Dilated cardiomyopathy (DCM) is an umbrella term used to describe a spectrum of myocardial disorders that result in an enlarged and poorly contractile left ventricle.1 Collectively, DCM underlies ≈30% to 40% of all heart failure (HF) cases and represents the second most common cause of HF after ischemic heart disease. Truncating variants in TTN-encoded titin (TTNtvs)—a sarcomeric protein with structural and signaling roles—are found in ≈10% to 25% of idiopathic DCM cases and represent the most common cause of familial DCM.1
Interestingly, TTNtvs are also observed in ≈10% of alcoholic2 and chemotherapy-induced cardiomyopathy cases,3 suggesting that secondary risk factors influence the penetrance and expressivity of TTNtvs. Therefore, we sought to determine the prevalence and clinical impact of secondary risk factors within a single-center cohort of unrelated patients with the American College of Medical Genetics and Genomics–graded pathogenic/likely pathogenic TTNtvs.
In this institutional review board–approved retrospective study, an institutionally developed natural language processing interface, Advanced Text Explorer, was used to identify all patients with a TTN variant identified via clinical genetic testing by performing a proximity search across unstructured text (clinical notes, genetic testing reports, etc) within the electronic medical record. After exclusion of patients with non-TTNtv variants, each TTNtv was then mapped to the TTN reference sequence (NM_133378.4) and classified according to the 2015 American College of Medical Genetics and Genomics guidelines.4 Following exclusion of patients without A-band localizing American College of Medical Genetics and Genomics pathogenic/likely pathogenic TTNtvs, available records were reviewed for evidence of secondary causes such as excess alcohol use, cardiotoxic chemotherapy, morbid obesity (body mass index >40), biopsy/imaging-confirmed myocarditis, peripartum/tachycardia-mediated cardiomyopathy, and valvular heart disease. In addition, to assess the prevalence of these risk factors in individuals without TTNtvs, a 1:2 age- and sex-matched cohort of patients evaluated and dismissed subsequently as normal from the Mayo Clinic Genetic Heart Rhythm Cohort was assembled. The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Overall, 35 unrelated, TTNtv-positive patients were identified (43% women; current median age, 53 [interquartile range (IQR), 36–64] years; median baseline left ventricular ejection fraction [LVEF], 35% [IQR, 20%–54%]; and 66% with a DCM family history). At presentation, 19 of 35 (54%) TTNtv-positive patients endorsed New York Heart Association class II or greater HF symptoms and 4 of 35 (12%) experienced either a sudden cardiac arrest or syncope/seizure secondary to sustained ventricular arrhythmia. The remaining 12 of 35 (34%) were asymptomatic. The diagnoses rendered are summarized in the Figure (A).
Figure.
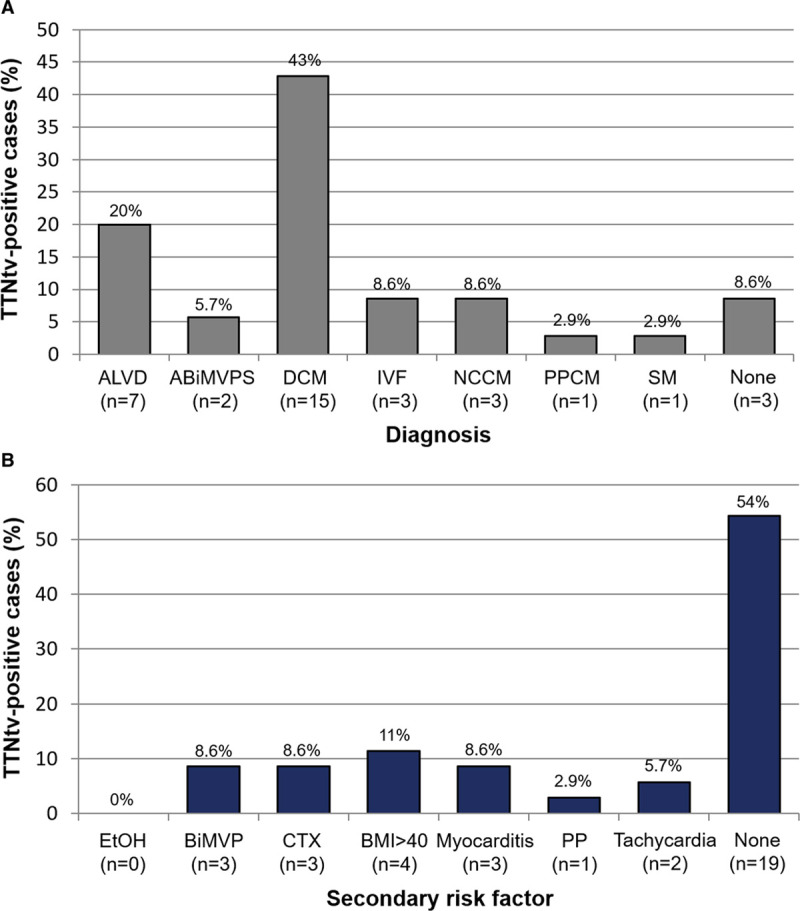
Secondary risk factors in titin-truncating variant (TTNtv)–positive patients. A, Clinical diagnoses rendered. B, Prevalence of prespecified secondary risk factors. ABiMVPS indicates arrhythmogenic bileaflet mitral valve prolapse; ALVD, asymptomatic left ventricular dysfunction; BiMVP, bileaflet mitral valve prolapse; BMI, body mass index; CTX, chemotherapy; DCM, dilated cardiomyopathy; EtOH, alcohol; IVF, idiopathic ventricular fibrillation; NCCM, noncompaction cardiomyopathy; PP, peripartum; PPCM, peripartum cardiomyopathy; and SM, skeletal myopathy.
