Supplemental Digital Content is available in the text.
Keywords: adult, atrial fibrillation, heart failure, myocardial infarction, stroke
Abstract
Background:
It is known that certain cardiovascular diseases (CVD) are associated, like atrial fibrillation and stroke. However, for other CVDs, the links and temporal trends are less studied. In this longitudinal study, we have investigated temporal epidemiological and genetic associations between different CVDs.
Methods:
The ULSAM (Uppsala Longitudinal Study of Adult Men; 2322 men aged 50 years) has been followed for 40 years regarding 4 major CVDs (incident myocardial infarction, ischemic stroke, heart failure, and atrial fibrillation). For the genetic analyses, publicly available data were used.
Results:
Using multistate modeling, significant relationships were seen between pairs of all of the 4 investigated CVDs. However, the risk of obtaining one additional CVD differed substantially both between different CVDs and between their temporal order. The relationship between heart failure and atrial fibrillation showed a high risk ratio (risk ratios, 24–26) regardless of the temporal order. A consistent association was seen also for myocardial infarction and atrial fibrillation but with a lower relative risk (risk ratios, 4–5). In contrast, the risk of receiving a diagnosis of heart failure following a myocardial infarction was almost twice as high as for the reverse temporal order (risk ratios, 16 versus 9). Genetic loci linked to traditional risk factors could partly explain the observed associations between the CVDs, but pathway analyses disclosed also other pathophysiological links.
Conclusions:
During 40 years, all of the 4 investigated CVDs were pairwise associated with each other regardless of the temporal order of occurrence, but the risk magnitude differed between different CVDs and their temporal order. Genetic analyses disclosed new pathophysiological links between CVDs.
It is well known that the presence of some cardiovascular diseases (CVD) will increase the risk for other CVDs. Examples of such well-established relationships are that atrial fibrillation is a powerful risk factor for both stroke1 and heart failure,2 and that a myocardial infarction (MI) is a strong risk factor for future heart failure.2 It is also known that prior CVD (except stroke) is associated with an increased risk of stroke,3 and that heart failure and MI are related to incident atrial fibrillation.4 Other links between the major CVDs, MI, stroke, heart failure, and atrial fibrillation are less well-known and the strength of associations between those CVDs are not well established.
Since the major common CVDs share many traditional risk factors, such as hypertension, hyperlipidemia, obesity, diabetes, and smoking, the links between pairs of these common CVDs might mainly be due to shared risk factors. It might also be that other pathophysiological pathways could be of importance.
The aim of the present study is 2-fold. First, to describe the temporal associations of the major CVDs; MI, stroke, heart failure, and atrial fibrillation. Second, to investigate shared genetics to investigate potential mechanisms linking these common diseases. Improved knowledge on the relationships between CVDs and mechanisms linking these common diseases might be useful in the secondary prevention following a first CVD.
For the first aim, we used data from the ULSAM (Uppsala Longitudinal Study of Adult Men) in which we have followed the development of these 4 major CVDs during 40 years in a population-based sample of middle-aged males.5 We investigated the life course temporal comorbidity between the major CVDs, MI, stroke, heart failure, and atrial fibrillation, by multistate modeling to calculate the risks of obtaining specific major CVDs given that the individual previously had experienced another specific major CVD.
For the second aim, we used the Mendelian randomization framework to study the genetic relationships between these 4 major CVDs using genetic data from already published genome-wide association studies (GWAS). We also evaluated the genetic overlap between the major CVDs in terms of pathway analysis of the genetic loci overlapping between CVDs.
Methods
The complete methods section is given in the beginning of the Data Supplement.
The study was approved by the Ethical Committee of Uppsala University, and each participant in ULSAM gave their informed consent.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Pairs of CVD
The incidence of the 4 investigated CVDs during the follow-up period were in the range from 340 (ischemic stroke) to 565 (atrial fibrillation) (Table 1 and Figure 1).
Table 1.
Pairwise Relationships Between 4 Major Cardiovascular Diseases
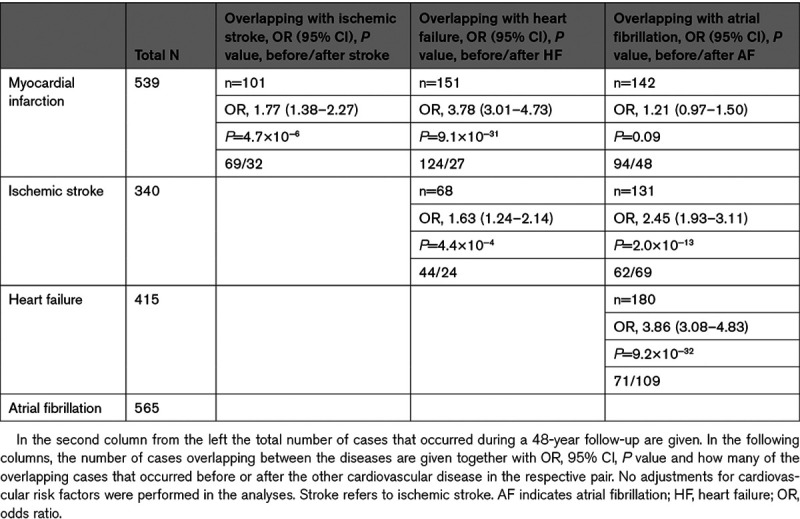
Figure 1.
