Keywords: motor function, neural plasticity, non-invasive brain stimulation, protection, repair, stroke, transcranial pulse current stimulation
Abstract
Previous studies have shown that transcranial pulse current stimulation (tPCS) can increase cerebral neural plasticity and improve patients’ locomotor function. However, the precise mechanisms underlying this effect remain unclear. In the present study, rat models of stroke established by occlusion of the right cerebral middle artery were subjected to tPCS, 20 minutes per day for 7 successive days. tPCS significantly reduced the Bederson score, increased the foot print area of the affected limbs, and reduced the standing time of affected limbs of rats with stroke compared with that before intervention. Immunofluorescence staining and western blot assay revealed that tPCS significantly increased the expression of microtubule-associated protein-2 and growth-associated protein-43 around the ischemic penumbra. This finding suggests that tPCS can improve the locomotor function of rats with stroke by regulating the expression of microtubule-associated protein-2 and growth-associated protein-43 around the ischemic penumbra. These findings may provide a new method for the clinical treatment of poststroke motor dysfunction and a theoretical basis for clinical application of tPCS. The study was approved by the Animal Use and Management Committee of Shanghai University of Traditional Chinese Medicine of China (approval No. PZSHUTCM190315003) on February 22, 2019.
Chinese Library Classification No. R454.1; R741; R338.1+3
Introduction
In recent years, there have been rapid advances in neuromodulation technologies, such as transcranial direct current stimulation (tDCS) (Yavari et al., 2018), transcranial pulse current stimulation (tPCS) (Ma et al., 2019), and transcranial magnetic stimulation (Burke et al., 2019). By increasing or decreasing synaptic plasticity, tDCS can regulate brain activity, affect brain function, and induce brain network remodeling (Ma et al., 2019; Barbati et al., 2020; Pilurzi et al., 2020). In stroke motor rehabilitation, neuromodulation technology could promote the recovery of brain dysfunction, primarily through inhibiting excitability of the contralesional hemisphere (Notturno et al., 2014) and exciting the cortex of the ipsilesional hemisphere (Kim and Han, 2017) to restore the balance between the bilateral hemispheres. Increasingly, studies have shown that these methods exhibit a lasting effect, thereby contributing to brain plasticity and motor re-learning (Fomenko and Lozano, 2019). tDCS has been shown to have long-term effects on cortical excitability (Dimyan and Cohen, 2011), which may in turn lead to long-term behavioral changes, and neuroplasticity is thought to underlie these effects. For example, several studies have indicated that, during acute and recovery periods, stimulation of the affected motor cortex with a current intensity of 0.2 mA can improve motor function by regulating the expression of axonal and dendritic growth proteins and promoting neural plasticity (Yoon et al., 2012).
tPCS induces transcranial electrical stimulation, which is essentially a non-constant direct current stimulation. The current transmits a weak pulse current to the cerebral cortex through the anode and cathode at a fixed stimulation amplitude, and regulates cortical excitability, thereby improving cortical-related functions (Jaberzadeh et al., 2014). Unidirectional tPCS regulates cerebral cortex excitability, which has the same polarity specificity as tDCS, with the anode inducing excitability and the cathode inducing inhibition (Fitzgerald, 2014). However, unlike tDCS, tPCS is not a continuous stimulus, and is interrupted at certain time intervals, which defines the pulse duration, frequency, and inter-pulse interval of the stimulus. A study using local field potentials to assess the effect of anode-tPCS on brain plasticity found that low-intensity current and long-term anode-tPCS can cause a large calcium response in astrocytes, thereby inducing an enhanced cortical excitability (Ma et al., 2019). tPCS not only improves brain function by regulating cerebral cortical plasticity, but also improves brain function recovery by regulating brain network connections. One study has shown that when tPCS stimulates the primary motor cortex (M1), it can weaken the connection between the M1 region and the surrounding cortex and increase the resting-state functional connectivity with the thalamus (Sours et al., 2014). The authors found that, after stimulation, the resting functional connection between M1 and both the cerebellum and the right insular increased. Another study placed the tPCS anode over the right M1 area. After 20 minutes of tPCS, the walking speed and step length of patients with moderate to severe Parkinson’s disease significantly improved, and fewer protective balance steps backward (Alon et al., 2012). This experiment demonstrated that tPCS can improve gait. Furthermore, all patients tolerated the treatment process and there were no adverse events, which indicates that tPCS is safe. At present, tPCS has few clinical applications and is mainly used for clinical tests of healthy control subjects. To further understand the mechanism of action of tPCS, and to provide more reliable neuromolecular theoretical support for its use in clinical treatment, this study explored the effect of tPCS on the motor function of a rat model of stroke.
