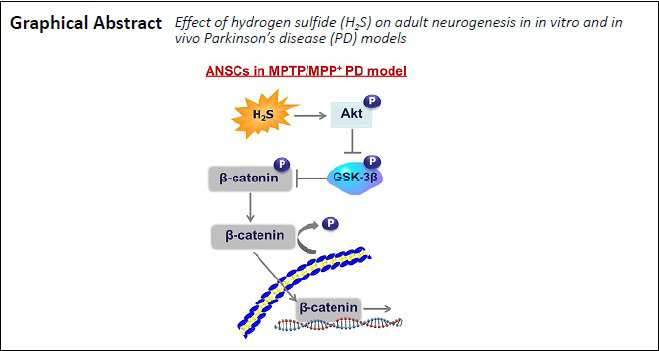
Keywords: factor, hydrogen sulfide, medical gas, neural stem cells, neurogenesis, neuroprotection, Parkinson's disease, pathways
Abstract
Hydrogen sulfide (H2S) is regarded to be a protectant against diseases of the central nervous system and cardiovascular system. However, the mechanism by which H2S elicits neuroprotective effects in the progression of Parkinson’s disease (PD) remains unclear. To investigate the role of H2S in delaying the pathological process of PD, we used the most common sodium hydrosulfide (NaHS) as an H2S donor and established a mouse model of PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid (MPTP/p) in the present study. Our results show that H2S reduced neuronal loss during the progression of PD. Notably, we found that H2S exhibited protective effects on dopaminergic neurons. Excitingly, H2S also increased the proliferation of neural stem cells in the subventricular zone. Next, we evaluated whether the neuroprotective effects of H2S on dopaminergic neurons in PD are dependent on adult nerve regeneration by treating primary adult neural stem cells cultured ex vivo with 1-methyl-4-phenylpyridine. Our results show that H2S could prevent nerve injury induced by 1-methyl-4-phenylpyridine, promote the growth of neurospheres, and promote neurogenesis by regulating Akt/glycogen synthase kinase-3β/β-catenin pathways in adult neural stem cells. These findings confirm that H2S can increase neurogenesis in an adult mouse model of PD by regulating the Akt/glycogen synthase kinase-3β/β-catenin signaling pathway. This study was approved by the Animal Care and Use Committee of Nanjing Medical University, China (IACUC Approval No. 1601153-3).
Chinese Library Classification No. R453; R741; TQ125.1+2
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease, which seriously affects the quality of life of older people (Aarsland et al., 2017; Baiano et al., 2020). Characteristics of PD include the progressive degeneration of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc) of the midbrain, which leads to dyskinesia such as muscle rigidity, bradykinesia, and tremors in PD patients (Aarsland et al., 2017; Benzagmout et al., 2019; Yakhine-Diop et al., 2019). At present, the most commonly accepted theory is that oxidative stress (Trist et al., 2019), mitochondrial dysfunction (Elfawy and Das, 2019), and accumulation of α-synuclein (Tan et al., 2020) are the primary factors inducing the loss of DA neurons. However, the precise mechanistic relationships that contribute to PD and the efficacy of therapeutics used to treat PD remain obscure. Thus, there is an urgent need to develop medications that can effectively relieve the symptoms of PD and slow the loss of DA neurons.
Hydrogen sulfide (H2S) can act in the body as a gasotransmitter, a gaseous signaling molecule that can transmit signals and induce physiological responses in various tissues, and exists widely in mammals (Cao et al., 2018; Pushchina et al., 2020). In recent decades, H2S has been connected with both physiological and pathological roles in multiple systems, such as the cardiovascular (Zhang et al., 2018), respiratory (Guan et al., 2020), and central nervous systems (Cao et al., 2018; Ma et al., 2019). Increasing evidence shows that H2S exerts anti-oxidant (Ali et al., 2019), anti-inflammatory (Wang et al., 2017), and anti-apoptotic effects in the brain (Wang et al., 2017, 2019). In the midbrain, the major enzymes involved in endogenous H2S biosynthesis are cystathionine β-synthase and cystathionine γ-lyase (Kimura et al., 2015). As the midbrain is the dominating brain region affected in the pathogenesis of PD, H2S has been suggested to be beneficial in attenuating PD-like neural damage (Cakmak, 2016; Yuan et al., 2018). Nevertheless, the relationship between protective mechanisms of H2S and the pathogenesis of PD remains largely elusive.
Adult neurogenesis is one method to combat the loss of DA neurons in the adult midbrain, which may prevent cell death and relieve dyskinesia in PD (Farzanehfar, 2018; Hain et al., 2018). The subgranular zone and subventricular zone (SVZ) are the two main neurogenic regions in the adult brain (Westerlund et al., 2005). Since the discovery of these important centers of regeneration, adult neurogenesis in the SVZ has been an area of active research (Fan et al., 2016; Dillen et al., 2020). Emerging evidence indicates that fresh DA neurons generated by neural stem cells (NSCs) in the SVZ could significantly enhance the functional performance of patients, suggesting that neurogenesis may be a feasible therapeutic strategy in adults (Le Grand et al., 2015; Fan et al., 2016; Farzanehfar, 2018). Previous studies have focused on DA neurons and how to delay neuronal degeneration by treatment with H2S (Wang et al., 2015; Yin et al., 2017). However, it remains unclear whether H2S therapy could induce neurogenesis in adults to alleviate the progression of PD.
Herein, we utilized sodium hydrosulfide (NaHS) as a donor of H2S (Cao et al., 2018; He et al., 2019), and further investigated the role of H2S in PD using a classical 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model.
Materials and Methods
Animals
All studies were approved by the Animal Care and Use Committee of Nanjing Medical University, China (IACUC Approval No. 1601153-3) on August 6, 2016. Thirty-six specific-pathogen-free adult male C57BL/6J mice aged 12–16 weeks and weighing 26–32 g, were applied in the in vivo study. Another 36 specific-pathogen-free male C57BL/6J mice aged 2–3 months were used for NSC isolation in an in vitro study. All mice were purchased from the Laboratory Animal Center of Nanjing Medical University (license No. SCXK (Su) 2013-0017). The mice were housed with free access to food and water in a room with an ambient temperature of 22 ± 2°C and a 12:12-hour light/dark cycle.
