The striatum is the primary input structure of the basal ganglia, which participates in motivational and goal-directed behaviors (Pisani et al., 2007). In physiological conditions, local cholinergic interneurons (ChIs) and dopaminergic afferents modulate basal ganglia output through striatal projection neurons, also called medium spiny neurons (MSNs). In general, the release of the neurotransmitters dopamine (DA) and acetylcholine (ACh) elicits contradictory effects on MSNs, which express their corresponding DA receptors (DARs) and muscarinic acetylcholine receptors (mAChRs), respectively (Ztaou and Amalric, 2019). Recently, we discovered a novel receptor-receptor interaction (i.e., heteromerization) between the dopamine D2 receptor (D2R) and the muscarinic acetylcholine M1 receptor (M1R), both expressed at striatopallidal MSNs (Crans et al., 2020). The putative striatal D2R-M1R complex coordinates a sophisticated interplay between the dopaminergic and cholinergic neurotransmission systems. Fuxe et al. (2012) foresaw that the existence of this heteromer within the striatum would mechanistically justify the use of anticholinergics in Parkinson’s disease (PD) treatment, thus opening up the development of novel pharmacotherapeutic strategies for PD management. As a proof of concept, we demonstrated that an M1R-selective antagonist (i.e., VU0255035, 10 mg/kg, i.p.) potentiated the antiparkinsonian-like efficacy of an ineffective D2R-selective agonist dose (i.e., sumanirole, 3 mg/kg, i.p.) in a rodent model of experimental Parkinsonism (Crans et al., 2020). Overall, the novel D2R-M1R heteromer could serve as a specific drug target to alleviate motor deficits in PD, whereas it may avoid major adverse effects associated with traditional pharmacotherapies.
The dorsal striatum is innervated by excitatory thalamic and cortical glutamatergic afferents and nigrostriatal DA-projecting neurons, where the latter is known to modulate the cortical-basal ganglia-thalamic circuit. Importantly, GABAergic MSNs are the only striatal efferent projections and descend to basal ganglia outputs (i.e., globus pallidus pars interna and substantia nigra pars reticulata) by two pathways, a direct (monosynaptic) connection and an indirect pathway through the globus pallidus pars externa and subthalamic nucleus (Figure 1A). While the MSNs of these two efferent pathways are anatomically and morphologically identical, the expression of key genes allows to differentiate between them. Thus, the MSNs of the direct pathway (i.e., striatonigral neurons) contain the neuropeptides substance P and dynorphin and express dopamine D1 receptors (D1Rs), which are coupled to Gs/aproteins. In contrast, the MSNs belonging to the indirect pathway (i.e., striatopallidal neurons) express D2R, coupled to Gi/oproteins, adenosine A2A receptor (A2AR) and the neuropeptide enkephalin. Activating D1R-MSNs or D2R-MSNs results in an opposite effect due to the stimulation or inhibition of adenylyl cyclase, respectively. Nevertheless, the release of striatal DA from nigrostriatal DA-projections increases thalamo-cortical activity by the direct or indirect inhibition of basal ganglia outputs and, thus, voluntary motor function (Lester et al., 2010). MSNs constitute 90–95% of all striatal neurons, while the remaining population consists of local ChIs and GABAergic interneurons in the striatum. Giant, aspiny ChIs only represent 1–3% of striatal neurons, although they are responsible for the highest concentration of ACh in the brain and collude with DA inputs to regulate motor function (Ztaou and Amalric, 2019). Indeed, more basal ganglia nuclei containing ChIs are also heavily innervated by dopaminergic terminals (i.e., nucleus accumbens and olfactory tubercle), which presents a similar functional interplay between the dopaminergic and cholinergic systems (Pisani et al., 2007). In contrast to other striatal neurons, ChIs possess an intrinsic firing activity in the absence of external stimuli. These autonomous pacemakers modulate the activities of neuronal afferents, but they are primarily targeting MSNs through their widely arborizing axons and dense terminal fields. The ChIs effects are controlled by two types of receptors, namely the mAChRs and ionotropic nicotinic ACh receptors (nAChRs). Five distinct mAChRs subtypes (M1R–M5R) have been identified and classified based on their pharmacological and molecular characteristics. The excitatory M1-like receptors (M1R, M3 R and M5R) transduce their signals through Gq/11proteins, whereas the inhibitory M2-like receptors (M2 R and M4R) are coupled to Gi/oproteins. Noteworthy, the D1R-MSNs express postsynaptic M4R, while M1R s are expressed at both D1R-MSNs and D2R-MSNs. The complexity of the striatal circuitry is characterized by the variety of DARs, mAChRs and nAChRs expression as well as their subcellular location at ChIs, MSNs, nigrostriatal DA-projecting neurons, thalamostriatal and corticostriatal glutamatergic afferents (Figure 1A).