Materials and Methods
Study design
Thirty-six clean-grade male Sprague-Dawley rats weighing 260 ± 20 g were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (license No. SCXK (Hu) 2017-0005). All experimental procedures were in accordance with animal welfare and ethics principles and were approved by the Animal Use and Management Committee of Shanghai University of Traditional Chinese Medicine on February 22, 2019 (approval No. PZSHUTCM190315003). The rats underwent adapted rearing and gait training for 10 days prior to the study. The 36 rats were allocated to the sham operation (sham), middle cerebral artery occlusion (MCAO), and tPCS (MCAO + tPCS) groups (n = 12/group) using the random number table method. The flow chart is shown in Figure 1.
Figure 1.
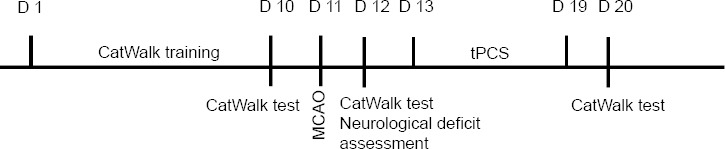
The study procedure.
MCAO: Middle cerebral artery occlusion; tPCS: transcranial pulse current stimulation.
Modeling of MCAO
MCAO was modeled using the Zea Longa method (Longa et al., 1989). Rats were anesthetized using 4% isoflurane (RWD Life Technology Co., Ltd., Guangdong, China) and were fixed on a sterilized operation table in the supine position. A 2–3 cm longitudinal incision from the submandibular midline to the right was made to expose the right common carotid artery, right external carotid artery, and right internal carotid artery. An 18-mm wire plug was inserted into the internal carotid artery to block the middle cerebral artery intersection. After 90 minutes of ischemia, the thread plug was removed, and cerebral ischemia and reperfusion was performed. The temperature of the animals was maintained at 37 ± 0.5°C using a feedback-adjusted heating blanket. The incision was sutured layer by layer. In the sham group, the right internal carotid artery, external carotid artery, and common carotid artery were also exposed, but the internal carotid artery was not plugged, and the incision was directly sutured layer by layer.
All rats were then returned to their cages and had ad libitum access to food and water.
Transcranial pulse current stimulation
At 48 hours after MCAO, rats in the tPCS group were placed under 4% isoflurane inhalation anesthesia and fixed on a stereotaxic instrument (RWD Life Technology Co., Ltd.). The scalp and underlying tissues were removed, and a round transparent hollow electrode base (3.4 mm in diameter) was placed onto the skull over the right cortex (anteroposterior 3.0 mm, mediolateral 2.0 mm (Paxinos and Watson, 2007; Yoon et al., 2012; Figure 2). The base was fixed to the skull with glass ionomer cement and the transparent hollow base was filled with electrolyte gel. Then, electrodes (3 mm in diameter; Soterix Medical, New York, NY, USA) were placed into the electrode base. A rubber pad (3.4 mm2) was attached to the rat’s chest and abdomen as a reference electrode. tPCS was performed under 4% isoflurane-induced anesthesia and 2.5% isoflurane-maintained anesthesia (RWD Life Technology Co., Ltd.) and was applied with an intensity of 0.2 mA, 10 Hz, 20 minutes per day for 7 days. The sham and MCAO groups were treated in the same way and anesthetized with isoflurane for 20 minutes without any other interventions.
Figure 2.
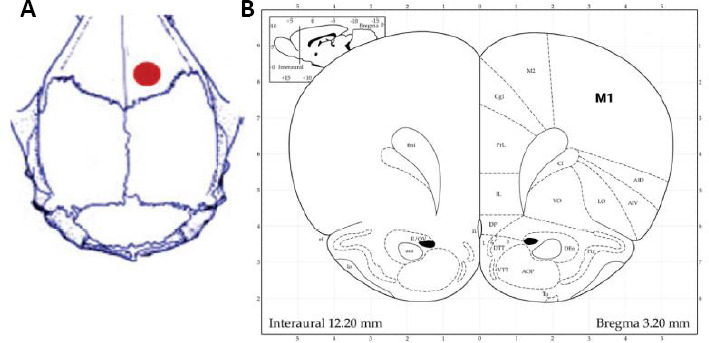
Positioning of transcranial pulse current stimulation.
(A) Positioning of transcranial pulse current stimulation on the skull. (B) The rat atlas (Paxinos and Watson, 2007).