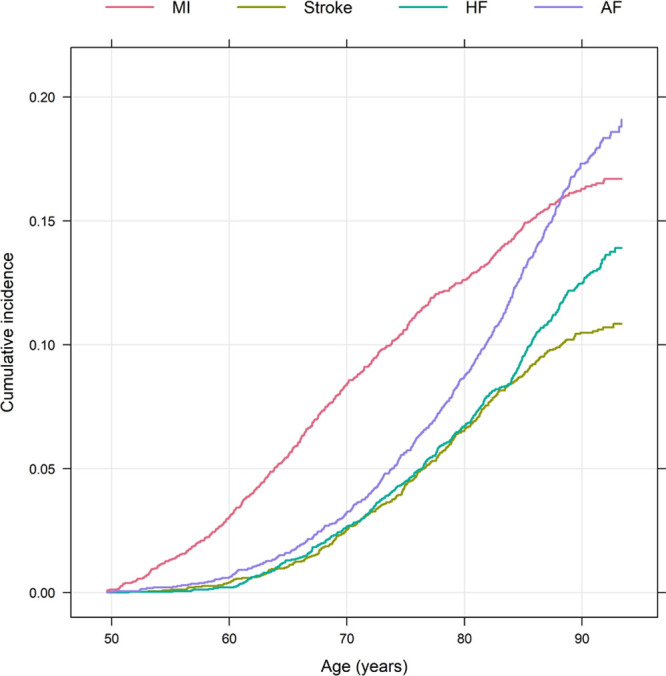
Cumulative incidence of the 4 major cardiovascular diseases, myocardial infarction (MI), ischemic stroke (Stroke), heart failure (HF), and atrial fibrillation (AF) from age 50 to 90 y.
Ignoring the temporal order of the CVDs, all of the 4 investigated CVDs were pairwise significantly associated with each other, except MI and atrial fibrillation (P=0.09). As could be seen in Table 1, the ORs ranged from 1.21 for MI versus atrial fibrillation to 3.86 for atrial fibrillation versus heart failure in the unadjusted analysis. No major differences in the strength of relationships were seen when adjusting for traditional cardiovascular risk factors (Figure 2).
Figure 2.
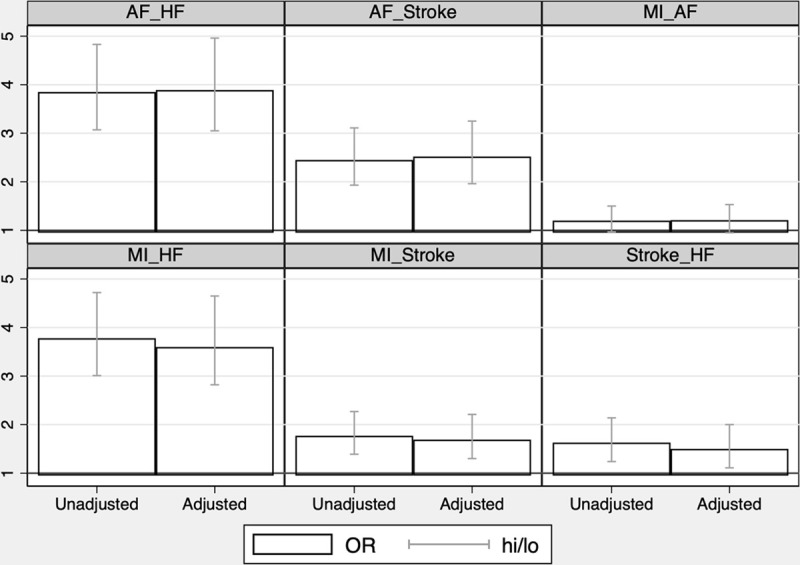
Relationships between the 4 major cardiovascular diseases during 40 y of follow-up in the longitudinal ULSAM (Uppsala Longitudinal Study of Adult Men) given as odds ratios (ORs) and 95% CI (hi/lo). No attention is paid to the temporal order of occurrence of the diseases in this evaluation. ORs are given for both an unadjusted analysis and an analysis adjusted for traditional cardiovascular risk factors at age 50. AF indicates atrial fibrillation; HF, heart failure; and MI, myocardial infarction.
