Peripheral nerve injuries (PNI) are common following blunt or penetrating trauma and can lead to disability and chronic pain in affected individuals, with limited options available to promote regeneration and functional recovery. From animal models, it is known that the regenerative capacity of the peripheral nervous system (PNS) is heavily dependent upon the remarkable ability of Schwann cells to undergo a phenotypic shift from a supportive/myelinating/maintaining phenotype to one that encourages neural regeneration. In rodents, a great deal is known about the molecular signals that control this process or mark the cells and cellular changes involved (Boerboom et al., 2017; Jessen and Mirsky, 2019). Effective translation of the wealth of animal model data into a human paradigm of nerve injury would be of great benefit in the development of improved clinical treatments. However, progress has been limited by ethical and practical challenges associated with studying human nerve injury (Hewitt et al., 2008; Wilcox et al., 2019). Moreover, the intricate anatomy and diverse range of injuries make PNI a heterogeneous pathology to study. To address this issue, in our recent article entitled Characterizing cellular and molecular features of human peripheral nerve degeneration, we analyzed nerve tissue retrieved from patients undergoing reconstructive nerve procedures (Wilcox et al., 2020). Since the patients had a range of differing time intervals between injury and surgery, it was possible to construct an impression of the phenotypic changes of Schwann cells within a population over acute and chronic time points of denervation. The findings reveal novel information about the cellular and molecular features that underpin human nerve degeneration. The patterns of changes seen in the human nerve samples were similar to those previously reported in rodent models of neural degeneration. Schwann cells adopted a repair phenotype in acutely injured nerve samples which faded over time with chronic denervation. This finding may assist clinicians to optimize the timing of surgical nerve repair, to understand how pharmacological interventions might be used to improve clinical outcomes following surgery, or perhaps to ensure that surgery is not necessary.
In order to overcome the limitations associated with current clinical treatment of nerve injury, improved understanding of nerve biology is essential. Surgical nerve repair generally involves restoring the connection between the proximal and distal segments of a damaged nerve in order to facilitate regeneration of truncated axons to their downstream target organs such as muscles and skin. Interventions are focused on the injury site, where microsurgical techniques can help provide safe passage for neurites as they extend from proximal to distal nerve stumps, reducing misdirection, tension and fibrosis. However, it is well accepted that the extent and quality of functional outcome depends not only on a robust number of axons successfully crossing the lesion, but on their subsequent journey downstream, supported by the environment within the degenerated distal nerve tissue. Extension of neurites is slow and inevitably the longer time required for the repair of more proximal lesions in longer nerves, or where there is a delay in repair, results in reduced functional recovery compared with shorter regeneration distances and rapid repair (He et al., 2014; M et al., 2014). Decisions about when and how to repair or reconstruct nerves must be made with these considerations in mind, yet there is little neurobiological information available that quantifies the extent and timing of the changes that occur in human nerve tissue following injury.
Meanwhile, the understanding of peripheral nerve biology in rodents has advanced considerably in recent decades, with particular attention being focused on unravelling the cellular and molecular events in the distal nerve following injury, which govern the extent to which it provides a permissive microenvironment for regeneration (Sulaiman and Gordon, 2000). Schwann cells are the predominant cellular component of peripheral nerves and rodent studies have shown that changes in these cells are likely to underpin the potential of denervated nerve tissue to support regeneration. Denervation in rodents causes affected Schwann cells to switch to a pro-repair phenotype, involving expression changes in many thousands of genes over a distinctive temporal sequence (Jessen and Arthur-Farraj, 2019). This repair phenotype is supportive of regeneration but is transient, persisting for about 2 months in most rodent studies then fading, with reduced regeneration support coinciding with a decline in key neurotrophic factors and repair cell markers (Jessen and Mirsky, 2019).
There is no doubt that rodent models have been extremely useful in helping to extend the boundaries of knowledge in this field, but attempts to extrapolate from rodent studies into human nerve repair scenarios are fraught with challenges (Standring, 2020). Animal models tend to use young healthy subjects with no additional damage or disease, little variability between individuals, standardized and homogenous injury patterns and shorter nerve regeneration lengths and durations than in humans. They also permit a range of outcome measures with which to probe cellular and molecular changes over time which cannot be performed in humans recovering from nerve injury (Rayner et al., 2020). To understand how the wealth of information gained from animal studies relates to the human situation, it is important to try and identify species-specific adaptions and similarities in the cellular and molecular responses seen following injury. In an attempt to start to address this we explored the presence of four biomarkers, associated with changes in rodent Schwann cell phenotype, in human tissue obtained from patients following nerve injury (Wilcox et al., 2020). These included the transcription factor c-Jun which is a pivotal mediator of the Schwann cell phenotypic shift (Arthur-Farraj et al., 2012), the neurotrophin receptor p75NTR which is upregulated in repair Schwann cells, the transcription factor EGR2 which is associated with myelination and the pan-Schwann cell marker SOX10.