Animal model preparation
A total of 36 mice were randomly divided into three groups: saline, MPTP, and MPTP + NaHS (n = 12 mice per group). One hour after intraperitoneal injection of probenecid (250 mg/kg; Jinan Times Pharmacology, Jinan, China), which was dissolved in dimethyl sulfoxide 30 minutes before injection, MPTP (20 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) was subcutaneously injected daily for 5 days to establish the PD model. In the NaHS group, mice were intraperitoneally injected with NaHS (5.6 mg/kg; Sigma-Aldrich) for 10 days (from days 4 to 8), 30 minutes before MPTP injection (Figure 1A). The saline group was injected with the same volume (about 0.4 mL) of normal saline for 10 days. At 3.5 days after the last administration of MPTP, mice were sacrificed by cervical dislocation or 5% chloral hydrate.
Figure 1.
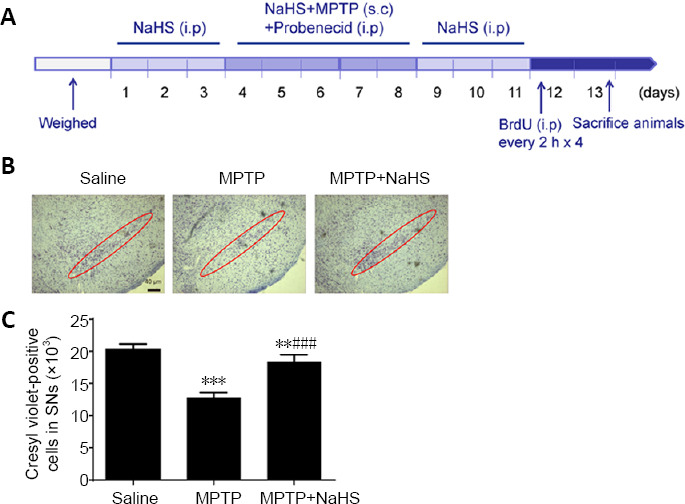
Effects of H2S on MPTP-induced PD mouse model.
(A) Schematic of protocol for establishing the MPTP mouse model of PD. (B, C) Cresyl violet staining and quantitative data for Nissl-positive neurons in the SNc (original magnification 10×, scale bar: 40 μm). Red circles represent the SNc brain region. Data are expressed as mean ± SEM (n = 6). **P < 0.01, ***P < 0.001, vs. saline group; ###P < 0.001, vs. MPTP group (one-way analysis of variance followed by Tukey’s post hoc test. All samples were detected repeatedly in three independent experiments. H2S: Hydrogen sulfide; i.p.: intraperitoneal injection; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NaHS: sodium hydrosulfide, a donor of H2S; PD: Parkinson’s disease; s.c: subcutaneously; SNc: substantia nigra pars compacta.
Nissl staining
Brain tissues were immobilized in 4% paraformaldehyde, which was prepared in 0.1 M phosphate buffer (pH 7.4) at 4°C. Midbrain tissue [according to the Atlas of Paxinos and Franklin (2013), from approximately –2.5 to –3.88 mm] was prepared into 30-μm-thick slices using a Leica cryostat (CM1860; Leica, Germany). Slices were stained with cresyl violet acetate, photographed, and counted by MicroBrightField Stereo Investigator software (MBF Bioscience, Williston, ND, USA). All histological section count data were obtained by three observers in a randomized double-blind controlled experiment.
Bromodeoxyuridine labeling
On the last day of the 5-day MPTP/probenecid cycle, mice were intraperitoneally injected with bromodeoxyuridine [BrdU; Sigma-Aldrich; 50 mg/kg four times with an interval of 2 hours (Sairanen et al., 2005)]. After the last BrdU treatment, mice were transcardially perfused with 0.1 M phosphate-buffered saline (PBS) for 5 minutes, followed by 4% paraformaldehyde. Four weeks after injection with BrdU, mice were sacrificed and perfused, and brain tissue was frozen at –70°C for subsequent calculation of the differentiation and survival of BrdU-labeled cells.
Immunofluorescence staining
The immunostaining method utilized has been described in previous literature (Qiao et al., 2017). Mice were infused with 4% paraformaldehyde after model preparation. Next, brain tissues were post-fixed in 4% paraformaldehyde at 4°C overnight, followed by transfer into 15% sucrose in PBS overnight, then 30% sucrose overnight, until the brain sank to the bottom of the tube. Brain tissue was sliced into 20-μm-thick slices using a cryostat. Brain slices were washed with PBS for 10 minutes, blocked with 5% bovine serum albumin in PBS for 1 hour, and then incubated with the appropriate primary antibody [anti-tyrosine hydroxylase (TH), a rate-limiting enzyme in the biosynthesis of dopamine, Cat# T1299, Sigma-Aldrich; anti-BrdU, a new-cell marker, Cat# MAB3510, Millipore, Boston, MA, USA; anti-Ki67, a proliferating cell marker, Cat# 610968, Becton Dickinson, Franklin Lakes, NJ, USA) overnight at 4°C. The second day, slices were incubated with the corresponding fluorescent secondary antibody [goat anti-mouse IgG-fluorescein isothiocyanate (Cat# ab150113, Abcam, Cambridge, UK) and goat anti-rabbit IgG-fluorescein isothiocyanate (Cat# ab150077) for 1 hour. Nuclei were stained with 4′,6-diamidino-2-phenylindole dye.
Image analysis
Images acquired under a confocal light microscope (Axiovert LSM510; Carl Zeiss Co., Weimar, Germany) were quantitatively analyzed for immunostaining signals using Optical fractionation and MicroBrightField Stereo Investigator software. Total numbers of Nissl+ neurons and TH+ neurons in the SNc, as well as Ki67+ cells and BrdU+ cells in the SVZ, were calculated from six different samples in each group. Before placing the coverslips, a fluorescent mounting medium was applied and allowed to dry in the dark. Specimens were examined with MicroBrightField Stereo Investigator software for counting and photography.
