6-Minute Walk Distance; BBS: Berg Balance Scale; FTSTS: Five Times Sit to Stand; MDS-UPDRS III: Movement Disorder Society-Unified Parkinson’s Disease Rating Scales III; MIRT: multidisciplinary intensive rehabilitation treatment; M-PAS: Modified Parkinson Activity Scale; n: number of patients; TUG: Timed Get Up and Go.
Figure 2.
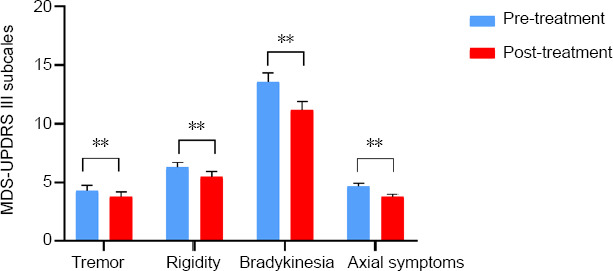
MDS-UPDRS III subscale changes between pre- and post-MIRT.
The MDS-UPDRS III subscales for tremor, bradykinesia, rigidity, and axial symptoms were evaluated. After 2 weeks of MIRT, the scores of all the subscales were significantly reduced. Data are reported as the mean ± standard deviation (n = 69; Wilcoxon signed-rank test). **P < 0.01. MDS-UPDRS III: Movement Disorder Society-Unified Parkinson’s Disease Rating Scales III; MIRT: multidisciplinary intensive rehabilitation treatment.
After 2 weeks of MIRT, MDS-UPDRS III, M-PAS, 10MT-comfortable gait speed, 10MT-fast gait speed, 6MWD, BBS, FTSTS, and TUG improved significantly in all three groups (P < 0.01 or P < 0.05), except for M-PAS in the TD group (P = 0.068; Table 3). After 2 weeks of MIRT, both axial and bradykinesia subscores were significantly decreased among the three groups (P < 0.05; Table 3). After 2 weeks of MIRT, there was a significant change in the tremor subscores of the indeterminate group only (P = 0.007). In addition, after 2 weeks of MIRT, rigidity subscores decreased significantly in the PIGD group (P = 0.003). There were no significant changes in rigidity subscores in the TD and indeterminate groups (P = 0.063 and P = 0.131, respectively; Table 3). After adjusting for baseline imbalanced factors (using multiple linear regression), the decrease in MDS-UPDRS III scores in the PIGD group was significantly greater than in the TD group (P = 0.021; Table 4), suggesting that MIRT is more effective at improving MS in the PIGD subtype (the changes in MDS-UPDRS III were –5.6 ± 4.9 and –3.5 ± 3.8 in the PIGD and TD groups, respectively). There were no significant differences in the score changes of any other scales between the groups (P > 0.05).
Table 3.
Changes in motor function among the three groups after MIRT
| Items | PIGD group (n = 36) | TH group (n = 19) | Indeterminate group (n = 14) | |||
|---|---|---|---|---|---|---|
| Pre-MIRT | Post-MIRT | Pre-MIRT | Post-MIRT | Pre-MIRT | Post-MIRT | |
| MDS-UPDRS IIIa | 29.1±11.2 | 23.5±11.7** | 28.1±10.7 | 24.6±9.3** | 30.1±10.0 | 25.8±8.8** |
| Axial subscoresb (scores) | 5(3) | 4(2)** | 3(2) | 3(2)** | 5(1) | 4(1)* |
| Bradykinesia subscoresb | 13.5(9) | 12(8)** | 12(9) | 10(7)** | 12(6) | 10(8)** |
| Tremor subscoresb | 0(3) | 0(3) | 7(6) | 7(3) | 5(5) | 4(3)** |
| Rigidity subscoresb | 7(6) | 6(6)** | 6(3) | 5(5) | 5(5) | 5(4) |
| M-PASb | 50(9) | 53(8)** | 54(5) | 56(3) | 52(10) | 54(5)* |
| BBSb | 53(4) | 54(4)** | 55(3) | 55(4)** | 55(2) | 56(3)* |
| 10MT-Comfortable gait speeda (m/s) | 1.09±0.17 | 1.21±0.21** | 1.19±0.17 | 1.30±0.16** | 1.13±0.14 | 1.25±0.15** |
