Abstract
Microglia are brain-resident immune cells that contribute to the maintenance of brain homeostasis. In the epileptic brain, microglia show various activation phenotypes depending on the stage of epileptogenesis. Therefore, it remains unclear whether microglial activation acts in a pro-epileptic or anti-epileptic manner. In mesial temporal lobe epilepsy, one of the most common form of epilepsies, microglia exhibit at least two distinct morphologies, amoeboid shape and ramified shape. Amoeboid microglia are often found in sclerotic area, whereas ramified microglia are mainly found in non-sclerotic area; however, it remains unclear whether these structurally distinct microglia share separate roles in the epileptic brain. Here, we review the roles of the two distinct microglial phenotypes, focusing on their pro- and anti-epileptic roles in terms of inflammatory response, regulation of neurogenesis and microglia-neuron interaction.
Keywords: epilepsy, epileptogenesis, inflammatory cytokines, microglia, neurogenesis, neuron-microglia interaction, seizure
Introduction
Microglia are brain-resident immune cells with highly motile processes that persistently survey surrounding environment. Microglial roles as immune cells such as the removal of cell debris as well as cytokine release are crucial to maintain brain homeostasis (Sierra et al., 2010). However, released proinflammatory cytokines from chronically activated microglia (Qin et al., 2007) as well as acutely activated microglia (Vezzani et al., 1999; Morin-Brureau et al. 2018) could also induce brain diseases. In the epileptic brain, microglia are often activated in the sclerotic areas. However, the role of ramified microglia, i.e., morphologically non-activated microglia, in the non-sclerotic or non-epileptic-focus regions in the epileptic brain remains largely unclear. Here we will briefly review the roles of two distinct microglial phenotypes, i.e., activated and non-activated microglia, focusing on their pro- and anti-epileptic roles (Figure 1).
Figure 1.
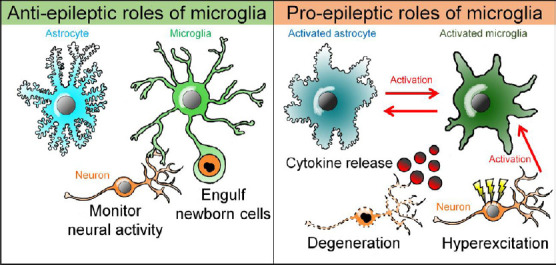
A dual role of microglia in the epileptic brain.
Microglia could play both anti-epileptic and pro-epileptic roles in mesial temporal lobe epilepsy. Microglia engulf excessively increased newborn cells and modulate neural activity and maintain brain homeostasis, which could act as antiepileptic. On the other hand, proinflammatory cytokines from activated microglia induce neuronal hyperactivation and death, which in turn activates microglia. Activated microglia also cause astrocytic activation through inflammatory and non-inflammatory mechanisms, and then, activated astrocyte in turn could induce microglial activation.
Microglial Activation in the Hippocampal Sclerotic Area
Epilepsy is a chronic neurological disorder with approximately 50 million patients in the world (WHO, 2019). Epilepsy is characterized by epileptic seizures including involuntary movement and loss of consciousness which result from synchronized hyperactivity of neurons. Among temporal lobe epilepsy, mesial temporal lobe epilepsy (MTLE), a syndrome in which clinical seizures originate in the medial limbic system of the hippocampus and amygdala and commonly accompanied with cases of hippocampal sclerosis, have been well-studied. The hippocampal sclerosis is primarily characterized by neuronal cell loss and chronic gliosis in hippocampal CA1 and CA3 areas. Morin-Brureau et al. (2018) investigated the difference of microglial properties between sclerotic and non-sclerotic areas in MTLE patients in terms of morphology, molecular marker, function and transcriptomic profiles, reporting that microglia were more activated with amoeboid morphology with a few processes in the sclerotic CA1 and CA3 areas than in non-sclerotic areas. The authors further examined microglial morphology and transcriptomic profile comparing between each brain region, but the relation between morphological differences and transcriptomic profile was not analyzed. Transcriptomic profile analysis also suggested that the expression level of proinflammatory cytokines were increased in microglia in the sclerotic areas, suggesting that activated microglia in sclerotic areas produce proinflammatory cytokines.
Properties of microglia in the epileptic brain have been extensively studied using well-established rodent models of MTLE which are developed by inducing status epilepticus (SE) with administration of convulsive drugs such as the kainite-class ionotropic glutamate receptor agonist kainic acid (KA). In KA models, the production and secretion of inflammatory cytokines and molecules including interleukin (IL)-1β, CXCL8, and TNF-α were elevated in microglia after seizures (Morin-Brureau et al., 2018). Further, it is possible that IL-1β released from microglia increased the excitability of neurons and prolonged KA-induced seizures, because the pre-treatment of IL-1β prolonged KA-induced seizures, a phenomenon blocked by co-treatment of N-methyl-D-aspartate antagonists (Vezzani et al., 1999).