Neurological deficit assessment
The Bederson score was used to record limb movements on the hemiplegia side 1 day before, 1 day after, and 9 days after the intervention (Bieber et al., 2019). The assessment method was as follows: (a) The rat’s tail was lifted 1 foot from the ground to observe the forelimb flexion. If both forelimbs were symmetrically extended to the ground, then a score of 0 was recorded. If there was shoulder flexion, elbow flexion, shoulder adduction, or both shoulder and elbow flexion and internal rotation on the forelimb contralateral to the operation, then a score of 1 was recorded; (b) The rat was placed on a smooth surface and its shoulders were pushed to move towards the opposite side to check resistance. If the bilateral resistance was equivalent and strong, then a score of 0 was recorded. If the resistance dropped when pushing to the side contralateral to the operated side, then a score of 1 was recorded; (c) The two forelimbs of the rat were placed on a metal net to observe muscle tension of the two forelimbs. If the bilateral muscle tension was equivalent, then a score of 0 was recorded. If the contralateral forelimb muscle tension was less than that of the ipsilateral forelimb, then a score of 1 was recorded; and (d) The rat’s tail was lifted 1 foot from the ground. If the rat turned to the side contralateral to the operation, then a score of 1 was recorded. The highest possible score was 4, whereby higher scores indicated a more severe behavioral dysfunction. Rats with a score of 0 or which were unable to walk were excluded from the experiment.
CatWalk gait analysis
CatWalk gait analysis is a reliable method to assess functional recovery after stroke (Hetze et al., 2012). Rat gait was tested by an experimenter who was blinded to group allocation. The rats passed through a 150-cm long Small animal gait CatWalk XT (Noldus, Wageningen, the Netherlands); food placed at the end of the treadmill was used as bait, and experiments were performed in a dark, quiet room. The changing paw images of rats were captured using a high-speed camera placed under the glass plate. The parameters obtained for each run were as follows: time parameters (running time, running speed, swing duration, standing time, and swing speed) and space parameters related to a single paw (paw print pressure, width, length, area, and maximum area). One day before ischemia, a baseline value was established for each rat. After the ischemia, rats were allowed to recover for 1 day before restarting behavioral testing. Therefore, 1 day after stroke and before the tPCS intervention, another gait test was performed to quantify the motor deficit caused by stroke. Gait testing was also performed 9 days after stroke.
Immunofluorescence staining
Rats were anesthetized with 4% isoflurane (RWD Life Technology Co., Ltd.) 9 days after stroke and cardiac-perfused with phosphate-buffered saline and 4% paraformaldehyde. The whole-brain tissues of 8 rats from each group were fixed with 4% paraformaldehyde for 48 hours and embedded in paraffin. The 5-μm paraffin sections were incubated with rabbit anti-microtubule-associated protein-2 (MAP-2) antibody (1:200; Cat# 32454; Abcam, Cambridge, MA, USA), rabbit anti-growth associated protein-43 (GAP-43) antibody (a neuronal marker; 1:200; Cat# 16053; Abcam), and rabbit anti-glial fibrillary acidic protein (GFAP) antibody (1:500; Cat# 7260; Abcam) overnight in a wet box at 4°C, and then rinsed with phosphate-buffered saline. Then, the sections were incubated with corresponding FITC 488-labeled goat anti-rabbit IgG (1:400; Cat# 4412S; Cell Signaling Technology, Danvers, MA, USA) at 37°C in the dark for 2 hours, then rinsed with phosphate-buffered saline. The sections were counter-stained with 4′,6-diamidino-2-phenylindole. The area around the infarct was selected under a 200× fluorescence microscope (Nikon Eclipse C1; Nikon, Tokyo, Japan), and the number of positive cells was counted.
Western blot assay
Four rats in each group were decapitated under 4% isoflurane-induced deep anesthesia 9 days after stroke. The ischemic area around the ischemic penumbra (approximately 100 mg) was quickly removed and frozen in liquid nitrogen. Brain tissue samples were sonicated in ice-containing lysate containing 50 mM Tris HCl, pH 7.2, 250 mM NaCl, 1% NP-40, and a protease inhibitor composed of Leupetin, Pepstatin A, Aprotinin, and E-64, followed by centrifugation at 12,000 × g for 15 minutes at 4°C. Protein concentration was determined by the Lowry assay (Bio-Rad, Hercules, CA, USA). Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and probed with specific MAP-2, GAP-43, and GFAP antibodies. The developer solution (wbkls0100; Millipore, Burlington, MA, USA) was applied to the film surface with a pipette and allowed to react for 1 minute.
Statistical analysis
SPSS 21.0 software (IBM SPSS, Chicago, IL, USA) was used for all statistical analyses. Data are expressed as the mean ± standard deviation (SD), and were analyzed using one-way analysis of variance followed by the least significant difference test. The significance level was set at α = 0.05.