| 10MT-Fast gait speeda (m/s) | 1.44±0.23 | 1.54±0.23** | 1.48±0.22 | 1.64±0.24** | 1.52±0.22 | 1.65±0.22** |
| FTSTSb (s) | 11.2(3.4) | 10.0(3.5)** | 10.6(3.2) | 9.1(3.5)** | 9.0(2.2) | 7.7(1.6)** |
| 6MWDb (m) | 463(111) | 490(93)** | 466(137) | 516(88)** | 473.5(139) | 524(81)** |
| TUGb (s) | 9.9(2.3) | 8.7(2.0)** | 9.3(2.6) | 8.4(1.5)** | 9.2(3.2) | 7.9(1.5)** |
The paired samples t-test was used for normally distributed data (MDS-UPDRS III, 10MT-comfortable gait speed, and 10MT-fast gait speed). The Wilcoxon signed-rank test was used for non-normal data (axial subscores, bradykinesia subscores, tremor subscores, rigidity subscores, M-PAS, BBS, FTSTS, 6MWD, and TUG). aNormally distributed data are expressed as the mean ± SD, and bnon-normal data are reported as the median (interquartile range). *P < 0.05, **P < 0.01, vs. pre-MIRT. 10MT: 10-Meter Walk; 6MWD: 6-Minute Walk Distance; BBS: Berg Balance Scale; FTSTS: Five Times Sit to Stand; MDS-UPDRS III: Movement Disorder Society-Unified PD Rating Scales III; MIRT: multidisciplinary intensive rehabilitation treatment; M-PAS: Modified Parkinson Activity Scale; n: number of patients; PIGD: postural instability/gait difficulty; SD: standard deviation; TD: tremor dominant; TUG: Timed Get Up and Go.
Table 4.
Comparison of ΔMDS-UPDRS III among the three groups
| Model | Coefficientsa | t | P | 95.0% confidence interval for β | R2 | ||
|---|---|---|---|---|---|---|---|
| Unstandardized coefficients | Standardized coefficients | ||||||
| β | SEM | β’ | |||||
| (Constant) | –7.493 | 3.131 | – | –2.393 | 0.02 | (–13.752 to –1.234) | 0.171 |
| H-Y stage | 2.029 | 1.236 | 0.240 | 1.642 | 0.106 | (–0.441 to 4.499) | |
| Axial subscores | 0.040 | 0.376 | 0.018 | 0.108 | 0.915 | (–0.710 to 0.791) | |
| Tremor subscores | –0.243 | 0.204 | –0.218 | –1.193 | 0.238 | (–0.651 to 0.164) | |
| TD group | 4.515 | 1.900 | 0.448 | 2.377 | 0.021# | (0.717 to 8.312) | |
| Indeterminate group | 2.493 | 1.561 | 0.223 | 1.597 | 0.115 | (–0.627 to 5.614) | |
| MDS-UPDRS III | –0.108 | 0.070 | –0.255 | –1.550 | 0.126 | (–0.248 to 0.031) | |
Multiple linear regression was used, and the basis for single-factor variables entering the regression model was P < 0.05. ΔMDS-UPDRS III: Changes in MDSUPDRS III after MIRT; adependent variable: ΔMDS-UPDRS III; #P < 0.05, vs. PIGD group. MDS-UPDRS III: Movement Disorder Society-Unified PD Rating Scales III; PIGD: postural instability/gait difficulty; TD: tremor dominant.
Outcomes at the 3-month follow-up
At the 3-month follow-up, the duration of efficacy and the improved grade of overall symptoms in each group are shown in Figure 3, as percentages. At the 3-month follow up, there was no difference in the duration of efficacy between the PIGD group and the TD or indeterminate group (P > 0.05). Furthermore, there was no significant difference in overall symptom grade effects between groups, as evaluated using ordinal logistic regression. The effect maintenance in the PIGD group appeared slightly longer than that in the TD group, but this difference was not significant (P > 0.05).