Human nerve tissue samples are challenging to obtain since most nerve repair procedures focus on preserving tissue during surgery and repairing nerves as soon as possible after damage. Our study capitalized on an opportunity to obtain acutely and chronically denervated tissue from patients undergoing nerve transfer procedures, in which excess tissue (which would normally be disposed of) was used for histological and gene expression analysis. For gene expression analysis using real-time quantitative polymerase chain reaction, we first needed to develop a protocol that enabled sufficient yields of mRNA to be extracted, which requires rapid processing and careful avoidance of contact with antiseptic reagents used in the operating theater (Wilcox et al., 2019). Rather than being a problematic uncontrollable variable, the heterogeneity in the duration of denervation experienced by the individuals in the study provided a way to start to understand the time course of changes in the biomarkers.
The hypothesis for the study was that characteristic changes in Schwann cells, specifically expression of c-Jun, p75NTR, ERG2 and SOX10, identified in rodent studies would also be present in denervated human nerves, and regulated after injury in a similar way to that seen in rodents. The study showed that markers associated with repair Schwann cells in rodents, c-Jun and p75NTR were upregulated in acutely injured human nerves and then declined during long term denervation. There were also changes in Schwann cell numbers, with increased Schwann cell density in acutely denervated tissue which then reduced in chronic denervation. These changes mirror the pattern seen in rodents, suggesting that basic molecular features associated with regeneration are conserved between these species. The timing for phenotypic changes in the Schwann cells of human nerve tissue appeared to follow a pattern whereby the initial switch to repair phenotype was detected at the earliest time points explored (4–50 days), then declined after 100 days (Figure 1). This decline is a significant reason for regeneration failure, and is also seen in rodents where it has been characterized in some detail. Repair cells do, however, appear to deteriorate rather faster in rodents than humans, most studies indicating that by 100 days there is already a substantial reduction in expression of c-Jun, p75NTR and trophic factors such as brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor, cell numbers and regenerative support.
Figure 1.
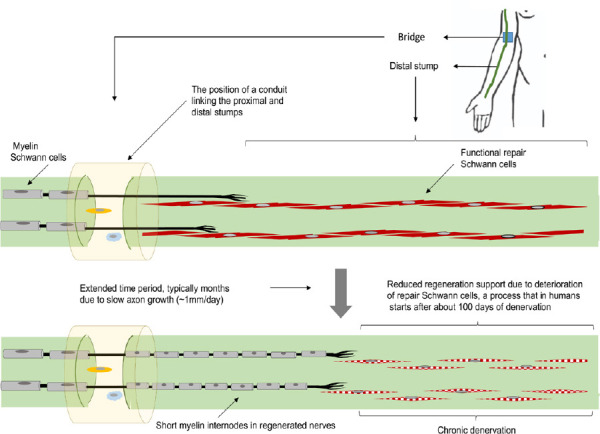
Changes in the Schwann cell population in chronically degenerated human nerve tissue.
Following nerve injury and repair, shown here as a nerve transection with a bridge to join the severed stumps, Schwann cells initially adopt a functional repair phenotype which is associated with regeneration support. Where there is chronic denervation of the distal nerve stump, deterioration of the repair Schwann cells begins, diminishing the capacity of the nerve tissue to support neuronal regeneration.
The use of surplus human tissue samples from a relatively small yet diverse patient population can only provide limited information, but this study helps to bridge between laboratory models and nerve injury in humans. The predicted clinical time frame for optimal functional recovery following nerve injury and repair in humans has largely been based on knowledge about what happens in denervated muscles, which have been shown to require sufficient reinnervation within 1 year. Our study indicates that the capacity to support regeneration of denervated nerve tissue distal to an injury may reduce over a considerably shorter time frame. Understanding this process, and assessment of individual variation in this, will guide clinical decision making between repair or reconstructive options for nerve injury. It will allow stratification of associated implications and risks versus likely mean outcome and allow greater patient education and shared decision making.