Results
tPCS improves the neurological function of cerebral infarction rats
The Bederson score
There were no significant differences in the Bederson scores between the MCAO and tPCS groups at 1 day after stroke (P > 0.05). At 9 days after stroke, the Bederson score in the tPCS group was significantly lower than that before the intervention (P < 0.01). No such significant change was seen within the MCAO group (P > 0.05). After 9 days of intervention, the Bederson score of the tPCS group was significantly lower than that of the MCAO group (P < 0.01; Table 1).
Table 1.
The effect of tPCS on the Bederson score in cerebral infarction rats
| Time after stroke | MCAO | tPCS |
|---|---|---|
| 1 d | 2.88±1.12 | 3.00±0.90 |
| 9 d | 2.13±0.83 | 1.50±0.53## |
Data are expressed as the mean ± SD (n = 8), ##P < 0.01, vs. MCAO group (one-way analysis of variance followed by the least significant difference test). MCAO: Middle cerebral artery occlusion; tPCS: transcranial pulse current stimulation.
CatWalk gait analysis
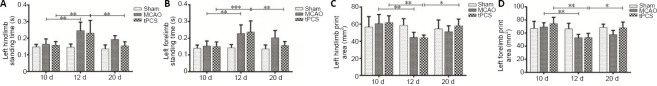
Compared with baseline, the average print area (P < 0.01) and average standing time (P < 0.01) of the affected limbs (left forelimb and left hindlimb) were significantly decreased within both the MCAO and tPCS groups at 1 day after stroke. At 9 days after stroke, the average print area increased (P < 0.05) and standing time of the affected limbs decreased (P < 0.01) compared with 1 day after stroke within the tPCS group (Figure 3). At 9 days after stroke, there was a significant difference in the left forelimb and left hindlimb standing time and the average left forelimb print area (P < 0.01) compared with 1 day after stroke. Compared with the MCAO group, the tPCS group exhibited improvements in the left forelimb and left hindlimb standing times and the left forelimb print area (P < 0.01; Table 2).
Figure 3.
The effect of tPCS on CatWalk gait in cerebral infarction rats.
(A) Left hindlimb standing time; (B) left forelimb standing time; (C) left hindlimb print area; (D) left forelimb print area. Data are expressed as the mean ± SD (n = 8). *P < 0.05, **P < 0.01 (one-way analysis of variance followed by the least significant difference test). MCAO: Middle cerebral artery occlusion; tPCS: transcranial pulse current stimulation.
Table 2.
Gait analysis of cerebral infarction rats 9 days after stroke
| Sham | MCAO | tPCS | |
|---|---|---|---|
| Left hindlimb standing time (s) | 0.14±0.02 | 0.19±0.02** | 0.15±0.02## |
| Left forelimb standing time (s) | 0.13±0.02 | 0.20±0.04** | 0.16±0.02## |
| Left forelimb print area (mm2) | 68.3±5.17 | 57.54±6.40** | 67.83±8.77## |
Data are expressed as the mean ± SD (n = 8). **P < 0.01, vs. sham group; ##P < 0.01, vs. MCAO group (one-way analysis of variance followed by the least significant difference test). MCAO: Middle cerebral artery occlusion; tPCS: transcranial pulse current stimulation.
tPCS increases the expression of nerve growth-associated proteins around the ischemic penumbra of the cerebral infarction in rats
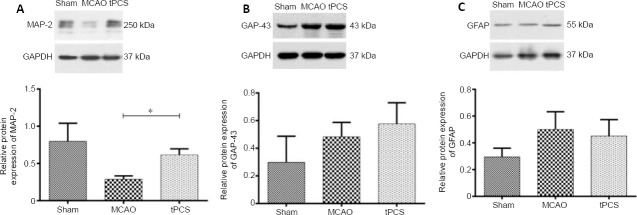
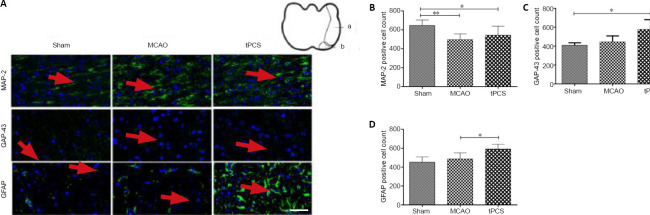
Western blot results showed that, at 9 days after stroke, the protein expression of MAP-2, GAP-43, and GFAP all increased to varying degrees compared with 1 day. The protein expression of MAP-2 in the tPCS group was significantly different from that in the MCAO group (P < 0.05). While the protein expression of GAP-43 in the tPCS group was higher than that in the MCAO group, this difference was not significant (P > 0.05). While the protein expression of GFAP in the tPCS group was lower than that in the MCAO group, this difference was not significant (P > 0.05; Figure 4). The trends towards immunopositivity of MAP-2, GAP-43, and GFAP that were identified using immunofluorescence staining were similar to the western blot results (Figure 5).
