Abstract
Chlamydia and gonorrhea are 2 of the most common bacterial sexually transmitted infections (STIs) worldwide. Rising chlamydia and gonorrhea rates along with increased closing of STI clinics has led many to seek STI testing in clinical settings such as urgent cares and walk-in clinics. However, with competing priorities, providing effective and efficient STI care can be difficult in these settings. This has left a growing need for the implementation of novel STI screening programs in other clinical settings. This review summarizes previous studies that have evaluated the clinical implementation of chlamydia and gonorrhea screening programs in these settings. Literature from January 2015 to February 2020 regarding the implementation or evaluation of STI screening programs in clinical settings was reviewed. Constructs from the Capability, Opportunity, Motivation, and Behavior model were used to organize results, as this model can aid in identifying specific strategies for behavior/process change interventions. We found that multiple STI screening programs have been implemented and evaluated in 5 different countries and multiple health care facilities including sexual health clinics, urgent cares, walk-in clinics, and university health clinics. When implementing new STI screening programs, sample-first, test-and-go services and molecular point-of-care (POC) testing approaches were found to be effective in increasing screening and reducing costs and time to treatment. At the health care systems level, these programs can help reduce STI screening costs and generate additional revenue for clinics. At the provider level, clear communication and guidance can help clinical and administrative staff in adopting new screening programs. Finally, at the patient level, new programs can reduce time to treatment and travel costs in visiting clinics multiple times for testing and treatment services.
Chlamydia trachomatis and Neisseria gonorrhoeae are 2 of the most commonly diagnosed bacterial sexually transmitted infections (STIs) in the United States and worldwide.1,2 An estimated 2.9 million cases of chlamydia and 820,000 cases of gonorrhea occur each year in the United States, with untreated infections leading to negative reproductive health outcomes, such as pelvic inflammatory disease and infertility among women.1 The annual medical costs for STIs are estimated to exceed $16 billion,3 which represents a significant public health burden of both financial costs and reproductive health outcomes.
Rising chlamydia and gonorrhea infection rates have increased the demand for testing and treatment services.4 However, although demand for services has increased, funding for STI and sexual health centers has decreased, leading to a gap in health care services.5,6 The lack of STI care centers has led many people to seek sexual health services at other clinics, such as primary care, urgent care, walk-in clinics, and emergency departments.7,8 There are multiple obstacles in providing effective STI diagnosis and treatment in these settings including insufficient time, lack of training, and challenges with patient follow-up.7 Clinic capacity has been stretched even further as health care providers have focused services on SARS-CoV2 response efforts over the last year, pointing to a great need of novel STI screening programs that can be effectively implemented in clinical settings.
Sexually transmitted infection screening programs are critical in identifying infections that do not result in symptoms and ensuring timely treatment. When implemented effectively, STI screening programs can decrease the time between diagnosis and treatment, limiting the transmission of future infections.9 In addition, with advancements in STI diagnostics, screening programs have the ability to guide treatment more accurately and support antimicrobial stewardship. Despite the advancement in screening and diagnostics programs, few clinics have implemented these novel STI screening technologies because of concerns related to clinic flow, funding for new processes, and staffing concerns. This has created a need to better understand how new STI screening technologies can be most effectively implemented in clinical settings.
We aimed to summarize work that has evaluated clinical implementation of chlamydia and gonorrhea screening programs and adoption of new technologies and have organized our review based on the theoretical constructs of the Capability, Opportunity, Motivation, and Behavior (COM-B) model to examine behavior and outcomes that would affect the implementation of chlamydia and gonorrhea screening programs with a focus on clinic flow changes and adoption of POC testing.10,11 The changes required to fully adopt new STI screening programs will need to occur at the levels of the health care system, provider/clinic, and patient. The outcomes of these process changes will have an impact at each of these levels.
METHODS
Theoretical Framework
Implementing new chlamydia and gonorrhea screening programs in a clinical setting requires behavior change at the system, provider, and patient levels for these new services to be effective in diagnosing infections in an acceptable and cost-effective manner. Theories such as the COM-B model aid in examining behaviors that will encourage the adoption of new screening programs. The COM-B model is a theory that posits that for any behavior to occur there is an interaction between 3 components: capability, opportunity, and motivation. In this review, we use this model to examine the interaction of these 3 components at the health care system, provider, and patient level. Capability comprises the psychological (e.g., knowledge on screening technologies) and physical abilities (e.g., skills in operating new screening technologies) that help facilitate the implementation of screening programs. Opportunity includes the social (e.g., impact on providing services) and physical (e.g., financial resources and clinic space) opportunities for implementing programs. Finally, motivation is the automatic (e.g., desire to decrease STI burden) and reflective (e.g., evaluation of screening programs) motivating factors behind implementing a screening program.12,13 These constructs each occur at 3 levels: the system level, which we will describe mainly in terms of health care systems and policies, the provider level (into which we include clinical processes), and the patient level. In addition, COM-B lies at the center of the behavior change wheel, which identifies specific strategies for behavior change interventions.12 COM-B has been applied to design behavior change interventions in many contexts including the identification of barriers and facilitators to chlamydia testing in general practice.11 Therefore, COM-B can aid in examining health behaviors and the implementation of behavior change interventions at both the individual and organizational levels and provide a framework for synthesizing evidence for this review.