Adult NSC culture
Mouse adult NSCs (ANSCs) were cultured according to an established primary culture system (Fan et al., 2016). In brief, coronal section microanatomy was performed on the brains of male C57BL/6J mice aged 2–3 months to obtain the periventricular rostral region. Tissues were gently chopped and incubated with papain hydrolysate at 37°C for 30 minutes. The supernatant was carefully discarded after centrifugation, and cells were re-suspended in a serum-free medium consisting of Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (1:1) (Gibco, Grand Island, NY, USA), 20 ng/mL epidermal growth factor (Peprotech, Rocky Hill, CT, USA), 2% B27 (Gibco), and 20 ng/mL basic fibroblast growth factor (Peprotech). The main cell culture technique used in this research was the neurosphere measure (Kong et al., 2008). Cell viability was evaluated by trypan blue exclusion (0.4%; Sigma-Aldrich), and exceeded 85%. In uncoated 24-well culture plates, the primary tissue was seeded at a rate of 10,000 cells per well. After 7 days of culture, the size of neurospheres and total number formed in each well were calculated. Self-renewal was also carried out by separating large numbers of neurospheres and re-culturing the cells at a constant density of 10,000 cells per well. After 7 days of culture in vitro, the number and size of second- or third-level spheres was evaluated. Under a confocal light microscope, neurospheres with less than a minimum diameter of 40 μm were excluded. Diameters of randomly selected neurospheres were measured by Image-Pro Plus software (Media Cybernetics, Inc., Bethesda, MD, USA).
Cell proliferation detection
Proliferation was measured using a BrdU-incorporation method (Fan et al., 2016). In brief, we collected neurospheres in control, 1-methyl-4-phenylpyridiniumion (MPP+), and MPP+ + NaHS groups, and gently mechanically separated them. Separated cells were cultured for 48 hours in 24-well plates pretreated with poly-L-ornithine (10 μg/mL) and laminin (5 μg/mL). Neurospheres were cultured with 10 μM BrdU for 60 minutes and then fixed. After washing, cells were treated with 2 M HCl at 37°C for 30 minutes to denature the DNA. After washing, cells were blocked with 5% bovine serum albumin for 40 minutes, incubated at 37°C for 1 hour with primary mouse anti-BrdU antibody (1:5000; Millipore), and then stained with anti-mouse antibody conjugated to fluorescein isothiocyanate (1:1000; Invitrogen, Carlsbad, CA, USA) at 37°C for 1 hour. Nuclei were stained by 4′,6-diamidino-2-phenylindole and mounted with anti-fade medium. Under a confocal light microscope, images were acquired of 10 randomly selected visual fields; fluorescence intensity of images was calculated with Image-Pro Plus software (Media Cybernetics, Inc.).
Western blot assay
Proteins within neurospheres were extracted with homogenate buffer. An equivalent amount of protein (50 μg) was transferred to a polyvinylidene difluoride membrane by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After blocking membranes with 5% skimmed milk in PBS, they were incubated with primary antibodies including rabbit anti-proliferating cell nuclear antigen [PCNA; 1:1000; Cat# 13110; Cell Signaling Technology (CST), Boston, MA, USA], rabbit anti-Akt (1:1000; Cat# 2920; CST), rabbit anti-pAkt (1:1000; Cat# 12694; CST), rabbit anti-H3 (1:500; CST), rabbit anti-β-catenin (1:1000; Cat# 8480; CST), rabbit anti-p-β-catenin (1:1000; Cat# 2009; CST), mouse anti-glycogen synthase kinase-3β (GSK-3β; 1:1000; Cat# 21301; SAB, Nanjing, China), and mouse anti-phosphorylated GSK-3β (p-GSK-3β; 1:1000; Cat# 11301; SAB) overnight at 4°C. According to the manufa cturer’s instructions, immunoreactive proteins were examined using horseradish peroxidase-labeled secondary antibodies and ECL kits (Amersham Biosciences, Piscataway, NJ, USA). Finally, we scanned and analyzed membranes by using an Omega 16ic Chemiluminescence Imaging System (Ultra-Lum, Claremont, CA, USA).
Statistical analysis
All the data are shown as mean ± standard error of the mean (SEM). One-way analysis of variance followed by Tukey’s post hoc test was used to evaluate the statistical significance of results using Graphpad Software 5.0 (GraphPad Software, San Diego, CA, USA). P-values less than 0.05 were considered statistically significant.
Results
H2S rescues the loss of DA neurons in an MPTP mouse model of PD
To determine the role of H2S in an MPTP mouse model of PD, we used Nissl staining to evaluate neuronal injury. As demonstrated in Figure 1B, compared with the saline group, MPTP treatment decreased the total number of neurons in the SNc by 39% (F(2, 15) = 106.2, n = 6, P < 0.0001), but treatment with 5.6 mg/kg NaHS led to a loss of just 9% of neurons (F(2, 15) = 106.2, n = 6, P =0.0075; Figure 1B and C). Furthermore, we observed damage to DA neurons using TH immunostaining. In this study, we found that MPTP observably reduced the number of TH-positive cells by 43% in the SNc (F(2, 15) = 81.51, n = 6, P < 0.0001; Figure 2A and B). Similar to the results of Nissl staining, NaHS treatment clearly decreased the loss of TH-positive cells caused by MPTP, leading to only a 16% loss relative to the Saline group (F(2, 15) = 81.51, N = 6, P = 0.0008; Figure 2B). These results indicate that H2S has protective effects in the MPTP mouse model of PD, especially with regard to the loss of DA neurons.
Figure 2.
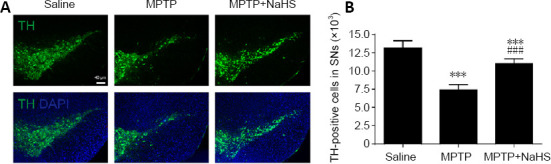
Effects of H2S on dopaminergic neurons in Parkinson’s disease mouse model.