Figure 3.
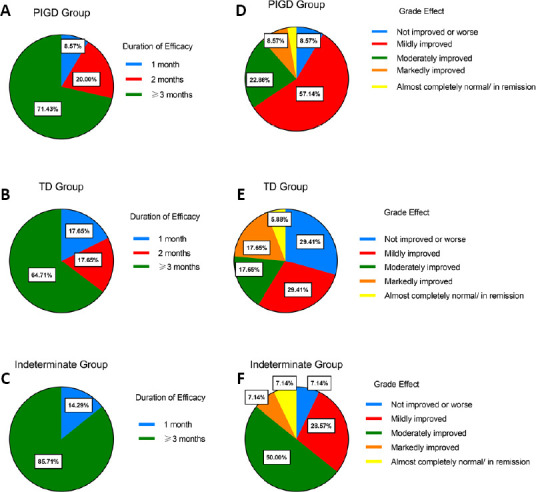
Results of the 3-month follow-up.
(A–C) The percentage of the duration of effects lasting less than 1 month, 2 months, or 3 months and above for each group. (D–F) The percentage of every grade effect in each group at the 3-month follow up. Data are reported as percentages; n = 35, 17, and 14 for the PIGD, TD, and indeterminate groups, respectively. PIGD: Postural instability/gait difficulty; TD: tremor dominant.
Discussion
This study demonstrated that MDS-UPDRS III improvement in the PIGD group was greater than that in the TD group in the short-term after MIRT, and that the beneficial effects on overall symptoms persisted for 1–3 months. This preliminary results appear to be in conflict with results from a previous study (Frazzitta et al., 2013a). One reason might be that the study by Frazzitta et al. (2013a) only included PD patients with H-Y stage 3, thus limiting the degree of dysfunction in patients with different motor subtypes. Patients with different motor subtypes exist in all of the different H-Y stages, which is one of the clinical features of PD. Compared with the TD and indeterminate subtypes, the PIGD subtype is associated with greater H-Y stage (Jankovic et al., 1990; Alves et al., 2006; Fereshtehnejad and Postuma, 2017), with patients mainly in H-Y stages of 2.5 and above. In contrast, TD subtype patients are usually in H-Y stages of 1–2, although they have been reported to reach stage 2.5 or 3 when tremor is severe (Jankovic et al., 1990; Alves et al., 2006). In the present pilot study, the PIGD group had greater H-Y stages than the other two groups, and their MS seemed to be more serious. Ritter and Bonsaksen (2019) reported that patients with PD who had a lower initial quality of life benefited more from rehabilitation treatment, whereas patients with PD who had more severe dysfunction seemed to respond better to MIRT treatment (Trend et al., 2002).
Although H-Y stage and cognitive status affected patients’ function and motor performance in previous studies, they did not influence the degree of improvement after MIRT (Ferrazzoli et al., 2016; Ortelli et al., 2018). In the present investigation, we used multiple linear regression to adjust for these factors and revealed that, after 2 weeks of MIRT, the PIGD group had greater improvements in MDS-UPDRS III scores compared with the TD group. This result may have been caused by the different physiological and pathological mechanisms of these two subtypes. It has been reported that PD patients in the PIGD group have significant alterations in the striatal-thalamo-cortical loop (Bergman et al., 1990; Lewis et al., 2011; Jiang et al., 2016), and the supplementary motor area plays a crucial role in the pathogenesis of bradykinesia and akinesia (Cunnington et al., 2002; Zhang et al., 2016). In contrast, resting tremor has been associated with dysfunction of the cerebello-thalamo-cortical circuit (Helmich et al., 2011; Lewis et al., 2011). In the future, we would like to combine functional magnetic resonance imaging or electroencephalogram with other methods to investigate the underlying mechanisms of MS improvement among the three groups. Although we have observed in clinical practice that MIRT appears to be more effective in improving MS in PIGD patients, the present study cannot strongly support this view, possibly because of the following limitations: the small sample size of this study, the inconsistent views around the different motor subtypes in PD, and the current lack of research in this area for reference.