Eligibility Criteria
Eligible studies had to explore implementation of chlamydia or gonorrhea POC testing in a clinical setting. A population was not specified as we wanted this review to incorporate implementation strategies for diverse groups. Studies had to be in English, be peer-reviewed, focus on chlamydia or gonorrhea, and include postimplementation quantitative or qualitative metrics. Implementation metrics pertaining to optimizing clinic flow or views toward POC testing were required. Studies conducted in countries where health care delivery systems were dissimilar to the United States were included in this study to provide additional perspectives as to how STI testing programs can be better implemented in various settings. Studies reporting research protocols were excluded from this study if postimplementation metrics were not provided.
Search Strategy
Six databases (PubMed, Embase, CINAHL, Scopus, Cochrane Library, and HSRProj) were searched from January 2015 to February 2020. Search terms, incorporating database-specific subject headings and title/abstract keywords, focused on the following concepts: implementation science, sexually transmitted diseases, mass screening, and office visits/primary care. Detailed search strategies are available upon request. Three authors (A.F., D.D., B.V.D.P.) independently screened all titles and abstracts against the prespecified inclusion and exclusion criteria and agreed on the selection of articles to be obtained for full text.
Data Synthesis and Analysis
Thematic analysis was used to identify prominent themes. Themes were refined through discussion and iterative comparison among the authors to ensure that they accurately reflected the material. Themes were organized based on constructs from the COM-B model (Fig. 1).
Figure 1.
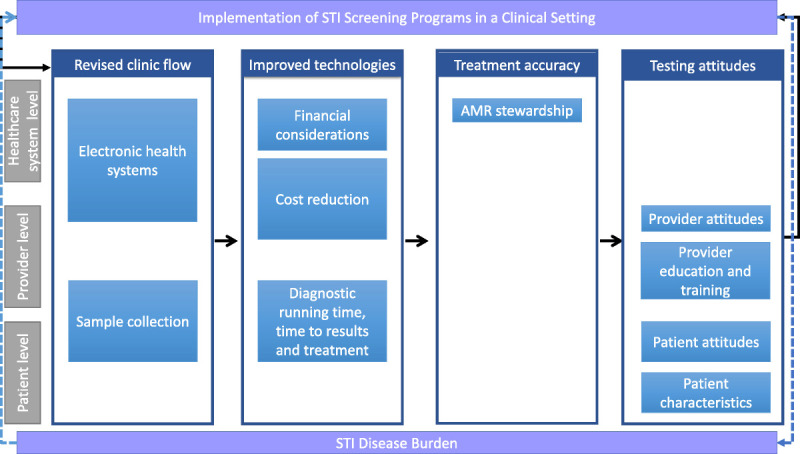
Outcomes associated with implementing POC testing at the societal, provider, and patient levels.
RESULTS
Search and Review Outcomes
We reviewed 590 citations and identified 541 abstracts or articles for further review after removing duplicates. Of those, 518 did not meet the required inclusion criteria, leaving 23 for further review. Full-text articles were read for articles that met the inclusion criteria, and 10 were excluded because they were not focused on evaluating a chlamydia or gonorrhea screening program in a clinical setting. One additional study was identified through bibliography search, resulting in a total of 14 articles being included for this review (Fig. 2). Six studies discussed adoption of improved technology, 3 included implementation effects on treatment accuracy, 8 described revised clinic flow, and 4 assessed testing attitudes. Several articles covered more than one of these topics.
Figure 2.
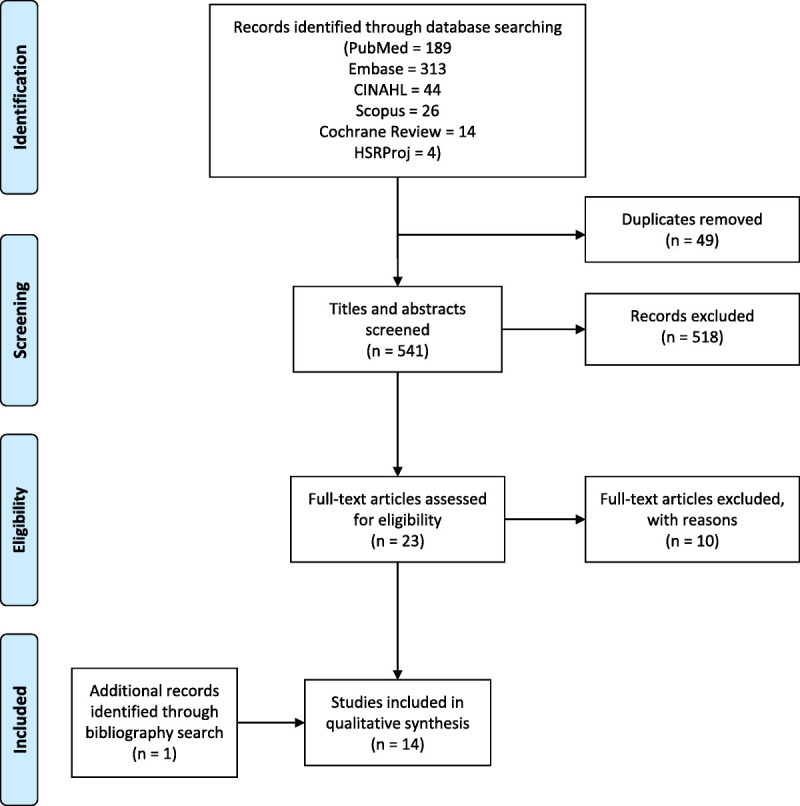
Flowchart of study selection.
