Alzheimer’s disease and mild cognitive impairment biomarkers: Alzheimer’s disease (AD) is a progressive neurodegenerative disease of advancing age. It affects around 47 million people in the world and the number is estimated to increase to 152 million by 2050. Someone develops dementia every three seconds and the current annual cost of dementia is estimated at US $1 trillion, a figure set to double by 2030 (Alzheimer’s Disease International, 2019).
Biomarkers for chronic neurodegenerative disorders such as AD hold diagnostic importance, since the often diffuse cognitive symptoms overlap with other disorders and the clinical progression is slow and variable even between patients with the same disease. Biomarkers for monitoring brain pathophysiology can be used for clinical diagnosis, especially in the early stages, to predict disease progression and monitor effects of novel drug candidates in clinical trials and basic research, to better understand the pathogenesis of the disease (Blennow et al., 2016).
The recent introduction of cerebrospinal fluid-based biomarkers (beta-amyloid, tau proteins) and neuroimaging (amyloid positron emission tomography, PET) has greatly improved diagnostic accuracy (Blennow et al., 2016). But because PET imaging is expensive and accessible only in specialized centers, it is unlikely to become a diagnostic tool in routine assessment. While cerebrospinal fluid (CSF) collection is a routine procedure in clinical neurology, and the cost for AD CSF biomarker tests is much lower than for PET studies, lumbar puncture can be complicated, time-consuming, and invasive (Wattamwar and Mathuranath, 2010). Blood samples are easier to collect than CSF, which makes screening of potential early diagnostic markers from blood an attractive alternative. A major challenge in developing blood biomarkers is that the concentration of brain-specific proteins that reflect AD molecular mechanisms are much lower in the blood than in the CSF. For example, the CSF level of the neuronal protein tau is around 2–300 pg/mL, whereas the plasma level is around 5 pg/mL, or about 100 times lower (Patel et al., 2011). Further, while amyloid beta and tau proteins reflect central pathogenic mechanisms of the disease, other emerging pathophysiological aspects of AD could suggest the use of novel markers that are able to monitor additional molecular mechanisms of AD development and can be obtained by non-invasive procedures (Patel et al., 2011; Blennow et al., 2016).
A key event in the pathogenesis of AD is neuroinflammatory processes characterized by microglia activation and increased pro-inflammatory cytokine production (Kinney et al., 2018). Increased expression of interleukin-1 β (IL-1β) in the brains of AD patients can promote the formation of amyloid plaques and the accumulation of neurofibrillary filaments (Lee et al., 2009), thus linking IL-1 β to the pathogenesis of the disease. The neuroinflammatory state accompanying AD and the concurrent involvement of the peripheral immune system, may promote leukocyte division and telomere shortening in relation to astrocyte and microglia proliferation (Liu et al., 2016).
Researchers and clinicians have increasingly recognized that it is essential not only to define a clinically and biologically well-characterized diagnosis for AD, but also to identify individuals at an increased risk of developing AD or in the prodromal stages of the disease so that prevention, early intervention, and monitoring can be implemented to achieve better clinical results. Mild cognitive impairment (MCI), considered the transition stage between normal ageing and dementia, is defined as a cognitive decline greater than that expected for age and educational level but that does not interfere notably with activities of daily life. While some biomarkers associated with the progression from MCI to AD have been identified (positive amyloid PET, abnormal CSF tau levels), research is still in its early stages and there is the need to identify novel and/or more specific predictive biomarkers (Jongsiriyanyong and Limpawattana, 2018). Novel potential markers of transition from MCI to AD include elevated levels of inflammatory cytokines (Kinney et al., 2018) and shortened leukocyte telomere length (LTL) (Scarabino et al., 2017). High levels of IL-18 in the CSF and a marked increase in circulating sIL-1R2 and free IL-18 are hallmark characteristics of MCI, and, as they decrease in AD, they may be suitable markers for assessing the progression from MCI to AD (Ojala et al., 2009). IL-1β and IL-18 are AD-associated cytokines. Their specificity for AD and MCI consists in the association between these two cytokines and shorter LTL, with a precise temporal modulation linked to the progression of the disease.
Telomeres, the terminal ends of chromosomes, play a role in preserving genome stability. Due to the “end replication problem”, telomeres may not be fully replicated when cells divide, so they tend to shorten over time (Blackburn et al., 2015). A cellular multi-protein complex, called telomerase, counteracts telomere shortening, but its activity, usually present in the early stages of embryonic development, is silenced in several human somatic tissues immediately after birth. As a consequence, in replicating cells of adult tissues, the telomeres shorten progressively with increasing age (Blackburn et al., 2015). This phenomenon may indicate cellular senescence and reflect an organism’s biological age. Leukocytes are an easily accessible source of cells to analyze telomere length. Shortened LTL has been associated with aging and various age-related diseases, including cardiovascular disease, AD, and stroke-associated dementia. Studies have demonstrated rapid leukocyte telomere shortening in conditions associated with increased systemic oxidative stress and chronic inflammation (Blackburn et al., 2015). When LTL was investigated in neurodegenerative diseases, shorter LTL was frequently found in association with cognitive decline/dementia and AD (Liu et al., 2016; Scarabino et al., 2017).