(A) Immunohistochemical staining of TH-positive neurons (green, stained by fluorescein isothiocyanate; original magnification 10×, scare bar: 40 μm). (B) Quantitative results of TH-positive neurons in the substantia nigra pars compacta (SNc). Data are expressed as mean ± SEM (n = 6). ***P < 0.001, vs. saline group; ###P < 0.001, vs. MPTP group (one-way analysis of variance followed by Tukey’s post hoc test). All samples were detected repeatedly in three independent experiments. DAPI: 4′,6-Diamidino-2-phenylindole, blue; H2S: hydrogen sulfide; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NaHS: sodium hydrosulfide a donor of H2S; TH: tyrosine hydroxylase.
H2S attenuates inhibition of SVZ neurogenesis in the MPTP mouse model
Neurogenesis is one potential mechanism to reverse DA cell loss in the SNc of MPTP-induced PD (Lindvall and Kokaia, 2009). To evaluate the protective effect of H2S on DA neurons, the extent of neurogenesis was evaluated. Using immunostaining for Ki67, an endogenous label of cellular proliferation (Fan et al., 2016; Farzanehfar, 2018), we discovered that Ki67-positive cells were enriched by 50% (F(2, 15) = 121.0, n = 6, P < 0.0001) in the SVZ following treatment with NaHS. However, Ki67-positive cells were decreased by 58% after MPTP treatment alone (F(2, 15) = 114.4, n = 6, P < 0.0001; Figure 3A and B). There was no significant change in Ki67 expression with NaHS treatment alone (Additional Figure 1 (2.2MB, tif) ). A BrdU immunoassay was used to further illustrate the role of H2S in increasing cell proliferation (Fan et al., 2016; Farzanehfar, 2018). We noticed a meaningful reduction of BrdU-positive cells in the SVZ following treatment with MPTP (F(2, 15) = 62.89, n = 6, P < 0.0001). However, consistent with the results of Ki67 staining, H2S treatment markedly increased the number of BrdU-positive cells in MPTP mice (F(2, 15) = 62.89, n = 6, P < 0.0001; Figure 3C and D). Thus, we found that H2S could promote neurogenesis and restore the loss of cell proliferation in the SVZ induced by MPTP.
Figure 3.
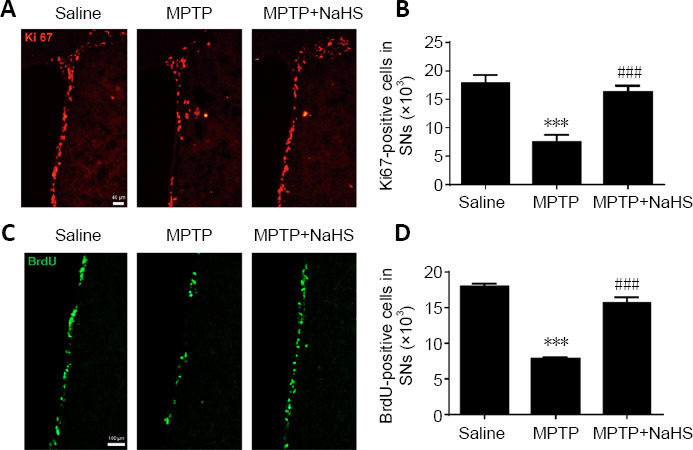
H2S promotes cell proliferation in the SVZ after MPTP-induced damage.
(A) Immunofluorescence staining of Ki67-positive (red, stained by fluorescein isothiocyanate) proliferating cells in the SVZ (original magnification 10×, scale bar: 40 μm). (B) Quantitative data for Ki67-positive cells. (C) Immunofluorescence staining of BrdU-positive (green, stained by fluorescein isothiocyanate) proliferating cells in the SVZ (original magnification 20×, scale bar: 100 μm). (D) Quantitative data for BrdU-positive cells. Data are expressed as mean ± SEM (n = 6). ***P < 0.001, vs. saline group; ###P < 0.001, vs. MPTP group (one-way analysis of variance followed by Tukey’s post hoc test). All samples were detected repeatedly in three independent experiments. BrdU: Bromodeoxyuridine; H2S: hydrogen sulfide; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NaHS: sodium hydrosulfide, a donor of H2S; SVZ: subventricular zone.
H2S promotes the proliferation of ANSCs injured by MPP+ in vitro
To further explore the role of H2S in promoting cell proliferation, we cultured adult ANSCs in vitro. As shown in Figure 4A, the number and diameter of neurospheres isolated from the SVZ brain region of WT mice were calculated. We observed no significance differences in the number of neurospheres between control and MPP+ -treated groups. In addition, NaHS treatment could slightly increase the number of neurospheres, but had no statistically significant effect (F(2, 9) = 1.386, P > 0.05; Figure 4B). Nevertheless, MPP+ inhibited the growth of neurospheres and reduced their diameter by 30% compared with the control group (F(2, 9) = 13.43, P = 0.0018). Unexpectedly, NaHS treatment alleviated the negative effects of MPP+ on neurospheres and restored the diameter to 91% of untreated groups (F(2, 9) = 13.43, P = 0.3636; Figure 4C).
Figure 4.
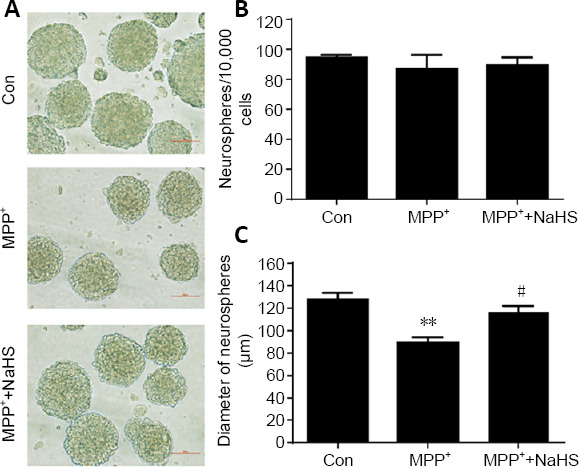
H2S relieves suppression of MPP+ -induced adult neurogenesis in vitro.