Study Locations
Studies in this review originate from 5 countries and were performed at 8 different health care facilities. One study was conducted in Australia at a sexual health clinic14; 1 in South Africa in an infectious disease clinic15; 2 in Canada, 1 at a walk-in clinic,16 and 1 at an STI clinic17; 4 studies were conducted in the United Kingdom: 2 in general practice settings,18,19 and 2 at a single sexual health clinic20,21; and 6 studies were conducted in the United States, with 2 at an HIV care clinic,22,23 1 at an urgent care,24 1 at a primary care clinic,25 1 at a university student health clinic,26 and 1 that used multiple sites including local clinics, private care practices, and a university setting.27
Revised Clinic Flow
Electronic Health Systems (Factors Related to Opportunity Building at the Health Care Systems Level)
As the use of electronic health systems grows in clinical settings, there is a need to understand how to use these systems to improve STI screening programs. Chadwick et al.16 created a standardized, computer-generated laboratory order sheet to reduce incomplete testing and changed their testing policy from “opt-in” to “opt-out.” This program led to an STI testing rate increase from 5.5% to 45.2%. In addition, Karas et al.25 implemented the use of an electronic health record–based clinical decision support tool to increase annual chlamydia screening in a primary care pediatric network. This system provided alerts for sexually active females presenting for well care visits who had not had an annual chlamydia screening. After the implementation of this program, adolescent girls were 2.143 times more likely to be screened for chlamydia. These studies illustrate the impact that electronic health systems can have on promoting STI screening programs both at the health care system and patient levels. The use of computer-generated laboratory sheets, electronic health records, and other electronic systems can help clinics in following STI screening recommendations by implementing reminders into their systems. Following screening recommendations will be especially beneficial for women, who are disproportionately burdened by STI sequelae.
Sample Collection (Factors Related to Capability at the Provider and Patient Levels)
Sample collection methods can serve as a barrier for many people in accessing and accurately diagnosing STIs. Self-testing programs provide patients with the confidentiality and comfort of collecting their own specimen and relieve discomfort experienced by both patient and provider in physical examinations. Barbee et al.22 implemented a self-sampling program in an HIV clinic and found that patients reported that availability of this program increased their frequency of being tested. In addition, through this program testing coverage increased, including a 32% increase in pharyngeal testing from 444 to 586 tests performed and a 33% increase in rectal testing from 390 to 520 tests performed. Overall, there were 47% and 50% increased yields for the detection of chlamydia and gonorrhea infections, respectively, when compared with baseline data. They also found that instructional posters with pictures that provided guidance on self-collection techniques were found to be extremely helpful for patients.
Tat et al.23 also implemented an STI self-testing program. In this program, patients could request self-testing without an appointment or clinicians could recommend the self-testing program at the end of a regular visit. Clinicians found this program saved them time from filling out paperwork and collecting samples, allowed more patient privacy, and increased patient access to testing. However, a lack of clarity regarding communicating directions about self-testing to patients created confusion and resulted in incorrect placement of labels when patients turned in their specimen.
Patients from both studies found self-testing programs increased STI testing availability and frequency of testing. When clinics are implementing these self-testing programs, it will be important to design policies that clearly state whom, whether clinical or administrative staff, is overseeing this process and providing instructions to patients. As seen with the study by Tat et al., lack of clear communication and guidance can create roadblocks in programs that have the potential to be beneficial and time saving. Overall, self-testing programs have the ability to increase patient engagement with STI services, the number of tests performed, and case detection.