When related to the other 3 CVDs, myocardial infarction occurred before all of the other 3 CVDs in the majority of cases. This could clearly be seen in Figure 1 and in Table 1. This pattern was most pronounced for heart failure, since it was quite uncommon that an individual received a diagnosis of heart failure before a diagnosis of MI. Atrial fibrillation usually occurred before heart failure, while there was no clear temporal trend for stroke versus heart failure, or stroke versus atrial fibrillation (Table 1).
Multistate Results
The flow of transitions from the healthy state to one of the 4 diseases to further other CVDs are shown in Figure 3 for the total follow-up period. No cases were censored due to withdrawal from study or loss of follow-up. No subject was censored because of death due to non-CVD causes before the first CVD diagnosis occurred in the sample. Of the individuals who had received a first CVD diagnosis, 395 were censored due to death from non-CVD causes before receiving a second CVD diagnosis. The analysis performed above, and in this step, only constitutes the first (from CVD healthy to first CVD) and second (form first CVD to second CVD) transition, since the number of subjects in further transitions is too limited for meaningful statistical evaluation. However, the total flow of transitions from one of the 4 diseases to combinations of the other CVDs over the 40 years are shown in Figure 3. It could be noted that 13 subjects received a diagnosis of all 4 disorders.
Figure 3.
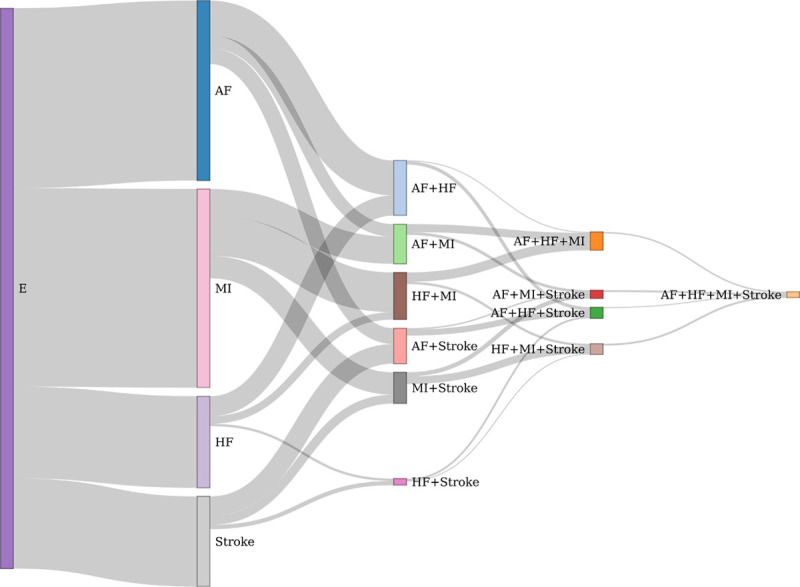
Flow chart of transitions from the healthy state (E) to a first event of atrial fibrillation (AF), myocardial infarction (MI), heart failure (HF), or ischemic stroke (Stroke). Thereafter follows the transitions to 2, 3, and 4 of these diseases. The thickness of the flow is proportional to the number of subjects.
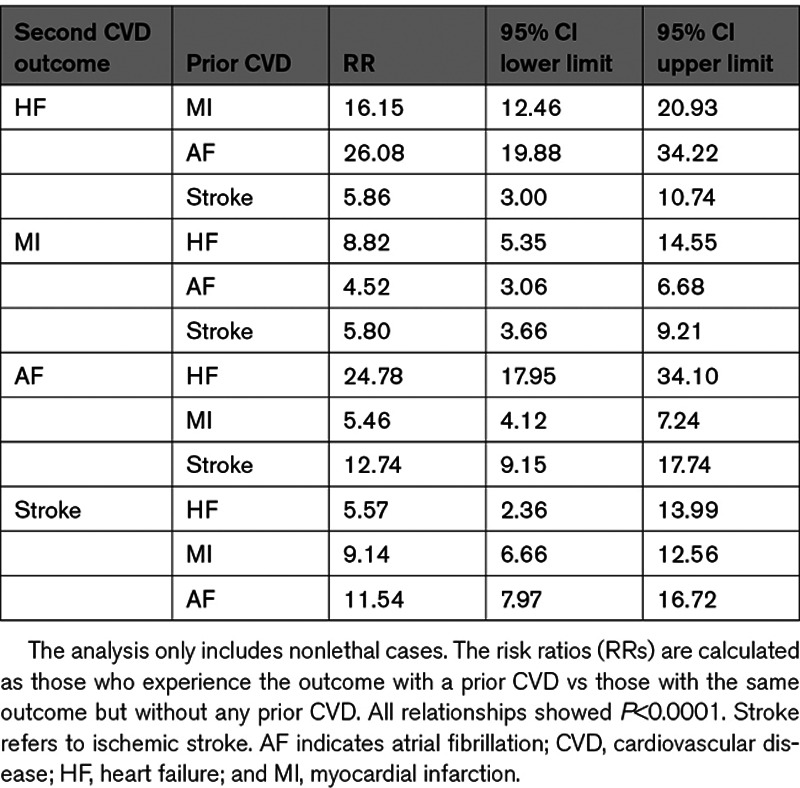
In the multistate modeling, taking the temporal order into account, significant relationships were seen between pairs of all of the 4 investigated CVDs regardless of the temporal order of the CVDs within each pair (Table 2). However, the relative risk of obtaining one CVD following another CVD differed substantially both between different CVDs and between the temporal order. For example, the relationship between heart failure and atrial fibrillation showed a very high risk ratio (RRs, 24 and 26) regardless of which of the conditions that appeared first. Also for the relationship between MI and atrial fibrillation, the risk was not dependent on the temporal order to a major extent, but in this case the relative risks (RRs, 4–5) were not as high as compared with the heart failure and atrial fibrillation relationship. In contrast, the risk of receiving a diagnosis of heart failure after a MI was almost double that compared with the reverse temporal order (RR, 16 versus 9). The 95% CIs are given in Table 2.