Figure 1.
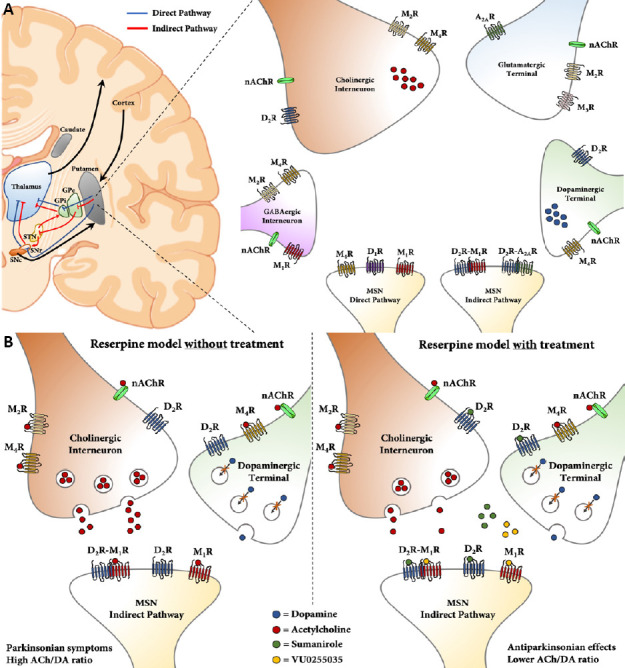
Schematic illustion of human basal ganglia circuitry and putative locations of neuronal DA and ACh receptors.
(A) The cortico-basal ganglia-thalamus circuit and striatal distribution of DA and ACh receptors at pre- and postsynaptic neurons. Schematic illustration (left) of the cortico-basal ganglia-thalamus circuit. DA-projecting neurons from the SNc release DA in the caudate/putamen (i.e., neostriatum) to activate D1R-MSNs of the direct pathway (blue lines) and inhibit D2R-MSNs of the indirect pathway (red lines). The basal ganglia output (GPi and SNr) descends their projections to the thalamus, which connects with the motor cortex. Simplified representation (right) of neurons and distribution of receptor subtypes within the striatum. The GABAergic interneuron (pink), ChI (orange), glutamatergic afferent (blue), DA-projecting neuron (green) and MSNs (yellow) express different mAChRs (M1R-M4R), nAChRs and DA receptor subtypes (D1R and D2R), emphasizing the complexity of the striatal circuitry. Multimodal strategy to alleviate parkinsonian symptoms in reserpinized mice. Reserpine depletes the DA levels within the striatum (left), resulting in a high striatal ACh/DA ratio and parkinsonian symptoms in mice. Our multimodal pharmacotherapy (right) with D2 R agonist (sumanirole) and M1R antagonist (VU0255035) alleviated parkinsonian symptoms. While targeting the putative D2R-M1R heteromer, sumanirole also actives D2 Rs at ChIs, thus contributing to the reinstatement of the ACh/DA ratio. A2AR: Adenosine A2Areceptor; ACh: acetylcholine; ChIs: cholinergic interneurons; DA: dopamine; GABA: gamma-aminobutyric acid; GPe: globus pallidus pars externa; GPi: globus pallidus pars interna; mAChR: muscarinic acetylcholine receptor; MSN: medium spiny neuron; nAChR: nicotinic acetylcholine receptors; SNc: substantia nigra pars compacta; SNr: substantia nigra pars reticulata; STN: subthalamic nucleus.