In settings where self-testing programs are not yet available, implementing new testing policies and programs can help in diagnosing infections quickly and reduce infection duration. Gratrix et al.17 implemented an express testing criterion at a Canadian STI clinic where patients who reported being asymptomatic, had not had a contact of an STI, had not had receptive anal intercourse since their last testing visit, and had no history of sexual assault in the last 2 weeks were able to forgo a physical examination and receive STI/HIV testing. Through this program, there was a significant increase in the diagnosis of chlamydia, gonorrhea, and syphilis infections compared with standard of care. This study was able to show that by eliminating the physical examination, clinics can appropriately treat those at lower risk for STIs. In addition, this method saved a median of 9 minutes per male visits and 13 minutes per female visit. The time saved could then be used to serve additional patients. Chow et al.14 implemented a test-and-go (TAG) service where a nurse collects blood and throat swabs, and the patient collects their own urine and rectal samples to drop-off and leave at the clinic. Testing results were then provided a week later. TAG decreased the median waiting time by about 44.5 minutes and the median consultation time by 8.7 minutes, allowing for 3 additional consultations per hour when compared with routine consultation service. However, this program did not allow treatment to occur any earlier, which could allow further STI transmission. A program such as this one could be extremely beneficial for populations with low STI prevalence levels or for populations trying to increase initial STI testing services appointments.
Improved Technologies
Financial Considerations (Factors Related to Opportunity at the Health Care System Levels)
Financial costs of new STI screening programs play a significant role in implementation of process change. One example is concern over POC tests, with many believing that new diagnostics and assay kits are more expensive than traditional laboratory-based testing and will be prohibitive for programs operating on a limited or controlled budget. Fisk et al.24 found the opposite to be true and that by using the GeneXpert (Cepheid, Sunnyvale, CA)28 nucleic acid amplification test (NAAT), on-site, at an urgent care, $18,0000–$25,000 could be generated in annual revenue from test reimbursement. Their analysis used both the minimum reimbursement projection and the median projection, which suggests that this may be a conservative estimate of the positive impact of adoption of a POC test for clinical settings with the capacity to bill third-party payers. This also suggests that public health departments might be able to adopt these tests because many publicly funded STI clinics now bill third-party payers. Thus, at both the health care system level and the individual practice level, these tests may prove to be cost-rational.
NAAT POC testing allows for same-day testing and treatment services, which could aid in lowering STI screening program costs by reducing repeat visits. Keizur et al. used the GeneXpert assay when implementing a same-day testing and treatment program among gay, bisexual, transgender, and homeless youth. They found that this program led to a decrease in reinfections from 20% before implementing this program to 12% (prevalence ratio, 0.60; 95% CI, 0.33–1.09) after implementation. Thus, return visits for initial treatment and visits due to reinfection were reduced. As a result, by providing same-day testing and treatment services, repeat visits are lowered and the overall costs to the health care system for STI care are reduced. At the patient level, access to STI services remains a barrier for many people, especially sexual minorities, who may have limited time and ability to visit health care centers multiple times. Therefore, by providing same-day testing and treatment services, and reducing patients need to return to clinic for treatment or repeat testing, patients could see a reduction in travel and medical costs.
For practices that are not equipped to implement POC NAAT tests, self-sampling has shown to be cost-effective when implementing new STI screening programs. Gratrix et al.17 reported on the implementation of triage criteria for express visits with self-sampling at a Canadian STI clinic. This program saved approximately CA$7.41 per male visit and CA$10.55 per female visits, resulting in cost savings estimated at CA$40,000 over the course of the 13-month study. Therefore, new STI programs can help in reducing costs, generating income, and lessen patient financial costs from traveling to clinics multiple times.
Diagnostic Running Time (Factors Related to Opportunity at the Provider and Motivation at the Patient Level)
To be implemented efficiently, NAAT POC tests must be effective in diagnosing STIs quickly, during a clinic visit, to provide treatment in one visit. Five studies used NAATs in their implementation program to determine the feasibility providing same-day diagnosis and treatment. Fisk et al., Harding-Esch et al., and Stime et al. used the GeneXpert assay, which has a 90-minute running time, Gratrix et al. used the Gen-Probe Aptima Combo-2 test (Gen-Probe Inc, San Diego),29 which has a 2-hour running time, and Gettinger et al. used the binx health io assay (binx Health LTD, Trowbridge, United Kingdom),30 which has a 30-minute run time. The diagnostic running time of these assays is substantially shorter than sending samples to an outside laboratory and waiting for results, which can take a week or longer. Therefore, we know that NAAT POC testing is available and can be implemented in a clinical setting. However, time to results and treatment is dependent on how these assays are implemented in the clinic setting, their availability, and general clinic flow.
Time to Results and Treatment (Factors Related to Opportunity at the Provider Level and Motivation at the Patient Level)
Large time gaps between STI sample collection, diagnostic results, and treatment continue to be a barrier in reducing STI transmission. Although NAAT POC tests have the potential to diagnose and treat STIs in one visit, patient willingness to wait for results and treatment remain a concern. Fuller et al.20 conducted a qualitative study to examine patient opinions on NAAT POC tests and AMR detection. They found that UK patients had mixed reactions about potentially waiting an extra 30 minutes in a clinic to receive results and treatment. Patients who reported that waiting an extra 30 minutes was acceptable also reported that waiting for results at the clinic was preferable than waiting at home. Other participants found the additional 30-minute wait time acceptable when they perceived themselves at risk for infection. Patients who did not find this added wait time acceptable reported that they had not had any problems with the previous wait times and that an additional 30-minute wait was too time consuming. This study highlights the importance of understanding different patient perspectives when making adoption decisions for new clinic strategies.