With this in mind, we evaluated the diagnostic and predictive value of a panel of blood biomarkers that reflect directly (cytokine) or indirectly (LTL) the inflammatory process in MCI and AD patients.
Serum interleukins and LTL as predictive biomarkers for MCI and AD: In a previous study (Scarabino et al., 2017), we analyzed LTL in MCI (n = 46) and AD (n = 61) patients and healthy matched controls (n = 56). We found that the mean LTL differed significantly (P < 0.0001) across the three groups (control LTL > MCI LTL > AD LTL), suggesting a definite relationship between telomere length reduction and AD development. This pattern was corroborated by the observation that progressive telomere shortening was associated with increasing cognitive impairment, as measured by Mini-Mental State Examination scores, with the lowest LTL values noted in the subjects with a clinical diagnosis of AD. This suggested that leukocyte telomere shortening could be a marker of disease progression, possibly reflecting the inflammatory state that accompanies disease development from prodromal states (MCI) to full-blown AD.
On the hypothesis that inflammation underlies leukocyte telomere attrition, in our most recent paper (Scarabino et al., 2020) we examined two proinflammatory cytokines, IL-1β and IL-18, as markers of the inflammasome activation that accompanies the neurodegenerative process, and we sought to determine their relationship with LTL in MCI and AD patients. Plasma IL-1β and IL-18 were assayed in 21 controls (age: 56.71 ± 4.4 years, sex: 42% females), 54 MCI (age: 76.1 ± 6.0 years, sex: 61.1% females) and 33 AD (age: 79.5 ± 6.2 years, sex: 58% females) patients. The proportion of subjects with detectable serum IL-1β was significantly higher in the AD (94.1%) and the MCI (79.6%) patients than in the controls (36.8%) (P < 0.0001) (Figure 1A).
Figure 1.
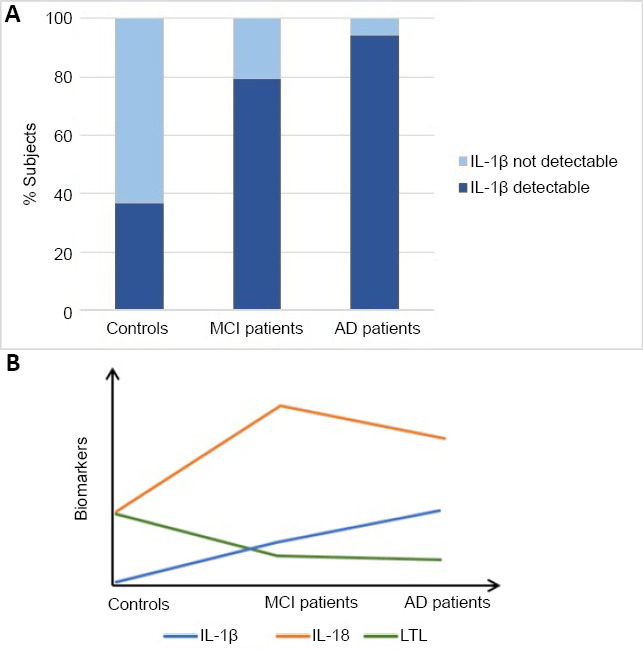
Proportion of subjects with detectable IL-1β and time course of biomarkers.
(A) % detectability of plasma IL-1β in controls, MCI and AD patients; (B) time course of the three biomarkers expression during disease progression. The LTL decreases during disease progression, the IL-18 levels show a peak during MCI and IL-1β increases during disease progression. The figure is sourced from the authors’ laboratory. AD: Alzheimer’s disease; IL: interleukin; LTL: leukocyte telomere length; MCI: mild cognitive impairment.
Plasma median IL-1β levels were higher in the MCI 2.2 pg/mL (0.07–2.9), P = 0.02) and the AD (4 pg/mL (2.4–7.4), P = 0.001) patients than in the controls 0 pg/mL (0.0–4.4), and higher in the AD than in the MCI patients (P = 0.02). Detectability of plasma IL-1β was associated with lower median Mini-Mental State Examination scores (n = 75, median 26.03, q1=21.2, q3 = 29.2) than in subjects in which IL-1β was not detectable (n = 12, median 29.03, q1 = 27.5, q3 = 30.0, P < 0.05) (Corbo RM, personal communication). A different picture emerged from the analysis of plasma IL-18: the highest median cytokine levels were observed in the MCI group (116.3 pg/mL (85.8–209.7), higher than in the AD group 85.8 pg/mL (29.3–158.5), P = 0.04), and much higher than in the controls 17.6 pg/mL (0.0–90.6), P = 0.0004.
LTL was determined in the subjects in which serum IL-1β and IL-18 levels were assayed. A significant difference in LTL between controls, MCI, and AD patients was observed (P < 0.0001), confirming previous observations (Scarabino et al., 2017) that LTL lower than age-matched controls could be a marker of MCI/AD and cognitive impairment.