After 2 weeks of MIRT, we revealed significant improvements in the MDS-UPDRS III, TUG, BBS, FTSTS, 10MT, and 6MWD in the three groups, suggesting that short-term MIRT has beneficial effects on MS, walking ability, and balance and posture control in patients with PD. However, compared with the other two groups, the improvement of M-PAS in the TD group was not statistically significant. This finding may be the result of better baseline motor function in the TD group. Previous studies have demonstrated that MIRT improves MS, reduces the risk of falling, improves quality of life, delays the progress of PD, and has neuroprotective effects in PD patients. Furthermore, a randomized controlled trial suggested that MIRT is useful in improving movement disorders, balance, activities of daily living, and quality of life (Monticone et al., 2015). In addition, the study by Ferrazzoli et al. (2018) suggested that MIRT could improve quality of life in patients with PD, and this improvement was maintained after a 3-month follow-up period. MIRT might also have a neuroprotective effect (Frazzitta et al., 2015). Moreover, MIRT can enhance brain-derived neurotrophic factor–tyrosine receptor kinase B signaling in lymphocytes (Fontanesi et al., 2016), which might lead to improved rigidity symptoms and reduce the frequencies of tremor in PD patients (Marusiak et al., 2015). MIRT has also been reported to improve the symptoms of individuals with PD over both short- and long-term periods (Frazzitta et al., 2012, 2013b, 2015, 2018; Monticone et al., 2015), with the efficacy of MIRT lasting up to 1 year (Frazzitta et al., 2013b; Monticone et al., 2015). In the present study, we found no differences in the duration or grade effects of efficacy among the three PD subtypes over the long-term period, which might be due to the short duration of follow-up and the evaluation methods we used.
Exercise is regarded as the basic element of rehabilitative programs, and the type, frequency, and intensity of exercise are important factors associated with rehabilitation effects in PD (Abbruzzese et al., 2016). Silva et al. (2019) performed a retrospective analysis of 236 clinical trials and found that the most common frequency of exercise was twice per week, with a mean intervention length of almost 13 weeks. A review by Mak et al. (2017) indicated that a minimum of 8 weeks of balance training or 4 weeks of gait training can have positive effects that last for 3–12 months after treatment. Sustained aerobic training, strength training, dance therapy, or tai chi lasting a minimum of 12 weeks also has beneficial long-term effects (Mak et al., 2017). Notably, MIRT has the advantages of a short intervention duration, improvements in both MS and NMS, and long-term effects, which indicates that PD patients have a better response to this multidisciplinary, comprehensive, and intensive rehabilitation treatment compared with the aforementioned single rehabilitation interventions. Trend et al. (2002) noted that MIRT provided immediate benefits for both patients with PD and their caregivers. Compared with usual care physiotherapy, specialized physiotherapy using the ParkinsonNet approach not only reduced the incidence of PD-related complications (17% vs. 21%) and decreased treatment sessions per year (33.72 vs. 47.97), but also lowered expenditure, both for direct costs (€933 vs. €1329) and total health-care expenditure (€2056 vs. €2586) (Ypinga et al., 2018). These findings imply that multidisciplinary rehabilitation has the potential to improve quality of care and health outcomes in patients with PD, while reducing healthcare costs (Tosserams et al., 2020).
In the present study, patients were divided into three subtypes according to their TD/PIGD ratio; this approach is more accurate and standardized compared with that of the study conducted by Frazzitta et al. (2013a). Compared with this previous study (in which all subjects were in H-Y stage 3), the present study included a wider range of subjects (H-Y stage 1.5–3). Moreover, we conducted a 3-month follow-up to assess any differences in the maintenance of efficacy among the three groups, which was not performed in the previous study. Compared with earlier MIRT studies, the length of the intervention was shorter in our study (the entire session lasted for 2 weeks). Additionally, our study focused on a “boost rehabilitative intervention”, which can reduce hospitalization costs for patients with PD and save time for their work and family.