Multiple studies have evaluated the real-world application of NAAT POC testing and its impact on clinic flow and patients' willingness to wait for results. Stime et al.15 conducted a time in motion study at the Prince Cyril Zulu Communicable Disease Centre (PCZ CDC) in Durban, South Africa, to evaluate clinic flow after implementing POC testing for STIs. Implementing the NAAT POC test resulted in a mean additional waiting time of 2 hours and 49 minutes, for a total of 4 hours and 56 minutes in clinic. Staffing shortages and lack of clinic space for all the doctors needed to see patients led to additional wait times when implementing this program. Providers interviewed in this study noted the importance of POC tests being implemented in an effective manner, stating that “It's important that POC tests are actually implemented as POC tests. If it ends up taking 24 hours to get results, then it makes no difference if the test is done at the PCZ CDC or at the central lab.” Overall, this study highlights that, although POC tests can be efficient and acceptable to patients, additional resources need to be allocated to improving patient flow and care models to maximize the impact of these diagnostics and reduce time to results and treatment.
In the United Kingdom, Harding-Esch et al.21 implemented a sample-first POC approach using the GeneXpert assay in a sexual health clinic. Seventy patients provided samples on arrival before clinician consultation. Of the 70 patients, only 15 (21%) received their results before leaving clinic, although the mean wait time beyond the end of the routine visit was only 46 minutes. Twenty-four of 70 participants completed a survey and more than 90% of participants were willing to give samples upon arrival and liked the idea of receiving tests results at the same clinical visit. The survey also found that participants were willing to wait 1 to 2 hours to receive clinical services but unwilling to wait more than 2 hours. Furthermore, even for those patients who did not choose to wait in clinic for their results, the mean time to treatment was reduced from 10 to 2 days after visit, resulting in shorter duration of infection, and potentially reducing transmission to sexual partners. Overall, a sample-first approach can be beneficial in decreasing the time between sample collection, diagnosis, and treatment, as patients provide their samples before they see their doctor, allowing their specimen to be tested while they undergo the rest of their visit.
Finally, in the United States, Gettinger and colleagues used the binx health io assay, a 30-minute test, and sample-first approach that minimized wait times, as the sample testing began before patient interaction with a clinical provider.26 In this highly efficient university student health clinic, waiting for results added an average of 11 minutes to the total clinical visit. In contrast to the Harding-Esch findings, in this study, 83% of the 108 participants were willing to wait for their results. For 22% of the participants, results were available by the end of the clinic visit, adding no additional wait times. Wait times ranged from 2 to 68 minutes, and longer wait times were usually associated with waiting for instrument availability as only a single instrument was available in this study. Overall, a sample-first approach can be highly efficient in a university setting and provide patients with timely results. However, availability of instruments can add to additional wait times for patients.
Treatment Accuracy
Antimicrobial Stewardship (Factors Related to Motivation at the Health Care System Level)
As antimicrobial resistance (AMR) in gonorrhea continues to rise, it becomes imperative to implement STI screening programs that can detect resistant strains for accurate treatment. Implementing diagnostic tools that can detect resistant gonorrhea strains or verify that only wild-type (susceptible) strains are present, can improve treatment accuracy and lower the chance of resistant strains being transmitted to other people. However, testing for resistance can add additional wait time. Fuller et al. found that, although participants were excited about the potential of NAAT POC testing, they had mixed feelings about waiting an additional 30 minutes for reflex testing for AMR detection and that the wait time would only be acceptable in specific circumstances and when patients felt at risk for specific infections.20
Even in the absence of AMR marker testing, confirming the presence or absence of specific pathogens can lead to improved treatment accuracy, which support antimicrobial stewardship efforts by reducing unnecessary treatment. Fisk et al.24 found that in the 90-day implementation study using the GeneXpert assay, appropriate treatment increased from 52% to 100% when compared with using traditional urogenital chlamydia and gonorrhea laboratory-based testing and treatment. This NAAT POC program provided results in 90 minutes and reduced both overtreatment and undertreatment of chlamydia and gonorrhea infections. Similarly, the Harding-Esch et al.21 study found that utilization of a POC assay for CT/NG changed the presumptive treatment decision for 20% of those who waited for their results by avoiding unnecessary treatment.21 Therefore, at the health care system level, implementing AMR marker testing or NAAT POC testing can help relieve issues related to undertreatment and overtreatment of chlamydia and gonorrhea infections, which can help in improving antimicrobial stewardship.