Interestingly, at least 1 prespecified DCM risk factor was identified in 16 of 35 (46%) TTNtv-positive patients as detailed in the Figure (B). In comparison to TTNtv-positive patients presenting without HF, those with New York Heart Association class II or greater HF symptoms had worse left ventricular function (median LVEF, 20% [IQR, 15%–33%] versus 54% [IQR, 41%–61%]; P<0.001) and were more likely to have ≥1 secondary risk factor (13/19 [68%] versus 4/16 [25%]; P=0.02). Furthermore, the proportion of TTNtv-positive patients diagnosed ultimately with a form of DCM (n=20; 35% women; current median, age 58 [IQR, 38–65] years; and median baseline LVEF, 21% [IQR, 15%–31%]) that possessed ≥1 secondary risk factor was significantly higher than those TTNtv-positive patients without a DCM diagnosis (14/20 [70%] versus 3/15 [20%]; P=0.006). Similarly, in comparison to an age- and sex-matched cohort (n=40; 50% women; current median age, 58 [IQR, 38–65] year; and median baseline LVEF, 63% [IQR, 61%–66%]), the burden of secondary risk factors observed in TTNtv-positive patients with DCM was significantly higher (7/40 [18%] versus 14/20 [70%]; P<0.001).
Importantly, the prevalence of TTNtvs in the Genome Aggregation Database (2585/141 456 [1.8%]; http://gnomad.broadinstitute.org) is not trivial and far exceeds the estimated prevalence of DCM in the general population (≈1/250 [≈0.4%]). When coupled with the overrepresentation of TTNtvs in acquired/secondary cardiomyopathies2,3 and lower LVEF in TTNtv-positive biobank participants,5 this observation suggests that many TTNtvs may lay quiescent until exacerbated by secondary risk factors.
However, the collective impact of these so-called second hits has yet to be studied in the context of an unselected cohort of TTNtv-positive patients. To this end, we note that most TTNtv-positive patients (14/20; 70%) with DCM evaluated at our institution possess ≥1 secondary risk factor. Although small cohort size limits our ability to adjust for the potential contributory effects of age, sex, or ancestry, this finding suggests that gene-environment interactions may play a substantial, collective role in determining the penetrance and expressivity of TTNtvs.
Of note, this study is limited by its retrospective nature, the potential for sampling bias inherent to rare disease cohorts, and statistical power secondary to small cohort size (n=35). Nevertheless, the burden of secondary risk factors observed within this cohort of unselected, TTNtv-positive patients provides further evidence of the potential importance of gene-environment interactions in TTN-mediated DCM. In the future, better powered, population-based genetic studies are needed to identify and determine the role of secondary risk factors in the clinical manifestation(s) and progression of genetic DCM.
Sources of Funding
This work was supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program.
Disclosures
Dr Ackerman is a consultant for Abbott, ARMGO, Audentes Therapeutics, Boston Scientific, Invitae, LQT Therapeutics, Medtronic, MyoKardia, and UpToDate. Dr Ackerman and Mayo Clinic are involved in an equity/royalty relationship with AliveCor, Blue Ox Health Corporation, and StemoniX. However, none of these entities were involved in this study in any manner. The other authors report no conflicts.
Footnotes
For Sources of Funding and Disclosures, see page 250.
Contributor Information
John R. Giudicessi, Email: giudicessi.john@mayo.edu.
Sanskriti Shrivastava, Email: Shrivastava.Sanskriti@mayo.edu.
Michael J. Ackerman, Email: ackerman.michael@mayo.edu.
References
- 1.Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012; 366:619–628. doi: 10.1056/NEJMoa1110186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ware JS, Amor-Salamanca A, Tayal U, Govind R, Serrano I, Salazar-Mendiguchía J, García-Pinilla JM, Pascual-Figal DA, Nuñez J, Guzzo-Merello G, et al. Genetic etiology for alcohol-induced cardiac toxicity. J Am Coll Cardiol. 2018; 71:2293–2302. doi: 10.1016/j.jacc.2018.03.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Pavia P, Kim Y, Restrepo-Cordoba MA, Lunde IG, Wakimoto H, Smith AM, Toepfer CN, Getz K, Gorham J, Patel P, et al. Genetic Variants Associated With Cancer Therapy-Induced Cardiomyopathy. Circulation. 2019; 140:31–41. doi: 10.1161/CIRCULATIONAHA.118.037934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. ; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haggerty CM, Damrauer SM, Levin MG, Birtwell D, Carey DJ, Golden AM, Hartzel DN, Hu Y, Judy R, Kelly MA, et al. Genomics-first evaluation of heart disease associated with titin-truncating variants. Circulation. 2019; 140:42–54. doi: 10.1161/CIRCULATIONAHA.119.039573 [DOI] [PMC free article] [PubMed] [Google Scholar]