When we examined the relationships between LTL and serum IL-1β and IL-18 levels, a significant negative linear relationship (P = 0.02) was observed between serum IL-1β levels and LTL (adjusted for age). Consistently higher mean LTL values (0.88 ± 0.13 T/S) were observed in the subjects in which IL-1β was not detectable compared to those in which it was detected (0.78 ± 0.16 T/S, P = 0.01). These results provide an in vivo link between telomere shortening and serum IL-1β levels, an inflammation marker that appears to be associated with Aβ plaque formation in AD brains already at the early stages (Lee et al., 2009). In contrast, no significant relationship was detected between plasma IL-18 levels and LTL in the whole sample, probably due to the non-progressive increasing trend of IL-18 in the three groups.
Taken together, our data seem to indicate that detectability of IL-1β, a sudden increase in IL-18, and a LTL shortening greater than expected for age, are typical signs of MCI and are detectable already at an early stage. Likewise, progressive LTL shortening, together with a further increase in IL-1β and a decrease in IL-18, could herald the progression of MCI toward AD (Figure 1B).
Conclusions and future perspectives: Our study findings suggest LTL and plasma IL-1β and IL-18 as novel blood-based markers of MCI onset and transition from MCI to AD. Analysis using these markers requires taking a peripheral blood sample, i.e. a simple procedure to follow disease evolution and to evaluate treatment efficacy. Further studies involving larger samples are desirable to confirm the present results. Studies with a follow-up design are needed to determine whether, and to what extent, LTL and/or IL-1β and IL-18 assays are comparable with the main CSF biomarkers used in the diagnosis of AD, such as amyloid-β42 (Aβ42), amyloid-ß 40 (Aβ40), total tau protein (t-tau), and phosphorylated tau protein (p -tau).
Finally, from a more general point of view, our data elucidate the relationships between LTL and inflammatory cytokines in MCI and AD, and lead to a partial reconceptualization of MCI and AD not only as neurodegenerative disorders but also as “total body” conditions characterized by accelerated cell aging.
The present work was supported by Sapienza University of Rome (2017/2018 grants allotted to RB and RMC).
We wish to thank E. Mantuano, M. Peconi, E. Maggi, F. Armeli and M. Morello for their contributions, K.A. Britsch for checking the manuscript style.
Footnotes
C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Blackburn EH, Epel ES, Lin J. Human telomere: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 2.Blennow K, Biscetti L, Eusebi P, Parnetti L. Cerebrospinal fluid biomarkers in Alzheimer’s and Parkinson’s diseases-From pathophysiology to clinical practice. Mov Disord Off J Mov Disord Soc. 2016;31:836–847. doi: 10.1002/mds.26656. [DOI] [PubMed] [Google Scholar]
- 3.International AD. World Alzheimer Report 2019: Attitudes to dementia | Alzheimer’s Disease International. [Accessed August 23, 2020]. Available at: https://www.alz.co.uk/research/worldreport-2019 .
- 4.Jongsiriyanyong S, Limpawattana P. Mild cognitive impairment in clinical practice: a review article. Am J Alzheimers Dis Other Demen. 2018;33:500–507. doi: 10.1177/1533317518791401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement Transl Res Clin Interv. 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KS, Chung JH, Choi TK, Suh SY, Oh BH, Hong CH. Peripheral cytokines and chemokines in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2009;28:281–287. doi: 10.1159/000245156. [DOI] [PubMed] [Google Scholar]
- 7.Liu M, Huo YR, Wang J, Wang C, Liu S, Liu S, Wang J, Ji Y. Telomere shortening in Alzheimer’s disease patients. Ann Clin Lab Sci. 2016;46:260–265. [PubMed] [Google Scholar]
- 8.Ojala J, Alafuzoff I, Herukka SK, van Groen T, Tanila H, Pirttilä T. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol Aging. 2009;30:198–209. doi: 10.1016/j.neurobiolaging.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Patel S, Shah RJ, Coleman P, Sabbagh M. Potential peripheral biomarkers for the diagnosis of Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011:572495. doi: 10.4061/2011/572495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarabino D, Broggio E, Gambina G, Corbo RM. Leukocyte telomere length in mild cognitive impairment and Alzheimer’s disease patients. Exp Gerontol. 2017;98:143–147. doi: 10.1016/j.exger.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Scarabino D, Peconi M, Broggio E, Gambina G, Maggi E, Armeli F, Mantuano E, Morello M, Corbo RM, Businaro R. Relationship between proinflammatory cytokines (Il-1beta, Il-18) and leukocyte telomere length in mild cognitive impairment and Alzheimer’s disease. Exp Gerontol. 2020;136:110945. doi: 10.1016/j.exger.2020.110945. [DOI] [PubMed] [Google Scholar]
- 12.Wattamwar PR, Mathuranath PS. An overview of biomarkers in Alzheimer’s disease. Ann Indian Acad Neurol. 2010;13:116. doi: 10.4103/0972-2327.74256. [DOI] [PMC free article] [PubMed] [Google Scholar]