Testing Attitudes
Patient Attitudes (Factors Related to Motivation at the Patient Level)
When implementing new programs, there is a need for patient buy-in and acceptance of new STI testing and treatment services. For self-sampling programs, McDonagh et al.19 interviewed 28 people aged 16 to 24 years in the United Kingdom. Participants noted several barriers to testing including physical capability, psychological capabilities, reflective motivation, automatic motivation, physical opportunity, and social opportunity. Participants from this study along with those from the Barbee et al. and Tat et al. found self-sampling to be acceptable and helped to alleviate some of the barriers and discomfort related to STI testing.19,22,23 However, to address additional patient concerns and increase testing among youth and young adults, there is a need to improve STI education and knowledge, as McDonagh et al. found that testing was not a high priority for many, and some were unaware about where to access testing services.19
Patient Characteristics (Factors Related to Motivation at the Patient Level)
Certain patient characteristics can have an impact on willingness to participate in new STI service programs and wait for results. Fuller at al. found that patients who were more experienced in receiving health services, such as frequent health screening, living with a chronic condition, or having a medical background were more prone to accept an additional 30-minute wait time, as they could compare this experience with other health care service experiences.20 In addition, this study saw that patients who felt they were at risk for an STI were willing to wait for results. Therefore, clinics will need to evaluate programs based on patient needs and characteristics and implement programs suited for their patient populations.
Provider Attitudes (Factors Related to Motivation at the Provider Level)
Similar to patients, provider attitudes related to STI testing and treatment services can impact the success of a program. For example, Chadwick et al.16 found that asking physicians to alter their individual practices by identifying patients to screen for STI testing, led to a decline in testing rates and negative physician feedback. However, shifting testing responsibilities to clinic nursing and administrative staff helped to increase testing, as they were better positioned to identify patients who presented with GU complaints that would be eligible for testing at their walk-in clinics. Therefore, at the provider level, when implementing a new STI program, it is important to identify clinical and administrative staff that have the time, resources, and expertise to support testing and treatment efforts.
Provider Education and Training (Factors That Can Impact Capability at the Provider Level)
When implementing new STI screening programs, in real-world settings, there can be difficulties for administrative and clinical staff in adhering to research protocols, as the information in these protocols can often be too general and lack information or tips when implementing programs in different settings. Allison et al.18 evaluated why an education intervention did not result in an increase in chlamydia screening in a general practice setting. They found that lack of time and resources, such as not having complete chlamydia kits or condoms in clinicians' rooms, and competing patient concerns, were barriers to implementing this education program where providers discussed chlamydia and chlamydia testing during a clinic visit. In addition, lack of privacy in the reception area served as a barrier for nonclinical staff in discussing chlamydia testing with patients. Therefore, although many of these studies offer solutions in improving or implementing new STI screening programs, there is a need to alter protocols when these programs are implemented in different clinical settings in order for them to be effective (Table 1).
TABLE 1.
Summary of Included Studies
| Authors (Year) | Country | Clinic Type | Revised Clinic Flow | Improved Technologies | Treatment Accuracy | Testing Attitudes |
|---|---|---|---|---|---|---|
| Allison et al. (2017)18 | UK | General practice | X | |||
| Barbee et al. (2016)22 | US | HIV care clinic | X | X | ||
| Chadwick et al. (2018)16 | CA | Walk-in clinic | X | X | ||
| Chow et al. (2018)14 | Australia | Sexual health clinic | X | |||
| Fisk et al. (2020)24 | US | Urgent care | X | X | ||
| Fuller et al. (2019)20 | UK | Sexual health clinic | X | X | X | |
| Gettinger et al. (2020)26 | US | University clinic | X | |||
| Gratrix et al. (2017)17 | CA | STI clinic | X | X | ||
| Harding-Esch et al. (2016)21 | UK | Sexual health clinic | X | X | ||
| Karas et al. (2016)25 | US | Primary care office | X | |||
| Keizur et al. (2016)27 | US | Multiple clinics | X | |||
| McDonagh et al. (2020)19 | UK | General practice | X | |||
| Stime et al. (2018)15 | SA | Urban infectious disease clinic | X | |||
| Tat et al. (2018)23 | US | HIV care clinic | X | X |
DISCUSSION
Although STI rates continue to rise every year, sexual health clinics continue to be less available because of financial constraints, pushing people to seek care at urgent care centers and other general health care clinics that are not always equipped to handle the increasing demand of STI testing and treatment services.6 To meet this demand, some clinics have started to implement STI screening programs that can easily be embedded in current clinical practices, including test-and-go services, NAAT POC testing, and sample-first approaches. These novel screening program can also help to address the suboptimal screening rates, where chlamydia screening is estimated to be lower than 50% in the private managed care sector.1 This review has provided evidence that the implementation of these programs can be successful in diagnosing and treating STI infections in a clinical setting. In addition, the use of behavior change theories such as the COM-B model allows for the identification of barriers and facilitators in implementing STI screening programs in a clinical setting at the health care systems, provider, and patient levels. Overall, these studies provide examples of how to implement novel STI screening programs that can be adapted by other health care clinics needing to meet the growing demand of providing STI services.