The disruption of the striatal circuitry result in basal ganglia dysfunction causing movement disorders, such as PD, dystonia, Huntington’s disease and Tourette syndrome (Pisani et al., 2007). PD is the second most common neurodegenerative disorder and is well-characterized by cardinal signs, including bradykinesia, muscular rigidity and resting tremors. The major PD pathophysiological hallmark is the progressive loss of dopaminergic afferents from the substantia nigra pars compacta, resulting in the reduction of striatal DA levels, increase in striatal ACh/DA ratio and dysregulation of ChIs transmission (McKinley et al., 2019). Early clinical studies show that both dopaminergic agonist and anticholinergic drugs provide a relief in parkinsonian rigidity and tremors, which led to the DA/ACh balance hypothesis (Ztaou and Amalric, 2019). Anticholinergics (e.g., benztropine and biperiden) were the main therapeutic agents in PD treatment before the discovery of L-3,4-dihydroxyphenylalanine (L-DOPA) and DAR agonists. Nowadays, their use is limited due to severe adverse effects (e.g., hallucinations, cognitive impairments, dry mouth, urinary retention and blurred vision) and only prescribed to relatively young patients in the early stages of PD. Indeed, DA replacement therapy with L-DOPA has been proven to be the “gold standard” to effectively manage motor deficits. However, long-term L-DOPA therapy is limited in most patients by the development of abnormal involuntary movements (i.e., L-DOPA-induced dyskinesia). Chronic L-DOPA treatment has been shown to enhance basal firing and induce stronger excitatory responses to DA in striatal ChIs (Ding et al., 2011). Overall, while many current dopaminergic and anticholinergic based treatments target multiple DARs and mAChRs subtypes simultaneously, selective drugs targeting D2 R and M1R within the striatum in a multimodal fashion may improve the patient’s quality of life during the course of the disease (McCall et al., 2005; Sheffler et al., 2009).
Interestingly, G protein-coupled receptor oligomerization has been shown to regulate receptor pharmacological responses due to receptor-receptor modulation (i.e., allosteric interaction), indirect downstream effectors (i.e., canonical interaction) and/or feedback control mechanisms. Many striatal D2R-containing heteromers have been described previously, wherein the functionality of the receptor is fine-tuned through a molecular interaction with another endogenously expressed G protein-coupled receptor (e.g., adenosine, cannabinoid and metabotropic glutamate receptors). In literature, the heteromerization between D2 R and A2A R has been well-characterized within the context of PD. Thus, a reciprocal antagonistic receptor-receptor interaction was demonstrated due to the capability of A2A R to tightly control D2 R activity and vice versa, both at allosteric and canonical interaction levels, which play a pivotal role to regulate motor function (Ferre et al., 2018). This functional interplay grounded the utility of A2A R blockade to alleviate motor deficits, which recently led to the approval of an A2AR-selective antagonist, istradefylline (Nourianz®), as an adjuvant drug in PD treatment. However, while a variety of D2R-containing complexes exist, only a few heteromers for M1R have been described in living cells (Fuxe et al., 2012). In our study, we described for the first time the existence of D2R-M1R heteromers through biophysical and biochemical cell-based assays. Subsequently, a co-distribution between these receptors in the mouse striatum was observed by double-immunofluorescence labeling. Moreover, we detected these D2R-M1R complexes with a new AlphaScreen-based assay in striatal membranes from wild-type mice, but not from D2R-deficient mice (Crans et al., 2020). The Alpha technology has recently been optimized and validated by our research group through the detection of D2R-A2A R heteromers in mice and post-mortem human brains. Importantly, our new AlphaScreen-based assay showed a high sensitivity, robustness and signal-to-background ratio, which is suitable to be implemented in high-throughput screenings (Fernandez-Duenas et al., 2019). Furthermore, the detection of D2R-A2A R heteromers was not influenced by necropsies or the variable time and conservation protocols of tissue extraction. Hence, the successful detection of the novel D2R-M1R interaction in the mouse striatum provides an opportunity to gain further insights of these complexes in healthy controls versus PD patients, whereas other receptor-receptor interaction assays (e.g., proximity ligation assay, immuno-electron microscopy and ligand fluorescence resonance energy transfer) have limitations in the assessment of heteromers due to poor signal-to-background ratio in