In addition to helping extrapolate findings from basic scientific animal studies towards application in human medicine, studies such as this help inform the direction of laboratory-based translational research. This is important to consider. New technologies emerging from the field of regenerative medicine have the potential to revolutionize treatment options for nerve injury, but there is a long history of potential new treatments that are effective in animal models failing to benefit patients (Standring, 2020). Increased understanding about how the distal nerve segment can provide regeneration support, and how this declines over time, presents researchers with an exciting array of new opportunities for therapeutic interventions. Such interventions are inevitably developed and tested in the well-established in vitro and in vivo research models that have proved fruitful in revealing details of nerve biology. However, for new approaches to translate into useful treatments they need to work effectively in humans, so studies which help to identify similarities and differences between rodent and human responses to nerve injury are an important contribution.
The study we report focuses on a small number of markers of Schwann cell phenotype and does not correlate this with functional recovery. It would be interesting in future studies to explore exactly how the duration of denervation and consequent changes in Schwann cell phenotype in the distal nerve influences motor and sensory recovery in patients. It would also be valuable to explore additional changes in human Schwann cells to understand the similarities and differences compared with rodents, e.g. by transcriptome analysis. The results of our study show a general pattern of change over time but if additional samples could be tested this could be refined and improved, for example to reveal details about the timing of initial molecular events leading to phenotypic switch. By understanding the cell signalling events that control the increase then decrease of the pro-regenerative environment in damaged nerves, pharmacological approaches to enhance or prolong the favorable environment can be developed. In conclusion, our exploration of human peripheral nerve regeneration revealed that hallmark changes in Schwann cell phenotype known to occur in rodents are also present in humans, providing an important translational link between fundamental nerve biology research and future applications in delivering improved patient care and clinical outcomes.
The present work was funded by the Royal National Orthopaedic Hospital Charitable Trust, England Golf Trust, Engineering and Physical Sciences Research Council (EP/R004463/1) and a UCL Graduate Research Scholarship (to MBW).
Footnotes
C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
Author statement: The abstract has been presented at a scientific meeting (International Symposium on Peripheral Nerve Repair (ISPNR)) in 2019.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boerboom A, Dion V, Chariot A, Franzen R. Molecular mechanisms involved in Schwann cell plasticity. Front Mol Neurosci. 2017;10:38. doi: 10.3389/fnmol.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He B, Zhu Z, Zhu Q, Zhou X, Zheng C, Li P, Zhu S, Liu X, Zhu J. Factors predicting sensory and motor recovery after the repair of upper limb peripheral nerve injuries. Neural Regen Res. 2014;9:661–672. doi: 10.4103/1673-5374.130094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hewitt SM, Lewis FA, Cao Y, Conrad RC, Cronin M, Danenberg KD, Goralski TJ, Langmore JP, Raja RG, Williams PM, Palma JF, Warrington JA. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2008;132:1929–1935. doi: 10.5858/132.12.1929. [DOI] [PubMed] [Google Scholar]
- 5.Jessen KR, Arthur-Farraj P. Repair Schwann cell update: adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia. 2019;67:421–437. doi: 10.1002/glia.23532. [DOI] [PubMed] [Google Scholar]
- 6.Jessen KR, Mirsky R. The success and failure of the Schwann cell response to nerve injury. Front Cell Neurosci. 2019;13:33. doi: 10.3389/fncel.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.M FG, M M, S H, Khan WS. Peripheral nerve injury: principles for repair and regeneration. Open Orthop J. 2014;8:199–203. doi: 10.2174/1874325001408010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayner MLD, Brown HL, Wilcox M, Phillips JB, Quick TJ. Quantifying regeneration in patients following peripheral nerve injury. J Plast Reconstr Aesthet Surg. 2020;73:201–208. doi: 10.1016/j.bjps.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Standring S. The history of nerve repair. In: Phillips JB, Hercher D, Hausner T, editors. Peripheral nerve tissue engineering and regeneration. Switzerland: Springer Nature; 2020. pp. 1–32. [Google Scholar]
- 10.Sulaiman OA, Gordon T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia. 2000;32:234–246. doi: 10.1002/1098-1136(200012)32:3<234::aid-glia40>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox M, Quick TJ, Phillips JB. The effects of surgical antiseptics and time delays on RNA isolated from human and rodent peripheral nerves. Front Cell Neurosci. 2019;13:189. doi: 10.3389/fncel.2019.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox MB, Laranjeira SG, Eriksson TM, Jessen KR, Mirsky R, Quick TJ, Phillips JB. Characterising cellular and molecular features of human peripheral nerve degeneration. Acta Neuropathol Commun. 2020;8:51. doi: 10.1186/s40478-020-00921-w. [DOI] [PMC free article] [PubMed] [Google Scholar]