Table 2.
Multistage Modeling of Cardiovascular Outcomes That Are Followed by Another CVD
Less than 1% of all transitions between the first and second CVD events occurred within 7 days, meaning that the few cases when a second event was taking place very soon after the first event were exceptions not contributing in any major degree to the multistage model results.
Mendelian Randomization
In the Mendelian randomization part of the study, the inverse-variance weighted method used as the primary analysis was highly significant (P<0.001) for all pairwise comparisons between the 4 CVDs regardless of the temporal order, except for atrial fibrillation (AF)->coronary heart disease (CHD) (P=0.31) when all single nucleotide polymorphism instruments were used (Table 3).
Table 3.
Bidirectional 2-Sample Mendelian Randomization Studies for Pairwise Relationships Between the 4 Major Cardiovascular Diseases
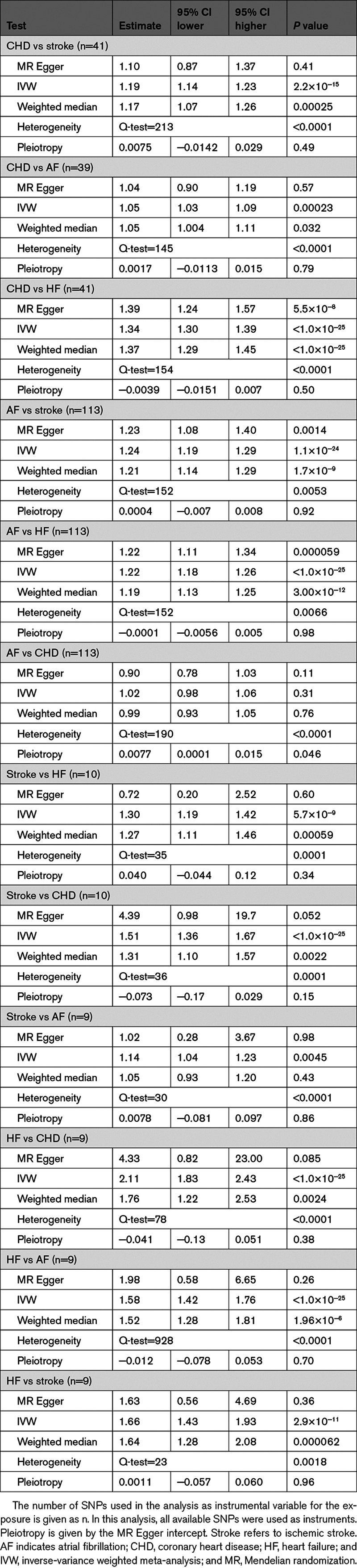
Similar results were obtained in the sensitivity analysis using the weighted median method, except for stroke->AF (P=0.43). Using the Mendelian randomization Egger method, significant relationships were only seen for CHD->heart failure (HF), AF->stroke, AF->HF, but for CHD->stroke, CHD->AF, HF->CHD, and HF->stroke, the estimate was similar for Mendelian randomization Egger compared with when using inverse-variance weighted and weighted median.
When only SNPs showing P>5×10−8 versus traditional risk factors were used as instruments, the estimates were fairly similar for the 3 pairwise relationships where atrial fibrillation was the exposure (Table I and Figure I in the Data Supplement). Regarding the 3 comparisons where coronary heart disease was the exposure, a slight reduction in the estimate was seen when SNPs being related to risk factors were removed, but in all the 3 cases, the P value was still <0.05. When heart failure was the exposure, a great reduction in the estimate was seen for HF->CHD and HF->AF but not for HF->stroke. When stroke was used as the exposure, reductions in the estimate were seen for stroke->CHD and stoke->HF, but not for stroke->AF.