human post-mortem brains (Fernandez-Duenas et al., 2019). Next, we demonstrated a functional interplay between the D2 R and M1R in reserpinized mice, mimicking parkinsonian motor and non-motor impairments. Reserpine irreversibly blocks the vesicular transporter of monoamines, which results in the presynaptic depletion of monoamines (DA, serotonin and noradrenaline) and, thus, an increase in striatal ACh/DA ratio (McKinley et al., 2019). The reserpine-induced motor disturbances (i.e., akinesia, catalepsy and tremulous jaw movements) were significantly alleviated by multimodal treatment with an M1R-selective antagonist (VU0255035) plus a D2R-selective agonist (sumanirole), both at suboptimal concentrations where these compounds were ineffective when administered as a stand-alone treatment (Crans et al., 2020). Noteworthy, we selected these compounds based on their high receptor subtype selectivity, where sumanirole is 200-fold more selective for D2 R and VU0255035 has a 75-fold higher selectivity for M1R over other DARs and mAChRs, respectively (McCall et al., 2005; Sheffler et al., 2009). This goal-oriented strategy aimed to target putative D2R-M1R complexes located at striatopallidal neurons in reserpinized mice, where striatal DA is depleted by more than 90% (Figure 1B). Moreover, D2 Rs are highly expressed at ChIs and their activation through sumanirole inhibits the release of ACh by ChIs. Although M4 R antagonists have proven to alleviate parkinsonian motor deficits, however, selectively targeting M1R s allows endogenously released ACh to activate M4 Rs at ChIs, which may further reinstate the ACh/DA balance in reserpinized mice (Pisani et al., 2007; McKinley et al., 2019; Ztaou and Amalric, 2019). Nevertheless, pharmacotherapeutic usefulness of targeting D2R-M1R complexes should be validated in preclinical models of parkinsonism involving dopaminergic neurodegeneration, including toxic lesion (e.g., 6-hydroxydopamine) and genetic (e.g., Pitx3ak/ak) models of PD.
Over the last decade, the interest to modulate striatal function by anticholinergic drugs has been renewed due to the development of improved pharmacological agents targeting specific mAChR subtypes (Sheffler et al., 2009; Ztaou and Amalric, 2019). Importantly, the pharmacological blockade of mAChR subtypes, more specifically M1R and M4R, have been demonstrated to alleviate antiparkinsonian deficits, whereas treatment of wild-type mice with M1R-selective agonist (i.e., telenzepine) reduced anxiety-like behaviors as well. Furthermore, M1R-deficient mice have an increased locomotor activity and elevated extracellular striatal DA levels, although these mice were not impaired in contextual fear condition, a test for hippocampus-dependent learning (Ztaou and Amalric, 2019). The D2R-MSNs are more efficiently suppressed than D1R-MSNs by M1R antagonists, which is suggested through their different subcellular expression of ion channels (e.g., potassium channels), regulated by M1R s, that modulate the MSN excitatory synaptic input. In our perspective, the putative striatal D2R-M1R formation might result in further differentiation of M1R signalization between the striatopallidal and striatonigral neurons. For instance, due to a reciprocal antagonistic D2R-M1R interaction, such as was previously described for the well-established D2R-A2A R heteromer (Ferre et al., 2018). Interestingly, the systemic administration of scopolamine (i.e., non-selective mAChR antagonist) and benztropine (i.e., moderate M1R-selective antagonist) reduced the affinity of raclopride and spiperone for D2 R in monkey brains, respectively (Tsukada et al., 2000). These findings may emphasize a reciprocal interaction between D2 Rs and M1R s, although this needs to be further studied more in-depth.
In summary, to restore the dopaminergic-cholinergic imbalance, prevalent in most movement disorders, a novel and complementary pharmacotherapeutic approach based on a multimodal strategy using selective D2 R agonist and M1R antagonist has been proposed. The use of suboptimal doses, which has no efficacy by itself, but with joint synergy as antiparkinsonians, will limit adverse effects (e.g., cognitive impairments) induced regularly by these dopaminergic and anticholinergic drugs used at optimal doses in PD treatment.
This work was supported by the Fonds Wetenschappelijk Onderzoek (FWO-SBO, Grant number 140028), Ministerio de Ciencia, Innovación y Universidades-Agencia Estatal de Investigación/FEDER (Grant number SAF2017-87349-R), Generalitat de Catalunya (Grant numbers 2017SGR1604 and 2017SGR595) and Fundació la Marató de TV3 (grant number 20152031). Moreover, RAJC was supported by an FWO Travel Grant for a Long Stay Abroad (grant number V420718N) and an EMBO Short-Term Fellowship (grant number 6735).