(A) Morphological observations of neurospheres in the SVZ of WT mice. Scale bar: 100 μm. (B, C) Statistical analysis showed that NaHS had no effect on the number of neurospheres, but could enlarge their diameter, which was suppressed by MPP+ stimulation. Data are expressed as mean ± SEM. **P < 0.01, vs. control group (Con); #P < 0.05, vs. MPP+ group (one-way analysis of variance followed by Tukey’s post hoc test). All experiments were repeated four independent times. H2S: Hydrogen sulfide; MPP: 1-methyl-4-phenylpyridiniumion; NaHS: sodium hydrosulfide, a donor of H2S; SVZ: subventricular zone; WT: wild type.
Furthermore, a BrdU proliferation assay was used to measure growth of the neurospheres (Fan et al., 2016; Farzanehfar, 2018). BrdU-positive cells were decreased by 52% in MPP+ -treated ANSCs (F(2, 9) = 25.61, P = 0.0003). However, NaHS treatment restored proliferation (F(2, 9) = 25.61, P = 0.0007), leading to only a 6% loss in positive cells relative to the control group (F(2, 9) = 25.61, P = 0.7154; Figure 5A and B). In conclusion, H2S had no effect on the number of cultured ANSCs, but dis increase cell proliferation to promote the growth of ANSCs.
Figure 5.
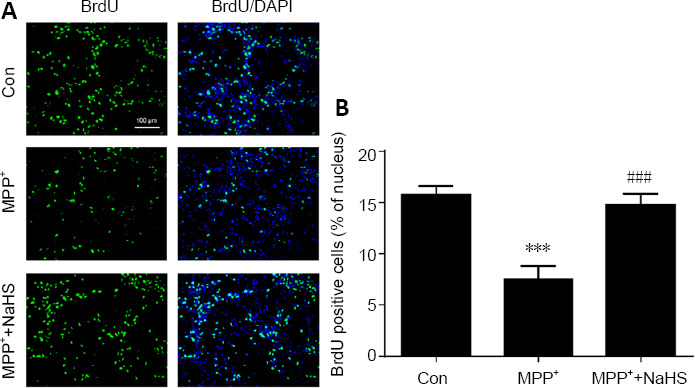
H2S restores BrdU expression in MPP+ -induced primary neurospheres.
(A) Immunofluorescence staining of BrdU-positive (green, stained by fluorescein isothiocyanate) proliferating adult neural stem cells (original magnification 20×, scale bar: 100 μm). (B) Quantitative data for BrdU-positive cells. Data are expressed as mean ± SEM. ***P < 0.001, vs. control group; ###P < 0.001, vs. MPP+ group (one-way analysis of variance followed by Tukey’s post hoc test). All experiments were repeated four independent times. BrdU: Bromodeoxyuridine; DAPI: 4′,6-diamidino-2-phenylindole; H2S: hydrogen sulfide; MPP: 1-methyl-4-phenylpyridiniumion; NaHS: sodium hydrosulfide, a donor of H2S.
H2S enhances ANSC proliferation via the Akt/GSK-3β/β-catenin pathway
To assess the role of H2S in proliferation of cultured ANSCs, protein was extracted from neurospheres and the concentration of PCNA was measured in the nucleus. PCNA was downregulated in the nuclei of MPP+ -treated ANSCs, however treatment with NaHS reversed this effect, leading to an increase in PCNA expression (F(2, 9) = 15.75, P = 0.0191; Figure 6A and B). Consistent with previous experiments, these observations corroborate the role of H2S in enhancing neurogenesis in the MPTP model of PD.
Figure 6.
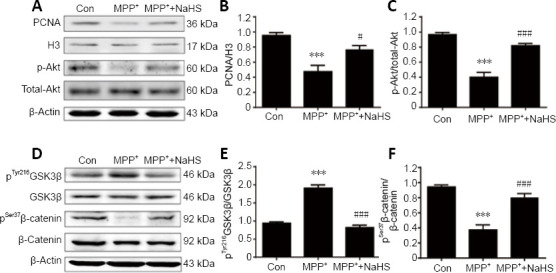
H2S enhances neurogenesis by upregulating the Akt/GSK-3β/β-catenin cascade in adult neural stem cells of the SVZ.
(A) Representative blots of PCNA, total-Akt, and p-Akt expression in adult neural stem cells. (B, C) Statistical analysis of PCNA and p-Akt expression in cell extracts. (D) Representative blots of GSK-3β, p-GSK-3β (Tyr216), β-catenin, and p-β-catenin (Ser37) expression in adult neural stem cells. (E, F) Statistical analysis of p-GSK-3β and p-β-catenin expression in cell extracts. Data are presented as mean ± SEM. ***P < 0.001, vs. control group; #P < 0.05, ###P < 0.001, vs. MPP+ group (one-way analysis of variance followed by Tukey’s post hoc test). All experiments were repeated four independent times. GSK-3β: Glycogen synthase kinase-3β; H2S: hydrogen sulfide; MPP: 1-methyl-4-phenylpyridiniumion; NaHS: sodium hydrosulfide, a donor of H2S; PCNA: proliferating cell nuclear antigen; SVZ: subventricular zone.
To explore the mechanisms by which H2S affects neurogenesis, western blotting was performed to identify differentially regulated signaling molecules. As PCNA is a target of Akt signaling, which is frequently activated in cell proliferation (Bertapelle et al., 2017), we hypothesized that the neuroprotective roles of NaHS may be promoted by Akt signaling. As shown in Figure 6A and C, MPP+ remarkably decreased expression of phosphorylated Akt (p-Akt) in NSCs by 59% (F(2, 9) = 43.84, P < 0.0001), however, this effect was attenuated by treatment with NaHS (F(2, 9) = 43.84, P = 0.0002; Figure 6C). This result supports the potential role of H2S in promoting neurogenesis through the Akt pathway.