Shared Genetic Loci
Using already published GWAS data about the 4 major CVDs, no locus showed an association versus all 4 traits at P<5×10−8 or false discovery rate <0.05. Three loci were related to 3 out of the 4 CVDs at P<5×10−8 (ABO and ATXN2 for the triplet CHD, stroke and atrial fibrillation and RP11-119H12.3 for the triplet heart failure, stroke, and atrial fibrillation). Another loci were related to heart failure, stroke, and atrial fibrillation using false discovery rate <0.05 (CDKN1A).
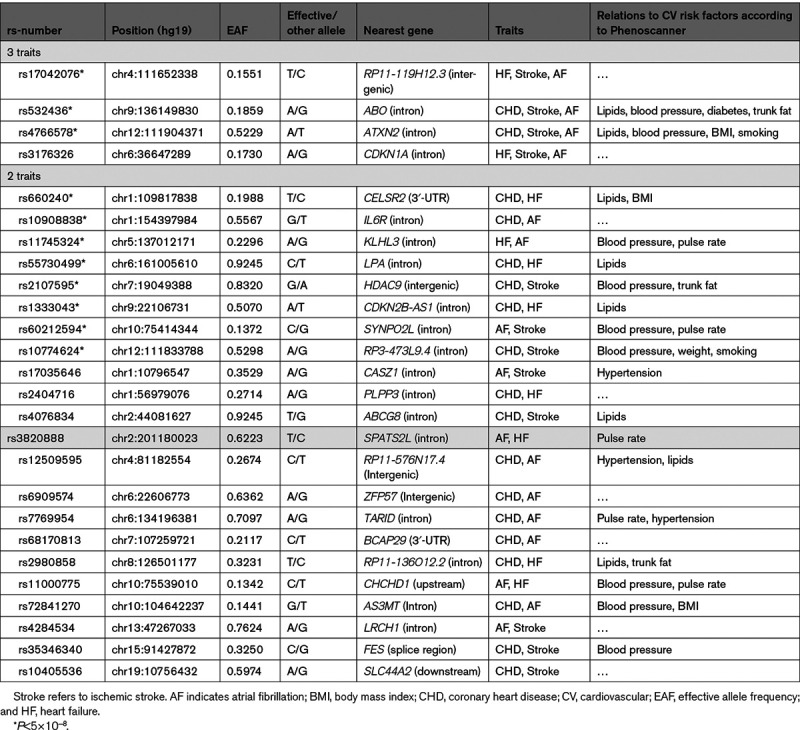
Eight loci were related to 2 out of the 4 CVDs using P<5×10−8, and another 14 using the more liberal false discovery rate <0.05 (Figure 4; Table 4).
Figure 4.
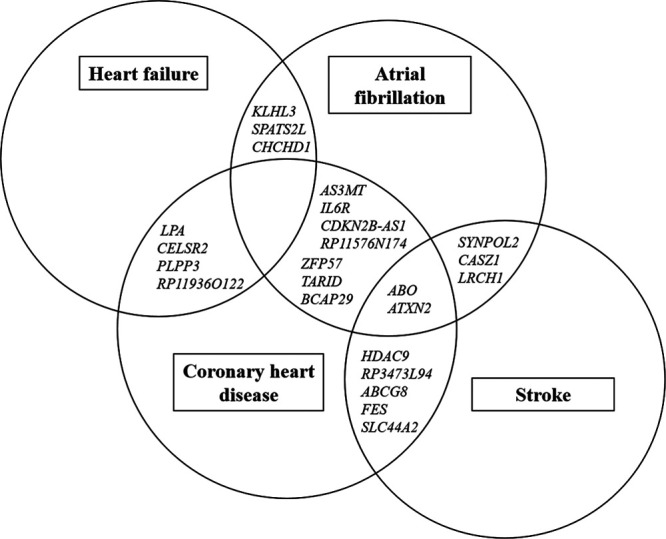
Venn diagram showing an overview of the genetic overlapping associations between 3 or 2 of the 4 cardiovascular disease traits when FDR<0.05 was used as cutoff for associations vs each trait. In addition, loci in or near RP11-119H12.3 and CDKN1A were related to the triplet heart failure, stroke, and atrial fibrillation. See Table 4 for further details. FDR indicates false discovery rate.
Table 4.
Genetic Loci Being Shared for 2 or 3 of the 4 Cardiovascular Traits; Coronary Heart Disease, Stroke, Heart Failure, or Atrial Fibrillation, at the FDR<0.05 Level
The pathway enrichment analysis highlighted blood group synthesis and glucose metabolism for the genes related to the triplet CHD, stroke and, atrial fibrillation and cell cycle regulation and cellular senescence for genes related to the triplet heart failure, stroke, and atrial fibrillation.
For genes related to both CHD and stroke, cholesterol metabolism and bile acid metabolism were the top ranked pathways but also neuronal differentiation, actin dynamics, notch signaling, and stem cell factor signaling were enriched pathways for these 2 CVDs. For genes related to both coronary heart disease and atrial fibrillation, mitogen-activated protein kinase activation and interleukin signaling were the top ranked enriched pathways, while for genes related to both coronary heart disease and heart failure, mainly lipid metabolism pathways were enriched. Other combinations of 2 coronary heart diseases did not show any significant enriched pathways.