Conceptual Model and Implications
By using the COM-B model, we were able to examine how factors related to capability, motivation, and opportunity interact to change behaviors necessary in adopting new screening programs at the health care system, provider, and patient levels. The use of electronic health systems, updates in sample collection, and provider education were all factors related to the capability of health care systems, providers, and patients. The use of electronic health systems can be implemented at the health care system level and remind providers about when an STI test is needed for a patient. Using different methods in collecting samples, such as the self-sampling program, can provide patients with the tools necessary in collecting their specimen while also saving clinicians time in having to collect samples.22,23
Factors related to opportunities for adopting new STI screening programs included the utilization of electronic health systems and financial considerations at the health care systems level. Electronic health records can serve as resources at the health care systems level by reminding providers about testing protocols and running algorithms to determine which patients need testing during their current visit. New screening technologies such as NAATs have also been shown to be cost effective by generating additional revenue for practices. Furthermore, because some of these tests can be performed on-site and provide results within 30 to 90 minutes, patients can receive results and treatment within a single visit, thus eliminating of the cost burden related to multiple clinic visits.24 The ability to test and treat at a single visit is not only cost-saving but also impacts the provider and the patient by improving their experiences and streamlining activities.
Treatment accuracy is an important motivator for adoption of new strategies because of the need to reduce the development of antibiotic-resistant gonorrhea. In addition, attitudes about new screening programs can affect providers drive to implement new methods. Programs that use both clinical and administrative staff to identify patients eligible for testing are beneficial in improving testing attitudes among providers by alleviating testing burden that often falls on them.16 At the patient level, new screening approaches that lower diagnostic running time and decrease the time between receiving results and treatment can help to improve patient motivation to seek testing.15,20,21,26 When implementing new screening processes, patient characteristics have a large effect on acceptance and willingness to seek testing. Studies in this review showed that self-sampling is generally acceptable to patients.19,22,23 However, patient characteristics such as previous interactions with the health care system were shown to affect willingness to wait for results during a visit. Therefore, it is important continue to evaluate the acceptability of these programs among patients and providers to understand how their willingness to promote and use new services impacts adoption and utilization new screening strategies.
Overall, the cost of STIs places a large strain on all health care systems. To reduce these costs, decrease the burden of STIs, and improve people's sexual health, there is a need for novel STI screening approaches. This review has provided examples of screening strategies implemented in multiple clinical settings and can provide guidance to those looking to adopt new testing and treatment services. When implementing new programs, it is critical to consider the population being served. Regardless of the setting where the program is being implemented, there is a need for strong evaluation reports from these programs to inform the STI control community about what has been successful and what needs further adjustments at the health care system, provider, and patient levels.
Footnotes
Conflict of Interest and Sources of Funding: Dr Van Der Pol receives research support to her institution from the following: Abbott Molecular, BD Diagnostics, BioFire Diagnostics, Hologic, Rheonix, Roche Diagnostics, and SpeeDx. For remaining authors, no potential conflicts were declared. The authors received no financial support for the research, authorship, or publication of this article.
Contributor Information
Alison Footman, Email: afootman@uab.edu;afootman@gmail.com.
Dorris Dagama, Email: ddagama@uab.edu.
Catherine Hogan Smith, Email: khogan@uab.edu.
REFERENCES
- 1.Centers for Disease Control and Prevention . Sexually Transmitted Disease Surveillance 2018. Atlanta, GA: US Department of Health and Human Services; 2019. [Google Scholar]
- 2.Rowley J Vander Hoorn S Korenromp E, et al. Global and regional estimates of the prevalence and incidence of four curable sexually transmitted infections in 2016. WHO Bulletin. 2019. Available at: https://www.who.int/bulletin/volumes/97/8/18-228486.pdf. Accessed January 10, 2021. [Google Scholar]
- 3.Owusu-Edusei K Jr. Chesson HW Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013; 40:197–201. [DOI] [PubMed] [Google Scholar]
- 4.Workowski KA, Bolan GA. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR. Recommendations and reports. Morb Mortal Wkly Rep Recommend Rep 2015; 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 5.Golden MR, Kerndt PR. Improving clinical operations: can we and should we save our STD clinics? Sex Transm Dis 2010; 37:264–265. [DOI] [PubMed] [Google Scholar]
- 6.Pearson WS Tao G Kroeger K, et al. Increase in urgent care center visits for sexually transmitted infections, United States, 2010–2014. Emerg Infect Dis 2017; 23:367–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schechter SB Romo DL Cohall AT, et al. Approach to human immunodeficiency virus/sexually transmitted infection testing for men at an urban urgent care center. Sex Transm Dis 2017; 44:255–259. [DOI] [PubMed] [Google Scholar]