According to the latest studies, GSK-3β is involved in the canonical Wnt signaling pathway and controls the level of β-catenin phosphorylation, thereby enhancing the self-renewal and proliferation potential of NSCs (Fidaleo et al., 2017; Horgusluoglu et al., 2017). Thus, our next step was to measure the contents of phosphorylated GSK-3β and β-catenin to evaluate whether H2S affects this pathway. In extracts of ANSCs, pGSK-3β was significantly increased after stimulation of MPP+ (F(2, 9) = 77.28, P < 0.0001; Figure 6D and E), but the level of p-β-catenin was markedly decreased (F(2, 9) = 34.63, P < 0.0001; Figure 6F). However, after treatment with H2S, expression of pGSK-3β and p-β-catenin was reversed, with a decrease in pGSK-3β (F(2, 9) = 77.28, P < 0.0001; Figure 6E) and an increase in p-β-catenin (F(2, 9) = 34.63, P = 0.0004; Figure 6F) expression compared with their total proteins (Figure 6D). These results support for the idea that H2S could influence the Akt/GSK-3β/β-catenin cascade to promote adult neurogenesis in PD.
Discussion
The SVZ is the main proliferative region of the adult brain. Accumulating evidence indicates that DA neurons produced by NSCs in the SVZ can measurably improve PD symptoms (Horgusluoglu et al., 2017). H2S is a gas that has been shown to have neuroprotective effects in PD. Nevertheless, the precise mechanisms by which H2S acts against PD remain unclear. Treatment with NaHS instantaneously generates H2S via pH-dependent salt dissociation in vivo and in vitro. In the present study, we used NaHS to produce H2S and showed that H2S could not only delay the loss of DA neurons in the SNc, but also further promoted adult neurogenesis in the SVZ of PD model animals. Further experiments suggested that H2S remarkably increased the proliferation and growth of ANSCs cultured in vitro. Excitingly, we found that H2S may regulate the Akt/GSK-3β/β-catenin cascade to enhance the growth of ANSCs. Together, these findings demonstrate a new neuroprotective role for H2S in promoting adult neurogenesis in PD.
H2S is an abundant gas in nature that plays a vital role as a signaling element in the central nervous system. The benefits of H2S in PD have recently been confirmed by accumulating reports from other labs (Cao et al., 2018; Shefa et al., 2018). Herein, we identified the molecular function of H2S in a PD model. Our results demonstrate that H2S rescued the loss of DA neurons following MPTP stimulation. Previous studies have demonstrated anti-apoptotic, anti-oxidative, and anti-inflammatory roles of H2S in PD models (Cao et al., 2018; Sarukhani et al., 2018). Nevertheless, the specific mechanism underlying the neuroprotective effects of H2S on PD has remained largely elusive.
Emerging evidence demonstrates that adult neurogenesis is beneficial for the recovery of neurons in the pathological process of PD. In the mammalian brain, the two major neurogenic zones are the SVZ of the lateral ventricles and subgranular zone of the dentate gyrus in the hippocampus (Horgusluoglu et al., 2017). Adult neurogenesis in the SVZ is mostly associated with migration of neuroblasts along the rostral migratory stream and generation of neurons in the striatum (Fidaleo et al., 2017). Expression of Ki67, a nuclear protein expressed primarily in proliferating cells (Fan et al., 2016), was fortified in the SVZ by H2S in the presence of MPTP stimulation. This result suggests that the protective effect of H2S on DA neurons may depend on promotion of nerve cell proliferation. We further found that after treatment with H2S, BrdU staining significantly increased, indicating an enhanced number of proliferating cells in the SVZ. To confirm the role of H2S in adult neurogenesis, we cultured mouse ANSCs from the SVZ under MPP+ stimulation. Following pre-treatment with H2S, we noted that MPP+ -induced inhibition of neurosphere growth was decreased, but there was no effect on the number of neurospheres. However, as expected, BrdU-labeled proliferating cells were significantly increased in cultured neurospheres following treatment with H2S. Accordingly, we clarified that H2S regulates adult neurogenesis to postpone DA neuronal degeneration in models of PD.
The Akt/GSK-3β/β-catenin cascade has recently emerged as a pivotal step in the proliferation of NSCs in the central nervous system (Fidaleo et al., 2017). Therefore, we hypothesized that H2S may control activity of the Akt/GSK-3β/β-catenin cascade to resist damage induced by MPP+ in ANSCs. Here, we found that the Akt pathway was activated and p-GSK-3β expression levels were decreased after H2S treatment. Notably, a recent study indicated that GSK-3β can be inactivated by p-Akt, and p-GSK-3β stabilizes β-catenin, which then migrates into the nucleus to mediate gene transcription enabling neurogenesis. In our study, we observed increased p-β-catenin levels in the group pre-treated with H2S compared with MPP+ treatment alone. Consistently, PCNA expression was highly upregulated along with p-β-catenin expression levels. Our findings provide evidence that H2S exerts a latent role in facilitating adult SVZ neurogenesis by activating the Akt/GSK-3β/β-catenin cascade to increase related gene transcription.
In conclusion, we demonstrated that H2S exerts neuroprotective roles in a pathological mouse model of PD. Furthermore, H2S promoted adult neurogenesis in areas of the SVZ that had been damaged by MPTP or MPP+ . Most notably, our study showed that the benefits of H2S in accelerating adult neurogenesis may be mediated by Akt/GSK-3β/β-catenin signaling activation. Further work is required to investigate whether proliferating ANSCs could be differentiated into DA neurons capable of making functional synaptic connections with neurons in a PD model or α-synuclein overexpression model. These findings will further increase our recognition of the potential of H2S as a new therapy for PD.
Additional files:
Additional Figure 1 (2.2MB, tif) : H2S promotes the expression of Ki67 in SVZ after MPTP-induced injury, but no significant effects on the expression of Ki67 in NaHS treatment alone.
H2S promotes the expression of Ki67 in SVZ after MPTP-induced injury, but no significant effects on the expression of Ki67 in NaHS treatment alone. (A) Immunofluorescence staining for Ki67-positive proliferating cells (red, stained by fluorescein isothiocyanate) in SVZ (original magnification 10×, scale bar: 40 µm). (B) Quantitative data for Ki67-positive cells. Data are presented as the mean ± SEM (n = 6). ***P < 0.001, vs. saline group; ###P < 0.001, vs. MPTP group (one-way analysis of variance followed by Tukey's multiple comparison test). All samples were detected repeatedly in three independent experiments. H2S: hydrogen sulfide; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NaHS: sodium hydrosulfide, a donor of H2S; SVZ: subventricular zone.