Figure 4.
The effect of tPCS on the expression of MAP-2 (A), GAP-43 (B), and GFAP (C) in the ischemic penumbra of cerebral infarction rats.
GAPDH was used as a loading control. The data are expressed as the mean ± SD (n = 4). *P < 0.05 (one-way analysis of variance followed by the least significant difference test). GAP-43: Growth-associated protein-43; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GFAP: glial fibrillary acidic protein; MAP-2: microtubule-associated protein-2; MCAO: middle cerebral artery occlusion; tPCS: transcranial pulse current stimulation.
Figure 5.
The effect of tPCS on the immunopositivities of MAP-2, GAP-43, and GFAP around the ischemic penumbra of cerebral infarction rats.
(A) The immunopositivities of MAP-2, GAP-43, and GFAP around the cerebral infarction in rats. Scale bar: 50 μm. Red arrows indicate a positive expression (green); a indicates the infarction area; b indicates the ischemic penumbra. (B–D) Quantitative results of MAP-2-, GAP-43-, and GFAP-positive cells in a 200× magnification area. The data are expressed as the mean ± SD (n = 8). *P < 0.05, **P < 0.01 (one-way analysis of variance followed by the least significant difference test). GAP-43: Growth-associated protein-43; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GFAP: glial fibrillary acidic protein; MAP-2: microtubule-associated protein-2; MCAO: middle cerebral artery occlusion; tPCS: transcranial pulse current stimulation.
Discussion
Ischemic stroke is caused by a disrupted blood to flow to the brain as a result of blockages of blood vessels in the brain, which can cause damage to brain tissues (Antipova et al., 2019; Iglesias-Rey and Castillo, 2020; Kaiser and West, 2020). Rehabilitation of ischemic stroke remains a challenge in clinical treatment. At present, conventional rehabilitation interventions are mainly traditional “bottom-up” methods, such as neurodevelopmental promotion technologies (proprioceptive neuromuscular facilitation, Brunnstrom’s stages of stroke recovery, and the Bobath concept), exercise relearning, constraint-induced therapy, physical factor therapy, and the application of new technology and new equipment, such as rehabilitation robots, but these methods have a poor efficacy. Some studies have confirmed that the recovery of functional deficits after cerebral ischemic injury depends on the compensatory capacity of functional reorganization that is, to a certain extent, inherent to the central nervous system following brain damage (Cassidy and Cramer, 2017; Lu et al., 2019). The reorganization observed in the area around the injury reflects this spontaneous regulation of nervous system plasticity. While the new tPCS method is used to treat stroke after neurological rehabilitation, its underlying mechanism of action is not yet clear.
The present results demonstrated that tPCS can promote the recovery of limb motor function in cerebral ischemia-reperfusion rats. MAP-2 is a promoter of microtubule assembly and is a static structural protein that, along with other cytoskeletal proteins, maintains neural structure (Yao et al., 2015; Kim et al., 2020). Recent studies have shown that MAP-2 has dynamic functions in neuronal growth, differentiation, and plasticity, and that it plays a key role in the response of neurons to growth factors, neurotransmitters, and synaptic activities (Milanovic et al., 2017; Ishikawa et al., 2019). These findings indicate that MAP-2 modification and rearrangement are essential early steps in many processes that modify neuronal function (Johnson and Jope, 1992). One study has suggested that all MAP-2 isoforms can induce tubulin assembly in vitro, and may promote microtubule growth by increasing the growth rate and repair frequency of microtubules (Goedert et al., 1991). MAP-2 only exists in the cell body and dendrites (Burgoyne and Cumming, 1983), and plays an important role in the extension and branching of dendrites (Farah and Leclerc, 2008). One previous study has shown that changes in the immune activity of MAP-2 are associated with changes in the cytoskeleton of dendritic cells; dendrites in MAP-2-deleted brain regions were found to collapse, and dendrites in MAP-2-increased brain regions increased (Harada et al., 2002). Considering that MAP-2 plays an important role in the growth of neuronal dendrites, it is often used as a growth marker for neuronal processes. Our results showed that, on the 9th day after stroke, the immune activity of MAP-2 in the surrounding area of the infarct in the tPCS group was significantly increased compared with the area at day 1 after stroke, which indicates that tPCS repairs synaptic dendritic sites through MAP-2 and induces changes in neuronal plasticity. These findings are similar to those of previous studies. For example, one tDCS study reported that an 0.2 mA current intensity over the M1 region in a rat model of stroke increased the expression of MAP-2, which suggests that anode tDCS may have the potential to modulate dendritic plasticity in the ischemic penumbra (Yoon et al., 2012). Another study found that compared with untreated rats with stroke, fully implantable cortical electrical stimulation induced a significant increase in the density of MAP-2 in the damaged peripheral cortex of a rat model of stroke and a shorter time for rats with stroke to return to preoperative weight levels (Zhou et al., 2010).