- 8.Weinick RM, Bristol SJ, DesRoches CM. Urgent care centers in the U.S.: Findings from a national survey. BMC Health Serv Res 2009; 9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rönn MM Tuite AR Menzies NA, et al. The impact of screening and partner notification on chlamydia in the United States, 2000 to 2015: Evaluation of epidemiological trends using a pair-formation transmission model. Am J Epidemiol 2019; 188:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cane J, O'Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012; 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonagh LK Saunders JM Cassell J, et al. Application of the COM-B model to barriers and facilitators to chlamydia testing in general practice for young people and primary care practitioners: A systematic review. Implement Sci 2018; 13:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michie S, Atkins L, West R. The Behaviour Change Wheel. A Guide to Designing Interventions 1st ed. Great Britain: Silverback Publishing, 2014:1003–1010. [Google Scholar]
- 13.Michie S. Implementation science: Understanding behaviour change and maintenance. BMC Health Serv Res 2014; 14(Suppl 2):O9. [Google Scholar]
- 14.Chow EPF Fortune R Dobinson S, et al. Evaluation of the implementation of a new nurse-led express “test-and-go” human immunodeficiency virus/sexually transmitted infection testing service for men who have sex with men at a sexual health center in Melbourne, Australia. Sex Transm Dis 2018; 45:429–434. [DOI] [PubMed] [Google Scholar]
- 15.Stime KJ Garrett N Sookrajh Y, et al. Clinic flow for STI, HIV, and TB patients in an urban infectious disease clinic offering point-of-care testing services in Durban, South Africa. BMC Health Serv Res 2018; 18:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chadwick RC McGregor K Sneath P, et al. STI initiative: Improving testing for sexually transmitted infections in women. BMJ Open Qual 2018; 7:e000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gratrix J Bergman J Brandley J, et al. Impact of introducing triage criteria for express testing at a canadian sexually transmitted infection clinic. Sex Transm Dis 2015; 42:660–663. [DOI] [PubMed] [Google Scholar]
- 18.Allison R Lecky DM Town K, et al. Exploring why a complex intervention piloted in general practices did not result in an increase in chlamydia screening and diagnosis: A qualitative evaluation using the fidelity of implementation model. BMC Fam Pract 2017; 18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonagh LK Harwood H Saunders JM, et al. How to increase chlamydia testing in primary care: A qualitative exploration with young people and application of a meta-theoretical model. Sex Transm Infect 2020; 96:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuller SS Pacho A Broad CE, et al. “It's not a time spent issue, it's a ‘what have you spent your time doing?’ issue…” A qualitative study of UK patient opinions and expectations for implementation of point of care tests for sexually transmitted infections and antimicrobial resistance. PLoS One 2019; 14:e0215380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding-Esch EM Nori AV Hegazi A, et al. Impact of deploying multiple point-of-care tests with a ‘sample first’ approach on a sexual health clinical care pathway. A service evaluation. Sex Transm Infect 2017; 93:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbee LA Tat S Dhanireddy S, et al. Implementation and operational research: Effectiveness and patient acceptability of a sexually transmitted infection self-testing program in an HIV care setting. J Acquir Immune Defic Syndr 2016; 72:e26–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tat S Dhanireddy S Marrazzo JM, et al. Health care provider perceptions of a sexually transmitted infection self-testing program in an HIV care clinic. Sex Transm Dis 2018; 45:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisk KM Derouin A Holm G, et al. Getting it right: The impact of point-of-care testing for gonorrhea and chlamydia in the urgent care setting. J Nurse Pract 2020; 16:388–393. [Google Scholar]
- 25.Karas D Sondike S Fitzgibbon J, et al. Using a clinical decision support tool to increase chlamydia screening across a large primary care pediatric network. Clin Pediatr (Phila) 2018; 57:1638–1641. [DOI] [PubMed] [Google Scholar]
- 26.Gettinger J Van Wagoner N Daniels B, et al. Patients are willing to wait for rapid sexually transmitted infection results in a university student health clinic. Sex Transm Dis 2020; 47:67–69. [DOI] [PubMed] [Google Scholar]
- 27.Keizur EM Goldbeck C Vavala G, et al. Safety and effectiveness of same-day Chlamydia trachomatis and Neisseria gonorrhoeae screening and treatment among gay, bisexual, transgender, and homeless youth in Los Angeles, California, and New Orleans, Louisiana. Sex Transm Dis 2020; 47:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geiger R Smith DM Little SJ, et al. Validation of the GeneXpert(R) CT/NG Assay for use with male pharyngeal and rectal swabs. Austin J HIV AIDS Res 2016; 3:1021. [PMC free article] [PubMed] [Google Scholar]
- 29.Chernesky MA Martin DH Hook EW, et al. Ability of new APTIMA CT and APTIMA GC assays to detect Chlamydia trachomatis and Neisseria gonorrhoeae in male urine and urethral swabs. J Clin Microbiol 2005; 43:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widdice LE Hsieh Y-H Silver B, et al. Performance of the Atlas Genetics Rapid Test for Chlamydia trachomatis and women's attitudes toward point-of-care testing. Sex Transm Dis 2018; 45:723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]