Additional file 1: Open peer review reports 1 (86.4KB, pdf) and 2 (86.8KB, pdf) .
Additional file 2 (51.4KB, pdf) : Original data of the experiment.
Footnotes
P-Reviewers: de Oliveira FF, Pal E; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Deusen AV, Yu J, Song LP; T-Editor: Jia Y
Conflicts of interest: The authors declare no conflict of interest.
Financial support: The study was supported by the National Natural Science Foundation of China, Nos. 81803505 (to CQ), 81803498 (to LXW); the Natural Science Foundation of Jiangsu Province of China, No. BK20170564 (to CQ), and Yong Talents Training Program of Jiangsu University of China, No. 5521471353 (to JY). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was approved by the Animal Care and Use Committee of Nanjing Medical University, China (IACUC Approval No. 1601153-3) on August 6, 2016. The animal experiment was reported according to the requirements of Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Fabricio Ferreira de Oliveira, Elysian Clinic, Brazil; Endre Pal, University of Pécs, Hungary.
Funding: The study was supported by the National Natural Science Foundation of China, Nos. 81803505 (to CQ), 81803498 (to LXW); the Natural Science Foundation of Jiangsu Province of China, No. BK20170564 (to CQ), and Yong Talents Training Program of Jiangsu University of China, No. 5521471353 (to JY).
References
- 1.Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, Ballard C. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017;13:217–231. doi: 10.1038/nrneurol.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali R, Pal HA, Hameed R, Nazir A, Verma S. Controlled release of hydrogen sulfide significantly reduces ROS stress and increases dopamine levels in transgenic C.elegans. Chem Commun (Camb) 2019;55:10142–10145. doi: 10.1039/c9cc05153h. [DOI] [PubMed] [Google Scholar]
- 3.Baiano C, Barone P, Trojano L, Santangelo G. Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: A meta-analysis. Mov Disord. 2020;35:45–54. doi: 10.1002/mds.27902. [DOI] [PubMed] [Google Scholar]
- 4.Benzagmout M, Boujraf S, Alami B, Amadou HA, El Hamdaoui H, Bennani A, Jaafari M, Rammouz I, Maaroufi M, Magoul R, Boussaoud D. Emotion processing in Parkinson’s disease: a blood oxygenation level-dependent functional magnetic resonance imaging study. Neural Regen Res. 2019;14:666–672. doi: 10.4103/1673-5374.247470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertapelle C, Polese G, Di Cosmo A. Enriched environment increases PCNA and PARP1 levels in octopus vulgaris central nervous system: first evidence of adult neurogenesis in lophotrochozoa. J Exp Zool B Mol Dev Evol. 2017;328:347–359. doi: 10.1002/jez.b.22735. [DOI] [PubMed] [Google Scholar]
- 6.Cakmak YO. Coffee consumption, smoking, and Parkinson’s disease. The beneficial role of hydrogen sulfide. Mov Disord. 2016;31:429. doi: 10.1002/mds.26526. [DOI] [PubMed] [Google Scholar]
- 7.Cao X, Cao L, Ding L, Bian JS. A new hope for a devastating disease: hydrogen sulfide in Parkinson’s disease. Mol Neurobiol. 2018;55:3789–3799. doi: 10.1007/s12035-017-0617-0. [DOI] [PubMed] [Google Scholar]
- 8.Dillen Y, Kemps H, Gervois P, Wolfs E, Bronckaers A. Adult neurogenesis in the subventricular zone and its regulation after ischemic stroke: implications for therapeutic approaches. Transl Stroke Res. 2020;11:60–79. doi: 10.1007/s12975-019-00717-8. [DOI] [PubMed] [Google Scholar]
- 9.Elfawy HA, Das B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019;218:165–184. doi: 10.1016/j.lfs.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Fan Z, Lu M, Qiao C, Zhou Y, Ding JH, Hu G. MicroRNA-7 enhances subventricular zone neurogenesis by inhibiting NLRP3/Caspase-1 axis in adult neural stem cells. Mol Neurobiol. 2016;53:7057–7069. doi: 10.1007/s12035-015-9620-5. [DOI] [PubMed] [Google Scholar]
- 11.Farzanehfar P. Comparative review of adult midbrain and striatum neurogenesis with classical neurogenesis. Neurosci Res. 2018;134:1–9. doi: 10.1016/j.neures.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Fidaleo M, Cavallucci V, Pani G. Nutrients, neurogenesis and brain ageing: From disease mechanisms to therapeutic opportunities. Biochem Pharmacol. 2017;141:63–76. doi: 10.1016/j.bcp.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Guan R, Wang J, Cai Z, Li Z, Wang L, Li Y, Xu J, Li D, Yao H, Liu W, Deng B, Lu W. Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway. Redox Biol. 2020;28:101356. doi: 10.1016/j.redox.2019.101356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hain EG, Sparenberg M, Rasińska J, Klein C, Akyüz L, Steiner B. Indomethacin promotes survival of new neurons in the adult murine hippocampus accompanied by anti-inflammatory effects following MPTP-induced dopamine depletion. J Neuroinflammation. 2018;15:162. doi: 10.1186/s12974-018-1179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He JT, Li H, Yang L, Mao CY. Role of hydrogen sulfide in cognitive deficits: Evidences and mechanisms. Eur J Pharmacol. 2019;849:146–153. doi: 10.1016/j.ejphar.2019.01.072. [DOI] [PubMed] [Google Scholar]
- 16.Horgusluoglu E, Nudelman K, Nho K, Saykin AJ. Adult neurogenesis and neurodegenerative diseases: A systems biology perspective. Am J Med Genet B Neuropsychiatr Genet. 2017;174:93–112. doi: 10.1002/ajmg.b.32429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura Y, Toyofuku Y, Koike S, Shibuya N, Nagahara N, Lefer D, Ogasawara Y, Kimura H. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci Rep. 2015;5:14774. doi: 10.1038/srep14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong H, Fan Y, Xie J, Ding J, Sha L, Shi X, Sun X, Hu G. AQP4 knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J Cell Sci. 2008;121:4029–4036. doi: 10.1242/jcs.035758. [DOI] [PubMed] [Google Scholar]