Microglial Role in Epileptogenesis without Inflammatory Activation
Above studies have suggested that microglia are activated and play pro-epileptic roles in the brain of rodent MTLE models. Thus, neuronal hyperactivation and neuronal loss result in the activation of microglia in the epileptic brain, and then, the secretion of proinflammatory cytokines from activated microglia in turn induces neuronal hyperactivation and neuronal loss. Whether neurons or microglia are activated first in the epileptic brain is an open question. The question of what causes the first seizure is one of the remaining questions in epilepsy research. For example, traumatic brain injury (TBI), which is thought to be one of the causes of epilepsy, could cause glial activation first, triggering neuronal excitation. In contrast, in case of epilepsy with a genetic cause that is related to neuronal hyperexcitation, neuronal hyperactivation may precede glial activation.
Zhao et al. (2018) showed that microglia could contribute to epileptogenesis without inflammatory activation, using Tsc1Cx3cr1 conditional knock out (CKO) mice, in which mTOR, serine/threonine kinase, signaling was selectively elevated in microglia. In Tsc1Cx3cr1 CKO mice, microglia exhibited reactive-like morphologies and increased proliferation. While proinflammatory cytokines were highly expressed in the hippocampus of Tsc1Cx3cr1 CKO mice, the expression of proinflammatory cytokines in purified microglia was decreased. This suggests that in Tsc1Cx3cr1 CKO mice, microglia did not contribute to inflammatory response and other cell types, such as neurons and astrocytes, produced proinflammatory cytokines. Moreover, noninflammatory changes in microglia induced the increase of reactive-like (GFAP and complement 3-positive) astrocytes. Although the detailed mechanisms remain unknown, Tsc1Cx3cr1 CKO mice developed spontaneous recurrent seizures by 5 weeks of age. These results suggest that the activation of microglial mTOR signaling may contribute to the induction of spontaneous seizures independent of microglia-derived proinflammatory cytokines.
Anti-Epileptic Role of Microglia
Although most studies have focused on pro-epileptic roles of microglia, microglia may play anti-epileptic roles in MTLE. In rodent models of MTLE, SE acutely and transiently enhances neurogenesis in the subgranular zone of the dentate gyrus. Increased newborn granule cells after SE display abnormal outgrowth of dendrites and axons as well as ectopic positioning of soma, leading to formation of hyperexcitable aberrant neural circuits (Zhou et al., 2019). Luo et al. (2016) reported that microglia increased the expression of lysosomal marker CD68 after SE induction by intraperitoneal KA-injection. The CD68-positive activated microglia engulfed excess newborn cells in the dentate gyrus shortly after SE. Pharmacological inhibition of microglial activation by minocycline reduced microglial engulfment of newborn cells and survived newborn cells frequently located in the ectopic position. Furthermore, Matsuda et al. (2015) reported that activation of microglial Toll-like receptor 9 (TLR9) after KA-induced seizures attenuated aberrant neurogenesis. They reported that self-DNA from degenerated neurons stimulates microglial TLR9, inducing microglial activation and TNF-α release, which alleviate aberrant neurogenesis. In addition to TLR9, microglia express various types of TLRs and downstream signaling of TLRs, such as IL-1β and IL-6, could affect neurogenesis. Thus, further studies on the roles of microglial TLRs in the neurogenesis after epileptic seizures should be conducted. These results suggest that microglia maintain dentate circuitry homeostasis in the epileptic brain. Because one of the most important functions of microglia is synaptic maintenance in the non-epileptic brain (Schafer et al., 2012), it is possible that microglia maintain circuitry homeostasis in the epileptic brain via regulation of synaptic pruning and formation.
Araki et al. (2019) also showed that KA application to mouse hippocampal slice cultures reproduced neuronal cell loss and microglial activation in CA3 areas, similar to the situation in in vivo KA-injected mice. Pharmacological depletion of microglia with either clodronate or CSF1 receptor inhibitor PLX3397 significantly promoted neuronal cell loss in KA-treated hippocampal slice cultures. These results suggest that microglia play a neuroprotective role against KA-induced neuronal death, and supports microglial antiepileptic role.