Discussion
Principal Findings
During 4 decades of follow-up, all of the 4 investigated CVDs were associated with each other regardless of the temporal order of occurrence, but the risk magnitude differed between different CVDs and their temporal order. Genetic analyses disclosed new pathophysiological links between CVDs.
Pairs of CVD
Significant relationships were seen between pairs of all of the 4 investigated CVDs when ignoring the temporal order, except for atrial fibrillation and MI. It could clearly be seen that MI most often is the first CVD to occur (Figure 1).
All of the 4 investigated CVDs share the traditional risk factors, although the impact of the different risk factors varied between the diseases.5 Thus, the most likely explanation for the relationships between the 4 CVDs would be these shared risk factors. However, the strength of the relationships between the CVDs was only marginally affected by including the traditional risk factors as confounders in the observational part of the study (Figure 3).
Multistate Results
It is well known that atrial fibrillation is a major risk factor for heart failure and stroke,1,2 and that heart failure often is preceded by a MI.2 However, the temporal order of other pairs of CVDs has not previously been extensively studied. In this study, it was clearly seen that the risk of obtaining one CVD following another differed substantially both between different CVDs and between their temporal order.
An MI as a complication following an acute ischemic stroke has been estimated to occur in around 2% of stroke cases.6,7 Also stroke as a complication to acute MI has been reported to be in the range of 2%.8,9 In the present study, not only events occurring during the acute phase of a disease were studied, but events occurring over 4 decades, and therefore the number of events reported in the present study were substantially higher.
Mendelian Randomization
Also the genetic analyses performed within the Mendelian randomization framework showed relationships between most pairs of CVDs. This was also seen following removal of SNPs linked to traditional risk factors. The exception from this was the attenuation found for the relationship between heart failure and later coronary heart disease or atrial fibrillation and stroke and later CVD after removal of SNPs. It must however be noticed that these relationships still showed P<0.05 also after removal of risk factor–associated SNPs (Figure 4).
The Mendelian randomization approach showed relationships for pairs of CVDs both when one disease was used as the exposure and the other as outcome, as well when this order was reversed. This is in accordance with the observational data and could be interpreted in 2 ways. Either is this due to etiological factors that are related to both the genetic instrument and the outcome, as for the pair of coronary heart disease and stroke could be atherosclerosis. Or it could be due to the fact that different mechanisms are involved depending on the temporal order between the CVDs. For example, atrial fibrillation could attenuate the pumping capacity of the left ventricle leading to heart failure, while heart failure with increased filling pressures could dilate the heart, including the left atrium, which might trigger atrial fibrillation. Therefore, it is in this study hard to tell if a significant estimate in Mendelian randomization in the present setting should be interpreted as causal or not.
Shared Genetic Loci
Analysis of shared genetic loci between these disorders showed that most such loci were linked to common traditional risk factors but also shared genetic loci with other functions were disclosed.
When using summary data from already published GWAS for the 4 major CVDs, no loci was related to all 4 CVDs, using either P<5×10−8 or the more liberal false discovery rate <0.05. Four loci were related to 3 out of the 4 CVDs, and 22 loci were associated with 2 of the 4 traits using the more liberal limit of significance. Generally, most of the identified loci have previously been associated with traditional risk factors for CVD, such as hypertension, hyperlipidemia, diabetes, or obesity (Table 4). This finding possibly represents that these 4 CVDs share the traditional risk factors, but for some loci no such known relationships versus traditional risk factors were found.
The single nucleotide polymorphism rs17042076, being intergenic with the nearest gene RP11-119H12.3, was related to heart failure, stroke, and atrial fibrillation, but has not been found in the GWASs versus traditional risk factors. Very little is known about the function of this locus, except that RP11-119H12.3 is expressed in the brain, heart and testis (according to Genotype – tissue expression project, https://gtexportal.org/home/).
Also rs3176326 in the intron of CDKN1A was related to the same triplet of CVDs, but not to traditional risk factors. In this case, however, other loci within this gene have been linked to hyperlipidemia and high blood pressure. Also for some of the loci being related to pairs of CVDs, associations versus traditional risk factors were not found between the single nucleotide polymorphism given in Table 4, but other SNPs within the gene show such relationships. This was however not found for PLPP3, LRCH1, and BCAP29, so it would be interesting to explore the functions of these genes more in detail to see if they could shed new light on the pathogenesis of CVDs.