The results of this study showed that tPCS could promote the expression of GAP-43 around the damaged cortex in rats with ischemic brain injury, demonstrating that tPCS could improve the motor function of rats. GAP-43 is the material basis of neural tissue and is involved in the growth, regeneration, and functional development of central and peripheral nerves (Harada et al., 2002). Therefore, GAP-43 is considered as an intrinsic determinant of the growth period of neurons and can be used as a marker of nerve growth and regeneration. Additionally, GAP-43 is an important mediator of axon development (Benowitz and Routtenberg, 1997). In the present study, we found that tPCS can significantly promote the expression and synthesis of GAP-43 in rat brains, further promote the growth and regeneration of axons in the injured area, and can enhance the recovery of injured nerves. On the 9th day after cerebral ischemia-reperfusion in the tPCS group, the immune activity of GAP-43 in the infarcted area was significantly higher than that in MCAO group, which increased during the early post-ischemic period. This suggests that from axonal sprouting to synapse formation, the immune response of GAP-43 in the ischemic penumbra and motor cortex increased significantly on the 9th day after focal cortical infarction. Moreover, this increase was most pronounced in the tPCS group, which indicates that tPCS promotes the development of axons within the ischemic penumbra and motor cortex by promoting GAP-43 expression and neuronal plasticity. This finding is similar to those of previous studies. For example, tDCS has been reported to enhance the expression of GAP-43 in peri-lesional areas in acute cerebral infarction rats, thereby promoting motor function recovery (Yoon et al., 2012).
GFAP is a major glial intermediate filament in mature astrocytes and an important component of the astrocyte cytoskeleton during development (Hol and Pekny, 2015). GFAP is mainly expressed in astrocytes of the central nervous system. Astrocytes play several key roles in supporting, guiding, and nourishing neuronal structures, as well as in signaling activities. GFAP is involved in many important central nervous system processes, including cellular communication and blood-brain barrier functions. GFAP has also been shown to be a potential biomarker for various diseases, such as traumatic brain injury and stroke, and some studies have suggested that GFAP expression is reduced following ischemic stroke (Parker et al., 2002). However, we found that the immune response around the infarction was increased following stroke, which is consistent with previous results (Sims and Yew, 2017). Previous studies have shown that low-intensity (0.1 mA) and 5-minute anode-tPCS can induce a calcium increase in astrocyte somatic cells and glial cell areas and can increase neural plasticity. It has also been reported that anode-tPCS of glial cells has a regulatory effect (Ma et al., 2019).
To conclude, our results indicate that tPCS can promote the recovery of motor function in ischemia-reperfusion rats, which may be related to tPCS-induced regulation of the expression of GAP-43, MAP-2, and GFAP, thereby promoting synaptic plasticity of neurons. However, our western blot analysis included a sample size of four, which may be the main reason why no significant difference was observed in the results. Our research group will continue to study the effect of tPCS in the treatment of stroke to provide a solid theoretical basis to explain its mechanism. At present, tPCS is seldom used in stroke treatment, and studies on influence on neuroplasticity in genomics, proteomics, and histochemistry have been lacking. We conducted an exploratory in vivo study of the effect of tPCS on the expression of nerve-related proteins around the infarct focus of cerebral infarction rats. To better understand the effect of tPCS on neurons, in vitro experiments will be necessary in the future.
Additional files:
Additional file 1: Open peer review report 1 (84.1KB, pdf) .
Additional file 2 (153.2KB, pdf) : Original data of the experiment.
Footnotes
P-Reviewer: Jin X; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Cason N, Yu J, Song LP; T-Editor: Jia Y
Conflicts of interest: All authors declare that there is no conflict of interest regarding the publication of this manuscript.
Financial support: This work was supported by the National Key R&D Program of China, No. 2018YFC2001600 (to CLS); the Shanghai Health Commission Accelerated the Development of Traditional Chinese Medicine Three-Year Action Plan Project, No. ZY(2018-2020)-CCCX-2001-06/2004-05 (to CLS); the Program of Shanghai Academic Research Leader, No. 19XD1403600 (to CLS); and the National Natural Science Foundation of China for the Youth Project, No. 81704163 (to JJZ). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was approved by the Animal Use and Management Committee of Shanghai University of Traditional Chinese Medicine on February 22, 2019 (approval No. PZSHUTCM190315003).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Xiaoming Jin, Indiana University School of Medicine, USA.