There are several limitations to our study. First, the sample size was relatively small, which might have led to statistical errors. Second, we did not assess MDS-UPDRS III, TUG, BBS, FTSTS, 10MT, or 6MWD at follow-up. Without these evaluations, we cannot know which aspects of motor function were improved at follow-up. Third, we used a patient self-reporting scale questionnaire to assess long-term effects, which may have led to bias in the results caused by differences in patient understanding. The questionnaire was also untargeted, which might have covered up any differences in long-term effects among the three groups. Fourth, the follow-up was relatively short. Finally, although excluding cognitively affected patients presumably ensured better adherence to the intervention, it has been noted that PIGD patients are more prone to developing cognitive impairment. When this impairment involves executive and visuospatial functions, it may be linked to a worse gait profile. As such, excluding cognitively affected patients from the study may be a relevant selection bias that limits the generalizability of our findings in real-life clinical practice. Further real-world studies with a greater sample size, covering a broad range of patients with PD over a longer follow-up period, should be explored.
In summary, the present results suggest that 2 weeks of MIRT can improve MS, walking disorders, balance, and postural control dysfunction in different PD subtypes. In total, 71.43% of patients in the PIGD group, 64.71% in the TD group, and 85.71% in the indeterminate group maintained their improvements at the 3-month follow-up. The rehabilitation effects of MIRT on the PIGD subtype appeared to be slightly better than those of the other subtypes. A neural network study on the rehabilitation effects of different motor subtypes in PD will be carried out in the future.
Footnotes
P-Reviewers: Comi C, Dushanova J; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Gardner B, Song LP; T-Editor: Jia Y
Conflicts of interest: The authors have no conflicts of interest to disclose.
Financial support: This study was supported by the National Key Research and Development Program Sub-project, No. 2018YFC0115405 (to BYF); the Start-up Fund for Scientific Research Talents of Beijing Rehabilitation Hospital, Capital Medical University of China, No. 2019R-006 (to ZHJ). The funding sources had no role in study design, statistical analysis and manuscript preparation.
Institutional review board statement: This study was performed in strict accordance with the Declaration of Helsinki formulated by the World Medical Association. This study was approved by the Ethics Committee of Beijing Rehabilitation Hospital of Capital Medical University of China (No. 2018bkky022) on May 7, 2018.
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: The writing and editing of the article was performed in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Statement statement.
Biostatistics statement: The statistical methods of this study were reviewed by the Peking University Clinical Research Institute, Beijing, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Key Research and Development Program Sub-project, No. 2018YFC0115405 (to BYF); the Start-up Fund for Scientific Research Talents of Beijing Rehabilitation Hospital, Capital Medical University of China, No. 2019R-006 (to ZHJ).
References
- 1.Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson’s disease: current outlook and future challenges. Parkinsonism Relat Disord. 2016;22(Suppl 1):S60–64. doi: 10.1016/j.parkreldis.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord. 2006;21:1123–1130. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- 3.Arie L, Herman T, Shema-Shiratzky S, Giladi N, Hausdorff JM. Do cognition and other non-motor symptoms decline similarly among patients with Parkinson’s disease motor subtypes. Findings from a 5-year prospective study. J Neurol. 2017;264:2149–2157. doi: 10.1007/s00415-017-8605-x. [DOI] [PubMed] [Google Scholar]