Microglia-Neuron Interaction in the Epileptic Brain
It is now well-confirmed that microglia continuously interact with neurons and monitor neural activity (Pósfai et al., 2019). However, it remains unknown whether and how microglia respond to neuronal hyperactivity in the epileptic brain. Neuronal activity is altered in the epileptic brain, but the changes in microglial-neuron interactions induced by the altered neuronal activity remain largely unclear. The studies presented below may help us to understand neuronal activity-induced changes in microglia-neuron interactions in the epileptic brain. Liu et al. (2012) have been reported that neural activity attracts microglial processes and induces the formation of microglial bulbous endings, which are crucial structures for microglia to interact with neuronal soma and synapses. Recently, a new form of neuron-microglia interaction was reported. Cserép et al. (2020) found that microglial processes directly interacted with neuronal somatic membrane, and the authors named this specialized nanoarchitecture as ‘somatic microglial junction’. Although most studies have focused on the neuronal axons and dendrites in microglia-neuron interactions, Cserép et al. (2020) showed that microglial processes contacted with soma more stably than with dendrites, and that microglia monitored neuronal activity through somatic microglial junction. Almost all microglia contacted neuronal somatic membranes at sites where the clusters of potassium channels Kv2.1 and Kv2.2 existed. Kv2.1 and Kv2.2 are associated with the formation of endoplasmic reticulum-plasma membrane junctions, therefore it is possible that microglia scan the neuronal state through signals released by exocytosis. Moreover, the formation of somatic microglial junction was dependent on the microglial purinergic receptors, P2Y12 receptors. Neuronal activity-dependent release of ATP stimulates P2Y12 receptors in microglia and promotes the outgrowth of microglial processes. Somatic coverage by microglial processes were rapidly increased when neuronal activity was enhanced by using the chemogenetic technique designer receptors exclusively activated by designer drugs (DREADD). These results suggest that microglia survey neuronal activity through somatic microglial junction. But it is also possible that microglia survey neuronal activity via other ways such as direct contact wish synapses.
Eyo et al. (2014) reported that ATP, released from neuron after SE, increased interaction between neurons and microglia through P2Y12 signals and that P2Y12 knock out mice displayed increase of seizure score and early onset of seizures after intraperitoneally KA injection. Avignone et al. (2008) reported that after KA-induced SE, microglial processes exhibited higher motility and microglial responses for P2Y12 receptor agonist were increased. Moreover, Li et al. (2012) reported that microglia more frequently contacted neuronal soma with higher spontaneous activity and that microglial contacts inhibited visually-evoked neuronal activity in zebrafish. It remains unclear whether this observation is the same somatic junction reported by Cserép et al. (2020) in which Kv2.1 protein clustering and mitochondrial accumulation were observed in neurons.
Perspectives on Glial Communication in the Epileptic Brain
Microglia may play both anti-epileptic and pro-epileptic roles in the epileptic brain. The role of microglia could change depending on the steps or stages of pathogenesis. Temporal activation of microglia after SE may exert anti-epileptic effects by inhibiting the formation of aberrant neural circuits, neuronal death and hyperactivation of neurons (Eyo et al., 2014; Luo et al., 2016). On the other hand, microglial chronic activation may exert pro-epileptic effects by inducing neuronal hyperactivity and neuronal cell loss through inflammatory responses (Vezzani et al., 1999). Most of the studies highlighted in the current perspective mainly utilized KA-induced rodent MTLE models and it should be carefully considered that microglial activation state is very likely affected by the methods to induce epileptic seizures. Although the origin of epileptic pathogenesis could be different between animal models and epilepsy patients, the common pathologies, such as gliosis and neuronal loss, are found in animal models. Moreover, changes of gene expression are partially similar. Common gene expression patterns of microglia between animal models and human samples have been reported; for example, the expression levels of IL-1β and TNF-α are commonly increased (Ravizza et al., 2008; Morin-Brureru et al., 2018). Investigation of epileptogenesis with human samples is difficult. Thus, proper combination of the data from animal models and human samples is important.
In addition to microglia-neuron interactions, it is also important to study the role of astrocytes and their interactions with neurons and microglia in epilepsy. Astrocytes are the major glial cells, and play critical roles in brain homeostasis through release of neurotrophic and inflammatory factors. For example, astrocytic thrombospondins mediate synaptogenesis and ephrin-B mediate neurogenesis. Although microglia acutely respond to SE, microglial activation is usually transient. Microglial activation could subsequently trigger astrocytic activation, and activated astrocytes release proinflammatory cytokines and increase neuronal excitability. For example, first, LPS-stimulated microglia activated astrocytes via P2Y1R by releasing ATP. Then, the release of glutamate from activated astrocytes enhanced neuronal excitability (Pascual et al., 2012). In various neuropathological conditions, microglia are often activated at an earlier stage than astrocytes during the process of disease development. Proinflammatory cytokines from activated microglia induce activation of astrocytes, and activated astrocytes further promote microglial activation (Alibhai et al., 2018). Therefore, activated astrocytes may induce chronic microglial activation in MTLE. It is also possible that glial communication between microglia and astrocytes is involved in microglial dual functions in epilepsy. We can only obtain a limited information when observing one brain cell type alone in the epileptic brain. To better understand the role of microglia in the epileptic brain, it is important to take a broader perspective that includes the surrounding environment of microglia, rather than focusing on microglia alone. Microglia regulate their own functions in response to the activation of other brain cells such as neurons, astrocytes, and oligodendrocytes in the surrounding environment. It is also possible that microglia form microglial network to transmit information each other to share the inflammatory state of remote brain regions. The removal and formation of synapses by microglia in the epileptic brain is an area of particular interest because the overexcitement of neuronal circuits, which is the cause of epilepsy, is caused by a disruption of the excitatory-inhibitory balance at the synapses.