- 19.Le Grand JN, Gonzalez-Cano L, Pavlou MA, Schwamborn JC. Neural stem cells in Parkinson’s disease: a role for neurogenesis defects in onset and progression. Cell Mol Life Sci. 2015;72:773–797. doi: 10.1007/s00018-014-1774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindvall O, Kokaia Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson’s disease. Trends Pharmacol Sci. 2009;30:260–267. doi: 10.1016/j.tips.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Ma M, Wang Y, Gao N, Liu X, Sun Y, Ren J, Qu X. A near-infrared-controllable artificial metalloprotease used for degrading amyloid-β monomers and aggregates. Chemistry. 2019;25:11852–11858. doi: 10.1002/chem.201902828. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Franklin KB. San Diego: Elsevier; 2013. The mouse brain in stereotaxic coordinates. [Google Scholar]
- 23.Pushchina EV, Varaksin AA, Obukhov DK, Prudnikov IM. GFAP expression in the optic nerve and increased H2S generation in the integration centers of the rainbow trout (Oncorhynchus mykiss) brain after unilateral eye injury. Neural Regen Res. 2020;15:1867–1886. doi: 10.4103/1673-5374.280320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao C, Zhang LX, Sun XY, Ding JH, Lu M, Hu G. Caspase-1 deficiency alleviates dopaminergic neuronal death via inhibiting Caspase-7/AIF pathway in MPTP/p mouse model of Parkinson’s disease. Mol Neurobiol. 2017;54:4292–4302. doi: 10.1007/s12035-016-9980-5. [DOI] [PubMed] [Google Scholar]
- 25.Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarukhani M, Haghdoost-Yazdi H, Sarbazi Golezari A, Babayan-Tazehkand A, Dargahi T, Rastgoo N. Evaluation of the antiparkinsonism and neuroprotective effects of hydrogen sulfide in acute 6-hydroxydopamine-induced animal model of Parkinson’s disease: behavioral, histological and biochemical studies. Neurol Res. 2018;40:523–531. doi: 10.1080/01616412.2017.1390903. [DOI] [PubMed] [Google Scholar]
- 27.Shefa U, Kim MS, Jeong NY, Jung J. Antioxidant and cell-signaling functions of hydrogen sulfide in the central nervous system. Oxid Med Cell Longev. 2018;2018:1873962. doi: 10.1155/2018/1873962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan Y, Sgobio C, Arzberger T, Machleid F, Tang Q, Findeis E, Tost J, Chakroun T, Gao P, Höllerhage M, Bötzel K, Herms J, Höglinger G, Koeglsperger T. Loss of fragile X mental retardation protein precedes Lewy pathology in Parkinson’s disease. Acta Neuropathol. 2020;139:319–345. doi: 10.1007/s00401-019-02099-5. [DOI] [PubMed] [Google Scholar]
- 29.Trist BG, Hare DJ, Double KL. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell. 2019;18:e13031. doi: 10.1111/acel.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M, Tang W, Zhu YZ. An Update on AMPK in Hydrogen Sulfide Pharmacology. Front Pharmacol. 2017;8:810. doi: 10.3389/fphar.2017.00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M, Zhu J, Pan Y, Dong J, Zhang L, Zhang X, Zhang L. Hydrogen sulfide functions as a neuromodulator to regulate striatal neurotransmission in a mouse model of Parkinson’s disease. J Neurosci Res. 2015;93:487–494. doi: 10.1002/jnr.23504. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Chi Q, Hu X, Cong Y, Li S. Hydrogen sulfide-induced oxidative stress leads to excessive mitochondrial fission to activate apoptosis in broiler myocardia. Ecotoxicol Environ Saf. 2019;183:109578. doi: 10.1016/j.ecoenv.2019.109578. [DOI] [PubMed] [Google Scholar]
- 33.Westerlund U, Svensson M, Moe MC, Varghese M, Gustavsson B, Wallstedt L, Berg-Johnsen J, Langmoen IA. Endoscopically harvested stem cells: a putative method in future autotransplantation. Neurosurgery. 2005;57:779–784. [PubMed] [Google Scholar]
- 34.Yakhine-Diop SMS, Martínez-Chacón G, Uribe-Carretero E, Niso-Santano M, González-Polo RA, Fuentes JM. The paradigm of protein acetylation in Parkinson’s disease. Neural Regen Res. 2019;14:975–976. doi: 10.4103/1673-5374.250575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin WL, Yin WG, Huang BS, Wu LX. Neuroprotective effects of lentivirus-mediated cystathionine-beta-synthase overexpression against 6-OHDA-induced parkinson’s disease rats. Neurosci Lett. 2017;657:45–52. doi: 10.1016/j.neulet.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Yuan YQ, Wang YL, Yuan BS, Yuan X, Hou XO, Bian JS, Liu CF, Hu LF. Impaired CBS-H(2)S signaling axis contributes to MPTP-induced neurodegeneration in a mouse model of Parkinson’s disease. Brain Behav Immun. 2018;67:77–90. doi: 10.1016/j.bbi.2017.07.159. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Yu J, Chen Y, Liu L, Xu M, Sun L, Luo H, Wang Y, Meng G. Exogenous hydrogen sulfide supplement attenuates isoproterenol-induced myocardial hypertrophy in a sirtuin 3-dependent manner. Oxid Med Cell Longev. 2018;2018:9396089. doi: 10.1155/2018/9396089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
H2S promotes the expression of Ki67 in SVZ after MPTP-induced injury, but no significant effects on the expression of Ki67 in NaHS treatment alone. (A) Immunofluorescence staining for Ki67-positive proliferating cells (red, stained by fluorescein isothiocyanate) in SVZ (original magnification 10×, scale bar: 40 µm). (B) Quantitative data for Ki67-positive cells. Data are presented as the mean ± SEM (n = 6). ***P < 0.001, vs. saline group; ###P < 0.001, vs. MPTP group (one-way analysis of variance followed by Tukey's multiple comparison test). All samples were detected repeatedly in three independent experiments. H2S: hydrogen sulfide; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NaHS: sodium hydrosulfide, a donor of H2S; SVZ: subventricular zone.