- 4.Bari BA, Chokshi V, Schmidt K. Locus coeruleus-norepinephrine: basic functions and insights into Parkinson’s disease. Neural Regen Res. 2020;15:1006–1013. doi: 10.4103/1673-5374.270297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27:27–36. [PubMed] [Google Scholar]
- 6.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 7.Bloem BR, de Vries NM, Ebersbach G. Nonpharmacological treatments for patients with Parkinson’s disease. Mov Disord. 2015;30:1504–1520. doi: 10.1002/mds.26363. [DOI] [PubMed] [Google Scholar]
- 8.Chase A. Parkinson disease: treatment needs vary between Parkinson disease subtypes. Nat Rev Neurol. 2015;11:123. doi: 10.1038/nrneurol.2015.19. [DOI] [PubMed] [Google Scholar]
- 9.Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage. 2002;15:373–385. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- 10.Czarnecki K, Thompson JM, Seime R, Geda YE, Duffy JR, Ahlskog JE. Functional movement disorders: successful treatment with a physical therapy rehabilitation protocol. Parkinsonism Relat Disord. 2012;18:247–251. doi: 10.1016/j.parkreldis.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Dani C, Proença IT, Marinho J, Peccin P, da Silva IRV, Nique S, Striebel V, Pochmann D, Elsner VR. Aquatic exercise program-modulated oxidative stress markers in patients with Parkinson’s disease. Neural Regen Res. 2020;15:2067–2072. doi: 10.4103/1673-5374.276337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorsey ER, Bloem BR. The parkinson pandemic-a call to action. JAMA Neurol. 2018;75:9–10. doi: 10.1001/jamaneurol.2017.3299. [DOI] [PubMed] [Google Scholar]
- 13.Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the parkinson pandemic. J Parkinsons Dis. 2018;8:S3–8. doi: 10.3233/JPD-181474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan RP, Leddy AL, Earhart GM. Five times sit-to-stand test performance in Parkinson’s disease. Arch Phys Med Rehabil. 2011;92:1431–1436. doi: 10.1016/j.apmr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fereshtehnejad SM, Postuma RB. Subtypes of Parkinson’s disease: what do they tell us about disease progression. Curr Neurol Neurosci Rep. 2017;17:34. doi: 10.1007/s11910-017-0738-x. [DOI] [PubMed] [Google Scholar]
- 16.Ferrazzoli D, Ortelli P, Maestri R, Bera R, Giladi N, Ghilardi MF, Pezzoli G, Frazzitta G. Does cognitive impairment affect rehabilitation outcome in parkinson’s disease. Front Aging Neurosci. 2016;8:192. doi: 10.3389/fnagi.2016.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrazzoli D, Ortelli P, Zivi I, Cian V, Urso E, Ghilardi MF, Maestri R, Frazzitta G. Efficacy of intensive multidisciplinary rehabilitation in Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2018;89:828–835. doi: 10.1136/jnnp-2017-316437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fontanesi C, Kvint S, Frazzitta G, Bera R, Ferrazzoli D, Di Rocco A, Rebholz H, Friedman E, Pezzoli G, Quartarone A, Wang HY, Ghilardi MF. Intensive rehabilitation enhances lymphocyte BDNF-TrkB signaling in patients with parkinson’s disease. Neurorehabil Neural Repair. 2016;30:411–418. doi: 10.1177/1545968315600272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C. Movement Disorder Society Evidence-Based Medicine C (2018) International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 33:1248–1266. doi: 10.1002/mds.27372. [DOI] [PubMed] [Google Scholar]
- 20.Frazzitta G, Abbruzzese G, Bertotti G, Boveri N, Pezzoli G, Maestri R. Effectiveness of an intensive rehabilitation treatment on different Parkinson’s disease subtypes. NeuroRehabilitation. 2013a;33:299–303. doi: 10.3233/NRE-130959. [DOI] [PubMed] [Google Scholar]
- 21.Frazzitta G, Bertotti G, Riboldazzi G, Turla M, Uccellini D, Boveri N, Guaglio G, Perini M, Comi C, Balbi P, Maestri R. Effectiveness of intensive inpatient rehabilitation treatment on disease progression in parkinsonian patients: a randomized controlled trial with 1-year follow-up. Neurorehabil Neural Repair. 2012;26:144–150. doi: 10.1177/1545968311416990. [DOI] [PubMed] [Google Scholar]