Footnotes
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported in part by a Grant-in-Aid for Scientific Research (B) (17H03988 to RK) from JSPS and JST PRESTO (JPMJPR18H4 to RK).
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported in part by a Grant-in-Aid for Scientific Research (B) (17H03988 to RK) from JSPS and JST PRESTO (JPMJPR18H4 to RK).
References
- 1.Alibhai JD, Diack AB, Manson JC. Unravelling the glial response in the pathogenesis of Alzheimer’s disease. FASEB J. 2018;32:5766–5777. doi: 10.1096/fj.201801360R. [DOI] [PubMed] [Google Scholar]
- 2.Araki T, Ikegaya Y, Koyama R. Microglia attenuate the kainic acid-induced death of hippocampal neurons in slice cultures. Neuropsychopharmacol Rep. 2020;40:85–91. doi: 10.1002/npr2.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avignone E, Ulmann L, Levavasseur F, Rassendren F, Audinat E. Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci. 2008;28:9133–9144. doi: 10.1523/JNEUROSCI.1820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cserép C, Pósfai B, Lénárt N, Fekete R, László ZI, Lele Z, Orsolits B, Molnár G, Heindl S, Schwarcz AD, Ujvári K, Környei Z, Tóth K, Szabadits E, Sperlágh B, Baranyi M, Csiba L, Hortobágyi T, Maglóczky Z, Martinecz B, et al. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science. 2020;367:528–537. doi: 10.1126/science.aax6752. [DOI] [PubMed] [Google Scholar]
- 5.Dubé CM, Ravizza T, Hamamura M, Zha Q, Keebaugh A, Fok K, Andres AL, Nalcioglu O, Obenaus A, Vezzani A, Baram TZ. Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci. 2010;30:7484–7494. doi: 10.1523/JNEUROSCI.0551-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J Neurosci. 2014;34:10528–10540. doi: 10.1523/JNEUROSCI.0416-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23:1189–1202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Luo C, Koyama R, Ikegaya Y. Microglia engulf viable newborn cells in the epileptic dentate gyrus. Glia. 2016;64:1508–1517. doi: 10.1002/glia.23018. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda T, Murao N, Katano Y, Juliandi B, Kohyama J, Akira S, Kawai T, Nakashima K. TLR9 signalling in microglia attenuates seizure-induced aberrant neurogenesis in the adult hippocampus. Nat Commun. 2015;6:6514. doi: 10.1038/ncomms7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morin-Brureau M, Milior G, Royer J, Chali F, Le Duigou C, Savary E, Blugeon C, Jourdren L, Akbar D, Dupont S, Navarro V, Baulac M, Bielle F, Mathon B, Clemenceau S, Miles R. Microglial phenotypes in the human epileptic temporal lobe. Brain. 2018;141:3343–3360. doi: 10.1093/brain/awy276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 2012;109:E197–E205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pósfai B, Cserép C, Orsolits B, Dénes Á. New Insights into Microglia-Neuron Interactions: A Neuron’s Perspective. Neuroscience. 2019;405:103–117. doi: 10.1016/j.neuroscience.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 13.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni MG. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Epilepsy Fact sheet No 999. 2012. [Accessed September 29, 2020]. Updated Jun 2019. http://www.who.int/mediacentre/factsheets/fs999/en/
- 19.Zhao X, Liao Y, Morgan S, Mathur R, Feustel P, Mazurkiewicz J, Qian J, Chang J, Mathern GW, Adamo MA, Ritaccio AL, Gruenthal M, Zhu X, Huang Y. Noninflammatory changes of microglia are sufficient to cause epilepsy. Cell Rep. 2018;22:2080–2093. doi: 10.1016/j.celrep.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou QG, Nemes AD, Lee D, Ro EJ, Zhang J, Nowacki AS, Dymecki SM, Najm IM, Suh H. Chemogenetic silencing of hippocampal neurons suppresses epileptic neural circuits. J Clin Invest. 2019;129:310–323. doi: 10.1172/JCI95731. [DOI] [PMC free article] [PubMed] [Google Scholar]