For IL6R and ZFP57, no SNPs in the gene were related to traditional risk factors, but links have been published versus proinflammatory markers in blood as well as inflammatory diseases, possibly highlighting the role of inflammation in CVD.10,11
Pathway enrichment analyses performed for the genes being related to pairs or triplet of the CVDs (Figure 4) highlighted also other pathways that might be of pathophysiological importance. For example, for loci being related to both coronary heart disease and stroke, bile acid metabolism was among the top ranked pathways but also neuronal differentiation, actin dynamics, notch signaling, and stem cell factor signaling were enriched pathways for these 2 CVDs. Thus, while the pathway analysis brought up pathways being related to traditional risk factors, mainly lipids, also pathways not well-known for CVDs were disclosed.
Clinical Implications
While connections between some CVDs, like atrial fibrillation and later stroke or heart failure is well-known for the clinician, the present study disclosed that also other CVDs are associated and the fact that one individual has a CVD diagnosis leads to an increased risk for also other CVDs. Thus, careful control of common traditional risk factors would not only lower the risk for a recurrent event but possibly also other CVDs, although shared risk factors only explained a part of the covariation of CVDs. The remaining part of the covariation was due to other pathophysiological mechanisms, and some of those were disclosed in the present pathway analysis, which might serve as a basis for future drug discovery efforts.
Strength and Limitations
The major strength of the study is the long follow-up period from 50 to 90 years of age in the population-based sample, allowing us to capture most of the CVDs that occur during a lifetime. Another strength is that we performed a longitudinal observational study and genetic studies in parallel. The obvious limitation is that we only have men of European descent in our sample, and therefore the results have to be confirmed in women, as well as in other ethnic groups. The study sample was also too small to study the transitions from 2 to 3 or 4 CVDs in terms of relative risk, and therefore only the transition from the first to the second CVD could be properly evaluated.
It should also be pointed out that the diagnostic criteria for the CVDs, especially MI, has changed during the 4 decades of follow-up, something that might have been affecting the results. This is however unavoidable during long follow-up periods.
Another limitation is that the number of SNPs that was used as instrumental variables for heart failure and stroke were small after removal of SNPs linked to risk factors, so those estimates might not be precise and likely underpowered. However, the largest GWASs to date published for stroke and heart failure were used to select the instrumental variables.
A significant interaction with time was seen in the multistate models meaning that the relative risks for a transition from one certain CVD to another do vary depending on the age of the subjects. Unfortunately, the sample is too small to be stratified into different age-groups, so the risk ratios presented should be regarded as average risk ratios over a 4-decade follow-up period. Further studies in far larger samples have to be conducted to produce age-specific risk ratios for the transitions given in Table 2.
Table 4 gives the overlap of the loci being related to 3 or 2 of the 4 CVDs. Also, the nearest gene is given for each locus and, based on those genes, pathway analyses were conducted. It must however be acknowledged that a locus is not always associated with the expression of the nearest gene. However, many of the loci presented in Table 4 are not a significant eQTL, or are associated with expression of different genes. Therefore, we have for the sake of simplicity used the nearest gene to describe the genetic region of interest, being fully aware that the nearest gene not always is the gene of interest.
Conclusions
During 40 years, all of the 4 investigated CVDs were pairwise associated with each other regardless of the temporal order of occurrence, but the risk magnitude differed between different CVDs and their temporal order. Genetic analyses disclosed new pathophysiological links between CVDs.
Sources of Funding
The study was funded by the Swedish Heart and Lung foundation and Uppsala University Hospital (ALF-medel).
Disclosures
None.
Supplemental Materials
Online Methods
Online Table I
Online Figure I
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- CHD
- coronary heart disease
- CVD
- cardiovascular disease
- GWAS
- genome-wide association studies
- HF
- heart failure
- MI
- myocardial infarction
- RR
- risk ratio
- ULSAM
- Uppsala Longitudinal Study of Adult Men
For Sources of Funding and Disclosures, see page 221.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCGEN.120.002963.
Contributor Information
Johan Sundström, Email: johan.arnlov@ki.se.
Johan Ärnlöv, Email: johan.arnlov@ki.se.
Martin Ingelsson, Email: martin.ingelsson@pubcare.uu.se.
Albert Henry, Email: albert.henry.16@ucl.ac.uk.