- 22.Frazzitta G, Bertotti G, Uccellini D, Boveri N, Rovescala R, Pezzoli G, Maestri R. Short- and long-term efficacy of intensive rehabilitation treatment on balance and gait in parkinsonian patients: a preliminary study with a 1-year followup. Parkinsons Dis. 2013b;2013:583278. doi: 10.1155/2013/583278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frazzitta G, Maestri R, Bertotti G, Riboldazzi G, Boveri N, Perini M, Uccellini D, Turla M, Comi C, Pezzoli G, Ghilardi MF. Intensive rehabilitation treatment in early Parkinson’s disease: a randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair. 2015;29:123–131. doi: 10.1177/1545968314542981. [DOI] [PubMed] [Google Scholar]
- 24.Gao H, Li YX, Wang D, Yang XL. Protective effects and the mechanism of lipoic acid on cell model of Parkinson’s disease induced by lipopolysaccharide. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:447–452. [Google Scholar]
- 25.Goetz CG, Tilley BC, Shaftman SR. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 26.Goulding SR, Sullivan AM, O’Keeffe GW, Collins LM. The potential of bone morphogenetic protein 2 as a neurotrophic factor for Parkinson’s disease. Neural Regen Res. 2020;15:1432–1436. doi: 10.4103/1673-5374.274327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol. 2011;69:269–281. doi: 10.1002/ana.22361. [DOI] [PubMed] [Google Scholar]
- 28.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 29.Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study. Group Neurology. 1990;40:1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 30.Jiang S, Wang M, Zhang L, Yuan Y, Tong Q, Ding J, Wang J, Xu Q, Zhang K. Regional homogeneity alterations differentiate between tremor dominant and postural instability gait difficulty subtypes of Parkinson’s disease. J Neural Transm (Vienna) 2016;123:219–229. doi: 10.1007/s00702-015-1490-5. [DOI] [PubMed] [Google Scholar]
- 31.Katz M, Luciano MS, Carlson K, Luo P, Marks WJ, Jr, Larson PS, Starr PA, Follett KA, Weaver FM, Stern MB, Reda DJ, Ostrem JL group CSPs. Differential effects of deep brain stimulation target on motor subtypes in Parkinson’s disease. Ann Neurol. 2015;77:710–719. doi: 10.1002/ana.24374. [DOI] [PubMed] [Google Scholar]
- 32.Keus SH, Nieuwboer A, Bloem BR, Borm GF, Munneke M. Clinimetric analyses of the modified Parkinson activity scale. Parkinsonism Relat Disord. 2009;15:263–269. doi: 10.1016/j.parkreldis.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Laboratories ATSCoPSfCPF (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 34.Lang JT, Kassan TO, Devaney LL, Colon-Semenza C, Joseph MF. Test-retest reliability and minimal detectable change for the 10-meter walk test in older adults with Parkinson’s disease. J Geriatr Phys Ther. 2016;39:165–170. doi: 10.1519/JPT.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 35.Lewis MM, Du G, Sen S, Kawaguchi A, Truong Y, Lee S, Mailman RB, Huang X. Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson’s disease. Neuroscience. 2011;177:230–239. doi: 10.1016/j.neuroscience.2010.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Xing Y, Martin-Bastida A, Piccini P, Auer DP. Patterns of grey matter loss associated with motor subscoress in early Parkinson’s disease. Neuroimage Clin. 2018;17:498–504. doi: 10.1016/j.nicl.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mak MK, Wong-Yu IS, Shen X, Chung CL. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol. 2017;13:689–703. doi: 10.1038/nrneurol.2017.128. [DOI] [PubMed] [Google Scholar]
- 38.Marusiak J, Zeligowska E, Mencel J, Kisiel-Sajewicz K, Majerczak J, Zoladz JA, Jaskolski A, Jaskolska A. Interval training-induced alleviation of rigidity and hypertonia in patients with Parkinson’s disease is accompanied by increased basal serum brain-derived neurotrophic factor. J Rehabil Med. 2015;47:372–375. doi: 10.2340/16501977-1931. [DOI] [PubMed] [Google Scholar]