Additional file: Open peer review report 1 (79.8KB, pdf) .
Footnotes
P-Reviewer: Banerjee A; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Anindita Banerjee, ICARE Institute of Medical Sciences and Research and Dr BC Roy Hospital, India.
References
- 1.Crans RAJ, Wouters E, Valle-Leon M, Taura J, Massari CM, Fernandez-Duenas V, Stove CP, Ciruela F. Striatal dopamine D2-muscarinic acetylcholine M1 receptor-receptor interaction in a model of movement disorders. Front Pharmacol. 2020;11:194. doi: 10.3389/fphar.2020.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding Y, Won L, Britt JP, Lim SA, McGehee DS, Kang UJ. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc Natl Acad Sci U S A. 2011;108:840–845. doi: 10.1073/pnas.1006511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Duenas V, Gomez-Soler M, Valle-Leon M, Watanabe M, Ferrer I, Ciruela F. Revealing adenosine A2A-dopamine D2 receptor heteromers in Parkinson’s disease post-mortem brain through a new AlphaScreen-based assay. Int J Mol Sci. 2019;20:3600. doi: 10.3390/ijms20143600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferré S, Bonaventura J, Zhu W, Hatcher-Solis C, Taura J, Quiroz C, Cai NS, Moreno E, Casadó-Anguera V, Kravitz AV, Thompson KR, Tomasi DG, Navarro G, Cordomí A, Pardo L, Lluís C, Dessauer CW, Volkow ND, Casadó V, Ciruela F, et al. Essential control of the function of the striatopallidal neuron by pre-coupled complexes of adenosine A2A-dopamine D2 receptor heterotetramers and adenylyl cyclase. Front Pharmacol. 2018;9:243. doi: 10.3389/fphar.2018.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Diaz-Cabiale Z, Rivera A, Ferraro L, Tanganelli S, Tarakanov AO, Garriga P, Narvaez JA, Ciruela F, Guescini M, Agnati LF. Extrasynaptic neurotransmission in the modulation of brain function. Focus on the striatal neuronal-glial networks. Front Physiol. 2012;3:136. doi: 10.3389/fphys.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lester DB, Rogers TD, Blaha CD. Acetylcholine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:137–162. doi: 10.1111/j.1755-5949.2010.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCall RB, Lookingland KJ, Bedard PJ, Huff RM. Sumanirole, a highly dopamine D2-selective receptor agonist: in vitro and in vivo pharmacological characterization and efficacy in animal models of Parkinson’s disease. J Pharmacol Exp Ther. 2005;314:1248–1256. doi: 10.1124/jpet.105.084202. [DOI] [PubMed] [Google Scholar]
- 8.McKinley JW, Shi Z, Kawikova I, Hur M, Bamford IJ, Sudarsana Devi SP, Vahedipour A, Darvas M, Bamford NS. Dopamine deficiency reduces striatal cholinergic interneuron function in models of Parkinson’s disease. Neuron. 2019;103:1056–1072. doi: 10.1016/j.neuron.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Sheffler DJ, Williams R, Bridges TM, Xiang Z, Kane AS, Byun NE, Jadhav S, Mock MM, Zheng F, Lewis LM, Jones CK, Niswender CM, Weaver CD, Lindsley CW, Conn PJ. A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Mol Pharmacol. 2009;76:356–368. doi: 10.1124/mol.109.056531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsukada H, Harada N, Nishiyama S, Ohba H, Kakiuchi T. Cholinergic neuronal modulation alters dopamine D2 receptor availability in vivo by regulating receptor affinity induced by facilitated synaptic dopamine turnover: positron emission tomography studies with microdialysis in the conscious monkey brain. J Neurosci. 2000;20:7067–7073. doi: 10.1523/JNEUROSCI.20-18-07067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ztaou S, Amalric M. Contribution of cholinergic interneurons to striatal pathophysiology in Parkinson’s disease. Neurochem Int. 2019;126:1–10. doi: 10.1016/j.neuint.2019.02.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