Funding: This work was supported by the National Key R&D Program of China, No. 2018YFC2001600 (to CLS); the Shanghai Health Commission Accelerated the Development of Traditional Chinese Medicine Three-Year Action Plan Project, No. ZY(2018-2020)-CCCX-2001-06/2004-05 (to CLS); the Program of Shanghai Academic Research Leader, No. 19XD1403600 (to CLS); and the National Natural Science Foundation of China for the Youth Project, No. 81704163 (to JJZ).
References
- 1.Alon G, Yungher DA, Shulman LM, Rogers MW. Safety and immediate effect of noninvasive transcranial pulsed current stimulation on gait and balance in Parkinson disease. Neurorehabil Neural Repair. 2012;26:1089–1095. doi: 10.1177/1545968312448233. [DOI] [PubMed] [Google Scholar]
- 2.Antipova D, Eadie L, Macaden A, Wilson P. Diagnostic accuracy of clinical tools for assessment of acute stroke: a systematic review. BMC Emerg Med. 2019;19:49. doi: 10.1186/s12873-019-0262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbati SA, Cocco S, Longo V, Spinelli M, Gironi K, Mattera A, Paciello F, Colussi C, Podda MV, Grassi C. Enhancing plasticity mechanisms in the mouse motor cortex by anodal transcranial direct-current stimulation: the contribution of nitric oxide signaling. Cereb Cortex. 2020;30:2972–2985. doi: 10.1093/cercor/bhz288. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 5.Bieber M, Gronewold J, Scharf AC, Schuhmann MK, Langhauser F, Hopp S, Mencl S, Geuss E, Leinweber J, Guthmann J, Doeppner TR, Kleinschnitz C, Stoll G, Kraft P, Hermann DM. Validity and reliability of neurological scores in mice exposed to middle cerebral artery occlusion. Stroke. 2019;50:2875–2882. doi: 10.1161/STROKEAHA.119.026652. [DOI] [PubMed] [Google Scholar]
- 6.Burgoyne RD, Cumming R. Characterisation of microtubule-associated proteins at the synapse: absence of MAP 2. Eur J Cell Biol. 1983;30:154–158. [PubMed] [Google Scholar]
- 7.Burke MJ, Fried PJ, Pascual-Leone A. Transcranial magnetic stimulation: Neurophysiological and clinical applications. Handb Clin Neurol. 2019;163:73–92. doi: 10.1016/B978-0-12-804281-6.00005-7. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy JM, Cramer SC. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res. 2017;8:33–46. doi: 10.1007/s12975-016-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farah CA, Leclerc N. HMWMAP2: new perspectives on a pathway to dendritic identity. Cell Motil Cytoskeleton. 2008;65:515–527. doi: 10.1002/cm.20284. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald PB. Transcranial pulsed current stimulation: a new way forward. Clin Neurophysiol. 2014;125:217–219. doi: 10.1016/j.clinph.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Fomenko A, Lozano AM. Neuromodulation and ablation with focused ultrasound - toward the future of noninvasive brain therapy. Neural Regen Res. 2019;14:1509–1510. doi: 10.4103/1673-5374.255961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goedert M, Crowther RA, Garner CC. Molecular characterization of microtubule-associated proteins tau and MAP2. Trends Neurosci. 1991;14:193–199. doi: 10.1016/0166-2236(91)90105-4. [DOI] [PubMed] [Google Scholar]
- 14.Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hetze S, Römer C, Teufelhart C, Meisel A, Engel O. Gait analysis as a method for assessing neurological outcome in a mouse model of stroke. J Neurosci Methods. 2012;206:7–14. doi: 10.1016/j.jneumeth.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32:121–130. doi: 10.1016/j.ceb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Iglesias-Rey R, Castillo J. New strategies for ischemic stroke: internal photobiomodulation therapy. Neural Regen Res. 2020;15:1658–1659. doi: 10.4103/1673-5374.276328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa Y, Okada M, Honda A, Ito Y, Tamada A, Endo N, Igarashi M. Phosphorylation sites of microtubule-associated protein 1B (MAP 1B) are involved in axon growth and regeneration. Mol Brain. 2019;12:93. doi: 10.1186/s13041-019-0510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaberzadeh S, Bastani A, Zoghi M. Anodal transcranial pulsed current stimulation: A novel technique to enhance corticospinal excitability. Clin Neurophysiol. 2014;125:344–351. doi: 10.1016/j.clinph.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Johnson GV, Jope RS. The role of microtubule-associated protein 2 (MAP-2) in neuronal growth, plasticity, and degeneration. J Neurosci Res. 1992;33:505–512. doi: 10.1002/jnr.490330402. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser EE, West FD. Large animal ischemic stroke models: replicating human stroke pathophysiology. Neural Regen Res. 2020;15:1377–1387. doi: 10.4103/1673-5374.274324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Han SJ. Anodal transcranial direct current stimulation provokes neuroplasticity in repetitive mild traumatic brain injury in rats. Neural Plast. 2017;2017:1372946. doi: 10.1155/2017/1372946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, Jang YN, Kim JY, Kim N, Noh S, Kim H, Queenan BN, Bellmore R, Mun JY, Park H, Rah JC, Pak DTS, Lee KJ. Microtubule-associated protein 2 mediates induction of long-term potentiation in hippocampal neurons. FASEB J. 2020;34:6965–6983. doi: 10.1096/fj.201902122RR. [DOI] [PubMed] [Google Scholar]
- 24.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 25.Lu Q, Huang G, Chen L, Li W, Liang Z. Structural and functional reorganization following unilateral internal capsule infarction contribute to neurological function recovery. Neuroradiology. 2019;61:1181–1190. doi: 10.1007/s00234-019-02278-x. [DOI] [PubMed] [Google Scholar]
- 26.Ma Z, Du X, Wang F, Ding R, Li Y, Liu A, Wei L, Hou S, Chen F, Hu Q, Guo C, Jiao Q, Liu S, Fang B, Shen H. Cortical plasticity induced by anodal transcranial pulsed current stimulation investigated by combining two-photon imaging and electrophysiological recording. Front Cell Neurosci. 2019;13:400. doi: 10.3389/fncel.2019.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milanovic D, Pesic V, Loncarevic-Vasiljkovic N, Avramovic V, Tesic V, Jevtovic-Todorovic V, Kanazir S, Ruzdijic S. Neonatal propofol anesthesia changes expression of synaptic plasticity proteins and increases stereotypic and anxyolitic behavior in adult rats. Neurotox Res. 2017;32:247–263. doi: 10.1007/s12640-017-9730-0. [DOI] [PubMed] [Google Scholar]
- 28.Notturno F, Pace M, Zappasodi F, Cam E, Bassetti CL, Uncini A. Neuroprotective effect of cathodal transcranial direct current stimulation in a rat stroke model. J Neurol Sci. 2014;342:146–151. doi: 10.1016/j.jns.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Parker MW, Chen Y, Hallenbeck JM, Ford BD. Neuregulin expression after focal stroke in the rat. Neurosci Lett. 2002;334:169–172. doi: 10.1016/s0304-3940(02)01126-6. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Academic Press/Elsevier; 2007. [Google Scholar]
- 31.Pilurzi G, Ginatempo F, Mercante B, Cattaneo L, Pavesi G, Rothwell JC, Deriu F. Role of cutaneous and proprioceptive inputs in sensorimotor integration and plasticity occurring in the facial primary motor cortex. J Physiol. 2020;598:839–851. doi: 10.1113/JP278877. [DOI] [PubMed] [Google Scholar]
- 32.Sims NR, Yew WP. Reactive astrogliosis in stroke: Contributions of astrocytes to recovery of neurological function. Neurochem Int. 2017;107:88–103. doi: 10.1016/j.neuint.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Sours C, Alon G, Roys S, Gullapalli RP. Modulation of resting state functional connectivity of the motor network by transcranial pulsed current stimulation. Brain Connect. 2014;4:157–165. doi: 10.1089/brain.2013.0196. [DOI] [PubMed] [Google Scholar]
- 34.Yao ZK, Wu ZP, Sun GX. Microtubule-associated protein-2:regulating neuronal development, structural stability, projection formation and synaptic plasticity. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:6010–6016. [Google Scholar]
- 35.Yavari F, Jamil A, Mosayebi Samani M, Vidor LP, Nitsche MA. Basic and functional effects of transcranial electrical stimulation (tES)-An introduction. Neurosci Biobehav Rev. 2018;85:81–92. doi: 10.1016/j.neubiorev.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Yoon KJ, Oh BM, Kim DY. Functional improvement and neuroplastic effects of anodal transcranial direct current stimulation (tDCS) delivered 1 day vs. 1 week after cerebral ischemia in rats. Brain Res. 2012;1452:61–72. doi: 10.1016/j.brainres.2012.02.062. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Q, Zhang Q, Zhao X, Duan YY, Lu Y, Li C, Li T. Cortical electrical stimulation alone enhances functional recovery and dendritic structures after focal cerebral ischemia in rats. Brain Res. 2010;1311:148–157. doi: 10.1016/j.brainres.2009.11.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.