R. Thomas Lumbers, Email: t.lumbers@ucl.ac.uk.
Erik Lampa, Email: erik.lampa@ucr.uu.se.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991; 22:983–988. doi: 10.1161/01.str.22.8.983 [DOI] [PubMed] [Google Scholar]
- 2.Goyal A, Norton CR, Thomas TN, Davis RL, Butler J, Ashok V, Zhao L, Vaccarino V, Wilson PW. Predictors of incident heart failure in a large insured population: a one million person-year follow-up study. Circ Heart Fail. 2010; 3:698–705. doi: 10.1161/CIRCHEARTFAILURE.110.938175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991; 22:312–318. doi: 10.1161/01.str.22.3.312 [DOI] [PubMed] [Google Scholar]
- 4.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013; 2:e000102. doi: 10.1161/JAHA.112.000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lind L, Sundström J, Ärnlöv J, Lampa E. Impact of aging on the strength of cardiovascular risk factors: a longitudinal study over 40 years. J Am Heart Assoc. 2018; 7:e007061. doi: 10.1161/JAHA.117.007061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alqahtani F, Aljohani S, Tarabishy A, Busu T, Adcock A, Alkhouli M. Incidence and outcomes of myocardial infarction in patients admitted with acute ischemic stroke. Stroke. 2017; 48:2931–2938. doi: 10.1161/STROKEAHA.117.018408 [DOI] [PubMed] [Google Scholar]
- 7.Seifi A, Carr K, Maltenfort M, Moussouttas M, Birnbaum L, Parra A, Adogwa O, Bell R, Rincon F. The incidence and risk factors of associated acute myocardial infarction (AMI) in acute cerebral ischemic (ACI) events in the United States. PLoS One. 2014; 9:e105785. doi: 10.1371/journal.pone.0105785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behar S, Tanne D, Abinader E, Agmon J, Barzilai J, Friedman Y, Kaplinsky E, Kauli N, Kishon Y, Palant A. Cerebrovascular accident complicating acute myocardial infarction: incidence, clinical significance and short- and long-term mortality rates. The SPRINT Study Group. Am J Med. 1991; 91:45–50. doi: 10.1016/0002-9343(91)90072-6 [DOI] [PubMed] [Google Scholar]
- 9.Naderi N, Masoomi H, Mozaffar T, Malik S. Patient characteristics and comorbidities associated with cerebrovascular accident following acute myocardial infarction in the United States. Int J Cardiol. 2014; 175:323–327. doi: 10.1016/j.ijcard.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kottoor SJ, Arora RR. The utility of anti-inflammatory agents in cardiovascular disease: a novel perspective on the treatment of atherosclerosis. J Cardiovasc Pharmacol Ther. 2018; 23:483–493. doi: 10.1177/1074248418778548 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen MT, Fernando S, Schwarz N, Tan JT, Bursill CA, Psaltis PJ. Inflammation as a therapeutic target in atherosclerosis. J Clin Med. 2019; 8:1109. doi: 10.3390/jcm8081109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010; 121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521 [DOI] [PubMed] [Google Scholar]
- 13.Merlo J, Lindblad U, Pessah-Rasmussen H, Hedblad B, Rastam J, Isacsson SO, Janzon L, Råstam L. Comparison of different procedures to identify probable cases of myocardial infarction and stroke in two Swedish prospective cohort studies using local and national routine registers. Eur J Epidemiol. 2000; 16:235–243. doi: 10.1023/a:1007634722658 [DOI] [PubMed] [Google Scholar]
- 14.Ingelsson E, Arnlöv J, Sundström J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005; 7:787–791. doi: 10.1016/j.ejheart.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 15.Bendix C, Martyn P. Using Lexis objects for multi-state models in R. J Stat Softw. 2011; 38:1–18 [Google Scholar]
- 16.Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, et al. ; CARDIoGRAMplusC4D Consortium. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013; 45:25–33. doi: 10.1038/ng.2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah S, Henry A, Roselli C, Lin H, Sveinbjörnsson G, Fatemifar G, Hedman ÅK, Wilk JB, Morley MP, Chaffin MD, et al. ; Regeneron Genetics Center. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020; 11:163. doi: 10.1038/s41467-019-13690-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten-Jacobs L, Giese AK, van der Laan SW, Gretarsdottir S, et al. ; AFGen Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium; International Genomics of Blood Pressure (iGEN-BP) Consortium; INVENT Consortium; STARNET; BioBank Japan Cooperative Hospital Group; COMPASS Consortium; EPIC-CVD Consortium; EPIC-InterAct Consortium; International Stroke Genetics Consortium (ISGC); METASTROKE Consortium; Neurology Working Group of the CHARGE Consortium; NINDS Stroke Genetics Network (SiGN); UK Young Lacunar DNA Study; MEGASTROKE Consortium. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018; 50:524–537. doi: 10.1038/s41588-018-0058-329531354 [Google Scholar]
- 19.Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, Almgren P, Alonso A, Anderson CD, Aragam KG, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018; 50:1225–1233. doi: 10.1038/s41588-018-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott RA, Scott LJ, Mägi R, Marullo L, Gaulton KJ, Kaakinen M, Pervjakova N, Pers TH, Johnson AD, Eicher JD, et al. ; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes. 2017; 66:2888–2902. doi: 10.2337/db16-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, et al. ; LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015; 518:197–206. doi: 10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, et al. ; Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013; 45:1274–1283. doi: 10.1038/ng.2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lind L. Genetic determinants of clustering of cardiometabolic risk factors in U.K. Biobank. Metab Syndr Relat Disord. 2020; 18:121–127. doi: 10.1089/met.2019.0096 [DOI] [PubMed] [Google Scholar]