- 39.Monticone M, Ambrosini E, Laurini A, Rocca B, Foti C. In-patient multidisciplinary rehabilitation for Parkinson’s disease: a randomized controlled trial. Mov Disord. 2015;30:1050–1058. doi: 10.1002/mds.26256. [DOI] [PubMed] [Google Scholar]
- 40.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 41.Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys Ther. 2001;81:810–818. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- 42.Opara J, Malecki A, Malecka E, Socha T. Motor assessment in Parkinson's disease. Ann Agric Environ Med. 2017;24:411–415. doi: 10.5604/12321966.1232774. [DOI] [PubMed] [Google Scholar]
- 43.Ortelli P, Ferrazzoli D, Bera R, Caremani L, Giladi N, Maestri R, Frazzitta G. Effectiveness of a goal-based intensive rehabilitation in Parkinsonian patients in advanced stages of disease. J Parkinsons Dis. 2018;8:113–119. doi: 10.3233/JPD-171247. [DOI] [PubMed] [Google Scholar]
- 44.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 45.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 46.Radder DLM, Nonnekes J, Bloem BR. Intensive inpatient rehabilitation for persons with Parkinson’s disease: last resort or pre-emptive strike. J Neurol Neurosurg Psychiatry. 2018;89:795–796. doi: 10.1136/jnnp-2017-317812. [DOI] [PubMed] [Google Scholar]
- 47.Rajput AH, Voll A, Rajput ML, Robinson CA, Rajput A. Course in Parkinson disease subtypes: a 39-year clinicopathologic study. Neurology. 2009;73:206–212. doi: 10.1212/WNL.0b013e3181ae7af1. [DOI] [PubMed] [Google Scholar]
- 48.Ritter VC, Bonsaksen T. Improvement in quality of life following a multidisciplinary rehabilitation program for patients with Parkinson’s disease. J Multidiscip Healthc. 2019;12:219–227. doi: 10.2147/JMDH.S202827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva CM, Travessa AM, Bouca-Machado R, Caldeira D, Ferreira JJ. Reporting and methodological quality of clinical trials on exercise therapy for Parkinson’s disease. Parkinsonism Relat Disord. 2019;69:150–156. doi: 10.1016/j.parkreldis.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord. 2013;28:668–670. doi: 10.1002/mds.25383. [DOI] [PubMed] [Google Scholar]
- 51.Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol. 2014;71:499–504. doi: 10.1001/jamaneurol.2013.6233. [DOI] [PubMed] [Google Scholar]
- 52.Tosserams A, de Vries NM, Bloem BR, Nonnekes J. Multidisciplinary care to optimize functional mobility in Parkinson disease. Clin Geriatr Med. 2020;36:159–172. doi: 10.1016/j.cger.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Trend P, Kaye J, Gage H, Owen C, Wade D. Short-term effectiveness of intensive multidisciplinary rehabilitation for people with Parkinson’s disease and their carers. Clin Rehabil. 2002;16:717–725. doi: 10.1191/0269215502cr545oa. [DOI] [PubMed] [Google Scholar]
- 54.Vervoort G, Bengevoord A, Nackaerts E, Heremans E, Vandenberghe W, Nieuwboer A. Distal motor deficit contributions to postural instability and gait disorder in Parkinson’s disease. Behav Brain Res. 2015;287:1–7. doi: 10.1016/j.bbr.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 55.Vervoort G, Leunissen I, Firbank M, Heremans E, Nackaerts E, Vandenberghe W, Nieuwboer A. Structural brain alterations in motor subtypes of Parkinson’s disease: evidence from probabilistic tractography and shape analysis. PLoS One. 2016;11:e0157743. doi: 10.1371/journal.pone.0157743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ypinga JHL, de Vries NM, Boonen L, Koolman X, Munneke M, Zwinderman AH, Bloem BR. Effectiveness and costs of specialised physiotherapy given via ParkinsonNet: a retrospective analysis of medical claims data. Lancet Neurol. 2018;17:153–161. doi: 10.1016/S1474-4422(17)30406-4. [DOI] [PubMed] [Google Scholar]
- 57.Zhang JR, Feng T, Hou YN, Chan P, Wu T. Functional connectivity of vim nucleus in tremor- and akinetic-/rigid-dominant Parkinson’s disease. CNS Neurosci Ther. 2016;22:378–386. doi: 10.1111/cns.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]


